Abstract
Inflammatory cytokines mediate inflammatory bowel diseases (IBDs) and cytokine blocking therapies often ameliorate the disease severity. IL-32 affects inflammation by increasing the production of IL-1, TNFα, and several chemokines. Here, we investigated the role of IL-32 in intestinal inflammation by generating a transgenic (TG) mouse expressing human IL-32γ (IL-32γ TG). Although IL-32γ TG mice are healthy, constitutive serum and colonic tissue levels of TNFα are elevated. Compared with wild-type (WT) mice, IL-32γ TG mice exhibited a modestly exacerbated acute inflammation early following the initiation of dextran sodium sulfate (DSS)-induced colitis. However, after 6 d, there was less colonic inflammation, reduced tissue loss, and improved survival rate compared with WT mice. Associated with attenuated tissue damage, colonic levels of TNFα and IL-6 were significantly reduced in the IL-32γ TG mice whereas IL-10 was elevated. Cultured colon explants from IL-32γ TG mice secreted higher levels of IL-10 compared with WT mice and lower levels of TNFα and IL-6. Constitutive levels of IL-32γ itself in colonic tissues were significantly lower following DSS colitis. Although the highest level of serum IL-32γ occurred on day 3 of colitis, IL-32 was below constitutive levels on day 9. The ability of IL-32γ to increase constitutive IL-10 likely reduces TNFα, IL-6, and IL-32 itself accounting for less inflammation. In humans with ulcerative colitis (UC), serum IL-32 is elevated and colonic biopsies contain IL-32 in inflamed tissues but not in uninvolved tissues. Thus IL-32γ emerges as an example of how innate inflammation worsens as well as protects intestinal integrity.
Keywords: innate immunity, intestinal barrier, Crohn's disease
Crohn's disease (CD) is a chronic inflammatory disorder of the gastrointestinal tract associated with multifactorial conditions and often occurs in genetically predisposed subjects (1). Although the mechanisms underlying CD remain unclear, a generally accepted hypothesis is that certain microorganisms trigger a dysregulated mucosal immune response, which results in intestinal tissue damage with loss of barrier function (2, 3). The treatment of CD with neutralizing antibodies to TNFα (4) has become the mainstay for therapy in steroid-dependent patients. Ulcerative colitis (UC) also responds to anti-TNFα treatment, although less than in CD (5). In addition to TNFα, IL-1, IL-18, and the antiinflammatory IL-10 are induced in the intestinal tract by Toll-like receptor (TLR) agonists.
IL-32 was first reported as an inducer of TNFα (6), particularly the IL-32γ isoform (7). IL-32 also induces IL-1β, IL-6, and MIP-2 in cell lines and primary cells (6, 8–12). Vector-mediated overexpression of IL-32 results in increased TNFα and IL-1β production (13, 14). On the other hand, silencing of endogenous IL-32 by either siRNA or shRNA results in decreased IL-1β, IL-8, and TNFα production (8, 12, 15) or IL-6 induced by IL-1β (16). Furthermore, IL-32 is present in the intestinal tissue of patients with CD (11) or UC (17).
There are six splicing variants; IL-32γ is the most complete and most active isoform (7). IL-32β is also active (18, 19). The presence of IL-32 protein has been reported in synovial tissues of rheumatoid arthritis (9, 20, 21) and Mycobacterium tuberculosis infections (22, 23). In lung biopsies from patients with emphysema, tissue levels of IL-32 and TNFα correlate (24). IL-32 synergizes with muramyl dipeptide (MDP) for the production of IL-1β and IL-6 via the nuclear oligiomerization domain (NOD) NOD1 and NOD2 in a caspase-1–dependent pathway (11). The effect of IL-32 was absent in the cells of patients with CD bearing the NOD2 polymorphism in CD (25). Because IL-32 may have a pivotal role in the pathogenesis of inflammatory bowel disease (IBD), we generated a strain of mice expressing human IL-32γ and challenged these mice with dextran sodium sulfate (DSS) colitis.
Results
Generation of Human IL-32γ Transgenic (TG) Mice.
Human IL-32γ was driven by the chicken β-actin promoter to express IL-32γ in all tissues (Figs. S1 A, B, and C and S2). Litters of IL-32γ TG mice were of normal size and developed without visible abnormalities or runting. Human IL-32γ was detected in the peripheral white blood cells of the TG mice (Fig. 1A) and in the liver, stomach, skeletal muscle, heart, and pancreas (Fig. S1C). However, expression of IL-32γ was low in cerebrum, lymph node, upper intestine, kidney, testis, and thymus.
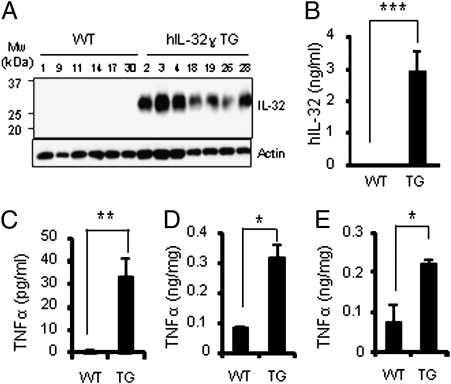
Fig. 1.
Expression of human IL-32γ in transgenic mice and spontaneous induction of TNFα. (A) Western blotting of human IL-32γ protein in peripheral blood leukocytes of transgenic mice (molecular weight of IL-32γ protein between 25 and 30 kDa). (B) Mean ± SEM level of IL-32γ in transgenic mouse serum. (C–E) Spontaneous induction of TNFα in IL-32γ transgenic mice sera (C), colon (D), and rectum (E). Values are means ± SEM (n = 6 per group). *P < 0.05; **P < 0.01; ***P < 0.001 WT versus IL-32γ TG mice (n = 24 per group).
In each of 24 IL-32γ TG mice tested, constitutive serum levels of IL-32γ were 3.1 ± 0.68 ng/mL (Fig. 1B) but absent in WT mice. Since previous studies demonstrated that recombinant IL-32 induced these cytokines in vitro, as shown in Figs. 1 and 3, constitutive production of TNFα was higher in IL-32γ TG mice than in WT mice. Constitutive TNFα and IL-10 in colonic explant cultures were also elevated compared with WT cultures. Thus, the IL-32γ TG mouse is a unique demonstration of endogenous IL-32γ induction of TNFα in vivo under nonstressed conditions.
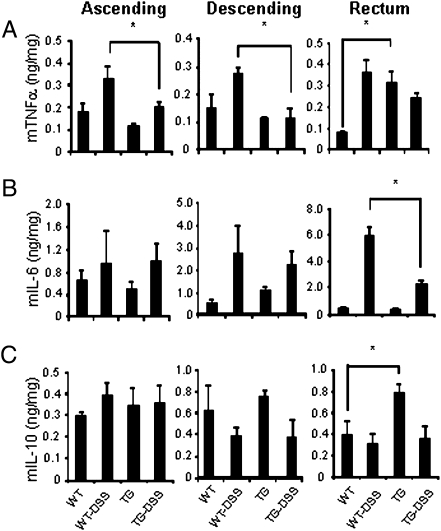
Fig. 3.
Colonic cytokine levels in DSS colitis. Day 6 colon segments were sliced, homogenized in lysis buffer, and centrifuged as described in Materials and Methods. (A–C) Mean ± SEM. TNFα, IL-6, and IL-10, respectively. (n = 6 per group). *P < 0.05.
Spectrum of Disease Severity in IL-32γ TG Mice Subjected to DSS Colitis.
IL-32γ TG mice and WT mice were fed a 3.5% solution of DSS in the drinking water for 5 d and observed for 14 d. All DSS-treated mice developed colitis, as indicated by weight loss and stool quality. In WT mice, DSS treatment resulted in weight loss or abnormal stools after day 5. In contrast, TG mice showed significant weight loss and rectal bleeding starting from day 4 (Fig. 2 A and B). However, this exacerbation in disease severity was short; after day 6, both DSS-treated groups showed similar clinical signs and weight loss as in WT.
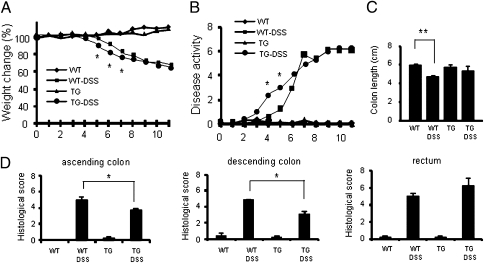
Fig. 2.
DSS-induced colitis in WT and IL-32γ TG mice. (A) Mean ± SEM loss in body weight loss (%). *P < 0.05 DSS-treated WT versus DSS-treated IL-32γ TG mice (n = 6 per group). (B) Disease activity index of colitis in DSS-treated WT and IL-32γ TG mice. The degree of colitis was quantified by the clinical disease features as described in Materials and Methods. Each point represents a mean value ± SEM. *P < 0.05 (n = 6 per group). (C) Mean ± SEM colon length in DSS-treated WT and IL-32γ TG mice on day 6. (D) Mean ± SEM. H&E histological scores. *P < 0.05 DSS-treated WT versus DSS-treated IL-32γ TG mice (n = 6 per group).
Mice were killed at day 6 and histological and immunological analyses performed. As expected, colon length in DSS-treated WT mice was significantly shorter (Fig. 2C, P < 0.01) compared with control mice. However, in DSS-treated IL-32γ TG mice, colon length was the same as non-DSS treated control mice. Histological scoring of colonic sections showed less severe disease in IL-32γ TG mice compared with WT mice (Fig. 2D). WT mice displayed increased inflammation upon histological examination (Fig. S3). By comparison, sections of the ascending and descending colon from DSS-treated IL-32γ TG mice revealed less edema, reduced epithelial cell damage, and a lower number of infiltrating leukocytes. Consistent with the histological data, WT mice died or had to be killed within 11 d because of severe loss of body weight (>30%), whereas 33% of IL-32γ TG mice survived and recovered (Fig. S4). These observations suggest that IL-32γ expression in DSS colitis accelerates the healing process.
Proinflammatory Cytokines and IL-10 Expression During DSS-Induced Colitis in IL-32γ TG Mice.
As shown in Fig. 3A, the tissue content of TNFα on day 6 was significantly greater in both ascending and descending colons of DSS-treated WT compared with DSS-treated IL-32γ TG mice. A similar difference was observed in the rectum but did not reach statistical significance. Unexpectedly, in IL-32γ TG mice not subjected to DSS colitis, levels of constitutive TNFα were higher in rectal tissue compared with WT mice (Fig. 3A, Right). Although the levels of IL-6 in colons increased by DSS treatment (Fig. 3B), the IL-6 concentration was comparable in IL-32γ TG to WT mice. However, in the rectum, there was a significant reduction in IL-6 in IL-32γ TG mice compared with WT mice, as was the case with TNFα (Fig. 3A).
In the case of IL-10 production, there was no difference in DSS-treated WT or IL-32γ TG mice in ascending or descending colons (Fig. 3C, Left and Center) as well as in the rectum (Fig. 3C, Right). However, constitutive IL-10 production was greater in the rectum of IL-32γ TG mice compared with WT mice (Fig. 3C, Right).
On day 6, explants of colon segments were incubated in serum-free RPMI1640 medium for 24 h and cytokine concentrations were determined in the supernatant. There were no differences in the secretion of TNFα in WT or IL-32γ TG mice subjected to DSS colitis. The level of IL-6 in supernatant from two parts of colons of DSS-treated IL-32γ TG mice was significantly lower than in DSS-treated WT mice (Fig. 4A).As shown in Fig. 4B, IL-10 in supernatant of cultured colon explants was higher in IL-32γ TG ascending colons following DSS treatment but was not statistically significant (Fig. 4B, Left). Spontaneous IL-10 secretion in descending colons of IL-32γ TG mice was significantly higher than WT mice (P < 0.01). These observations suggest that IL-32γ appears to increase constitutive IL-10 production (also Fig. 3C, Right), which may account for improved outcome as well as lower TNFα and IL-6 production.
Fig. 4.
Spontaneous release of cytokines from colonic explant cultures. Means ± SEM supernatant (A) IL-6 and (B) IL-10, respectively, following a 24-h incubation. *P < 0.05; **P < 0.01 (n = 6 per group).
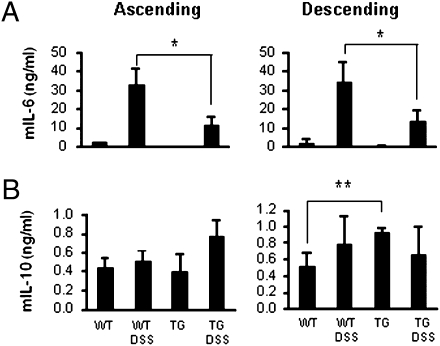
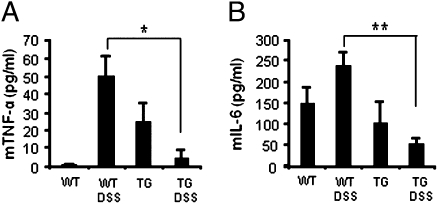
Support for this concept can be found in Fig. 5. We determined circulating cytokines in DSS-treated mice at day 6. As shown in Fig. 5 A and B, TNFα and IL-6 levels were significantly increased in DSS-treated WT mice but impressively reduced in IL-32γ TG mice. IL-10 was not detected in these sera.
Fig. 5.
Circulating cytokines in DSS colitis. Six days after DSS colitis, serum from untreated or DSS-treated WT and IL-32γ TG mice were collected. Mean ± SEM (A) TNFα and (B) IL-6. *P < 0.05; **P < 0.01 (n = 6 per group).
IL-32 Production in IL-32 TG Mice Decreases with DSS Colitis.
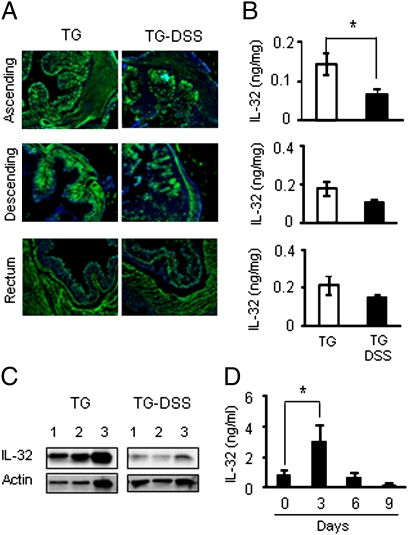
On day 6, after 5 d of DSS, ascending, descending, and rectal colons were stained with anti–IL-32 antibodies. As shown in Fig. 6A, IL-32γ was expressed in the mucosa, muscular layers, and in the lamina propria. In the lamina propria, IL-32γ was observed in macrophages and T cells. Similar to the decreased levels of TNFα, staining of IL-32γ was less in DSS-treated IL-32γ TG mice (P < 0.05) compared with non-DSS IL-32γ TG mice (Fig. 6A). IL-32γ was ≈50% lower in the ascending colon of DSS-treated TG mice compared with TG mice not subjected to the inflammatory stimulus of the colitis (Fig. 6B). The decrease in IL-32γ associated with DSS is similar to the decrease observed in TNFα (Fig. 3A).
Fig. 6.
The effect of DSS induced colon injury in IL-32γ TG mice. (A) Immunofluorescence staining of colons from untreated or DSS-treated transgenic mice. Anti–huIL-32 antibody (green) and DAPI (blue). Representative photographs (magnification, 100×) are shown. (B) Mean ± SEM. IL-32γ on day 6 in colon lysates of untreated or DSS-treated TG mice. *P < 0.05 (n = 6 per group). (C) Day 6 Western blotting of colons in untreated or DSS-treated transgenic mice. Lane 1, ascending colon; lane 2, descending colon; lane 3, rectum. (D) Serum IL-32γ levels in DSS-treated transgenic mice. The serum of mice was collected at day 0, 3, 6, and 9. *P < 0.05 (n = 6 per group).
By Western blotting, there was a similar down-regulation of IL-32γ associated with the inflammatory process compared with constitutive expression of IL-32γ. Consistent with these data, serum levels of IL-32γ during DSS-induced colitis progressively decreased from a maximal elevation on day 3 to levels below those of constitutive expression (Fig. 6D).
IL-32 Expression in Patients with Ulcerative Colitis.
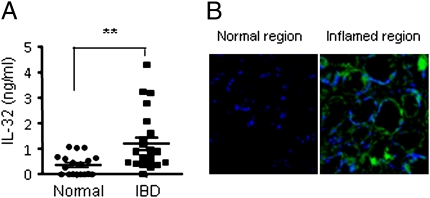
Although we and others reported the immunohistochemical presence of IL-32 in epithelial cells of colonic biopsies of patients with CD (11, 17), we investigated IL-32 in patients with ulcerative colitis. Plasma levels of IL-32 were elevated (mean 1.25 ng/mL) compared with levels from healthy, age-matched subjects (Fig. 7A). Immunohistochemical staining of inflamed tissue from these patients revealed the presence of IL-32 but its absence in uninvolved areas of the colon of the same patients (Fig. 7B).
Fig. 7.
IL-32 in UC patients. (A) Plasma levels of IL-32γ from healthy subjects (controls) compared with UC patients. Horizontal bars represent mean values ± SEM. **P < 0.01 controls versus UC patients (n = 18, controls; n = 24, patients). (B) IL-32 expression in the epithelial cells of a UC patient biopsy from a macroscopically inflamed region is shown stained with affinity-purified goat anti–IL-32γ antibody (green) and DAPI (blue). In same patient, anti–IL-32 staining of a tissue taken from macroscopically noninvolved region is shown. Representative photographs are shown (original magnification, 200×) are shown.
Discussion
Characterization of the IL-32γ TG Mouse.
The disadvantage of constitutive expression of a proinflammatory transgene in vivo is an exaggerated effect of the gene. Nevertheless, we generated mice using constitutive expression of IL-32γ to confirm its TNFα-inducing effects in a whole animal. Although the IL-32γ TG mice appear healthy, levels of constitutive TNFα were elevated in the circulation and tissues of IL-32γ TG mice. In many ways, thus is a confirmation of the ability of recombinant IL-32γ in inducing cytokines such as TNFα, an observation of the original report on the properties of IL-32 (6). Subsequently, several studies have confirmed the ability of recombinant IL-32 in the induction of TNFα as well as IL-1β and chemokines either alone but also with costimulants such as other cytokines or TLR2 agonists, but particularly with the NOD2 ligand MDP (11). In a recent study, using an adenoviral delivery vector, IL-32γ induced TNFα and IL-1β but also increased expression of NOD2 and TLR2 (13).
Role of Endogenous IL-32 in Regulating Innate Responses in DSS Colitis.
Despite the constitutive expression of TNFα in these mice, the innate immune/inflammatory response must be balanced by the coexpression of IL-10. Several studies have also shown the regulation of inflammatory cytokines by reducing endogenous IL-32 with siRNA or shRNA. For example, in stable clones of IL-32 shRNA in the human macrophage cell line THP-1, both LPS and IL-1β–induced production of TNFα and IL-8 were markedly reduced compared with clones of scrambled shRNA (15). Suppression of endogenous IL-32 in THP-1 cells also resulted in decreased cytokines induced by live infection (8). When endogenous IL-32 was silenced in freshly isolated human peripheral blood mononuclear cells (PBMC) with IL-32 siRNA, IL-6, IFNγ, and TNFα were reduced by 57, 51, and 36%, respectively (12). In that study, the inhibitory activity of IL-32 siRNA targeted Th1 cytokines and the chemokines MIP-1α and MIP-1β (12). In human endothelial cells, silencing of endogenous IL-32 resulted in a reduction of constitutive as well as IL-1β–induced intercellular adhesion molecule-1 by 55 and 54%, respectively (16). In addition, there was a decrease of constitutive as well as IL-1β–induced IL-1α, (of 62 and 43%), IL-6 (of 53 and 43%), and IL-8 (of 46 and 42%) (16).
The injection of stable porcine kidney PK-15 cells expressing human IL-32β inhibited the cytotoxic T lymphocyte following cellular xenografts into mice (26), suggesting an immunosuppressive property of IL-32β. In support of this concept, monocytes from human PBMC were differentiated into dendritic cells (DC) and stimulated with lypopolysaccharides (LPS). Gene expression of IL-32β paralleled the secretion of IL-10 reaching levels of 5–6 ng/mL between 24 and 48 h (18). Silencing of endogenous IL-32 in LPS-stimulated DC cells resulted in a 50% decrease in IL-10 production compared with scrambled siRNA (18).
DSS Colitis in IL-32γ TG Mice.
In the present study, we sought to characterize a role for IL-32 in IBD because IL-32 has been implicated in CD (11) as well as in UC (17). Because CD is often treated with neutralizing anti-TNFα antibodies and because of the role of endogenous IL-32 in the induction of TNFα, we expected that IL-32γ TG mice would exhibit an exaggerated course of colitis when subjected to DSS. We postulated that the responses to DSS in the inflamed intestinal epithelium would be amplified by IL-32γ–induced TNFα secretion from monocytes stimulated by IL-32γ from the intestinal epithelial cells (17). Indeed, the severity of the model during the early phase of DSS colitis was modestly exacerbated. Unexpectedly, however, disease severity indices were lower in the transgenic mice in the last days compared with colitis in the WT mice. With prolonged exposure to DSS, survival in IL-32γ TG mice was greater than WT mice. Tissue injury and healing were also improved and levels of TNFα, IL-6, and IL-32γ itself were lower. It became clear that constitutive expression of IL-32γ in these mice had two different properties: one expected induction of constitutive TNFα and the other unexpected constitutive elevation in IL-10.
Role of IL-10 in IL-32γ TG Mice.
IL-10 reduced inflammatory cytokine production (27) and a CD-like colitis spontaneously developed in mice deficient in IL-10 (22). That constitutive levels of IL-10 were observed in IL-32γ TG mice may explain the protection in DSS colitis. Thus the reduction in inflammation in IL-32γ TG mice may be due to IL-10 suppression of TLR-induced TNFα and IL-6. Although LPS-induced endogenous IL-32β in human DCs increases IL-10 production (18), it is unlikely that administration of recombinant IL-32β can be exploited therapeutically to induce IL-10 without its proinflammatory effects (19).
Protective Role for TNFα in the Innate Response of the Intestinal Barrier.
Although TNFα is considered clinically causative in IBD, mice deficient in TNFα exhibit enhanced intestinal inflammation during DSS colitis and 40% more deaths compared with no deaths in WT mice (28). Also, the absence of TNFα results in a lack of colonic glucocorticoid synthesis and there is exacerbation of DSS-induced colitis (29). TNFα-deficient mice also had increased infiltration of mucosal neutrophils and disruption of the epithelial cell layer (28). In support of these studies, mice deficient in TNF receptor type 1 exhibited greater inflammation with DSS colitis, indicating that signaling from this receptor suppresses acute damage and provides homeostasis of colonic epithelial cells (30).
Role for IL-32 in NOD2 Protection of the Intestinal Barrier.
NOD2 is a CD susceptibility gene and IL-32 participates in the intracellular signal pathway through peptidoglycan motifs (11). IL-32 acts with MDP for the induction of IL-1β and IL-6 production, which is mediated by caspase-1 (11). As stated above, using an adenoviral delivery vector, IL-32γ increased expression of NOD2 and TLR2 (13). Silencing of endogenous NOD1 resulted in a reduction of IL-32 but also of IL-1β, IL-6, IL-8, and ICAM-1 (31). In summary, the in vivo effect of IL-32γ expression during DSS colitis exhibits the dual function of innate responses in the intestine. The induction of constitutive TNFα as well as IL-10 by IL-32 appears to be consistent with control of inflammatory cytokines by NOD2 and protection of the barrier (32).
Materials and Methods
Generation of IL-32γ TG Mice.
The ORF of IL-32γ cDNA was transferred into pCAGGS (Fig. S1). The entire sequence was then linearized with SalI and microinjected into mouse zygotes of the C57BL/6 strain. The transgenic mice showed no obvious physical abnormalities and were screened by RT-PCR of whole blood RNA (Qiagen). The peripheral blood leukocytes were separated from heparinized blood using RBC lysis buffer (0.17 M Tris-Cl pH 7.4, 0.83% NH4Cl). Control mice were the wild-type littermates. All animal procedures were reviewed and approved by the Konkuk University Animal Care Committee.
Induction of Colitis and Analysis.
Male mice of 10–12 wk were used. DSS (MP Biomedicals) was supplied in the drinking water as a 3.5% solution for 5 d. At regular time intervals, the mice were evaluated as described previously (33). Mice were anesthetized, killed, and large intestines (from cecum to anus) were quickly removed. Segments from each tissue were excised and immediately frozen and stored at −80 °C until thawed later for homogenization. Segments were also stored in OCT compound for cryosection. Sections were scored as described (33).
Colonic Tissue Cytokines.
The colons were homogenized in lysis buffer (Cell Signaling) using a tissue tearer (BioSpec Products). Following clarification by centrifugation, cytokine levels were measured by cytokine-specific ELISA (R&D Systems). Protein concentrations of the homogenate were quantified by a BCA assay (Thermo). Details of the ELISA for human IL-32γ are given in SI Materials and Methods.
Colon Explant Cultures.
Segments of the colon (2 cm) were removed, cut open longitudinally, and washed in PBS, placed in 6-well culture plates containing 2 mL fresh RPMI (Invitrogen) supplemented with penicillin and streptomycin, and incubated at 37 °C for 24 h. Culture supernatants were harvested and assayed for cytokines.
Western Blotting.
Samples were immunoblotted with affinity-purified goat anti-human IL-32γ antibody and a rabbit antibody against β-actin (Santa Cruz Biotechnology) as described (7).
Patient Plasma and Tissue Samples.
These studies were approved by Konkuk University Hospital Institutional Review Board. Blood from patients was collected in heparinized tubes, the plasma separated by centrifugation and stored at –80 °C. After informed consent, biopsy samples were obtained at colonoscopy. Tissue samples were frozen in OCT (Ames), cut, air dried, fixed, and used for histological evaluation.
Statistical Analysis.
Statistical significance was analyzed by unpaired, two-tailed Student's t test. Significance of Kaplan and Meier survival curves was calculated with the log-rank test using Prism 5 (Graphpad).
Supplementary Material
Acknowledgments
This work is supported by the Korea Science and Engineering Foundation (WCU: R33-2008-000-10022-0 and KRF-2008-313-C00644). D.Y. is partially supported through the National Research Foundation of Korea. These studies were also supported by National Institutes of Health Grants AI-15614, CA-04 6934, and Juvenile Diabetes Research Foundation 26-2008-893 (to C.A.D.) and Amgen, Inc., Thousand Oaks, CA.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015418107/-/DCSupplemental.
References
- 1.Pizarro TT, Cominelli F. Cytokine therapy for Crohn's disease: Advances in translational research. Annu Rev Med. 2007;58:433–444. doi: 10.1146/annurev.med.58.121205.100607. [DOI] [PubMed] [Google Scholar]
- 2.Hibi T, Ogata H. Novel pathophysiological concepts of inflammatory bowel disease. J Gastroenterol. 2006;41:10–16. doi: 10.1007/s00535-005-1744-3. [DOI] [PubMed] [Google Scholar]
- 3.Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514–521. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Derkx B, et al. Tumour-necrosis-factor antibody treatment in Crohn's disease. Lancet. 1993;342:173–174. doi: 10.1016/0140-6736(93)91375-v. [DOI] [PubMed] [Google Scholar]
- 5.Pastorelli L, Pizarro TT, Cominelli F, Vecchi M. Emerging drugs for the treatment of ulcerative colitis. Expert Opin Emerg Drugs. 2009;14:505–521. doi: 10.1517/14728210903146882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim SH, Han SY, Azam T, Yoon DY, Dinarello CA. Interleukin-32: A cytokine and inducer of TNFalpha. Immunity. 2005;22:131–142. doi: 10.1016/j.immuni.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Choi JD, et al. Identification of the most active interleukin-32 isoform. Immunology. 2009;126:535–542. doi: 10.1111/j.1365-2567.2008.02917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bai X, et al. IL-32 is a host protective cytokine against Mycobacterium tuberculosis in differentiated THP-1 human macrophages. J Immunol. 2010;184:3830–3840. doi: 10.4049/jimmunol.0901913. [DOI] [PubMed] [Google Scholar]
- 9.Joosten LA, et al. IL-32, a proinflammatory cytokine in rheumatoid arthritis. Proc Natl Acad Sci USA. 2006;103:3298–3303. doi: 10.1073/pnas.0511233103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim S, et al. Proteinase 3-processed form of the recombinant IL-32 separate domain. BMB Rep. 2008;41:814–819. doi: 10.5483/bmbrep.2008.41.11.814. [DOI] [PubMed] [Google Scholar]
- 11.Netea MG, et al. IL-32 synergizes with nucleotide oligomerization domain (NOD) 1 and NOD2 ligands for IL-1beta and IL-6 production through a caspase 1-dependent mechanism. Proc Natl Acad Sci USA. 2005;102:16309–16314. doi: 10.1073/pnas.0508237102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nold MF, et al. Endogenous IL-32 controls cytokine and HIV-1 production. J Immunol. 2008;181:557–565. doi: 10.4049/jimmunol.181.1.557. [DOI] [PubMed] [Google Scholar]
- 13.Heinhuis B, et al. IL-32gamma and Streptococcus pyogenes cell wall fragments synergise for IL-1-dependent destructive arthritis via upregulation of TLR-2 and NOD2. Ann Rheum Dis. 2010;69:1866–1872. doi: 10.1136/ard.2009.127399. [DOI] [PubMed] [Google Scholar]
- 14.Shoda H, et al. Interactions between IL-32 and tumor necrosis factor alpha contribute to the exacerbation of immune-inflammatory diseases. Arthritis Res Ther. 2006;8:R166. doi: 10.1186/ar2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong J, et al. Suppressing IL-32 in monocytes impairs the induction of the proinflammatory cytokines TNFα and IL-1β. Cytokine. 2009;49:171–176. doi: 10.1016/j.cyto.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Nold-Petry CA, et al. IL-32-dependent effects of IL-1beta on endothelial cell functions. Proc Natl Acad Sci USA. 2009;106:3883–3888. doi: 10.1073/pnas.0813334106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shioya M, et al. Epithelial overexpression of interleukin-32alpha in inflammatory bowel disease. Clin Exp Immunol. 2007;149:480–486. doi: 10.1111/j.1365-2249.2007.03439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang JW, et al. A proinflammatory cytokine interleukin-32beta promotes the production of an anti-inflammatory cytokine interleukin-10. Immunology. 2009;128(1, Suppl):e532–e540. doi: 10.1111/j.1365-2567.2008.03025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi H, et al. Interleukin-32beta propagates vascular inflammation and exacerbates sepsis in a mouse model. PLoS ONE. 2010;5:e9458. doi: 10.1371/journal.pone.0009458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cagnard N, et al. Interleukin-32, CCL2, PF4F1 and GFD10 are the only cytokine/chemokine genes differentially expressed by in vitro cultured rheumatoid and osteoarthritis fibroblast-like synoviocytes. Eur Cytokine Netw. 2005;16:289–292. [PubMed] [Google Scholar]
- 21.Mun SH, et al. Tumor necrosis factor alpha-induced interleukin-32 is positively regulated via the Syk/protein kinase Cdelta/JNK pathway in rheumatoid synovial fibroblasts. Arthritis Rheum. 2009;60:678–685. doi: 10.1002/art.24299. [DOI] [PubMed] [Google Scholar]
- 22.Kundu M, Basu J. IL-32: An emerging player in the immune response network against tuberculosis? PLoS Med. 2006;3:e274. doi: 10.1371/journal.pmed.0030274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Netea MG, et al. Mycobacterium tuberculosis induces interleukin-32 production through a caspase- 1/IL-18/interferon-gamma-dependent mechanism. PLoS Med. 2006;3:e277. doi: 10.1371/journal.pmed.0030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calabrese F, et al. IL-32, a novel proinflammatory cytokine in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178:894–901. doi: 10.1164/rccm.200804-646OC. [DOI] [PubMed] [Google Scholar]
- 25.Netea MG, et al. NOD2 3020insC mutation and the pathogenesis of Crohn's disease: Impaired IL-1beta production points to a loss-of-function phenotype. Neth J Med. 2005;63:305–308. [PubMed] [Google Scholar]
- 26.Chae JI, et al. Downregulation of immune response by the human cytokines Interleukin-32alpha and beta in cell-mediated rejection. Cell Immunol. 2010;264:47–53. doi: 10.1016/j.cellimm.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 27.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 28.Naito Y, et al. Enhanced intestinal inflammation induced by dextran sulfate sodium in tumor necrosis factor-alpha deficient mice. J Gastroenterol Hepatol. 2003;18:560–569. doi: 10.1046/j.1440-1746.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 29.Noti M, Corazza N, Mueller C, Berger B, Brunner T. TNF suppresses acute intestinal inflammation by inducing local glucocorticoid synthesis. J Exp Med. 2010;207:1057–1066. doi: 10.1084/jem.20090849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mizoguchi E, et al. TNF receptor type I-dependent activation of innate responses to reduce intestinal damage-associated mortality. Gastroenterology. 2008;134:470–480. doi: 10.1053/j.gastro.2007.11.055. [DOI] [PubMed] [Google Scholar]
- 31.Cho KA, et al. Orientia tsutsugamushi induced endothelial cell activation via the NOD1-IL-32 pathway. Microb Pathog. 2010;49:95–104. doi: 10.1016/j.micpath.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi KS, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 33.Munitz A, et al. Resistin-like molecule alpha decreases glucose tolerance during intestinal inflammation. J Immunol. 2009;182:2357–2363. doi: 10.4049/jimmunol.0803130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.