Abstract
Vibrio cholerae is a human pathogen that causes the life-threatening diarrheal disease cholera. A type VI secretion system (T6SS) was recently shown to be required for full virulence in the O37 serogroup strain V52, which causes only sporadic human disease, but T6SS is not expressed in seventh pandemic O1 El Tor strains under standard laboratory conditions. In this study, we show that in the O1 El Tor strain C6706, T6SS is repressed by both quorum sensing and the uncharacterized protein VC0070 (TsrA). Disruption of TsrA and the quorum sensing regulator LuxO induces expression and secretion of the T6SS substrate Hcp, and this is dependent on the downstream regulator HapR, which directly binds to the promoter region of the T6SS genes hcp1 and hcp2 to induce expression. The activated T6SS in C6706 is functional and can translocate the effector protein VgrG-1 into macrophage cells, and T6SS activation leads to fecal diarrhea and intestinal inflammation in infant rabbits. Using an infant mouse infection model, we show that deletion of tsrA results in a 9.3-fold increase in intestinal colonization compared with wild type. TsrA functions as a global regulator to activate expression of hemagglutinin protease and repress cholera toxin and toxin coregulated pilus. Our findings provide significant insight into the molecular mechanism of T6SS and ToxT regulon gene regulation by quorum sensing and TsrA.
Cholera is a severe, life-threatening diarrheal disease caused by Vibrio cholerae, a Gram-negative bacterium that is transmitted to the human host following consumption of contaminated food or water (1). Orally ingested bacteria that survive passage through the stomach use flagellar motility to make contact with the small bowel epithelium (2) and coordinately express the ctx and tcp operons, which encode cholera toxin (CT) and toxin coregulated pilus (TCP), respectively. TCP pili are essential for successful colonization in mice (3, 4) and humans (5, 6), and CT is the main factor responsible for generating the severe diarrhea that is a hallmark of the disease. The genes involved in TCP and CT production are directly controlled by the transcriptional activator ToxT, whose expression is in turn regulated by two membrane-localized protein complexes, ToxR/S and TcpP/H (7, 8). In addition, the expression of TcpP/H is dependent on another two cytoplasmic activators, AphA and AphB (9).
The expression of virulence genes in V. cholerae is also influenced by cell density through quorum sensing (10, 11), a population density-dependent regulatory system that utilizes small molecules to control a wide range of phenotypes in many different bacteria (12). At low cell density when the extracellular concentration of autoinducers is low, the membrane bound sensor kinases CqsS and LuxQ initiate a phosphorylation cascade, which results in activation of the response regulator LuxO. Together with the alternative σ-factor RpoN (σ-54), phosphorylated LuxO activates the expression of four small RNAs (qrr1–4), which prevent expression of HapR by destabilizing its mRNA (13). HapR represses virulence gene expression by directly blocking aphA transcription and therefore tcpP/H expression (11, 14). Thus, hapR down-regulation under low cell density conditions induces virulence gene expression. In contrast, the elevated concentration of autoinducers at high cell density causes CqsS and LuxQ to function as phosphatases to render LuxO inactive. The subsequent accumulation of HapR shuts off virulence gene expression and simultaneously induces the expression of HapA, a secreted hemagglutinin (HA) protease that may serve as a “detachase” during colonization (15).
V. cholerae possesses a recently defined type VI secretion system (T6SS). T6SS genes are clustered in pathogenicity islands found in around 25% of all sequenced Proteobacteria and have been implicated as a virulence factor in many bacteria including V. cholerae (16), Pseudomonas aeruginosa (17), Edwardsiella tarda (18, 19), and Burkholderia species (20–22). The hallmarks of T6SS include secretion of the conserved hexameric protein Hcp into the external medium and the presence of both a AAA+ ATPase and a homolog of IcmF, a protein that confers stability to the Legionella T4SS (23–25). In V. cholerae, T6SS studies have focused mainly on the O37 serogroup strain V52 where T6SS is required for full virulence toward the amoebae Dictyostelium discoideum and J774 macrophages (16) and for the inflammatory diarrhea in infant mice (26). In V52, T6SS is responsible for the secretion of Hcp as well as three VgrG proteins (VgrG-1–3) (16), which interact to form a phage spike-like complex that likely perforates the host cell membrane (27, 28). VgrG-1, which functions as both a T6SS structural element and an effector protein (27), contains a C-terminal domain that shows strong homology to the actin cross-linking domain (ACD) of V. cholerae RtxA or MARTX toxin (29). Indeed, VgrG-1 was shown to cause actin cross-linking in host cells following its T6SS-dependent translocation during V52 infection (26, 27, 30). Interestingly, T6SS is constitutively expressed in V52 under both in vitro laboratory conditions and during infection, but T6SS expression is strongly repressed by an unknown mechanism in the seventh pandemic O1 El Tor strains N16961 and C6706 (16, 31).
In this article, we show that the global regulator TsrA (VC0070) coordinates with the quorum sensing system to control T6SS in the seventh pandemic O1 El Tor strain C6706. Simultaneous disruption of the quorum sensing regulator LuxO and TsrA led to Hcp secretion into culture supernatant and VgrG-1 translocation into J774 macrophages, and TsrA disruption induced T6SS-dependent fecal diarrhea and intestinal inflammation in infant rabbits. In addition, we found that the tsrA mutant displayed an increased fitness for colonization in infant mice. As a global regulator, TsrA controls the expression of both T6SS and the main virulence factors in V. cholerae.
Results
Transposon Mutation of VC0070 Results in Hcp Induction and Secretion.
V. cholerae seventh pandemic O1 El Tor strain C6706 has a complete T6SS gene cluster, but Hcp could not be detected in either the culture supernatant or bacterial pellet when cultured in LB medium (Fig. 1A). We used a colony lift immunoassay (32) to screen a sequence-defined C6706 transposon library (33) for mutants that express Hcp. Among the 3,156 nonredundant mutants examined, we found one mutant (EC8403) that produced Hcp, which was detected in both the bacterial pellet and cell supernatant by Western blot (Fig. 1A). This mutant contained a transposon insertion in the hypothetical gene VC0070 (Fig. S1) (33), which we have named type VI secretion system regulator A (tsrA). TsrA is conserved in Vibrio species and shows a weak similarity to the N-terminal sequence of heat-stable nucleoid-structuring (H-NS) protein of Escherichia coli as predicted by HHPRED (21% identity and 43% similarity to H-NS in E. coli over amino acids 38–76 of TsrA).
Fig. 1.
Quorum sensing and TsrA control T6SS. (A) Western blot analysis of Hcp in bacterial pellet (P) and culture supernatant (S) from various C6706 strains. The Western blot was probed with anti-Hcp serum. (B) Effect of mutations in various regulators on the expression of T6SS substrate hcp1 and the T6SS structural element VCA0107 using chromosomal transcriptional lacZ fusions. Bacteria were cultured in LB medium. Values given are means ± SD. (C) Deletion of hapR in ΔluxO and ΔluxO tsrA (C6706) decreased the expression of Hcp. Proteins from bacterial pellet were analyzed by Western blot with anti-Hcp serum.
Quorum Sensing and TsrA Repress T6SS.
Deletion of tsrA in C6706 did not reproduce the Hcp expression and secretion profile seen in EC8403 (Fig. 1A). Single colony purification of EC8403 on LB plates found at least two different morphologies: one that resembled the wild-type parental strain (EC8403-W) and another that was smoother (EC8403-S). Multiple isolates of both colony morphologies all contained the annotated transposon insertion in tsrA, but Hcp expression and secretion was only detected in colonies displaying the smooth morphology (Fig. 1A). We hypothesized that the smooth colony morphology was due to altered extracellular polysaccharide production, which is known to be controlled by the quorum sensing regulators luxO and hapR (10, 11). DNA sequencing of luxO and hapR genes in EC8403-S found that luxO contained a point mutation in the 40th codon (GAG to TAG), which caused premature translation termination. Deletion of luxO in a clean wild-type background led to an increase in Hcp expression but not secretion, and deletion of both luxO and tsrA caused a larger increase in Hcp expression and allowed for Hcp secretion (Fig. 1A). As expected, complementation with tsrA in trans in the ΔluxO tsrA strain reduced Hcp expression and abolished its secretion (Fig. 1A). The Hcp expression and secretion profile of this ΔluxO tsrA double mutant closely matched the profile of EC8403-S (Fig.1A), suggesting that these two mutations together are responsible for the EC8403-S phenotype.
Deletion of luxO or tsrA alone each caused a small but significant increase in transcription of hcp1 and the T6SS structural gene VCA0107, and simultaneous deletion of both genes (ΔluxO tsrA) caused a further increase in their expression (Fig. 1B). The expression of hcp2 and the T6SS structural gene VCA0120 also increased after disruption of luxO alone (Fig. S2), suggesting that quorum sensing controls expression of both T6SS effectors and the T6SS secretion apparatus. Hcp expression was dramatically decreased when hapR was disrupted in either the ΔluxO or ΔluxO tsrA strains (Fig. 1C), suggesting that quorum sensing activates T6SS through HapR. HapR expression was not affected in ΔtsrA (Fig. S3A), suggesting that TsrA acts independently of the quorum sensing system to affect T6SS expression. Interestingly, V. cholerae strains V52, SCE226, and SCE223, which constitutively express T6SS, all have nonsense mutations in hapR, which lead to the loss of its dimerization domain (Fig. S3B) (16, 34). Deletion of the truncated hapR in V52 (hapRV52) also abolished Hcp expression (Fig. S3C), suggesting that the truncated HapR in this strain is functional and is involved in controlling T6SS expression. However, we could not restore Hcp production in ΔluxO tsrA hapR in C6706 or hapRV52 in V52 by expressing hapR or hapRV52 expression in trans (Fig. 1C and Fig. S3C), suggesting that HapR needs to be expressed at a specific time or protein level to appropriately control T6SS.
C6706 Secretes Hcp and Translocates VgrG-1 upon T6SS Activation.
To test whether the activation of T6SS in C6706 resembles that in V52, we constructed several in-frame deletions in T6SS apparatus genes in the ΔluxO tsrA background. As shown in Fig. 2A, deletion of vasK, vgrG-1, and the conserved T6SS gene VCA0111 did not affect Hcp expression but abolished its secretion. When provided in trans, vasK restored Hcp secretion into the culture supernatant, and this was not due to cytoplasmic leakage because RNA polymerase was not found in the supernatant fraction. In agreement with previous studies in V52 (16), T6SS in C6706 is also under the control of the σ-54–dependent regulator VasH, as deletion of vasH abolished Hcp expression.
Fig. 2.
The secretion of Hcp and translocation of VgrG-1 by C6706 after T6SS activation. (A) Western blot analysis of Hcp in bacterial pellet (P) and culture supernatant (S) from various C6706 strains. (B) Actin cross-linking in J744 macrophage cells infected with various C6706 strains. The Western blot was probed with anti–G-actin.
V52 uses T6SS to translocate the effector protein VgrG-1 into macrophage J774 cells, where the ACD domain of VgrG-1 causes actin cross-linking (27, 30). Accordingly, we sought to determine whether C6706 could cause actin cross-linking in infected J774 cells following T6SS activation in the ΔluxO tsrA strain. To eliminate any background actin cross-linking activity, rtxA was disrupted in all of the V. cholerae strains tested. Consistent with previous reports (27, 30), V52 induced massive cross-linking of cytosolic actin in J774 cells after a 3-h incubation (Fig. 2B). Actin cross-linking was not observed in J774 cells infected with wild-type C6706 (ΔrtxA), but a band corresponding to a G-actin dimer (near 100 kDa) was detected in the ΔrtxA luxO tsrA strain (Fig. 2B). Disruption of vgrG-1 or the T6SS apparatus gene vasK in this mutant completely abrogated the actin cross-linking (Fig. 2B). Taken together, these results indicate that the T6SS in C6706 is functionally capable of secreting Hcp and translocating VgrG-1 into the cytosol of J774 cells, but C6706 does not do so under standard infection conditions due to repression of T6SS expression by TsrA and LuxO.
T6SS Activation Induces Fecal Diarrhea in Infant Rabbits.
To investigate the role of T6SS in the pathogenesis of V. cholerae C6706 infection, we used the infant rabbit as a model for V. cholerae-induced diarrheal disease (35, 36). To determine whether T6SS activation causes an increase in diarrhea production, we used a strain of C6706 that lacks the cholera toxin genes ctxAB and the flagellin genes flaABCDE (Δctx fla) because this strain colonizes the rabbit intestine but induces very little diarrhea compared with wild-type C6706 (35). As expected, most rabbits infected with the Δctx fla strain remained healthy and did not show any symptoms of fecal diarrhea, but 11 out of 12 rabbits infected with the Δctx fla tsrA strain developed fecal diarrhea around 24–72 h postinfection (Fig. 3A). These animals had loosely adherent fecal material around their perianal areas, hind limbs, and tails. Inactivation of T6SS in this strain (Δctx fla tsrA vasK) abolished the diarrhea phenotype in most (6 out of 7) of the infant rabbits (Fig. 3A), suggesting that the fecal diarrhea seen in the Δctx fla tsrA strain was due to T6SS activation. It is important to note that we were not able to directly examine the role of luxO in T6SS activation in this animal model because of the essential role that luxO plays in activation of other virulence genes during intestinal colonization (11).
Fig. 3.
T6SS activation by tsrA deletion leads to fecal diarrhea in infant rabbits. Infant rabbits were orogastrically infected with sodium bicarbonate buffer, containing the indicated V. cholerae strains and observed for 3 d. (A) The frequency of infected rabbits that exhibited diarrhea. (B) H&E staining of the large intestine of infected infant rabbits. The arrows indicate signs of edema, congestion, and hemorrhage. (C) The expression of IL-8 in infected infant rabbits was examined with qRT-PCR. Each data point represents an individual animal infected with V. cholerae, and the horizontal bars indicate the mean.
Histopathology studies demonstrated that the large intestine of rabbits infected with the Δctx fla tsrA strain showed signs of edema, congestion, and hemorrhage and contained increased numbers of heterophils infiltrated into the lumen compared with controls (Fig. 3B). Furthermore, IL-8 mRNA levels were consistently elevated in the large intestines of Δctx fla tsrA-infected rabbits compared with Δctx fla-infected rabbits (Fig. 3C). These findings suggest that T6SS activation by TsrA disruption causes fecal diarrhea, possibly induced by an innate inflammatory response.
TsrA Mutant Shows Hypercolonization in the Infant Mouse.
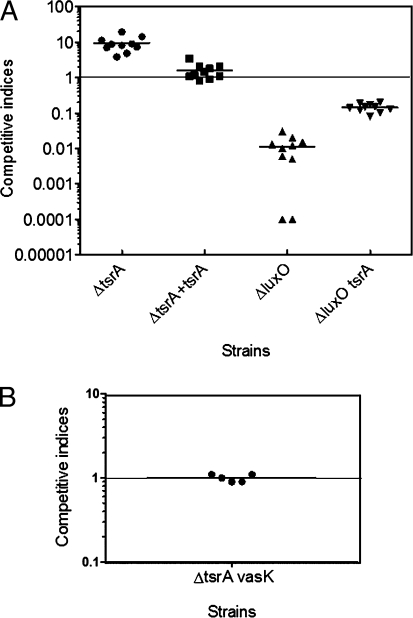
To further examine the role of tsrA in virulence, we used the infant mouse infection model to compare the intestinal colonization ability of the single deletion mutants (ΔtsrA and ΔluxO) and the double deletion mutant (ΔluxO tsrA) to wild-type C6706. As shown in Fig. 4, ΔtsrA showed a 9.3-fold increase in intestinal colonization at 20 h postinfection, and tsrA provided in trans returned the colonization rate to wild-type levels. The increased colonization in the ΔtsrA mutant is unlikely to be due to an altered growth rate because it showed a comparable growth to wild type in liquid culture (Fig. S4). Consistent with previous reports (11), the ΔluxO strain showed a very strong colonization defect, but deletion of tsrA in the ΔluxO strain significantly increased its colonization fitness (Fig. 4). Interestingly, the hypercolonization phenotype seen in the ΔtsrA strain was not due to increased T6SS expression because the ΔtsrA vasK strain did not show any colonization defect relative to ΔtsrA (Fig. 4).
Fig. 4.
Competitive indices of various regulatory deletion mutants (ΔtsrA, ΔtsrA + tsrA, ΔluxO, and ΔluxO tsrA) (LacZ−) compared with wild-type C6706 (LacZ+) (A) and T6SS mutant ΔtsrA vasK (LacZ+) compared with ΔtsrA (LacZ−) (B) in the infant mouse model. Each data point represents an individual animal infected with V. cholerae, and the horizontal bars indicate the mean.
TsrA and Quorum Sensing Fine Tune the Expression of Virulence Factors.
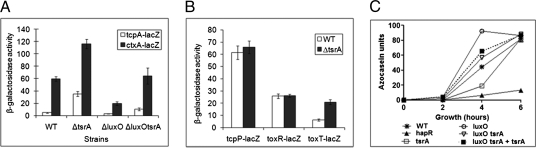
To determine whether TsrA affects mouse colonization by controlling virulence gene expression, we used chromosomal lacZ transcriptional reporters to measure transcription of V. cholerae virulence genes. We found that when grown in LB medium, disruption of tsrA in wild-type C6706 increased tcpA expression by sevenfold, and its deletion in a ΔluxO strain background also increased tcpA expression approximately fourfold (Fig. 5A). Mutation of tsrA in wild-type and ΔluxO strains was shown to increase ctxA expression by two- and threefold, respectively (Fig. 5A). Furthermore, the expression of toxT but not tcpP or toxR increased by approximately threefold in the absence of tsrA (Fig. 5B). Finally, none of these transcriptional changes were affected by disruption of toxR or tcpP (Fig. S5), suggesting that TsrA controls virulence gene expression by regulating toxT expression independently of TcpP/H and ToxR/S.
Fig. 5.
TsrA and quorum sensing regulate the expression of virulence factors. Effect of deletion mutants in various regulators on expression of the virulence factors TCP and CT (A) and their regulators TcpP, ToxR, and ToxT (B) using chromosomal transcriptional lacZ fusion. Bacteria were cultured in LB medium. Values given are means ± SD. (C) The regulation of TsrA on HA protease production determined by azocasein activity.
To determine whether TsrA affects other quorum sensing regulated factors, we examined the affect of ΔtsrA on the production of the HA protease HapA, which is directly regulated by quorum sensing (15). Azocasein digestion analysis of bacterial supernatant demonstrated that ΔtsrA displayed a significant decrease in protease activity compared with wild type during 2–6 h incubation (Fig. 5C), suggesting that TsrA positively controls HapA. Deletion of tsrA from ΔluxO also decreased protease activity to wild-type level, which could be partially complemented by tsrA provided in trans (Fig. 5C). Taken together, these results suggest that quorum sensing and TsrA work together to control V. cholerae virulence factors.
Discussion
V. cholerae C6706 is a clinical isolate belonging to the O1 El Tor biotype that is responsible for the ongoing seventh pandemic of cholera. In this report, we provided evidence that T6SS in C6706 is under the negative control of both the quorum sensing system and the regulator TsrA (Fig. 6). In C6706, mutation of the quorum sensing regulator luxO increased the expression of T6SS structural genes and the secretion substrates Hcp1 and Hcp2, but further disruption of tsrA was required for Hcp secretion to occur. Importantly, T6SS in this ΔluxO tsrA strain appears to be functional because it is able to translocate the effector protein VgrG-1 into J774 macrophages, and activation of T6SS by tsrA disruption causes increased fecal diarrhea production in the infant rabbit infection model.
Fig. 6.
A model for quorum sensing and TsrA regulation on V. cholerae T6SS and virulent factors. Solid line arrows or T bars denote the characterized positive or negative regulation. Dot line arrows or T bars denote the newly defined positive or negative regulations.
Our results demonstrate that the seventh pandemic strain C6706 contains a T6SS that is fully functional, but its expression is normally repressed under standard laboratory growth conditions. This result is similar to those found in many other bacteria where T6SS is tightly controlled to prevent premature or inappropriate expression (37). In P. aeruginosa, the T6SS substrate Hcp is not expressed in vitro but is abundantly expressed and secreted during long-term lung infection in cystic fibrosis patients (17). Similarly, T6SS is strongly up-regulated in Burkholderia pseudomallei and Pectobacterium atrosepticum during macrophage invasion or when exposed to host cell extracts (38, 39). These regulatory patterns suggest that bacteria use environmental cues to trigger T6SS expression and activation, but the specific signals remain unknown.
In V. cholerae, quorum sensing is known to function during intestinal colonization to control virulence gene expression (40). High cell densities are common during the late stage of infection, and HapR-mediated repression of virulence genes is thought to help V. cholerae detach from the intestinal wall to find new sites of infection or exit the host and initiate a new infection cycle (41). The finding that T6SS activation also causes diarrhea in infant rabbits suggests that it might contribute to the exit of V. cholerae from the host during the late stage of infection. The T6SS locus is well conserved among V. cholerae strains and is known to be responsible for the inflammatory diarrhea caused by V. cholerae strain V52 (26), but the role of T6SS in V. cholerae strains that cause more widespread disease was unclear. In this study, a possible role for T6SS in the pathogenesis of the seventh pandemic O1 El Tor isolate C6706 was successfully demonstrated after removal of the proinflammatory genes flaABCDE and the primary toxin genes ctxAB (35). These results suggest that the effects of T6SS on bacterial pathogenesis in other V. cholerae strains may be obscured in vivo by the predominant effect of other virulence factors and proinflammatory molecules. Interestingly, the fecal diarrhea that is induced by ctx deletion strains in infant rabbits is thought to mimic the reactogenic diarrhea experienced by human volunteer subjects orally immunized with certain flagellated live attenuated cholera vaccines (35). Our results suggest that in addition to eliminating production of flagella, it may be necessary to inactivate type VI secretion or the proinflammatory effector VgrG-1 (26) to fully suppress reactogenic diarrhea induced by live attenuated ctx deletion strains derived from V. cholerae of the El Tor biotype (35).
The master regulator of the quorum sensing pathway is the TetR-family transcriptional regulator HapR (42). HapR forms a dimer with its C-terminal dimerization domain and binds to the promoter of its target gene, thereby repressing or activating its expression (34). It appears that in V. cholerae, quorum sensing controls T6SS through HapR as deletion of hapR in ΔluxO or ΔluxO tsrA abolished or strongly decreased Hcp expression (Figs. 1C and 6). The positive regulation of Hcp by HapR has also been seen in V. cholerae strain A1552 (31). The binding of HapR to the promoters of hcp1 and hcp2 has been demonstrated (43), suggesting that its effect on T6SS expression may occur in part at the level of Hcp expression. Interestingly, the truncated version of HapR in V52 is also functional and controls T6SS expression, suggesting that HapR dimerization is not required for its effect on T6SS. Besides HapR and VasH, RpoN is a third regulator involved in T6SS regulation (Fig. 6). Transposon mutation of rpoN in V52 is attenuated toward Dictyostelium (16), and deletion of rpoN in A1552 abolished the expression of hcp1 and hcp2 (31). We found that a deletion of rpoN in C6706 caused increased HapR expression and decreased Hcp expression (Fig. S6 A and B), suggesting that RpoN controls T6SS through some mechanism other than simply modulating HapR expression. The finding of RpoN binding sites in the hcp1 and hcp2 promoter regions in A1552 (31) and C6706 (Fig. S6C) argues for direct binding of RpoN to the hcp promoters. Therefore, we propose a mechanism in which HapR, VasH, and RpoN collaborate to activate the expression of T6SS by directly binding to the promoter regions of the T6SS structural elements and secretion substrate genes.
TsrA is not strongly related to any characterized regulator at the amino acid level. It is conserved in Vibrio species but is rarely found in other bacteria. However, using the HHPRED program, we found that it is structurally similar to the N terminus of H-NS protein from bacteria such as E. coli and V. cholerae. H-NS protein contains an N-terminal oligomerization domain and a C-terminal DNA binding domain (44) and has been shown to have a pleiotropic effect on gene expression in V. cholerae; disruption of H-NS causes increased cholera toxin expression in nonpermissive LB medium, enhanced resistance to low pH and hydrogen, reduced flagellar motility, and reduced colonization efficiency (45, 46). H-NS has been demonstrated to silence virulence gene expression at multiple steps in the ToxR regulatory cascade (47) and activate the HA protease gene hapA (45, 46). Overexpression of a truncated H-NS lacking the C-terminal DNA binding domain resulted in diminished expression of HapA (46). In this study, TsrA was seen to have both negative (repression) and positive (activation) effects on its downstream targets (Fig. 6). It repressed TCP and CT in nonpermissive LB medium while it activated HapA production (Fig. 6). On the basis of these phenotypes, TsrA seems to have a similar function to H-NS and probably does not act as a dominant-negative inhibitor of H-NS. TsrA has limited similarity to the N terminus of H-NS and is not predicted to form a coiled-coil domain by the Lupas algorithm (48). As no DNA binding domain was found with bioinformatics analysis, TsrA might require another transcriptional regulator to control its target genes.
In summary, we have demonstrated that T6SS in the V. cholerae seventh pandemic O1 El Tor strain C6706 is tightly controlled by quorum sensing and the global regulator TsrA. T6SS was functional after activation and caused inflammatory fecal diarrhea in infant rabbits. We conclude that TsrA-regulated T6SS might contribute to the pathogenesis of pandemic V. cholerae strains and might cause some of the residual reactogenicity seen in live attenuated cholera vaccines that no longer express cholera toxin.
Materials and Methods
Strains and Culture Conditions.
V. cholerae O1 El Tor biotype strain C6706 and C6706lacZ, a streptomycin-resistant spontaneous lacZ derivative of C6706, were used in this study. Unless specified, C6706lacZ was used as parental wild-type strain for the mutant construction. E. coli strains DH5α λpir and SM10 λpir or BW20767 λpir were used for cloning and mating, respectively.
Mutants and lacZ Reporter Strains Construction.
In-frame deletion mutants were generated by the sacB-based allelic exchange as described previously (49). V. cholerae reporter strains were generated using promoterless lacZ reporter suicide plasmid as described (50).
Colony Lift Immunoassay and Protein Assays.
Colony lift immunoassays were performed as described (32). Secreted proteins were isolated from midlog cultured V. cholerae (OD600 = 0.4–0.5) in LB medium. Proteins were precipitated with trichloroacidic acid (TCA) and subjected to 10–20% gradient SDS/PAGE.
Enzyme Activity Assays.
β-Galactosidase activity was measured with cells permeabilized with detergent and chloroform as described by Miller (51). The amount of HapA secreted to the medium was measured using an azocasein assay as described previously (52). One azocasein unit is the amount of enzyme that produces an increase of 0.01 optical density units in this assay.
Actin Cross-Linking Assay.
This assay was performed as previously described (16). Briefly, J774 cells were seeded into six-well tissue culture plates at a density of 106 cells per well. After 16 h incubation at 37 °C, cells were infected with various V. cholerae strains at a multiplicity of infection of 10 for 3 h. Cells were harvested in 50 μL of 2× loading buffer. Twenty-five microliters of each sample was analyzed by Western blot.
Infant Rabbit Model.
Infant rabbits were infected with various V. cholerae as described previously (35, 36). Briefly, 2- to 3-d-old infant rabbits were given cimetidine 2–3 h before orogastric inoculation of V. cholerae (1 × 1010 cfu/rabbit). Rabbits were monitored twice daily for signs of illness. Fecal material consistent of loose adherent stools, accumulated in the areas of perineum, hind legs, and tail, was defined as fecal diarrhea. At day 3 postinoculation, rabbits were anesthetized and intestinal tissues were collected and fixed in 10% neutral-buffered formalin for H&E staining.
For quantitative RT-PCR (qRT-PCR), cDNA was synthesized with ImProm-II kit (Promega). Quantification of cDNA was performed by qRT-PCR (Applied Biosystems) using Sybrgreen PCR mix (Biorad). Relative expression levels were calculated using the ΔΔCt method, with normalization of individual expression to that of a housekeeping gene HPRT.
Infant Mouse Colonization Competition Assay.
The infant mouse colonization assay was performed as described (53). V. cholerae mutant strains (LacZ−) were mixed with the wild-type strain (LacZ+), and ≈105 cells were inoculated into 5-d-old CD-1 suckling mice. To examine the function of T6SS in colonization, ΔtsrAvask (LacZ−) and ΔtsrA (LacZ+) were used for infection.
Supplementary Material
Acknowledgments
Special thanks to Haopeng Rui and Matthew Waldor (Brigham and Women’s Hospital, Boston) for providing strain Δctx fla and to Jennifer Ritchie for providing training in the use of the infant rabbit model. This work was supported by grant AI-018045 to J.J.M. from the National Institute of Allergy and Infectious Diseases.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1014998107/-/DCSupplemental.
References
- 1.Faruque SM, Albert MJ, Mekalanos JJ. Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. Microbiol Mol Biol Rev. 1998;62:1301–1314. doi: 10.1128/mmbr.62.4.1301-1314.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freter R, O'Brien PC, Macsai MS. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: In vivo studies. Infect Immun. 1981;34:234–240. doi: 10.1128/iai.34.1.234-240.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor RK, Miller VL, Furlong DB, Mekalanos JJ. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci USA. 1987;84:2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thelin KH, Taylor RK. Toxin-coregulated pilus, but not mannose-sensitive hemagglutinin, is required for colonization by Vibrio cholerae O1 El Tor biotype and O139 strains. Infect Immun. 1996;64:2853–2856. doi: 10.1128/iai.64.7.2853-2856.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herrington DA, et al. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J Exp Med. 1988;168:1487–1492. doi: 10.1084/jem.168.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tacket CO, et al. Investigation of the roles of toxin-coregulated pili and mannose-sensitive hemagglutinin pili in the pathogenesis of Vibrio cholerae O139 infection. Infect Immun. 1998;66:692–695. doi: 10.1128/iai.66.2.692-695.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiRita VJ. Co-ordinate expression of virulence genes by ToxR in Vibrio cholerae. Mol Microbiol. 1992;6:451–458. doi: 10.1111/j.1365-2958.1992.tb01489.x. [DOI] [PubMed] [Google Scholar]
- 8.Häse CC, Mekalanos JJ. TcpP protein is a positive regulator of virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci USA. 1998;95:730–734. doi: 10.1073/pnas.95.2.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skorupski K, Taylor RK. A new level in the Vibrio cholerae ToxR virulence cascade: AphA is required for transcriptional activation of the tcpPH operon. Mol Microbiol. 1999;31:763–771. doi: 10.1046/j.1365-2958.1999.01215.x. [DOI] [PubMed] [Google Scholar]
- 10.Miller MB, Skorupski K, Lenz DH, Taylor RK, Bassler BL. Parallel quorum sensing systems converge to regulate virulence in Vibrio cholerae. Cell. 2002;110:303–314. doi: 10.1016/s0092-8674(02)00829-2. [DOI] [PubMed] [Google Scholar]
- 11.Zhu J, et al. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci USA. 2002;99:3129–3134. doi: 10.1073/pnas.052694299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jayaraman A, Wood TK. Bacterial quorum sensing: Signals, circuits, and implications for biofilms and disease. Annu Rev Biomed Eng. 2008;10:145–167. doi: 10.1146/annurev.bioeng.10.061807.160536. [DOI] [PubMed] [Google Scholar]
- 13.Lenz DH, et al. The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell. 2004;118:69–82. doi: 10.1016/j.cell.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Kovacikova G, Skorupski K. Regulation of virulence gene expression in Vibrio cholerae by quorum sensing: HapR functions at the aphA promoter. Mol Microbiol. 2002;46:1135–1147. doi: 10.1046/j.1365-2958.2002.03229.x. [DOI] [PubMed] [Google Scholar]
- 15.Finkelstein RA, Boesman-Finkelstein M, Chang Y, Häse CC. Vibrio cholerae hemagglutinin/protease, colonial variation, virulence, and detachment. Infect Immun. 1992;60:472–478. doi: 10.1128/iai.60.2.472-478.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pukatzki S, et al. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc Natl Acad Sci USA. 2006;103:1528–1533. doi: 10.1073/pnas.0510322103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mougous JD, et al. A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science. 2006;312:1526–1530. doi: 10.1126/science.1128393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng J, Leung KY. Dissection of a type VI secretion system in Edwardsiella tarda. Mol Microbiol. 2007;66:1192–1206. doi: 10.1111/j.1365-2958.2007.05993.x. [DOI] [PubMed] [Google Scholar]
- 19.Rao PS, Yamada Y, Tan YP, Leung KY. Use of proteomics to identify novel virulence determinants that are required for Edwardsiella tarda pathogenesis. Mol Microbiol. 2004;53:573–586. doi: 10.1111/j.1365-2958.2004.04123.x. [DOI] [PubMed] [Google Scholar]
- 20.Aubert DF, Flannagan RS, Valvano MA. A novel sensor kinase-response regulator hybrid controls biofilm formation and type VI secretion system activity in Burkholderia cenocepacia. Infect Immun. 2008;76:1979–1991. doi: 10.1128/IAI.01338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schell MA, et al. Type VI secretion is a major virulence determinant in Burkholderia mallei. Mol Microbiol. 2007;64:1466–1485. doi: 10.1111/j.1365-2958.2007.05734.x. [DOI] [PubMed] [Google Scholar]
- 22.Pilatz S, et al. Identification of Burkholderia pseudomallei genes required for the intracellular life cycle and in vivo virulence. Infect Immun. 2006;74:3576–3586. doi: 10.1128/IAI.01262-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Filloux A. The type VI secretion system: A tubular story. EMBO J. 2009;28:309–310. doi: 10.1038/emboj.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bingle LE, Bailey CM, Pallen MJ. Type VI secretion: A beginner's guide. Curr Opin Microbiol. 2008;11:3–8. doi: 10.1016/j.mib.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Cascales E. The type VI secretion toolkit. EMBO Rep. 2008;9:735–741. doi: 10.1038/embor.2008.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma AT, Mekalanos JJ. In vivo actin cross-linking induced by Vibrio cholerae type VI secretion system is associated with intestinal inflammation. Proc Natl Acad Sci USA. 2010;107:4365–4370. doi: 10.1073/pnas.0915156107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pukatzki S, Ma AT, Revel AT, Sturtevant D, Mekalanos JJ. Type VI secretion system translocates a phage tail spike-like protein into target cells where it cross-links actin. Proc Natl Acad Sci USA. 2007;104:15508–15513. doi: 10.1073/pnas.0706532104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leiman PG, et al. Type VI secretion apparatus and phage tail-associated protein complexes share a common evolutionary origin. Proc Natl Acad Sci USA. 2009;106:4154–4159. doi: 10.1073/pnas.0813360106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheahan KL, Cordero CL, Satchell KJ. Identification of a domain within the multifunctional Vibrio cholerae RTX toxin that covalently cross-links actin. Proc Natl Acad Sci USA. 2004;101:9798–9803. doi: 10.1073/pnas.0401104101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma AT, McAuley S, Pukatzki S, Mekalanos JJ. Translocation of a Vibrio cholerae type VI secretion effector requires bacterial endocytosis by host cells. Cell Host Microbe. 2009;5:234–243. doi: 10.1016/j.chom.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishikawa T, Rompikuntal PK, Lindmark B, Milton DL, Wai SN. Quorum sensing regulation of the two hcp alleles in Vibrio cholerae O1 strains. PLoS ONE. 2009;4:e6734. doi: 10.1371/journal.pone.0006734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ingram DT, et al. Development of a colony lift immunoassay to facilitate rapid detection and quantification of Escherichia coli O157:H7 from agar plates and filter monitor membranes. Clin Diagn Lab Immunol. 1998;5:567–573. doi: 10.1128/cdli.5.4.567-573.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cameron DE, Urbach JM, Mekalanos JJ. A defined transposon mutant library and its use in identifying motility genes in Vibrio cholerae. Proc Natl Acad Sci USA. 2008;105:8736–8741. doi: 10.1073/pnas.0803281105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Silva RS, et al. Crystal structure of the Vibrio cholerae quorum-sensing regulatory protein HapR. J Bacteriol. 2007;189:5683–5691. doi: 10.1128/JB.01807-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rui H, et al. Reactogenicity of live-attenuated Vibrio cholerae vaccines is dependent on flagellins. Proc Natl Acad Sci USA. 2010;107:4359–4364. doi: 10.1073/pnas.0915164107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ritchie JM, Rui H, Bronson RT, Waldor MK. Back to the future: Studying cholera pathogenesis using infant rabbits. MBio. 2010;1:e00047–e00010. doi: 10.1128/mBio.00047-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Filloux A, Hachani A, Bleves S. The bacterial type VI secretion machine: Yet another player for protein transport across membranes. Microbiology. 2008;154:1570–1583. doi: 10.1099/mic.0.2008/016840-0. [DOI] [PubMed] [Google Scholar]
- 38.Shalom G, Shaw JG, Thomas MS. In vivo expression technology identifies a type VI secretion system locus in Burkholderia pseudomallei that is induced upon invasion of macrophages. Microbiology. 2007;153:2689–2699. doi: 10.1099/mic.0.2007/006585-0. [DOI] [PubMed] [Google Scholar]
- 39.Mattinen L, Nissinen R, Riipi T, Kalkkinen N, Pirhonen M. Host-extract induced changes in the secretome of the plant pathogenic bacterium Pectobacterium atrosepticum. Proteomics. 2007;7:3527–3537. doi: 10.1002/pmic.200600759. [DOI] [PubMed] [Google Scholar]
- 40.Zhu J, Mekalanos JJ. Quorum sensing-dependent biofilms enhance colonization in Vibrio cholerae. Dev Cell. 2003;5:647–656. doi: 10.1016/s1534-5807(03)00295-8. [DOI] [PubMed] [Google Scholar]
- 41.Nielsen AT, et al. RpoS controls the Vibrio cholerae mucosal escape response. PLoS Pathog. 2006;2:e109. doi: 10.1371/journal.ppat.0020109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jobling MG, Holmes RK. Characterization of hapR, a positive regulator of the Vibrio cholerae HA/protease gene hap, and its identification as a functional homologue of the Vibrio harveyi luxR gene. Mol Microbiol. 1997;26:1023–1034. doi: 10.1046/j.1365-2958.1997.6402011.x. [DOI] [PubMed] [Google Scholar]
- 43.Tsou AM, Cai T, Liu Z, Zhu J, Kulkarni RV. Regulatory targets of quorum sensing in Vibrio cholerae: Evidence for two distinct HapR-binding motifs. Nucleic Acids Res. 2009;37:2747–2756. doi: 10.1093/nar/gkp121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dorman CJ. H-NS: A universal regulator for a dynamic genome. Nat Rev Microbiol. 2004;2:391–400. doi: 10.1038/nrmicro883. [DOI] [PubMed] [Google Scholar]
- 45.Ghosh A, Paul K, Chowdhury R. Role of the histone-like nucleoid structuring protein in colonization, motility, and bile-dependent repression of virulence gene expression in Vibrio cholerae. Infect Immun. 2006;74:3060–3064. doi: 10.1128/IAI.74.5.3060-3064.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silva AJ, Sultan SZ, Liang W, Benitez JA. Role of the histone-like nucleoid structuring protein in the regulation of rpoS and RpoS-dependent genes in Vibrio cholerae. J Bacteriol. 2008;190:7335–7345. doi: 10.1128/JB.00360-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nye MB, Pfau JD, Skorupski K, Taylor RK. Vibrio cholerae H-NS silences virulence gene expression at multiple steps in the ToxR regulatory cascade. J Bacteriol. 2000;182:4295–4303. doi: 10.1128/jb.182.15.4295-4303.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- 49.Zheng J, Tung SL, Leung KY. Regulation of a type III and a putative secretion system in Edwardsiella tarda by EsrC is under the control of a two-component system, EsrA-EsrB. Infect Immun. 2005;73:4127–4137. doi: 10.1128/IAI.73.7.4127-4137.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalogeraki VS, Winans SC. Suicide plasmids containing promoterless reporter genes can simultaneously disrupt and create fusions to target genes of diverse bacteria. Gene. 1997;188:69–75. doi: 10.1016/s0378-1119(96)00778-0. [DOI] [PubMed] [Google Scholar]
- 51.Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1972. pp. 352–355. [Google Scholar]
- 52.Mel SF, Fullner KJ, Wimer-Mackin S, Lencer WI, Mekalanos JJ. Association of protease activity in Vibrio cholerae vaccine strains with decreases in transcellular epithelial resistance of polarized T84 intestinal epithelial cells. Infect Immun. 2000;68:6487–6492. doi: 10.1128/iai.68.11.6487-6492.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gardel CL, Mekalanos JJ. Alterations in Vibrio cholerae motility phenotypes correlate with changes in virulence factor expression. Infect Immun. 1996;64:2246–2255. doi: 10.1128/iai.64.6.2246-2255.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.