Abstract
Mismatch repair (MMR) of replication errors requires DNA ends that can direct repair to the newly synthesized strand containing the error. For all but those organisms that use adenine methylation to generate nicks, the source of these ends in vivo is unknown. One possibility is that MMR may have a “special relation to the replication complex” [Wagner R, Jr., Meselson M (1976) Proc Natl Acad Sci USA 73:4135–4139], perhaps one that allows 5′ or 3′ DNA ends associated with replication to act as strand discrimination signals. Here we examine this hypothesis, based on the logic that errors made by yeast DNA polymerase α (Pol α), which initiates Okazaki fragments during lagging-strand replication, will always be closer to a 5′ end than will be more internal errors generated by DNA polymerase δ (Pol δ), which takes over for Pol α to complete lagging-strand replication. When we compared MMR efficiency for errors made by variant forms of these two polymerases, Msh2-dependent repair efficiencies for mismatches made by Pol α were consistently higher than for those same mismatches when made by Pol δ. Thus, one special relationship between MMR and replication is that MMR is more efficient for the least accurate of the major replicative polymerases, exonuclease-deficient Pol α. This observation is consistent with the close proximity and possible use of 5′ ends of Okazaki fragments for strand discrimination, which could increase the probability of Msh2-dependent MMR by 5′ excision, by a Msh2-dependent strand displacement mechanism, or both.
Keywords: replication fork, DNA repair, infidelity, mutagenesis, base substitutions
Various types of DNA repair, including base excision repair, nucleotide excision repair, double-strand DNA break repair, and mismatch repair (MMR), are differentiated into subpathways that can be distinguished by substrate specificity and enzymology (1). This is particularly true for DNA mismatch repair (reviewed in refs. 2–6), which can involve any of several different protein complexes at sequential steps in the pathway. For example, repair of mismatches generated during eukaryotic DNA replication is initiated upon mismatch binding by Msh2-Msh6 or Msh2-Msh3, and subsequent steps can involve three different MutL heterodimers (reviewed in ref. 5). Starting from a nick in the nascent DNA strand, mismatches can be removed by excision. Recent evidence suggests that mismatches may also sometimes be removed by a strand displacement mechanism (7). In either case, removal of the mismatch requires a DNA strand discontinuity. Studies in vitro demonstrate that this discontinuity can be a nick located either 3′ or 5′ to the mismatch, with the protein requirements for MMR differing somewhat depending on the location of the nick relative to the mismatch (see refs. 2 and 7 and references therein).
Early experiments in Escherichia coli revealed that one signal that directs MMR to the nascent strand is transient under-methylation of adenine in GATC sequences, allowing MutH endonuclease to nick that strand to direct MMR. However, many other bacteria and eukaryotes do not methylate adenine and lack MutH, such that strand discrimination depends on other, yet to be determined signals. One general possibility for signaling was suggested over 30 years ago by Wagner and Meselson (8), who wrote that mismatch repair may occur with particularly high efficiency on the newly synthesized DNA strand because of “a special relation to the replication complex.” Since that time, it has become clear that replication and MMR use many of the same proteins. This finding includes functionally asymmetric sliding clamps [i.e., the bacterial β-clamp and eukaryotic proliferating cell nuclear antigen (PCNA)]. These clamps stimulate processive DNA synthesis, and PCNA is also required for early steps in MMR that precede resynthesis of the nascent strand [(9) and also see ref. 10]. Sliding clamp involvement in replication and MMR led to the suggestion that these two processes may be coupled (9), such that the 3′ ends of the growing DNA chains at the replication fork (11, 12) and the numerous 5′ ends of Okazaki fragments present during lagging-strand replication (13), may act in coordination with clamps to direct MMR to the nascent strand (see ref. 3 for further discussion).
The possibility that replication and MMR may be coupled implies that an understanding of the contribution of MMR to replication fidelity would be enhanced by examining repair of mismatches actually generated by replicative DNA polymerases and emerging from a replication fork in a cell. Doing so is complicated by the fact that at least three different DNA polymerases generate errors at the eukaryotic replication fork (14). Nuclear DNA replication is initiated at origins when the RNA primase activity associated with Pol α synthesizes RNA primers of ∼10 bases. Pol α then extends these primers by incorporating about 20 to 30 deoxynucleotides. Recent evidence [(15, 16) and reviewed in ref. 17] suggests that once replication is initiated at origins, the leading-strand template in Saccharomyces cerevisiae is primarily copied by highly processive Pol ε. The lagging-strand template is copied slightly thereafter and discontinuously, as a series of Okazaki fragments of ∼200–300 base pairs. Each Okazaki fragment is initiated by Pol α-primase, again involving incorporation of about 10 ribonucleotides plus 20 to 30 deoxynucleotides, representing perhaps 10% of lagging-strand replication. Recent evidence in S. cerevisiae (16, 17) suggests that after Okazaki fragments are initiated, Pol δ has a major role in lagging-strand synthesis.
Pol ε and Pol δ both harbor intrinsic proofreading exonucleases that act in series with MMR (18–21) to enhance genome stability. The mutation rates of yeast strains harboring mutator alleles of Pol α are also synergistically increased upon inactivation of MMR (22, 23), demonstrating that MMR corrects replication errors made by all three replicative polymerases. Here we examine whether the efficiency of MMR depends on which of the two lagging-strand DNA polymerases generates the error. If the 5′ end of an Okazaki fragment can serve as a signal for MMR, then Pol α errors may be corrected by MMR more efficiently than Pol δ errors, because the former are closer to the 5′ end. If so, this would support a second special relationship between MMR and replication suggested by early studies of MMR in E. coli (24–26): namely, that MMR has evolved to most efficiently correct mismatches that most often emerge from the replication fork. In eukaryotes, these are mismatches made by exonuclease-deficient Pol α, which is less accurate than exonuclease-proficient Pol δ.
Here we examine these ideas using yeast strains with polymerase alleles (pol1-L868M and pol3-L612M) that have elevated spontaneous mutation rates, thereby identifying the polymerase responsible for generating the lagging-strand replication error in vivo (16, 22, 23). MMR efficiency is determined by comparing specific base substitution mutation rates in MMR-proficient yeast strains to those that lack MMR because of inactivation of MSH2. We find that mismatches made by both lagging-strand polymerases are corrected with high efficiency that varies depending on the identity of the mismatch and the surrounding sequence context. These variations in MMR efficiency place some genomic sequences at higher-than-average risk of replicational mutagenesis. We also observe that L868M Pol α and L612M Pol δ can generate the same mismatch at the same genomic position. When this occurs, the mismatch is repaired with higher apparent efficiency if it is made by Pol α, compared to Pol δ. This observation supports the idea that MMR has evolved to most efficiently correct the most frequently generated replication errors, and is consistent with the hypothesis that the 5′ ends of Okazaki fragments may serve as signals to direct Msh2-dependent MMR to the newly synthesized strand in vivo.
Results
Mutation Rates and Specificity in Wild-Type, pol1-L868M, and pol3-L612M Strains.
We began by comparing spontaneous mutation rates in MSH2 and msh2Δ derivatives of yeast strains with wild-type DNA polymerases to strains with pol1-L868M (Pol α) or pol3-L612M (Pol δ) mutant alleles. Mutation rates were measured using the URA3 reporter gene present in either of two orientations, in close proximity to ARS306, a frequently used, early-firing replication origin on chromosome III. Compared to strains expressing wild-type polymerases (Table 1, Top), the pol1-L868M strains (Table 1, Middle and ref. 22) and the pol3-L612M strains (Table 1, Bottom, reproduced from ref. 16) have elevated spontaneous mutation rates (irrespective of the orientation of the URA3 reporter), both when the strains are MMR-proficient (MSH2) and when they are defective (msh2Δ) for MMR. When independent ura3 mutants collected from each of the 12 different strains were sequenced, the majority contained transitions, transversions, or single-base deletions (Table 1). The mutations were distributed throughout the URA3 ORF (Figs. S1–S3), and the distributions differed depending on the polymerase allele, the location of the mutation, and the URA3 orientation (OR1 spectrum above the coding strand, OR2 spectrum below). These results were used to calculate mutation rates for single-base mutations (Figs. 1 and 2).
Table 1.
Mutation rates and sequencing data for the yeast strains used in this study
| URA3 orientation | OR1 | OR2 | OR1 | OR2 |
| Strain | Wild Type | msh2Δ | ||
| Mutation rate (×10−7) | 0.32 | 0.47 | 24 | 29 |
| 95% CI | 0.20–0.51 | 0.37–0.51 | 12–38 | 19–62 |
| ura3 mutants sequenced | 191 | 239 | 180 | 181 |
| Transitions | 47 | 44 | 38 | 20 |
| Transversions | 72 | 93 | 17 | 39 |
| Single base deletions | 11 | 18 | 100 | 94 |
| Others* | 9 | 15 | 13 | 17 |
| Strain | pol1-L868M | pol1-L868M msh2Δ | ||
| Mutation rate (×10−7) | 1.7 | 3.1 | 1,000 | 920 |
| 95% CI | 1.2–4.9 | 1.8–7.6 | 960–2,100 | 800–1,600 |
| ura3 mutants sequenced | 175 | 185 | 170 | 167 |
| Transitions | 52 | 26 | 125 | 53 |
| Transversions | 36 | 89 | 27 | 101 |
| Single base deletions | 12 | 6 | 18 | 12 |
| Others* | 6 | 8 | 0 | 6 |
| Strain | pol3-L612M | pol3-L612M msh2Δ | ||
| Mutation rate (×10−7) | 2.7 | 3.3 | 560 | 510 |
| 95% CI | 1.9–7.3 | 2.9–3.9 | 500–750 | 360–790 |
| ura3 mutants sequenced | 217 | 196 | 174 | 168 |
| Transitions | 97 | 76 | 85 | 57 |
| Transversions | 28 | 63 | 4 | 19 |
| Single base deletions | 17 | 24 | 77 | 86 |
| Others* | 8 | 2 | 8 | 4 |
*Other mutations include single-base additions (indicated as closed triangles in Figs. S1–S3) and mutations involving multiple bases (deletions, duplications and complex mutations; Table S1). A number of 5-FOA-resistant mutants had no sequence change in the 804 base pair URA3 ORF. These mutants were not investigated further, but they may result from epigenetic silencing, they may contain sequence changes in the promoter or 3′ untranslated region of URA3, or they may contain mutations in other genes that result in 5-FOA resistance.
Fig. 1.
Mutation rates and MMR efficiencies for total transitions and transversions. (A) Mutation rates for transitions and transversions are shown for MSH2 (white bars, scale on left axis) and msh2Δ (black bars, scale on right axis in white font on black background) strains that harbor wild-type polymerase genes (WT), or pol1-L868M (α) or pol3-L612M (δ) alleles. (B) MMR efficiencies (determined by dividing the msh2Δ mutation rate by the MSH2 mutation rate) for mutations in A. The ratios of the MMR efficiencies for L868M Pol α mutations relative to L612M Pol δ mutations are shown at the bottom (α/δ).
Fig. 2.
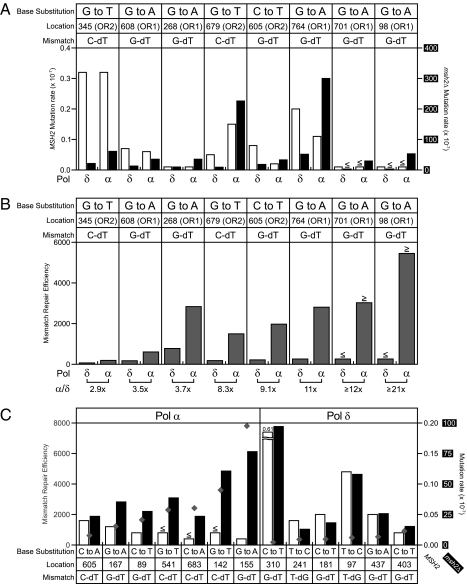
Mutation rates and MMR efficiencies for mutations at specific URA3 base pairs. (A) Mutation rates are displayed as described in the legend to Fig. 1A for the specific base substitutions indicated. For each base substitution, the URA3 base pair at which the mutation occurred, as well as the URA3 orientation in which that substitution was preferentially observed, is specified. Shown below each substitution is the inferred lagging-strand mismatch (template strand-incoming incorrect deoxynucleotide). (B) MMR efficiencies (determined by dividing the msh2Δ mutation rate by the MSH2 mutation rate) for mutations in A. The ratios of the MMR efficiencies for L868M Pol α mutations relative to L612M Pol δ mutations are shown at the bottom (α/δ). (C) Mutation rates are displayed for seven base substitutions primarily observed in the pol1-L868M strains (Pol α, Left) and six base substitutions primarily observed in the pol3-L612M strains (Pol δ, Right). Mutation rates for both MSH2 (white bars) and msh2Δ (black bars) strains are shown on the right axis in black font and white font on a black background, respectively. For each base substitution, the URA3 base pair at which the mutation occurred is indicated. Shown below each substitution is the inferred lagging-strand mismatch (template strand-incoming incorrect deoxynucleotide). MMR efficiencies (determined by dividing the msh2Δ mutation rate by the MSH2 mutation rate) are indicated as gray diamonds for each base substitution (scale in gray, Left). Note that MMR efficiencies for Pol α mutations at base pairs 541, 683 and 142 are greater than or equal to the values shown.
MMR Efficiency in Strains Expressing Wild-Type DNA Polymerases.
The strains that were wild type for polymerases and MMR exhibited the broadest distributions of mutations across URA3 (Fig. S1A). This finding is consistent with the idea that many different DNA transactions contribute to spontaneous mutagenesis (e.g., ref. 27 and references therein). In contrast, the spectra in the msh2Δ strains with wild-type polymerases were dominated by sequence changes at certain locations (Fig. S1B). The most obvious locations were the three longest runs of A•T base pairs, where single-base deletions were observed in both URA3 orientations. These hotspots are consistent with the role of MMR in stabilizing repetitive sequences that are prone to strand slippage and are inefficiently proofread during replication (reviewed in ref. 28). The spectra in the msh2Δ strains likely reflect replication errors that escape proofreading, but the identity of the polymerase(s) responsible for generating these mutations is uncertain. Even so, the mutation rates can be used to calculate the efficiency with which MMR corrects replication errors in strains with wild-type polymerases. From the data in Table 1, we calculate that the overall average mutation rates for single-base deletions in the msh2Δ strains are 820-fold higher in orientation 1 and 430-fold higher in orientation 2 than in the corresponding MSH2 strains, indicating that MMR corrects about 99.8% of all single-base insertion/deletion mismatches arising during replication of the URA3 gene. We also calculate that the overall rates of single base substitutions are 35-fold higher in the msh2Δ strains (both orientations) than in the MSH2 strains, indicating that MMR corrects at least 97% of single base mismatches generated by wild-type polymerases.
Base Substitution Errors Made by L868M Pol α and by L612M Pol δ.
Overall mutation rates (Table 1) and rates for transitions and transversions (Fig. 1A) were elevated in the strains with polymerase mutator alleles relative to strains with wild-type polymerases. This finding indicates that the majority of the ura3 mutants in the pol1-L868M and pol3-L612M strains are a result of inaccurate replication by L868M Pol α and L612M Pol δ, respectively. The pol1-L868M msh2Δ spectra (Fig. S2B) primarily contain single-base changes (336 of 353 events). Among these, 90% are base substitutions, mostly G•C to A•T (183 occurrences, 54%; predominantly in orientation 1) and G•C to T•A (88 occurrences, 26%; predominantly in orientation 2). In contrast, very few single-base deletions were observed (Table 1 and Fig. S2), especially in comparison with the single-base deletion hotspots at three runs of A•T base pairs observed in the pol3-L612M msh2Δ spectra (Fig. S3B). The proportion of single-base deletions in the pol1-L868M msh2Δ spectra is smaller than observed in studies of the error specificity of L868M Pol α in vitro (13). This paucity of single-base deletions in the pol1-L868M msh2Δ spectra led us to focus here on the efficiency of MMR of single-base substitutions. Single-base deletions made by Pol δ at the hotspots were analyzed earlier (16), and additional details of Pol δ deletion specificity at other locations will be presented elsewhere.
Average MMR Efficiency for Transition and Transversion Mismatches Made by Pol α and Pol δ.
Mutation rates for transitions and transversions were calculated in the pol1-L868M and pol3-L612M strains for both URA3 orientations (Fig. 1A). Dividing these rates in the msh2Δ strain by the corresponding rates in the MSH2 strain provides the apparent MMR efficiency (Fig. 1B). For example, in OR1 the rate of transitions in the pol1-L868M strain is 740 × 10−7 in the msh2Δ background and 0.51 × 10−7 in the MSH2 background (Fig. 1A), yielding a MMR correction factor of 1,500-fold (Fig. 1B). A similar calculation for OR1 transition mutations in the pol3-L612M strains yields a MMR correction factor of 220-fold. Thus, transition mismatches made by both lagging-strand polymerases are efficiently repaired, but those made by L868M Pol α are corrected 6.5-fold more efficiently than those made by L612M Pol δ. Measurements in OR2 strains confirm that transition mismatches made by L868M Pol α are corrected more efficiently than transition mismatches made by L612M Pol δ, in this case by 4.8-fold (Fig. 1B). Similar calculations reveal that transversion mismatches are corrected less efficiently than transition mismatches (Fig. 1B). Nonetheless, MMR efficiencies are 12-fold (OR1) and 6.9-fold (OR2) higher for transversion mismatches made by L868M Pol α as compared to those made by L612M Pol δ.
MMR of Site-Specific Mismatches Made by Pol α and Pol δ.
To eliminate the possibility that differences in mismatch composition or surrounding sequence context are responsible for the higher apparent MMR efficiency for Pol α errors, we compared MMR efficiencies for the same mismatch in the same sequence context. Pol α and Pol δ generated base substitutions at eight common locations in URA3 (Fig. 2 A and B, and Figs. S2 and S3). Six mutations were G•C to A•T transitions at base pairs 608, 268, 605, 764, 701, and 98, and two mutations were G•C to T•A transversions at base pairs 345 and 679. These substitutions were biased for one orientation over the other (Fig. 2 A and B, and Figs. S2 and S3). In each case, the orientation bias was the same for both Pol α and Pol δ, consistent with the proposal that both polymerases have primary roles in lagging-strand replication. In relation to the closest replication origin (ARS306), these preferred orientations imply that the responsible mismatches are G-dT (template-nascent base) for the G•C to A•T transitions, and C-dT for the G•C to T•A transversions (Fig. 2 A and B). Calculation of specific mutation rates (Fig. 2A) and MMR efficiencies (Fig. 2B) at these locations indicates that (i) unrepaired replication error rates for the same mismatch made by either polymerase vary by severalfold depending on location (Fig. 2A, black bars); (ii) MMR efficiency varies by severalfold for the same mismatch made by the same polymerase but at different locations (Fig. 2B); and (iii) MMR efficiencies for the same mismatch in the same sequence context are consistently higher (by 2.9- to 21-fold) for mismatches made by L868M Pol α as compared to those made by L612M Pol δ (Fig. 2B). These three interpretations are reinforced by a similar analysis of base substitutions observed multiple times at 13 other locations in URA3; 7 primarily observed in the Pol α spectra, and 6 primarily observed in the Pol δ spectra (Fig. 2C, and Figs. S2 and S3). Calculations of specific mutation rates and MMR efficiencies at these 13 locations support the trend of higher MMR efficiency (gray diamonds in Fig. 2C) for lagging-strand replication errors made by Pol α as compared to Pol δ.
Discussion
This study provides information on repair of mismatches made by, and emerging from, a replication fork in S. cerevisiae. The present focus on lagging-strand replication is partly based on studies (13, 29) suggesting that template 8-oxo-G•dA mismatches generated during lagging-strand replication are repaired more efficiently than 8-oxo-G•dA mismatches generated during leading-strand replication. We considered the idea that the high density of both 5′ ends of Okazaki fragments and PCNA present during lagging-strand replication might be used as strand discrimination signals for MMR. This hypothesis conforms to the suggestion that there might be a special relationship between MMR and the replication fork (8). The present study further develops this idea by comparing the MMR efficiency of errors made by Pol α close to the 5′ ends of Okazaki fragments to more internal errors generated by Pol δ as it continues lagging-strand replication.
The results reveal that one special relationship between replication and MMR is reciprocity, wherein the most frequently generated replication errors are those that are also the most efficiently corrected by MMR. First proposed based on studies of E. coli MMR (24–26), reciprocity is most clearly observed in eukaryotes as the frequent loss or gain of nucleotides in repetitive sequences (i.e., microsatellite instability, MSI), most notoriously in the genomes of tumors from persons with defective MMR (30). The mismatches responsible for MSI result from frequent DNA strand slippage and inefficient proofreading during replication of repetitive sequences (reviewed in ref. 28), leaving MMR as the major guardian against this type of genome instability. The present study reveals that one source of MSI is unrepaired lagging-strand replication errors, as manifest by the many single-base deletions in the L612M Pol δ spectra (Fig. S3B). The unexpectedly low proportion of single-base deletions in the L868M Pol α spectra suggests that Pol α could have a more restricted role in generating MSI, possibly because synthesis by Pol α is limited to initiating Okazaki fragments within sequences that are generally less repetitive, or because misalignments arising during initiation of Okazaki fragments are less stable than misalignments arising during the bulk of chain elongation. More novel here is the demonstration that MMR of base substitution mismatches is more efficient for errors made by Pol α than Pol δ. Pol α, which is naturally exonuclease-deficient, is ∼10- to 100-fold less accurate than Pol δ and Pol ε, both of which have proofreading exonucleases (31). Thus, eukaryotic MMR appears to have evolved to most efficiently repair errors made by the least accurate of the three major replicative polymerases.
The results further demonstrate that, on average, purine-pyrimidine mismatches that result in transitions are repaired more efficiently than purine-purine or pyrimidine-pyrimidine mismatches that result in transversions (Fig. 1B). MMR in E. coli exhibits a similar bias (26, 32, 33). This finding may partly reflect less efficient repair of mismatches arising during bypass of lesions in DNA (33). For example, the contribution of MutSα to repair of 8-oxo-G•dA mismatches in ogg1Δ yeast strains (13, 34) is typically less than 10-fold, substantially lower than the average efficiency of Msh2-dependent repair of spontaneous replication errors made by L868M Pol α and L612M Pol δ. An additional conclusion is that MMR efficiency is dependent on sequence context. For the same mismatch made by the same polymerase during replication of the same strand, site-to-site variations in MMR efficiency can differ by more than 10-fold: for example, for G-dT mismatches made by Pol α at base pairs 608 and 98 (Fig. 2B). Several explanations for this can be explored in the future, including the effects of flanking DNA sequences. For example, the efficiency with which E. coli repairs transversion mismatches in phage λ increases with increasing G•C content in neighboring nucleotides (32), and recognition of certain mismatches by MutSα is influenced by a six-nucleotide region surrounding the mismatch (35). It may be that flanking sequences influence eukaryotic MMR efficiency by modulating (i) mismatch binding by MutSα, which contacts several base pairs on either side of the mismatch (36); (ii) base stacking; and/or (iii) DNA flexibility, because MutSα-bound mismatched DNA is kinked, and because a transition between bent and unbent DNA may be critical for limiting MMR to processing of mismatched, as compared to matched, base pairs (37). Variations in MMR efficiency could also involve proteins that operate downstream of mismatch binding or reflect the timing of nucleosome reloading or chromatin remodeling behind the replication fork. A recent study reported that the presence of nucleosomes on mismatched DNA reduces Msh2-Msh6 function (38).
The fact that replication errors at some locations are repaired with lower efficiency than at others indicates that some sites in the yeast genome are at greater risk than others of mutagenesis due to replication infidelity, even when there is no genetic defect in MMR. Thus, in addition to increased mutagenesis resulting from mutations in MMR genes, from silencing Mlh1 expression, from imbalanced expression of Msh3 or Mlh1, from cadmium inhibition of MMR, and from saturation of MMR, this study indicates that the intrinsic inefficiency of MMR can also contribute to mutagenesis in vivo at certain locations.
As mentioned above, a previous study (13) suggested that template 8-oxo-G•dA mismatches generated during lagging-strand DNA replication in S. cerevisiae may be repaired more efficiently than 8-oxo-G•dA mismatches generated during leading-strand replication (although other models were also considered). More recently, a study using a different experimental approach has reported an asymmetry for the functions of yeast Msh2-Msh6 and Msh2-Msh3 on the leading and lagging strands (39). In light of those studies and the present study of lagging-strand errors, we have initiated studies to determine MMR efficiency for errors made by a mutator derivative of Pol ε, which is implicated in replicating the leading-strand template (15). Experiments are also underway to examine MMR efficiency in strains with defects in individual MutS heterodimers that may selectively inactivate MMR subpathways with differing substrate specificities.
During initiation of Okazaki fragments, Pol α synthesizes ∼20–30 nucleotides: that is, about 5% of the nuclear genome (Fig. 3). This is a substantial workload for a polymerase that cannot proofread its own errors and has an average error rate of ∼10−4 (40), which is increased approximately fivefold by the L868M substitution (23). To maintain genome stability when initiating ∼50,000 Okazaki fragments during replication of the yeast genome, Pol δ (but not Pol ε) has been suggested to “extrinsically” proofread errors made by Pol α (23, 41). Extrinsic proofreading may occur largely after Pol α incorporates enough deoxynucleotides (e.g., 5–10 nucleotides) to provide Pol δ with a fully DNA-DNA duplex, as Pol δ may not function well nearer to the 5′ end, where an RNA-DNA duplex initially exists (Fig. 3). Based on the reciprocity mentioned above, this may place a particularly heavy burden on the MMR machinery to protect the genome against Pol α replication infidelity near the 5′ ends of Okazaki fragments. Consistent with this idea, mutation rates in the pol1-L868M msh2Δ strains are even higher than those in the pol3-L612M msh2Δ strains (Table 1), despite the fact that Pol α is thought to have a much smaller workload than Pol δ in lagging-strand replication. The nonrandom distribution of unrepaired L868M Pol α mutations (Fig. S2B) may partly reflect locations in the URA3 gene where Pol α is more likely to initiate Okazaki fragments. Our attempts to identify a “signature” for Okazaki fragment initiation within these mutational spectra has not been informative, but further analyses are planned as additional information accumulates.
Fig. 3.
Possible explanations for DNA polymerase-dependent and position-dependent differences in MMR of lagging-strand replication errors. A lagging-strand Okazaki fragment is shown that contains a mismatch made by Pol α (red X) as it initiates DNA synthesis from an RNA primer, a more internal mismatch made by Pol α (purple X) as it nearly completes synthesis of 20 to 30 nucleotides, and a mismatch made by Pol δ (green X) after it takes over for Pol α to continue lagging-strand replication. See text for explanation of the possible contributions of proofreading and MMR to correcting these mismatches.
The higher repair efficiency for base-base mismatches made by Pol α as compared to Pol δ might be explained if L868M Pol α only functions during replication, whereas L612M Pol δ occasionally generates mismatches during DNA transactions that are not subject to MMR (e.g., during gap-filling synthesis required for repair of spontaneous lesions). The large differences in MMR efficiency between Pol α and Pol δ errors (Fig. 2B) disfavor this hypothesis, but it cannot be completely excluded. A second explanation is that the proximity of a Pol α error to a 5′ DNA end may result in higher efficiency for the general Msh2-dependent MMR machinery (implied by the gradient for “General MMR” increasing toward the 5′ end in Fig. 3), just as the efficiency of E. coli MMR is proportional to the distance between the mismatch and the strand discrimination signal (reviewed in ref. 2). This idea in turn implies that the 5′ ends of Okazaki fragments may indeed serve as a strand discrimination signal. It is also possible that mismatches made by both Pol α and Pol δ during lagging-strand replication are equally well repaired by the general Msh2-dependent MMR pathway, but that mismatches close to the 5′ ends of Okazaki fragments can also be repaired by an additional Msh2-dependent pathway that is specialized to protect the genome against particularly abundant replication errors made by Pol α (Fig. 3). This process could involve mismatch removal via strand displacement, a normal mode of synthesis required for maturation of Okazaki fragments (14, 42), but in this case involving Msh2. In fact, MMR via stand displacement synthesis has recently been observed using mammalian MMR proteins (7). As noted in that study, and in support of the importance of strand displacement to MMR, the modest mutator effect resulting from deletion of yeast Exo1, which is implicated in the excision step of general MMR, is synergistically increased by deleting Pol32 (43), which encodes a Pol δ accessory protein required for strand displacement synthesis of the type needed for maturation of the 5′ ends of Okazaki fragments. Hypothetically, a specialized MMR pathway could also involve exonucleolytic removal of the mismatch by a nuclease other than Exo1; for example, by the exonuclease activity of yeast Fen1 (Rad27/Rth1), a nuclease involved in Okazaki fragment maturation that has also been implicated in Msh2-Msh6-dependent MMR (44). Whether by excision or strand displacement, if mismatches close to the 5′ ends of Okazaki fragments are subject to repair by a specialized Msh2-dependent pathway, the efficiency of this pathway may be proportional to the distance between the 5′ end and the mismatch (Fig. 3, and also see ref. 3). Theoretically, this could explain why some errors (e.g., G-dT mismatch at base pair 98 or 701) (Fig. 2B) are repaired more efficiently if made by Pol α very near the 5′ end, whereas another, perhaps more internal error (e.g., G-dT mismatch at base pair 608) (Fig. 2B) is repaired only 3.5-fold more efficiently than for the same mismatch made by Pol δ.
Experimental Procedures
Strains, Mutation Rates, and Analysis of ura3 Mutants.
Strains were isogenic derivatives of strain Δl(-2)l-7B-YUNI300 (MATa CAN1 his7-2 leu2-Δ::kanMX ura3-Δ trp1-289 ade2-1 lys2-ΔGG2899-2900) (29). Introduction of the pol1-L868M and pol3-L612M mutations and manipulations of the resulting strains, including measurements of spontaneous mutation rates by fluctuation analysis using 12 independent spore colonies obtained by dissection of diploid strains, were as described previously (16). For each ura3 mutant that was sequenced, an independent colony was patched to YPDA and then replica plated to media containing 5-fluoro-orotic acid (5-FOA). Genomic DNA from a single 5-FOA-resistant colony from each patched spore colony was then isolated, and the ura3 gene was PCR-amplified and sequenced.
Statistical Analysis.
To identify locations in the ura3 spectra where base substitutions occurred at rates significantly higher than expected for a random distribution, obvious hotspots were first identified using the Dixon Q-test (45). This test indicated that locations at which 11 or more mutations were observed are obvious hotspots (P < 0.01). Subtracting these mutations and URA3 locations left different numbers of mutants in each spectra, in each case distributed over a known number of locations where the mutation being considered would result in resistance to 5-FOA (database available upon request). This information was used to determine which of the remaining locations where substitutions occurred should also be considered hotspots, using the following calculation. If errors occur at random, the binomial probability of observing x or more mutations at the same location when there are N errors distributed over L locations is:
 |
Calculations using this equation indicated that, depending on the spectrum, the substitution, and the URA3 location, two to four mutations were required to assign a hotspot with a probability of ≥ 0.05. When the appropriate number of substitutions was observed at a URA3 location in the pol1-L868M msh2Δ or pol3-L612M msh2Δ spectra (four events in all but one instance), site-specific MMR efficiencies were analyzed at these locations (shown in Fig. 2).
Supplementary Material
Acknowledgments
We thank Jana Stone and Zachary Pursell for contributing to the mutation spectra in the wild-type yeast strains, the National Institute of Environmental Health Sciences DNA Sequencing Core and Molecular Genetics Core for technical support, Dmitry Gordenin for advice, and Mercedes Arana and Scott Lujan for helpful comments on the manuscript. This work was supported by Project Z01 ES065089 (to T.A.K.) in the Division of Intramural Research of the National Institute of Environmental Health Sciences, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
See Commentary on page 20851.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1013048107/-/DCSupplemental.
References
- 1.Friedberg EC, et al. DNA Repair and Mutagenesis. 2nd Ed. Washington, D.C.: ASM Press; 2006. [Google Scholar]
- 2.Iyer RR, Pluciennik A, Burdett V, Modrich PL. DNA mismatch repair: Functions and mechanisms. Chem Rev. 2006;106:302–323. doi: 10.1021/cr0404794. [DOI] [PubMed] [Google Scholar]
- 3.Kunkel TA, Erie DA. DNA mismatch repair. Annu Rev Biochem. 2005;74:681–710. doi: 10.1146/annurev.biochem.74.082803.133243. [DOI] [PubMed] [Google Scholar]
- 4.Jiricny J. The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol. 2006;7:335–346. doi: 10.1038/nrm1907. [DOI] [PubMed] [Google Scholar]
- 5.Hsieh P, Yamane K. DNA mismatch repair: Molecular mechanism, cancer, and ageing. Mech Ageing Dev. 2008;129:391–407. doi: 10.1016/j.mad.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li GM. Mechanisms and functions of DNA mismatch repair. Cell Res. 2008;18:85–98. doi: 10.1038/cr.2007.115. [DOI] [PubMed] [Google Scholar]
- 7.Kadyrov FA, et al. A possible mechanism for exonuclease 1-independent eukaryotic mismatch repair. Proc Natl Acad Sci USA. 2009;106:8495–8500. doi: 10.1073/pnas.0903654106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner R, Jr, Meselson M. Repair tracts in mismatched DNA heteroduplexes. Proc Natl Acad Sci USA. 1976;73:4135–4139. doi: 10.1073/pnas.73.11.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Umar A, et al. Requirement for PCNA in DNA mismatch repair at a step preceding DNA resynthesis. Cell. 1996;87:65–73. doi: 10.1016/s0092-8674(00)81323-9. [DOI] [PubMed] [Google Scholar]
- 10.Johnson RE, et al. Evidence for involvement of yeast proliferating cell nuclear antigen in DNA mismatch repair. J Biol Chem. 1996;271:27987–27990. doi: 10.1074/jbc.271.45.27987. [DOI] [PubMed] [Google Scholar]
- 11.López de Saro FJ, O'Donnell M. Interaction of the beta sliding clamp with MutS, ligase, and DNA polymerase I. Proc Natl Acad Sci USA. 2001;98:8376–8380. doi: 10.1073/pnas.121009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleczkowska HE, Marra G, Lettieri T, Jiricny J. hMSH3 and hMSH6 interact with PCNA and colocalize with it to replication foci. Genes Dev. 2001;15:724–736. doi: 10.1101/gad.191201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pavlov YI, Mian IM, Kunkel TA. Evidence for preferential mismatch repair of lagging strand DNA replication errors in yeast. Curr Biol. 2003;13:744–748. doi: 10.1016/s0960-9822(03)00284-7. [DOI] [PubMed] [Google Scholar]
- 14.Burgers PM. Polymerase dynamics at the eukaryotic DNA replication fork. J Biol Chem. 2009;284:4041–4045. doi: 10.1074/jbc.R800062200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pursell ZF, Isoz I, Lundström EB, Johansson E, Kunkel TA. Yeast DNA polymerase epsilon participates in leading-strand DNA replication. Science. 2007;317:127–130. doi: 10.1126/science.1144067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nick McElhinny SA, Gordenin DA, Stith CM, Burgers PM, Kunkel TA. Division of labor at the eukaryotic replication fork. Mol Cell. 2008;30:137–144. doi: 10.1016/j.molcel.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunkel TA, Burgers PM. Dividing the workload at a eukaryotic replication fork. Trends Cell Biol. 2008;18:521–527. doi: 10.1016/j.tcb.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison A, Bell JB, Kunkel TA, Sugino A. Eukaryotic DNA polymerase amino acid sequence required for 3′–5′ exonuclease activity. Proc Natl Acad Sci USA. 1991;88:9473–9477. doi: 10.1073/pnas.88.21.9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrison A, Johnson AL, Johnston LH, Sugino A. Pathway correcting DNA replication errors in Saccharomyces cerevisiae. EMBO J. 1993;12:1467–1473. doi: 10.1002/j.1460-2075.1993.tb05790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrison A, Sugino A. The 3′—>5′ exonucleases of both DNA polymerases δ and ε participate in correcting errors of DNA replication in Saccharomyces cerevisiae. Mol Gen Genet. 1994;242:289–296. doi: 10.1007/BF00280418. [DOI] [PubMed] [Google Scholar]
- 21.Albertson TM, et al. DNA polymerase epsilon and delta proofreading suppress discrete mutator and cancer phenotypes in mice. Proc Natl Acad Sci USA. 2009;106:17101–17104. doi: 10.1073/pnas.0907147106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niimi A, et al. Palm mutants in DNA polymerases alpha and eta alter DNA replication fidelity and translesion activity. Mol Cell Biol. 2004;24:2734–2746. doi: 10.1128/MCB.24.7.2734-2746.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pavlov YI, et al. Evidence that errors made by DNA polymerase alpha are corrected by DNA polymerase delta. Curr Biol. 2006;16:202–207. doi: 10.1016/j.cub.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Kramer B, Kramer W, Fritz HJ. Different base/base mismatches are corrected with different efficiencies by the methyl-directed DNA mismatch-repair system of E. coli. Cell. 1984;38:879–887. doi: 10.1016/0092-8674(84)90283-6. [DOI] [PubMed] [Google Scholar]
- 25.Dohet C, Wagner R, Radman M. Repair of defined single base-pair mismatches in Escherichia coli. Proc Natl Acad Sci USA. 1985;82:503–505. doi: 10.1073/pnas.82.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaaper RM. Base selection, proofreading, and mismatch repair during DNA replication in Escherichia coli. J Biol Chem. 1993;268:23762–23765. [PubMed] [Google Scholar]
- 27.Drake JW. General antimutators are improbable. J Mol Biol. 1993;229:8–13. doi: 10.1006/jmbi.1993.1002. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Diaz M, Kunkel TA. Mechanism of a genetic glissando: Structural biology of indel mutations. Trends Biochem Sci. 2006;31:206–214. doi: 10.1016/j.tibs.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Pavlov YI, Newlon CS, Kunkel TA. Yeast origins establish a strand bias for replicational mutagenesis. Mol Cell. 2002;10:207–213. doi: 10.1016/s1097-2765(02)00567-1. [DOI] [PubMed] [Google Scholar]
- 30.Umar A, et al. Defective mismatch repair in extracts of colorectal and endometrial cancer cell lines exhibiting microsatellite instability. J Biol Chem. 1994;269:14367–14370. [PubMed] [Google Scholar]
- 31.Nick McElhinny SA, Pursell ZF, Kunkel TA. Mechanisms for High Fidelity DNA Replication. Cambridge, United Kingdom: Royal Society of Chemistry; 2009. pp. 86–111. [Google Scholar]
- 32.Jones M, Wagner R, Radman M. Repair of a mismatch is influenced by the base composition of the surrounding nucleotide sequence. Genetics. 1987;115:605–610. doi: 10.1093/genetics/115.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaaper RM, Dunn RL. Spontaneous mutation in the Escherichia coli lacI gene. Genetics. 1991;129:317–326. doi: 10.1093/genetics/129.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ni TT, Marsischky GT, Kolodner RD. MSH2 and MSH6 are required for removal of adenine misincorporated opposite 8-oxo-guanine in S. cerevisiae. Mol Cell. 1999;4:439–444. doi: 10.1016/s1097-2765(00)80346-9. [DOI] [PubMed] [Google Scholar]
- 35.Marsischky GT, Kolodner RD. Biochemical characterization of the interaction between the Saccharomyces cerevisiae MSH2-MSH6 complex and mispaired bases in DNA. J Biol Chem. 1999;274:26668–26682. doi: 10.1074/jbc.274.38.26668. [DOI] [PubMed] [Google Scholar]
- 36.Warren JJ, et al. Structure of the human MutSalpha DNA lesion recognition complex. Mol Cell. 2007;26:579–592. doi: 10.1016/j.molcel.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 37.Wang H, et al. DNA bending and unbending by MutS govern mismatch recognition and specificity. Proc Natl Acad Sci USA. 2003;100:14822–14827. doi: 10.1073/pnas.2433654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li F, Tian L, Gu L, Li GM. Evidence that nucleosomes inhibit mismatch repair in eukaryotic cells. J Biol Chem. 2009;284:33056–33061. doi: 10.1074/jbc.M109.049874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kow YW, Bao G, Reeves JW, Jinks-Robertson S, Crouse GF. Oligonucleotide transformation of yeast reveals mismatch repair complexes to be differentially active on DNA replication strands. Proc Natl Acad Sci USA. 2007;104:11352–11357. doi: 10.1073/pnas.0704695104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kunkel TA, Hamatake RK, Motto-Fox J, Fitzgerald MP, Sugino A. Fidelity of DNA polymerase I and the DNA polymerase I-DNA primase complex from Saccharomyces cerevisiae. Mol Cell Biol. 1989;9:4447–4458. doi: 10.1128/mcb.9.10.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nick McElhinny SA, Pavlov YI, Kunkel TA. Evidence for extrinsic exonucleolytic proofreading. Cell Cycle. 2006;5:958–962. doi: 10.4161/cc.5.9.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossi ML, Purohit V, Brandt PD, Bambara RA. Lagging strand replication proteins in genome stability and DNA repair. Chem Rev. 2006;106:453–473. doi: 10.1021/cr040497l. [DOI] [PubMed] [Google Scholar]
- 43.Amin NS, Nguyen MN, Oh S, Kolodner RD. exo1-Dependent mutator mutations: Model system for studying functional interactions in mismatch repair. Mol Cell Biol. 2001;21:5142–5155. doi: 10.1128/MCB.21.15.5142-5155.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson RE, Kovvali GK, Prakash L, Prakash S. Requirement of the yeast RTH1 5′ to 3′ exonuclease for the stability of simple repetitive DNA. Science. 1995;269:238–240. doi: 10.1126/science.7618086. [DOI] [PubMed] [Google Scholar]
- 45.Dixon WJ. Analysis of extreme values. Ann Math Stat. 1950;21:488–506. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.