Abstract
Two-component signal transduction mediates a wide range of phenotypes in microbes and plants. The sensor transmitter module controls the phosphorylation state of the cognate-response-regulator receiver domain. Whereas the two-component autokinase and phosphotransfer reactions are well-understood, the mechanism by which sensors accelerate the rate of phospho-response regulator dephosphorylation, termed “transmitter phosphatase activity,” is unknown. We identified a conserved DxxxQ motif adjacent to the phospho-accepting His residue in the HisKA_3 subfamily of two-component sensors. We used site-specific mutagenesis to make substitutions for these conserved Gln and Asp residues in the nitrate-responsive NarX sensor and analyzed function both in vivo and in vitro. Results show that the Gln residue is critical for transmitter phosphatase activity, but is not essential for autokinase or phosphotransfer activities. The documented role of an amide moiety in phosphoryl group hydrolysis suggests an analogous catalytic function for this Gln residue in HisKA_3 members. Results also indicate that the Asp residue is important for both autokinase and transmitter phosphatase activities. Furthermore, we noted that sensors of the HisKA subfamily exhibit an analogous E/DxxT/N motif, the conserved Thr residue of which is critical for transmitter phosphatase activity of the EnvZ sensor. Thus, two-component sensors likely use similar mechanisms for receiver domain dephosphorylation.
Keywords: histidine kinase, DHp domain, DesK
Two-component signal transduction systems, found in all three domains of life, are widespread signaling pathways in nature. Bacterial species have numerous, distinct two-component systems regulating physiological activities such as chemotaxis, central metabolism, environmental stress responses, virulence, and pathogenesis. Most systems consist of a sensor protein that exerts both positive and negative control on a cognate response regulator. Well-studied examples include nitrate regulation by NarX-NarL, general nitrogen regulation by NtrB-NtrC (also termed NRII-NRI), osmoregulation by EnvZ-OmpR (1), and membrane fluidity regulation by DesK-DesR (2).
Signal transduction involves phosphotransfer between a His residue on the transmitter module of the sensor and an Asp residue on the receiver domain of its cognate response regulator. Sensor positive function, which results in receiver phosphorylation, triggers a receiver conformational change that controls output, typically DNA binding. Sensor negative function, which results in phospho-receiver dephosphorylation, is essential not only for setting the baseline level of signal transduction (3, 4), but also for suppressing crosstalk between noncognate two-component systems (5). Therefore, the ratio between sensor positive and negative function determines the overall phospho-receiver concentration, and defects in either function result in aberrant output (1).
In a prototypical sensor, signal is transduced from an amino-terminal sensory module to a carboxyl-terminal transmitter module. Many sensors also have central PAS (Per, Arnt, Sim) or GAF (cGMP-specific phosphodiesterase, adenylyl cyclase, FhlA) domains between the sensory and transmitter modules. The transmitter module comprises an amino-terminal DHp (dimerization and histidine phosphotransfer) domain connected to a carboxyl-terminal CA (catalytic and ATP-binding) domain (6).
The transmitter module displays three distinct reactions (7, 8): (i) transmitter autokinase in response to input stimulus, (ii) phosphotransfer to the receiver domain, and (iii) dephosphorylation of the phospho-receiver domain. In transmitter autokinase, the invariant His residue on the DHp domain accepts the phosphoryl group from ATP bound to the CA domain. The phosphotransfer reaction involves receiver domain autophosphorylation, using the phospho-transmitter module as a high-affinity substrate (6).
The phospho-receiver catalyzes autodephosphorylation through in-line attack by a nucleophilic water molecule (9). In the absence of stimulus, most sensors accelerate this rate of phospho-receiver dephosphorylation. In stark contrast to the well-studied transmitter autokinase and phosphotransfer reactions, the mechanism of the transmitter-mediated phosphatase reaction is unknown. A prominent question is whether the sensor simply stimulates the intrinsic autodephosphorylation or catalyzes the phosphatase reaction. For simplicity, the sensor-mediated phosphatase reaction is termed “transmitter phosphatase activity” (10).
The well-studied NarX-NarL two-component system in Escherichia coli controls thetranscriptional response to nitrate, the preferred anaerobic respiratory oxidant. Genetic assays are established for NarX-positive and -negative functions. Biochemical assays for NarX transmitter activities also have been optimized. Finally, the overall features of signal transduction, including nitrate binding to the NarX periplasmic domain and transcriptional output by the NarL response regulator, are understood in detail. Therefore, NarX is a tractable model for analyzing sensor function (ref. 11 and references therein).
We identified conserved residues that are immediately adjacent to the phospho-accepting His in the NarX DHp domain, with the potential of mediating sensor negative function. Both genetic and biochemical data demonstrate the requirement of residues Gln-404 and Asp-400 for NarX transmitter phosphatase activity. Studies with chemotaxis auxiliary phosphatases and Ras-like GTPases have revealed that conserved Gln or Asn residues catalyze phosphoryl group hydrolysis by coordinating the nucleophilic water (12–14). We therefore propose that Gln-404 catalyzes this reaction in NarX and related sensors. This provides a mechanistic explanation for the previously unknown transmitter phosphatase function in two-component signal transduction.
Results
Sequence and Structural Analyses.
The Pfam database (15) delineates five DHp sequence subfamilies. The HisKA subfamily (pfam00512), exemplified by EnvZ and NtrB, comprises the majority of sequences, whereas the HisKA_3 subfamily (pfam07730), covering about 10% of DHp domain sequences (16), corresponds to the HPK7 transmitter subfamily of Grebe and Stock (17). Well-studied examples of HisKA_3 members include E. coli NarX; Mycobacterium tuberculosis DosS and DosT, which control dormancy (18); Staphylococcus aureus VraS, which mediates cell-envelope stress response (19); and Bacillus subtilis DesK with available transmitter module X-ray structures (2, 16).
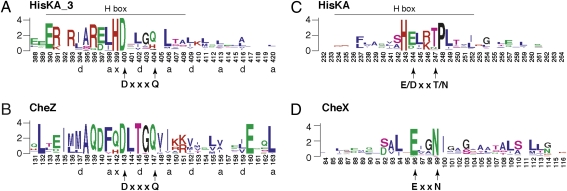
The DesK DHp domain is a dimeric four-helix antiparallel coiled-coil containing two helices from each subunit (2). The DHp domain sequences of NarX and other HisKA_3 family members are colinear, indicating that they share virtually identical structures. Sequence conservation for helix α1, which includes the H-box motif, is summarized in Fig. 1A. Heptad repeat patterns of hydrophobic residues define the coiled-coil structure (21). Immediately adjacent to the invariant His-399 (numbering according to NarX), which is the site of autophosphorylation, is the invariant Asp-400. Other conserved positions include charged residues in the amino-terminal portion that are involved in interactions with the CA domain (16). Residues in the carboxyl-terminal portion of helix α1, which are not conserved, determine interaction specificity with the cognate receiver domain (5). The α2 helix, which includes the variable X-box region (22), exhibits little sequence conservation beyond the heptad repeat pattern.
Fig. 1.
Sequence conservation for DHp and Che phosphatase helices. These sequence logos display the relative frequency and information content at each position in a collection of aligned sequences (20). Numbering is according to the (A) E. coli NarX (HisKA_3), (B) CheZ, (C) EnvZ (HisKA), and (D) B. burgdorferi CheX sequences. Hydrophobic residues that define coiled-coil heptad repeats in the HisKA_3 and CheZ sequences are denoted by lowercase letters; residues marked “x” represent “skip” positions (21). The H-box motif is indicated for the DHp sequences. The conserved DxxxQ, E/DxxT/N, and ExxN motifs are described in the text.
Examining HisKA_3 DHp domain sequence alignments, we recognized the conserved Gln-404 (NarX numbering), which is substituted by the structurally similar His in about 30% of the sequences or by Gly, Ala, or Ser in a small minority of sequences (Fig. 1A). Thus, Asp-400 and Gln-404 form an apparent DxxxQ motif.
The structure and sequence of the HisKA_3 DHp domains are related to those of the auxiliary phosphatase CheZ in chemotaxis. The catalytic core of CheZ adopts a four-helix bundle structure and interacts similarly with the cognate receiver domain (5, 23). More pertinently, CheZ sequences also feature a conserved DxxxQ motif (Fig. 1B) (23), the conserved Gln residue from which was previously shown to catalyze the auxiliary phosphatase reaction (12). The Asp and Gln residues in both HisKA_3 and CheZ motifs are one turn apart on their respective helical surfaces.
In the CheZ DxxxQ motif, the conserved Gln-147 residue inserts directly into the active site of the response regulator CheY, where it orients a water molecule for in-line nucleophilic attack on the acyl-phosphate. Residue Asp-143 stabilizes an invariant Lys residue in the CheY receiver domain (12, 23). By analogy, the HisKA_3 DxxxQ motif could be critical for transmitter phosphatase activity.
Positive and Negative Function Phenotypes.
NarX positive function, which reflects transmitter autokinase activity followed by phosphotransfer to NarL, is monitored in a narL+ Φ(narG-lacZ) gene fusion strain. Following the convention established for EnvZ and OmpR regulation (22), we denote positive-function phenotypes as K+ for near-wild type and as K− for deficient.
NarX-negative function, which reflects transmitter phosphatase activity, is monitored in a narL505 Φ(narG-lacZ) gene fusion strain. The narL505-encoded NarL (V88A) protein activates Φ(narG-lacZ) expression even in narX null strains (24). As NarL (V88A) binds efficiently only to DNA upon phosphorylation, Nar-independent mechanisms must exist to phosphorylate NarL (V88A). During growth in the absence of nitrate, the narX+ allele lowers Φ(narG-lacZ) expression in narL505 strains, reflecting NarX-negative activity. We denote negative-function phenotypes as P+ for near-wild type and as P– for deficient (22). Reporter strains for both negative- and positive-function assays carry a null allele of narQ, encoding the paralogous nitrate sensor (11).
In Vivo Phenotypes of Gln-404 Mutants.
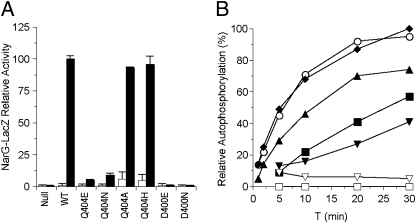
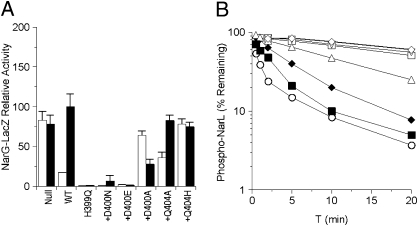
We introduced Ala, His, Asp, and Glu substitutions for Gln-404. Because Ala is small and conducive to helix formation and the other residues are structurally related to Gln, none of these substitutions was expected to disrupt helical packing and structure. The Q404A and Q404H mutants showed essentially wild-type levels of nitrate-induced Φ(narG-lacZ) expression (Fig. 2A; K+ phenotype). The Q404N and Q404E mutants exhibited readily detectable activities, about 5–10% of wild type, which we also denote as K+. Overall, these results indicate that Gln-404 is not essential for NarX-positive function.
Fig. 2.
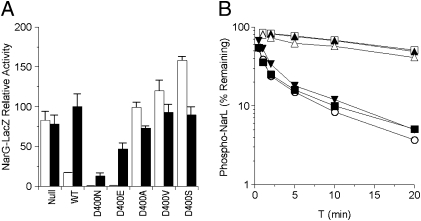
Positive-function assays for Asp-400 and Gln-404 substitution mutants. (A) In vivo Φ(narG-lacZ) expression in a narL+ strain. Activities from cultures grown in the absence and the presence of nitrate are indicated as open and solid bars, respectively. Data are averaged from three to five independent experiments. Error bars show SD values. The 100% activity value corresponds to about 1,200 Miller units. (B) In vitro transmitter autokinase activities. Phospho-MBP-NarX was measured by filter binding as described in Materials and Methods. The 100% activity value corresponds to about 2 pmol of phospho-MBP-NarX. Data are averaged from two independent experiments. ○, wild-type; ◆, Q404H; ▲, Q404A; ■, Q404N; ▼, Q404E; ▽, D400E; □, D400N.
The absence of negative function is revealed in the narX null narL505 strain, in which Φ(narG-lacZ) expression was at similarly high levels irrespective of added nitrate (Fig. 3A). The presence of the narX+ allele lowered the uninduced level of Φ(narG-lacZ) expression, reflecting NarX-negative activity toward the NarL (V88A) protein. The Q404N mutant appeared similar to wild type (P+ phenotype). By contrast, the other mutants were deficient in negative function to varying degrees. The Q404A mutant retained weak negative function, whereas the Q404E mutant was similar to the narX null strain. Finally, the Q404H mutant displayed elevated Φ(narG-lacZ) expression in the uninduced culture. Collectively, we denote these mutants as exhibiting the P– phenotype. Overall, these results indicate that Gln-404 is critical for NarX-negative function.
Fig. 3.
Negative-function assays for Gln-404 substitution mutants. (A) In vivo Φ(narG-lacZ) expression in a narL505 strain. Activities from cultures grown in the absence and presence of nitrate are indicated as open and solid bars, respectively. Data are averaged from three to five independent experiments. Error bars show SD values. The 100% activity value corresponds to about 1,500 Miller units. (B) In vitro transmitter phosphatase activities. Phospho-NarL was measured by filter binding as described in Materials and Methods. Data are averaged from two independent experiments. □, no sensor; ▼, Q404E; ▲, Q404A; ◆, Q404H; ■, Q404N; ○, wild type.
In Vitro Autokinase and Phosphotransfer Assays with Gln-404 Mutants.
We used soluble MBP-NarX fusion protein in which maltose-binding protein (MBP) has replaced the amino-terminal nitrate-binding and transmembrane domains. MBP-NarX retains an intact GAF domain and transmitter module and displays transmitter autokinase, phosphotransfer, and transmitter phosphatase activities (11, 25).
All Q404 mutant proteins exhibited considerable transmitter autokinase activity. The rates for the wild-type and Q404H mutant were comparable, whereas the rates for the Q404A, Q404N, and Q404E mutants were progressively slower (Fig. 2B). The relative autokinase rates for these mutants are congruent with their respective levels of Φ(narG-lacZ) expression (Fig. 2A). Together, these results indicate that Gln-404 is not essential for transmitter autokinase activity.
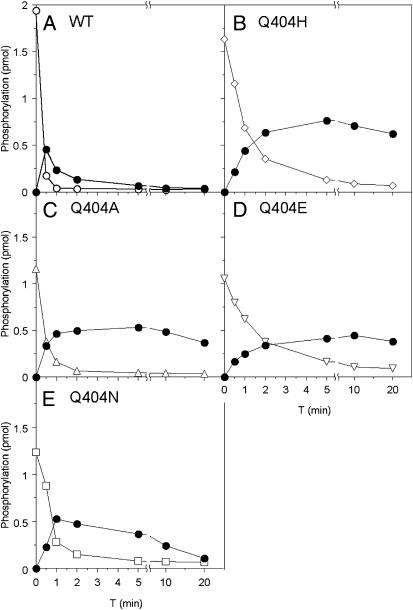
Adding His6-NarL to phospho-MBP-NarX results in fast phosphotransfer. However, the resulting phospho-NarL is rapidly depleted due to transmitter phosphatase activity by unphosphorylated MBP-NarX (11). Therefore, this experiment monitors both phosphotransfer and transmitter phosphatase activities (Fig. 4). All four Q404 mutant proteins exhibited only slightly slower phosphotransfer rates compared with wild-type MBP-NarX. However, in the reactions with Q404A, Q404E, and Q404H mutants, the level of phospho-NarL remained relatively constant. The Q404N mutant dephosphorylated phospho-NarL, but at a much slower rate than the wild type (Fig. 4). These in vitro results are in concordance with in vivo negative-function assays. In vivo, Q404A, Q404H, and Q404E mutants were severely diminished for negative activity, and in vitro, they failed to reduce phospho-NarL. Both assays reflect a severe impairment in phosphatase activity. Furthermore, the Q404N mutant exhibited readily detectable transmitter phosphatase activities in both in vivo and in vitro assays.
Fig. 4.
Phosphotransfer assays for Gln-404 substitution mutants. Phospho-MBP-NarX was isolated and mixed with NarL. Proteins were resolved by Laemmli gel electrophoresis, and phosphorylation was measured by phosphorimaging as described in Materials and Methods. ●, phospho-NarL; (A) ○, phospho-MBP-NarX wild type; (B) ◇, phospho-MBP-NarX Q404H; (C) △, phospho-MBP-NarX Q404A; (D) ▽, phospho-MBP-NarX Q404E; (E) □, phospho-MBP-NarX Q404H. Data are representative of at least two independent experiments for each mutant.
Direct Phosphatase Assays with Gln-404 Mutants.
We next investigated the transmitter phosphatase activity in direct assays by adding MBP-NarX to 32P-phospho-NarL. Wild-type MBP-NarX accelerated phospho-NarL dephosphorylation by at least 25-fold during the first 2 min and continued at a faster rate than autodephosphorylation for at least 20 min (Fig. 3B).
The Q404A and Q404E mutant rates were indistinguishable from the autodephosphorylation rate, whereas the Q404H and Q404N mutants exhibited progressively faster rates. Therefore, all three assays—in vivo negative function, phosphotransfer, and direct phosphatase—were in line for common patterns. All Gln-404 substitutions diminished transmitter phosphatase activity, with the most severe effects exhibited by the Q404A, Q404H, and Q404E mutants.
In Vivo Phenotypes of Asp-400 Mutants.
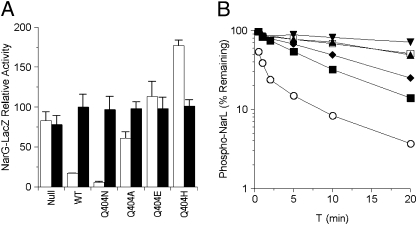
We generated Ala, Ser, and Val substitutions and the conservative Asn and Glu subsitutions for Asp-400. None of the D400 mutants exhibited detectable Φ(narG-lacZ) induction (K− phenotype; Fig. 2A shows data for the D400E and D400N mutants). This demonstrates that Asp-400 is essential in transmitter positive function.
The D400A, D400V, and D400S substitutions were devoid of activity (P– phenotype; Fig. 5A). In contrast, the D400N and D400E mutants exhibited strong in vivo negative activity (P+ phenotype). Therefore, except for the conservative Asn and Glu, all other substitutions abrogated negative activity. These results suggest that Asp-400 is important for transmitter phosphatase function.
Fig. 5.
Negative-function assays for Asp-400 substitution mutants. (A) In vivo Φ(narG-lacZ) expression in a narL505 strain, as described for Fig. 3. (B) In vitro transmitter phosphatase activities, as described for Fig. 3. □, no sensor; ▲, D400A; △, D400S; ▼, D400E; ■, D400N; ○, wild type.
In Vitro Assays with Asp-400 Mutants.
The MBP-NarX D400N and D400E mutant proteins exhibited undetectable activity for both autokinase (Fig. 2B) and phosphotransfer assays. These results are consistent with the absence of in vivo positive function of D400 mutants and indicate that the D400 mutant defect in Φ(narG-lacZ) induction is a consequence of impaired transmitter autokinase activity. Together, these assays suggest an essential role for Asp-400 in autokinase function.
We also examined the in vitro phosphatase reactions for several D400 mutant proteins. The phosphatase rate by the D400S mutant was indistinguishable from the autodephosphorylation rate, and the rate for the D400A mutant was only slightly faster (Fig. 5B). In contrast, the rates by the D400N and D400E mutants approached that of wild-type NarX. Therefore, both the Asn and Glu substitution mutants retained in vitro transmitter phosphatase activity, whereas the Ala and Ser substitutions abolished the function. These results again are consistent with in vivo negative-function data, and both assays together indicate the complex involvement of Asp-400 in NarX transmitter phosphatase function.
In Vivo Phenotypes of Mutants with the H399Q Substitution.
As NarX His-399 is the site of autophosphorylation, the H399Q mutant is devoid of positive function. In contrast, this mutant exhibited a strong negative function, lowering NarL (V88A)-dependent Φ(narG-lacZ) expression even in the presence of nitrate (K− P+ phenotype; Fig. 6A). Therefore, the two activities are genetically separable. Accordingly, single substitutions that directly perturb transmitter phosphatase activity are expected to affect the strong P+ phenotype of the H399Q mutant.
Fig. 6.
Negative-function assays for Asp-400 and Gln-404 plus H399Q double-substitution mutants. (A) In vivo Φ(narG-lacZ) expression in a narL505 strain, as described for Fig. 3. Mutants carrying the H399Q substitution are indicated by plus sign (“+”). (B) In vitro transmitter phosphatase activities, as described for Fig. 3. □, no sensor; ▽, Q404A+H399Q; ◇, Q404H+H399Q; △, D400A+H399Q; ◆, H399Q; ■, D400N+H399Q; ○, wild type.
As expected, none of these mutants exhibited detectable positive activity for Φ(narG-lacZ) induction, reflecting the absolute requirement of His-399 for NarX autophosphorylation. For in vivo negative assays, the H399Q+Q404A mutant retained a residual activity, whereas the H399Q+Q404H mutant had virtually none (Fig. 6A), corresponding to the respective levels of Q404A and Q404H single-mutant activities. Because the P– Q404A and Q404H single substitutions abrogated negative activity, the double-substitution phenotypes indicate the dominant effect of these mutations on the H399Q substitution and emphasize their severe defects for phosphatase function. Among the D400 mutants, H399Q+D400A also lost phosphatase activity. In contrast, both H399Q+D400N and H399Q+D400E mutants exhibited strong phosphatase activity irrespective of the inducer, thus mimicking the H399Q phenotype (Fig. 6A). Therefore, the P– D400A substitution was dominant over H399Q, whereas the P+ D400N and D400E phosphatase functions were slightly enhanced by H399Q.
Direct Phosphatase Assays for Mutants with the H399Q Substitution.
The K− P+ H399Q mutant dephosphorylated His6-NarL at ≈50% of the initial wild-type rate (Fig. 6B), although both the wild-type and the H399Q mutant reactions progressed to comparable extents after 60 min. The rates for the H399Q+Q404A and H399Q+Q404H mutants were indistinguishable from the autodephosphorylation rate. The rate for the H399Q+D400A mutant was only slightly faster, and the rate for the H399Q+D400N mutant was intermediate between wild type and the H399Q mutant. These results confirm the in vivo phenotypes of double substitutions. The Q404A and Q404H substitutions abrogated the H399Q phosphatase activity. The P– D400 mutants, represented by D400A, also had a similar effect, whereas the P+ D400 mutants, represented by D400N, either retained or enhanced the H399Q function.
Discussion
Our sequence analysis revealed a DxxxQ sequence motif conserved in HisKA_3 subfamily DHp domains, reminiscent of the similar motif in the CheZ auxiliary phosphatase (23). We examined NarX mutants with missense substitutions at the conserved Gln-404 and the invariant Asp-400 residues. Both in vivo and in vitro assays for all three two-component reactions—transmitter autokinase, phosphotransfer, and transmitter phosphatase—were in striking correspondence. Results strongly support the catalytic role of Gln-404 in transmitter phosphatase and reveal a complex role of Asp-400 for both transmitter autokinase and phosphatase reactions.
Catalytic Role of Gln-404 in HisKA_3 Transmitter Phosphatase Activity.
The mechanism for phosphoryl group hydrolysis is well-documented in chemotaxis and Ras-like GTPases. In CheZ, the conserved Gln-147 orients the nucleophilic water for an in-line hydrolysis of the phosphoryl group (12). The auxiliary phosphatase CheX employs a virtually identical mechanism with an analogous role for Asn-99 (14). Likewise, Ras-like GTPases rely on a conserved Gln or Asn for the correct alignment of the nucleophilic water in GTP hydrolysis (13). These examples illustrate a catalytic role for an amide group on a conserved Gln or Asn in phosphatase reactions.
The NarX Gln-404 residue corresponds to the catalytic residues Gln-147 in CheZ and Asn-99 in CheX. The NarX Q404A substitution abrogated phosphatase function, as did the analogous CheZ Q147A and CheX N99A substitutions (12, 14). The NarX Q404E substitution likewise eliminated phosphatase activity, whereas the Q404N mutant exhibited appreciable activity. These phenotypes thus align with the requirement of an amide group for the in-line water hydrolysis of the phosphoryl group. The retention of phosphotransfer activity in all Q404 mutants indicates normal interactions with the cognate response regulator NarL. Therefore, Gln-404 is specifically involved in the transmitter phosphatase activity.
The position corresponding to NarX Gln-404 is occupied by His in about 30% of HisKA_3 DHp sequences (Fig. 1A), suggesting that the amide group of Gln can be substituted by an imidazole ring for hydrogen bonding with the nucleophilic water. Nevertheless, the NarX Q404H mutant was severely defective for phosphatase function in vivo and in the phosphotransfer assay, although it displayed measurable activity in the direct phosphatase assay. Further analysis is required to examine the role of His in other members of the HisKA_3 subfamily.
Complex Role of Asp-400 in HisKA_3 Function.
The invariant CheZ Asp-143 residue makes a salt bridge with the invariant Lys-109 residue in the CheY response regulator (12). This interaction is suggested to have a subsidiary function in CheZ phosphatase (23). We were interested in examining the role of the analogous NarX Asp-400 residue in transmitter phosphatase activity, recognizing also a probable role for NarX-positive function. As a negatively charged residue immediately adjacent to the phospho-accepting His-399, Asp-400 is likely to be essential for determining the suitable pKa for His-399 protonation. In addition, the X-ray structures of the DesK transmitter reveal critical salt bridges for the interactions between Asp-400 (NarX numbering) and invariant charged residues in the CA domain (2, 16). Indeed, all NarX substitutions for Asp-400 abolished autokinase activity, as did substitutions for the analogous NtrB residue, Glu-140 (26).
The NarX D400A, D400S, and D400V substitutions nullified NarX phosphatase function, as did the analogous CheZ D143V and D143G substitutions (27). However, the conservative NarX D400N and D400E substitutions retained nearly wild-type activity, in contrast to the analogous substitutions in CheZ, which eliminate activity (28, 29). As noted previously (12), the corresponding position in the phosphotransfer protein Spo0B is occupied by Asn-34, which makes a hydrogen bond with the invariant Lys in the receiver domain active site (30). The permissibility of the NarX D400N substitution perhaps reveals an analogous hydrogen bond rather than a salt bridge as seen for CheZ. In addition, the tolerance of the D400E substitution may reflect greater structural flexibility at this position in NarX. Perhaps the stringency of CheZ Asp-143 is related to the relative rate of phospho-receiver autodephosphorylation, which for phospho-CheY is one to three orders of magnitude faster than most response regulators (31). Overall, these results indicate a complex role of Asp-400 in HisKA_3 function.
DxxxQ Motif in the HisKA_3 Subfamily.
The structural resemblance between the HisKA_3 DHp domain and the CheZ catalytic core, as well as the presence of a conserved DxxxQ motif in both, prompted us to postulate that the HisKA_3 DHp displays a similar mechanism for phospho-receiver dephosphorylation. We experimentally demonstrate here the critical role of the conserved Gln-404. We also present evidence for the ancillary involvement of the invariant Asp-400.
There are also structural and functional distinctions between CheZ and the HisKA_3 DHp domains. Whereas CheZ possesses only phosphatase function, DHp domains display all three signal transduction activities: transmitter autokinase, phosphotransfer, and transmitter phosphatase. In addition, whereas cognate receiver domains bind to CheZ via a carboxyl-terminal helix separate from the four-helix bundle core, they bind directly to the DHp domain four-helix bundle (5, 23). These differences are reflected in the complex role of Asp-400 as described above.
Missense substitutions and amino acid deletions conferring the K+ P– phenotype have been identified throughout the DHp and CA domains (11, 22, 32). Most of these substitutions change nonconserved residues and likely influence transmitter phosphatase function indirectly by affecting interactions between the DHp and CA domains, DHp and receiver domains, or transmitter and auxiliary proteins (such as NtrB-PII). In contrast, the conserved Gln-404 residue appears to be involved directly in transmitter phosphatase.
In addition, Gln-404 may be catalytic only upon a correct transmitter conformation for phosphatase activity. The X-ray structures of the DesK DHp domain reveal a helical phase shift between the autokinase-competent and phosphatase-competent states. The imidazole ring of the phospho-accepting His is fully exposed only in the autokinase-competent state (2). By analogy, perhaps the Gln-404 side chain adopts a catalytic geometry only in the phosphatase-competent conformation, as previously described for Ras-like GTPases (13).
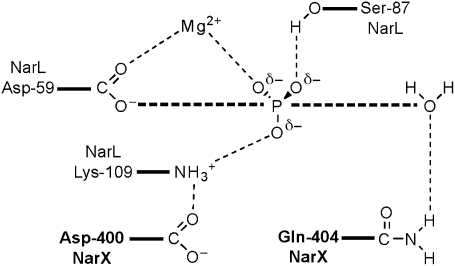
We therefore propose a model for the HisKA_3 family transmitter phosphatase mechanism (Fig. 7), modified from that revealed for the CheZ auxiliary phosphatase (12). In this model, the conserved Gln residue forms a hydrogen bond with the nucleophilic water, and the invariant Asp residue forms a hydrogen bond with the receiver domain invariant Lys residue.
Fig. 7.
Hypothetical mechanism for transmitter phosphatase. Modified and redrawn from ref. 23. NarX and NarL residues are indicated in boldface and roman typeface, respectively.
E/DxxN/T Motif in the HisKA Subfamily.
This study examined the DxxxQ motif in HisKA_3 transmitter phosphatase activity. However, this motif is absent from the HisKA family, which constitutes the majority of two-component sensor DHp domain sequences. Sequence conservation for helix α1 of HisKA DHp sequences is shown in Fig. 1C. Conserved residues distal to the phospho-accepting His are distinct from those in the HisKA_3 family. Instead, we found a conserved E/DxxT/N motif adjacent to the phospho-accepting His residue (Fig. 1C). This motif is reminiscent of the ExxN motif documented in the auxiliary phosphatase CheX (Fig. 1D) (23). The CheX conserved Asn-99 and Glu-96 residues within this motif function in virtually identical manner to CheZ Gln-147 and Asp-143 (14).
Among HisKA members, little mutational analysis has been conducted on the conserved Glu/Asp residue, although both the NtrB E140A and the E140Q mutants exhibit the K− P+ phenotype (26). In contrast, most substitutions at the highly conserved Thr-247 residue for EnvZ [HPK2b subfamily (17)] abolish phosphatase activity, with little effect on autokinase and phosphotransfer activities (33). However, the EnvZ T247Q, T247N, and T247S mutants are progressively phosphatase competent, demonstrating flexibility among Thr, Ser, Gln, and Asn residues at this position. Moreover, the analogous position is occupied by Asn in NtrB and other members of the HPK4 subfamily (17). [The HPK1-4 transmitter module subfamilies delineated by Grebe and Stock (17) are aggregated to the HisKA subfamily of DHp domains.] Because all four residues are conducive to hydrogen bond formation, this position may coordinate the catalytic water molecule similarly to CheX. Therefore, the conserved Thr/Asn residue in HisKA family members is likely to be involved directly in phosphatase activity.
Materials and Methods
Sequence Comparisons.
Sequence logos (21) were prepared with WebLogo (34). Sequence analyses used databases and programs made available through the National Center for Biotechnology Information (35). Sequences with the HisKA_3 annotation were extracted from completed geneome sequences for 17 representative bacterial species representing actinomycetes, cyanobacteria, firmicutes, and proteobacteria. Additional HisKA_3 sequences were identified with BLAST followed by visual inspection to identify those with unusual sequence features, such as nonconserved residues at position Gln-404. Orthologs (such as NarX) and closely related paralogs (such as DevS and DosT) were identified, and only one example of each was retained for inclusion in the sequence comparison. The HisKA_3 logo in Fig. 1A is derived from 70 different sequences. The CheZ logo in Fig. 1B is derived from 24 different sequences from diverse species, identified from a BLAST-P search with the E. coli K-12 protein sequence from positions 131–163. The resulting logo is similar to one developed recently from a larger number of sequences (29). The HisKA logo shown in Fig. 1C is derived from 36 different sequences (21 from E. coli K-12 and 15 from B. subtilis 168). The CheX logo in Fig. 1D is derived from 25 different sequences from diverse species, identified from a BLAST-P search with the Borrelia burgdorferi protein sequence from residues 84–116.
Assays in Vivo.
NarX-positive and -negative functions were examined by monitoring Φ(narG-lacZ) expression as described previously (36). Relevant genetic markers for the strains used are VJS5054 [ΔnarX242 narQ251::Tn10d(Tc) narL+ pcnB1] and VJS4033 [ΔnarX242 narQ251::Tn10d(Tc) narL505 pcnB+].
Assays in Vitro.
Protein purification, autokinase, and phosphotransfer assays followed protocols described previously (11, 25). For the phosphatase reaction protocol, we used a phosphatase-deficient form of MBP-NarX, carrying the M411T substitution (5), to prepare [32P]-phospho-His6-NarL in Hepes reaction buffer (5). For phosphatase reactions, phospho-NarL was preincubated at 19 °C for 2 min before the addition of 0.5-μM dimers of MBP-NarX. Time-point samples were added to stop solution (20 mM Hepes, pH 7.0, 100 mM EDTA, 0.01% SDS). The remaining phospho-NarL was quantified by filter binding and plotted as the percentage of the initial value.
Acknowledgments
Li-Ling Chen made invaluable contributions to the experiments reported here and provided much helpful advice and support. Michele Igo and Chet Price offered constructive comments on a draft version of this manuscript. This study was supported by Public Health Service Grant GM036877 from the National Institute of General Medical Sciences.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Hoch JA, Silhavy TJ, editors. Two-Component Signal Transduction. Washington, DC: ASM Press; 1995. [Google Scholar]
- 2.Albanesi D, et al. Structural plasticity and catalysis regulation of a thermosensor histidine kinase. Proc Natl Acad Sci USA. 2009;106:16185–16190. doi: 10.1073/pnas.0906699106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McFarland N, McCarter L, Artz S, Kustu S. Nitrogen regulatory locus “glnR” of enteric bacteria is composed of cistrons ntrB and ntrC: Identification of their protein products. Proc Natl Acad Sci USA. 1981;78:2135–2139. doi: 10.1073/pnas.78.4.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacNeil T, Roberts GP, MacNeil D, Tyler B. The products of glnL and glnG are bifunctional regulatory proteins. Mol Gen Genet. 1982;188:325–333. doi: 10.1007/BF00332696. [DOI] [PubMed] [Google Scholar]
- 5.Szurmant H, Hoch JA. Interaction fidelity in two-component signaling. Curr Opin Microbiol. 2010;13:190–197. doi: 10.1016/j.mib.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao R, Stock AM. Biological insights from structures of two-component proteins. Annu Rev Microbiol. 2009;63:133–154. doi: 10.1146/annurev.micro.091208.073214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ninfa AJ, Magasanik B. Covalent modification of the glnG product, NRI, by the glnL product, NRII, regulates the transcription of the glnALG operon in Escherichia coli. Proc Natl Acad Sci USA. 1986;83:5909–5913. doi: 10.1073/pnas.83.16.5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keener J, Kustu S. Protein kinase and phosphoprotein phosphatase activities of nitrogen regulatory proteins NTRB and NTRC of enteric bacteria: Roles of the conserved amino-terminal domain of NTRC. Proc Natl Acad Sci USA. 1988;85:4976–4980. doi: 10.1073/pnas.85.14.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolanin PM, Webre DJ, Stock JB. Mechanism of phosphatase activity in the chemotaxis response regulator CheY. Biochemistry. 2003;42:14075–14082. doi: 10.1021/bi034883t. [DOI] [PubMed] [Google Scholar]
- 10.Song Y, Peisach D, Pioszak AA, Xu Z, Ninfa AJ. Crystal structure of the C-terminal domain of the two-component system transmitter protein nitrogen regulator II (NRII; NtrB), regulator of nitrogen assimilation in Escherichia coli. Biochemistry. 2004;43:6670–6678. doi: 10.1021/bi049474r. [DOI] [PubMed] [Google Scholar]
- 11.Noriega CE, Lin HY, Chen LL, Williams SB, Stewart V. Asymmetric cross-regulation between the nitrate-responsive NarX-NarL and NarQ-NarP two-component regulatory systems from Escherichia coli K-12. Mol Microbiol. 2010;75:394–412. doi: 10.1111/j.1365-2958.2009.06987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao R, Collins EJ, Bourret RB, Silversmith RE. Structure and catalytic mechanism of the E. coli chemotaxis phosphatase CheZ. Nat Struct Biol. 2002;9:570–575. doi: 10.1038/nsb816. [DOI] [PubMed] [Google Scholar]
- 13.Li G, Zhang XC. GTP hydrolysis mechanism of Ras-like GTPases. J Mol Biol. 2004;340:921–932. doi: 10.1016/j.jmb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Pazy Y, et al. Identical phosphatase mechanisms achieved through distinct modes of binding phosphoprotein substrate. Proc Natl Acad Sci USA. 2010;107:1924–1929. doi: 10.1073/pnas.0911185107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finn RD, et al. The Pfam protein families database. Nucleic Acids Res. 2010;38(Database issue):D211–D222. doi: 10.1093/nar/gkp985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trajtenberg F, Graña M, Ruétalo N, Botti H, Buschiazzo A. Structural and enzymatic insights into the ATP binding and autophosphorylation mechanism of a sensor histidine kinase. J Biol Chem. 2010;285:24892–24903. doi: 10.1074/jbc.M110.147843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grebe TW, Stock JB. The histidine protein kinase superfamily. Adv Microb Physiol. 1999;41:139–227. doi: 10.1016/s0065-2911(08)60167-8. [DOI] [PubMed] [Google Scholar]
- 18.Roberts DM, Liao RP, Wisedchaisri G, Hol WG, Sherman DR. Two sensor kinases contribute to the hypoxic response of Mycobacterium tuberculosis. J Biol Chem. 2004;279:23082–23087. doi: 10.1074/jbc.M401230200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jordan S, Hutchings MI, Mascher T. Cell envelope stress response in Gram-positive bacteria. FEMS Microbiol Rev. 2008;32:107–146. doi: 10.1111/j.1574-6976.2007.00091.x. [DOI] [PubMed] [Google Scholar]
- 20.Schneider TD, Stephens RM. Sequence logos: A new way to display consensus sequences. Nucleic Acids Res. 1990;18:6097–6100. doi: 10.1093/nar/18.20.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lupas AN, Gruber M. The structure of alpha-helical coiled coils. Adv Protein Chem. 2005;70:37–78. doi: 10.1016/S0065-3233(05)70003-6. [DOI] [PubMed] [Google Scholar]
- 22.Hsing W, Russo FD, Bernd KK, Silhavy TJ. Mutations that alter the kinase and phosphatase activities of the two-component sensor EnvZ. J Bacteriol. 1998;180:4538–4546. doi: 10.1128/jb.180.17.4538-4546.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silversmith RE. Auxiliary phosphatases in two-component signal transduction. Curr Opin Microbiol. 2010;13:177–183. doi: 10.1016/j.mib.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egan SM, Stewart V. Mutational analysis of nitrate regulatory gene narL in Escherichia coli K-12. J Bacteriol. 1991;173:4424–4432. doi: 10.1128/jb.173.14.4424-4432.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noriega CE, Schmidt R, Gray MJ, Chen LL, Stewart V. Autophosphorylation and dephosphorylation by soluble forms of the nitrate-responsive sensors NarX and NarQ from Escherichia coli K-12. J Bacteriol. 2008;190:3869–3876. doi: 10.1128/JB.00092-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atkinson MR, Ninfa AJ. Mutational analysis of the bacterial signal-transducing protein kinase/phosphatase nitrogen regulator II (NRII or NtrB) J Bacteriol. 1993;175:7016–7023. doi: 10.1128/jb.175.21.7016-7023.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boesch KC, Silversmith RE, Bourret RB. Isolation and characterization of nonchemotactic CheZ mutants of Escherichia coli. J Bacteriol. 2000;182:3544–3552. doi: 10.1128/jb.182.12.3544-3552.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blat Y, Eisenbach M. Mutants with defective phosphatase activity show no phosphorylation-dependent oligomerization of CheZ. The phosphatase of bacterial chemotaxis. J Biol Chem. 1996;271:1232–1236. doi: 10.1074/jbc.271.2.1232. [DOI] [PubMed] [Google Scholar]
- 29.Paphavee L, Karen MO. A remote CheZ orthologue retains phosphatase function. Mol Microbiol. 2010;77:225–235. doi: 10.1111/j.1365-2958.2010.07200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zapf J, Sen U, Madhusudan, Hoch JA, Varughese KI. A transient interaction between two phosphorelay proteins trapped in a crystal lattice reveals the mechanism of molecular recognition and phosphotransfer in signal transduction. Structure. 2000;8:851–862. doi: 10.1016/s0969-2126(00)00174-x. [DOI] [PubMed] [Google Scholar]
- 31.Thomas SA, Brewster JA, Bourret RB. Two variable active site residues modulate response regulator phosphoryl group stability. Mol Microbiol. 2008;69:453–465. doi: 10.1111/j.1365-2958.2008.06296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atkinson MR, Ninfa AJ. Characterization of Escherichia coli glnL mutations affecting nitrogen regulation. J Bacteriol. 1992;174:4538–4548. doi: 10.1128/jb.174.14.4538-4548.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dutta R, Yoshida T, Inouye M. The critical role of the conserved Thr247 residue in the functioning of the osmosensor EnvZ, a histidine kinase/phosphatase, in Escherichia coli. J Biol Chem. 2000;275:38645–38653. doi: 10.1074/jbc.M005872200. [DOI] [PubMed] [Google Scholar]
- 34.Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: A sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sayers EW, et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2010;38(Database issue):D5–D16. doi: 10.1093/nar/gkp967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stewart V, Chen LL. The S helix mediates signal transmission as a HAMP domain coiled-coil extension in the NarX nitrate sensor from Escherichia coli K-12. J Bacteriol. 2010;192:734–745. doi: 10.1128/JB.00172-09. [DOI] [PMC free article] [PubMed] [Google Scholar]