Abstract
A large proportion of the global soil carbon pool is stored in soils of high-latitude ecosystems in which microbial processes and production of greenhouse gases proceed during the winter months. It has been suggested that microorganisms have limited ability to sequester substrates at temperatures around and below 0 °C and that a metabolic shift to dominance of catabolic processes occurs around these temperatures. However, there are contrary indications that anabolic processes can proceed, because microbial growth has been observed at far lower temperatures. Therefore, we investigated the utilization of the microbial substrate under unfrozen and frozen conditions in a boreal forest soil across a temperature range from −9 °C to +9 °C, by using gas chromatography-isotopic ratio mass spectrometry and 13C magic-angle spinning NMR spectroscopy to determine microbial turnover and incorporation of 13C-labeled glucose. Our results conclusively demonstrate that the soil microorganisms maintain both catabolic (CO2 production) and anabolic (biomass synthesis) processes under frozen conditions and that no significant differences in carbon allocation from [13C]glucose into [13C]CO2 and cell organic 13C-compounds occurred between +9 °C and −4 °C. The only significant metabolic changes detected were increased fluidity of the cell membranes synthesized at frozen conditions and increased production of glycerol in the frozen samples. The finding that the processes in frozen soil are similar to those in unfrozen soil has important implications for our general understanding and conceptualization of soil carbon dynamics in high-latitude ecosystems.
Keywords: soil organic matter mineralization, 13CO2, 13C-NMR
About 40% of the earth's soil carbon pool is stored in high-latitude ecosystems (1), and boreal forest covers a large part of this area. Microbial processes and production of greenhouse gases continue during the winter months in soils of high-latitude regions (2–6), and a significant fraction of the carbon fixed during the growing season can be lost during the following winter (7, 8). Hence, knowledge about winter microbial processes is essential for understanding carbon mineralization in these ecosystems and its coupling to climate change.
The amount of unfrozen water in frozen soil has been found to be an important control for microbial processes at subzero temperatures (3) because low liquid water contents, like dry conditions, also affect the diffusion rates, and thus the supply, of substrates (9). When soil freezes, the liquid water content is reduced (10), and the solutes in the liquid water phase must be concentrated for the unfrozen water to reach the required water potential for a specific subzero temperature (11). That soil microbial catabolic processes occur in frozen soils is known through the detection of biogenic CO2 production (2, 3, 12), but knowledge about anabolic processes and the partitioning of the carbon flow between catabolic and anabolic processes in frozen soil is less complete.
It has been suggested that at temperatures below their optimum microbes have increasing difficulty in sequestering substrates from their environment (13) and that the soil microbial community undergoes a shift from growth to maintenance-related metabolism when the soil freezes. This shift would markedly reduce the metabolic carbon allocation to biomass synthesis through anabolic processes (14). However, microbial CO2 production has been detected at temperatures down to −39 °C in frozen surface horizons of tundra (4), and anabolic activity has been determined in bacterial populations from permafrost down to −6 °C (15) and −20 °C (16). It also has been suggested that there is no evidence of a minimum temperature for the metabolism of microbes in permafrost and ice (17). For cell membranes to be physiologically active under such extreme conditions, the acyl chains of the membrane lipids must maintain a liquid-like state to keep the membrane proteins active. Both bacteria and fungi adjust their membrane composition in response to changes in temperature >0 °C in ways that maintain a lamellar liquid crystalline phase (18). This adjustment requires anabolic processes.
Several studies have investigated differences in soil microbial biomass and soil metabolic activity (e.g., incorporation of 14C-labeled acetate into lipids) at temperatures ≤0 °C (16, 19), but, as far as we know, none of these studies has distinguished between catabolic and anabolic processes. The objectives of the this study were to determine the potential metabolic activity of soil microbial communities in frozen soils to assess the relative rates of anabolic and catabolic processes and to identify compounds synthesized across a temperature range from −9 °C to +9 °C. To do so, we used gas chromatography-isotopic ratio mass spectrometry (GC-IRMS) and 13C magic-angle spinning NMR (13C MAS NMR) spectroscopic evaluation of the microbial turnover of 13C-labeled glucose, together with appropriate amounts of nitrogen and phosphorus, in samples of the organic surface layer of a boreal forest soil incubated at freezing and nonfreezing temperatures. We hypothesized that both catabolic and anabolic processes could proceed under frozen conditions.
Results
[13C]CO2 Production Rates.
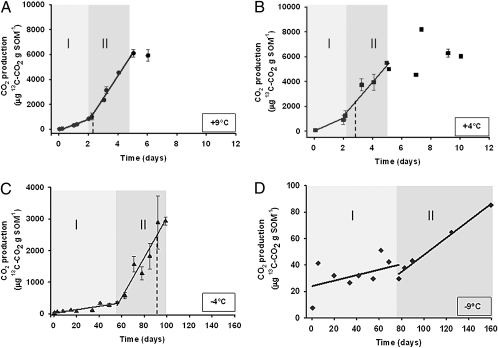
Biogenic [13C]CO2 was produced in soil samples incubated at +9 °C, +4 °C, and −4 °C, but at −9 °C production rates bordered on the detection limits of the experimental set-up. Two phases with different rates of [13C]CO2 production were identified at the three higher temperatures, but with differing time constants (Fig. 1 and Table S1). Production rates in phase 1 were 0.44, 0.52, and 0.006 mg 13C g soil organic matter (SOM) · d−1 at +9 °C, +4 °C, and −4 °C, respectively. Corresponding rates for phase 2 were 1.82, 1.36, and 0.06 mg 13C g SOM · d−1. Phase 1 lasted for about 2 d at temperatures above 0 °C and for ca. 60 d at −4 °C. The relative increase in production rates in phase 2 was 4.1, 2.6, and 11.4 times that of phase 1 at +9 °C, +4 °C, and −4 °C, respectively. The relative increase is based on exact figures (Table S1).
Fig. 1.
(A–D) Accumulation of [13C]CO2 from the incubated boreal forest soil at each of the four incubation temperatures [+9 °C (A); +4 °C (B); −4 °C (C); and −9 °C (D)] as a function of time. Areas shaded in gray indicate the different phases (Methods) identified during incubation at each of the temperatures, and the solid lines represent the linear regressions used to derive the [13C]CO2 production rates. The dotted lines indicate the times when 50% of the glucose remained. Data on the r2 and P values of the linear regressions are presented in Table S1.
Compounds Derived from Biogenic Glucose Utilization.
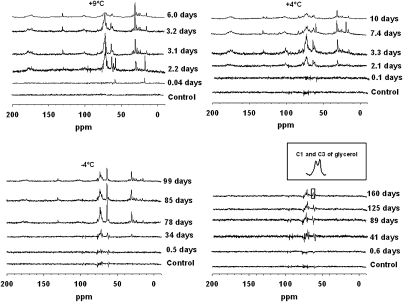
Several anabolic 13C-compounds were synthesized in the incubated soil samples. These compounds were mainly glycerol, phospholipids, polymeric carbohydrates (e.g., glycogen), protein compounds, and ethanol. The chemical shifts of the acyl region of the lipids (SI Methods) in the spectra we acquired showed that the formed fatty acids were ester bound to a backbone (Fig. 2). The chemical shifts corresponded well to those of the phospholipid dioleoylphosphatidylcholine but did not coincide with shifts of free fatty acids. At all the investigated temperatures formation of glycerol was observed (at ∼63.6 ppm; Fig. 2). At +9 °C, ethanol (∼C1 58.5 ppm and C2 17.9 ppm) and glycerol (∼63.6 ppm and ∼72.4 ppm) were formed after 2–3 d, but both these compounds decreased at the end of the incubation period. At +4 °C, glycerol was formed after ca. 3 d but decreased after 7 d, when ethanol was detected; ethanol subsequently declined to subdetectable levels by the end of the incubation period. At −4 °C, glycerol was first detected after 34 d and was still present at the end of the incubation period (day 99). At −9 °C, formation of glycerol was detected at the end of the incubation period (day 160, Fig. 2), but it could not be quantified significantly because of the low signal-to-noise ratio. Signals corresponding to glycogen were observed (∼60–100 ppm), especially in the final +9 °C spectrum, indicating that glycogen was formed as a storage compound.
Fig. 2.
13C MAS NMR spectra of the incubated soil samples acquired during the course of the incubations, after subtracting the glucose signal in the 60- to100-ppm region. Negative and dispersive signals originate from imperfect subtraction of a glucose reference spectrum (SI Methods) and should be ignored. The Inset in the −9 °C figure (Lower Right) shows the doublet signal at ∼63.6 ppm representing C1 and C3 of glycerol.
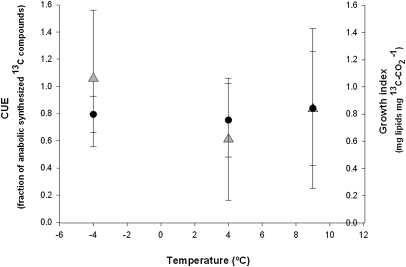
The time corresponding to the consumption of 50% of the added glucose was estimated to be 2.5 d at +9 °C, 2.9 d at +4 °C, and 93.7 d at −4 °C. At this point, the partitioning of glucose into [13C]CO2 and organic 13C-compounds (Fig. 3) did not differ significantly among the investigated temperatures, although the proportions of glycerol were significantly higher in the samples incubated at −4 °C than in those incubated at temperatures >0 °C (P ≤ 0.05; 95% t test; Table 1). The carbon use efficiency (CUE) index and growth index (amount of membrane phospholipids formed per amount of [13CO2]carbon produced) were determined at the different temperatures at the time when 50% of the glucose had been consumed. These indexes were not significantly different, and no shift in the carbon allocation pattern was observed between the unfrozen and frozen samples (Fig. 3).
Fig. 3.
Interpolated values of CUE and growth index at +9 °C, +4 °C, and −4 °C at the time when 50% of the glucose was consumed. Black dots indicate CUE indexes, and gray triangles indicate growth indexes. Error bars show statistical estimated SE; n = 3.
Table 1.
The proportion of synthesized 13C-compounds detected by NMR at the time of 50% glucose consumption
| Temperature (°C) | Time (days) | Glycerol % (SE)*,† | Phospholipids % (SE)*,‡ | Polymeric carbohydrates % (SE)* | Proteins % (SE)* | Ethanol % (SE)* | Total %¶ | 13C (SE)§ {from[13C]CO2) |
| 9 | 2.5 | 11 (0.003) | 16 (0.009) | 63 (< 0.001) | 4 (0.007) | 3 (< 0.001) | 97 | 1.57 (0.75) |
| 4 | 2.9 | 10 (0.252) | 20 (0.144) | 57 (0.179) | 9 (0.281) | 2 (0.282) | 99 | 2.87 (0.90) |
| −4 | 93.7 | 21 (< 0.001) | 28 (< 0.001) | 54 (< 0.001) | 5 (< 0.001) | 2 (< 0.001) | 110 | 2.53 (0.08) |
SEs are estimated.
*Percent of the total synthesized 13C-compounds at the time when 50% of the added glucose was left.
†Corresponds to C1 and C3 in glycerol.
‡Corresponds to the acyl chain of phospholipids. The synthesized 13C-compounds at the time when 50% of the added glucose was left could not be estimated at −9 °C.
¶Total % of the interpolated fractions of synthesized 13C-compounds at the time when 50% of the added glucose was left.
§mg 13C (from [13C]CO2 /g SOM).
Length of Acyl Chains and Degree of Unsaturation in Lipids.
The average number of double bonds (C = C) per acyl chain in the samples at the end of the incubations (± SE) was estimated to 0.43 ± 0.04, 0.32 ± 0.05, and 0.69 ± 0.05 at +9 °C, +4 °C, and −4 °C, respectively. Hence, the degree of unsaturation in the membrane lipids was significantly higher at −4 °C than at either +4 °C or +9 °C (95% t test). At +9 °C and +4 °C the acyl chains were, on average, significantly (95% t test) shorter than at −4 °C (13.7 ± 0.44, 13.0 ± 0.07, 16.6 ± 0.36 at +9 °C, +4 °C, and −4 °C, respectively).
Carbon Budget.
To assess the recovery of added [13C]glucose as either [13C]CO2 or 13C-organic compounds, [13C]carbon mass balances were calculated for the samples incubated at each of the temperatures (Table S2). The mean ratio of measured 13C/added 13C for all samplings was 1.07 ± 0.05. However, on three sampling occasions the sum of measured 13C-compounds accounted for less than 80% of the initially added amount of [13C]glucose.
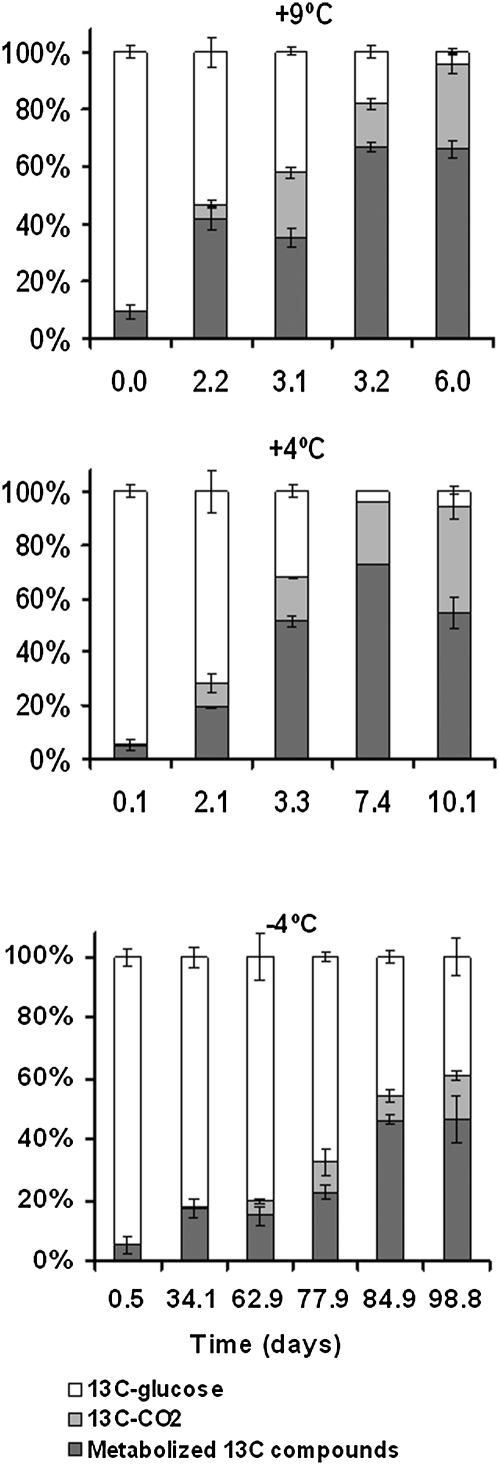
At +9 °C and +4 °C most of the added glucose was consumed during incubation (Fig. 4). At the end of the incubation at +9 °C, 30% of the added [13C]glucose had been respired as [13C]CO2, 66% had been incorporated in organic 13C-compounds, and 4% remained as glucose. At the end of the +4 °C incubation, 39% of the added glucose had been respired as [13C]CO2, 56% had been incorporated in 13C-compounds, and 6% remained as glucose. At the end of the −4 °C incubation, 14% of the added glucose had been respired as [13C]CO2, 47% had been used for organic 13C-compounds, and 39% remained as glucose (Fig. 4). Given the low activities at −9 °C, no significant consumption of added glucose could be detected.
Fig. 4.
The proportions of [13C]glucose, [13C]CO2 (originating from catabolic processes), and new 13C-compounds (originating from anabolic processes) in samples during incubation at 9 °C, +4 °C, and −4 °C. Error bars indicate SE.
Discussion
Our results provide conclusive evidence that not only catabolic processes (CO2 production) but also anabolic microbial processes (synthesis of biomass) proceed below 0 °C in frozen boreal forest soils. It has been suggested that no (or highly limited) microbial growth can take place at temperatures <0 °C because the severely limited fluidity of the cell membrane at low temperatures inhibits the utilization of substrates from the environment (13). It also has been suggested that the soil microbial community undergoes a shift from growth to survival-related metabolism, with decreased carbon allocation to anabolic processes, when subjected to various stresses, including freezing (14). This suggestion is refuted by our results, which show that the soil microorganisms in frozen soil readily use substrates from their environment for both catabolic and anabolic purposes.
[13C]CO2 Production.
At temperatures ranging −4 °C to +9 °C, the determined [13C]CO2 production rates from the added glucose are in the same range as previously reported CO2 production rates after sugar additions to soil incubations at similar temperatures (6, 20). However, the [13C]CO2 production rate at −4 °C increased after 60 d to about 11 times the initial rate. This result strongly suggests that experiments with commonly applied incubation times (12) of days or weeks may severely underestimate the potential for CO2 production at freezing temperatures. Conceivably, the period of low respiration represents an induction period during which the microbial community adapts to the degradation of glucose at temperatures <0 °C. Winter soil respiration has been shown to respond rapidly to increases in labile carbon availability (21), and this response also was observed in our study, because utilization of the added glucose commenced within days at −4 °C. Under field conditions, roots contribute significantly to the production of readily available carbon substrates (exudates) (22), and the soil sucrose concentration has been shown to be eight times higher during the winter (beneath snow) than in summer and spring (23). It has been suggested that this increased sucrose concentration depends on mechanical damage from soil frost of shallow roots, in which sucrose is produced as a low-temperature protectant for sensitive tissues (23). The fact that the microbial community was able to adapt to a colder environment indicates that more of the winter CO2 flux may come from carbon mineralization of soils than estimated from short-term incubation experiments.
Our results indicate that carbon mineralization in boreal forest soils at −9 °C is insignificant in relation to carbon mineralization at −4 °C, supporting previous investigations reporting strong temperature sensitivities in CO2 production in frozen soils (7, 24).
Glucose Transformation and Synthesis of 13C-Compounds.
At the time when 50% of the added glucose remained, we observed no significant differences in relative 13C allocation patterns during metabolism between samples incubated at +9 °C and −4 °C, although the processes were slower in the frozen soil matrix. This finding contradicts the general view of microbial processes in low-temperature soil, i.e., that catabolic processes become more prominent, in relation to anabolic processes, as the soil freezes and substrate diffusion and membrane fluidity become limited (6, 13, 14). However, we observed a response in the phospholipid composition as a result of freezing. The ability of the microorganisms to continue to grow in frozen soil may be related to the observed responses in their phospholipid composition to freezing. Common features of these temperature responses include changes in the ratio of saturated to unsaturated fatty acids in the membrane lipids and in the length of the fatty acid chains (18, 25). These features can be discerned in our results showing that the average degree of unsaturation of the acyl chains in the membrane lipids was higher at −4 °C than at temperatures above 0 °C, indicating that fluidity of the membrane lipids was maintained at −4 °C.
Based on data obtained from the samples incubated at −4 °C, we estimated (see SI Methods for details) that the membrane lipids in the samples would be transformed into a more rigid gel state at ca. −24 °C. Small changes in the composition of the membrane lipids can change the transition temperature over a range of 100 °C (26); hence, minor adjustments of membrane composition should allow microbial activity to occur even at extremely low temperatures (4, 17).
The observed changes in lipid composition in response to temperature changes could be caused by adaptations in one or a few specific organisms. Alternatively, the changes could be caused by the selection of microorganism species occurring at lower temperatures. We cannot couple the observed change in lipid composition to specific organisms or populations and therefore cannot distinguish between these possibilities. However, we have shown a significant shift in the lipid composition of the cell membrane as a result of freezing, and this shift may explain the observed lag-phase in the frozen samples.
In addition to changes in the membrane lipid composition, we also observed differences in the production of glycerol. Glycerol is best known as an antifreeze medium, but it also serves as a substrate for certain microorganisms at low temperatures (27, 28). The production of glycerol at −4 °C coincided with the increase in CO2 production and the synthesis of phospholipids that occurred after the lag-phase. However, the level of glycerol was insignificant at the start of the incubation period at −4 °C, when only catabolic processes (CO2 production) were detected. The high level of glycerol detected at the end of the incubation at −4 °C could reflect its use as an antifreeze agent that limits intracellular cell damage caused by freezing and hence maintains microbial activity and CO2 production at subzero temperatures. This use would induce a positive feedback reaction of microbial activity under frozen condition and illustrates the important ability of the soil microbial community to maintain metabolic activity at frozen conditions.
However, the temperature is likely to decrease more gradually under field conditions than in our experiments, and adaptation to gradual changes is likely to be easier than adaptation to sudden shifts conceivably affecting the length of the lag-phase.
Carbon Budget.
The mean ratio of the measured 13C to the added 13C for all incubation temperatures indicated that we closed the 13C mass balance and were able to keep track of all 13C-compounds with an uncertainty (SE) of about 5%. When half the glucose remained, the proportion of phospholipids in the synthesized 13C-compounds was higher at −4 °C than at temperatures >0 °C, suggesting that the viable microbial community is capable of cell membrane synthesis in frozen soil. It is possible that the total amount of phospholipids might change without changes in total microbial biomass. However, measurements of total amounts of phospholipids (or the amount of phosphate in the phospholipid fraction) in soil are well recognized as reflecting the microbial biomass (29–31). These observations of the active synthesis of cell membrane fatty acids also support other studies in which both fungal and bacterial total biomass values have been found to be higher under snow-covered soil than in summer, reflecting significant anabolic activity but also suggesting microbial growth during the winter months (5, 19, 32). Previous studies also have indicated that patterns of trace gas fluxes from soils during winter are consistent with a transition in metabolism from aerobic to anaerobic (33, 34), a transition that indicates an active response of the microbial community involving changes in enzymatic expressions and metabolic pathways requiring anabolic processes. However, depending on the insulating capacity of the accumulated snowpack, winter conditions do not necessarily imply frozen soils. Nonetheless, we can conclude that the previously observed increases in microbial biomass under snow also may occur if the soil is frozen. In addition, our findings contradict earlier studies suggesting that the microbes shift resources to predominately catabolic processes at subzero temperatures (14).
We conclude that both catabolic and anabolic processes can proceed in boreal forest soils at typical winter soil temperatures. Furthermore, no clear differences in carbon allocation during glucose metabolism were observed at the various temperatures, refuting the conclusion from previous investigations that a shift toward catabolic processes occurs when soil is subjected to freezing. However, the extent to which the microbial community can use more complex substrates (polymers) and the extent to which freezing may select for certain organisms or groups of organisms remain to be resolved. The ability for microbial biosynthesis and growth to continue, in combination with the strong temperature sensitivity at temperatures <0 °C (9, 24), also has implications for how microbial communities (e.g., those in permafrost soils) may respond to relatively small increases in temperature.
Methods
Soil Samples.
The materials examined in this investigation were collected from a site in the central boreal climate zone of northern Sweden (64°11′ N, 19°35′ E) at the end of October 2008. The site was dominated by spruce (Picea abies, L.) with sparse pine (Pinus sylvestris, L.). The soil type was Typic Haplocryods (35). The investigations were confined to the organic (O)-horizon (96% OM). A more detailed description of the methods is found in the SI Methods.
Soil Incubation.
On collection, roots, coarse debris, and contemporary (i.e., green) plant debris were removed manually. The soils were homogenized by passing through a cutting sieve (6 × 3.5 mm mesh) and stored at −20 °C until sample preparation. The frozen soil was thawed to ca. +4 °C, and field-moist samples of soil were transferred to glass bottles. A [13C]glucose solution with nitrogen and phosphorus (1, 1/13, 1/18) was prepared and added to the soil samples in amounts corresponding to 40 mg [13C]glucose/g dry SOM (36, 37), and the water content of the soil was adjusted to 550% H2O/g SOM (38). We incubated the samples at +9 °C and +4 °C for 6 and 10 d, respectively, and the samples at −4 °C and −9 °C for 99 d and 160 d, respectively. The [13C]CO2 content in gas samples from the incubations was analyzed by GC-IRMS. No evidence of nonbiological processes was detected after sterilization with NaN3.
NMR.
The synthesized anabolic compounds formed after addition of [13C]glucose were studied using 13C MAS NMR spectroscopy. All NMR spectra were acquired with a 500 spectrometer (Avance DRX; Bruker). After each CO2 sampling, the microbial activity (n = 3) was terminated by adding 0.5% NaN3, and 13C NMR spectra were obtained on wet samples. Spectra of the metabolites formed in the unsterilized samples were obtained by subtracting a glucose spectrum from them. A sample from the −4 °C incubation was analyzed by 13C-solution NMR to identify a 13C MAS peak at 63.6 ppm (C1 and C3 of glycerol).
Identification of Compounds Measured by 13C MAS NMR.
We defined the total microbial synthesized compounds as the 13C-compounds synthesized from the added [13C]glucose and measurable by NMR. Several references were used to identify the newly synthesized 13C-compounds derived from microbial [13C]glucose transformation (39–43). The chemical shifts of our spectra were compared with the chemical shifts in the references (see above) and identified with respect to chemical shifts, intensities, and coupling patterns. We identified the NMR-measured lipids as membrane phospholipids. More information about the identification of the synthesized 13C-compounds and the chemical shifts of the acyl region of the lipids can be found in the SI Methods. The average degree of unsaturation of the membrane lipids and the average length of the acyl chains in the lipids were determined from the NMR data.
Data Evaluation.
The [13C]CO2 production rates of the initial rate (phase 1) and the highest observed rate (phase 2) at each temperature were determined from linear regressions. Changes in the allocation of carbon between catabolic and anabolic processes over time at each investigated temperature were estimated by calculating the relative (%) proportions of [13C]glucose, [13C]CO2, and 13C-compounds formed from anabolic processes as a function of time. The proportions of the synthesized 13C-compounds measured by 13C MAS NMR were estimated by linear interpolation at the times when 50% of the added glucose was left in the samples incubated at each of the temperatures (Table 1). We estimated the variances (reported as SEs) for the interpolated values of each compound, and the effect of covariance between the data points were included in these estimations. The CUE of the microbial populations incubated at each of the temperatures was then determined at the times when 50% glucose remained.
Supplementary Material
Acknowledgments
We acknowledge financial support from the Center for Environmental Research, the Swedish Research Council, the Swedish Reachers Council Formas, and the Kempe Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008885107/-/DCSupplemental.
References
- 1.IPCC . Land Use, Land-Use Change and Forestry—Summary for Policymakers. Cambridge: Cambridge Univ Press; 2000. [Google Scholar]
- 2.Oquist MG, et al. Nitrous oxide production in a forest soil at low temperatures - processes and environmental controls. FEMS Microbiol Ecol. 2004;49:371–378. doi: 10.1016/j.femsec.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Oquist MG, et al. Water availability controls microbial temperature responses in frozen soil CO2 production. Glob Change Biol. 2009;15:2715–2722. [Google Scholar]
- 4.Panikov NS, Flanagan PW, Oechel WC, Mastepanov MA, Christensen TR. Microbial activity in soils frozen to below -39 degrees C. Soil Biol Biochem. 2006;38:785–794. [Google Scholar]
- 5.Schadt CW, Martin AP, Lipson DA, Schmidt SK. Seasonal dynamics of previously unknown fungal lineages in tundra soils. Science. 2003;301:1359–1361. doi: 10.1126/science.1086940. [DOI] [PubMed] [Google Scholar]
- 6.Schimel JP, Mikan C. Changing microbial substrate use in Arctic tundra soils through a freeze-thaw cycle. Soil Biol Biochem. 2005;37:1411–1418. [Google Scholar]
- 7.Monson RK, et al. Winter forest soil respiration controlled by climate and microbial community composition. Nature. 2006;439:711–714. doi: 10.1038/nature04555. [DOI] [PubMed] [Google Scholar]
- 8.Sagerfors J, et al. Annual CO2 exchange between a nutrient-poor, minerotrophic, boreal mire and the atmosphere. Journal of Geophysical Research-Biogeosciences. 2008;113:1–15. [Google Scholar]
- 9.Davidson EA, Janssens IA. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature. 2006;440:165–173. doi: 10.1038/nature04514. [DOI] [PubMed] [Google Scholar]
- 10.Sparrman T, Öquist M, Klemedtsson L, Schleucher J, Nilsson M. Quantifying unfrozen water in frozen soil by high-field 2H NMR. Environ Sci Technol. 2004;38:5420–5425. doi: 10.1021/es0493695. [DOI] [PubMed] [Google Scholar]
- 11.Harrysson Drotz S, et al. Contributions of matric and osmotic potentials to the unfrozen water content of frozen soils. Geoderma. 2009;148:392–398. [Google Scholar]
- 12.Mikan CJ, Schimel JP, Doyle AP. Temperature controls of microbial respiration in arctic tundra soils above and below freezing. Soil Biol Biochem. 2002;34:1785–1795. [Google Scholar]
- 13.Nedwell DB. Effect of low temperature on microbial growth: Lowered affinity for substrates limits growth at low temperature. FEMS Microbiol Ecol. 1999;30:101–111. doi: 10.1111/j.1574-6941.1999.tb00639.x. [DOI] [PubMed] [Google Scholar]
- 14.Schimel J, Balser TC, Wallenstein M. Microbial stress-response physiology and its implications for ecosystem function. Ecology. 2007;88:1386–1394. doi: 10.1890/06-0219. [DOI] [PubMed] [Google Scholar]
- 15.Bergholz PW, Bakermans C, Tiedje JM. Psychrobacter arcticus 273-4 uses resource efficiency and molecular motion adaptations for subzero temperature growth. J Bacteriol. 2009;191:2340–2352. doi: 10.1128/JB.01377-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rivkina EM, Friedmann EI, McKay CP, Gilichinsky DA. Metabolic activity of permafrost bacteria below the freezing point. Appl Environ Microbiol. 2000;66:3230–3233. doi: 10.1128/aem.66.8.3230-3233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price PB, Sowers T. Temperature dependence of metabolic rates for microbial growth, maintenance, and survival. Proc Natl Acad Sci USA. 2004;101:4631–4636. doi: 10.1073/pnas.0400522101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rilfors L, Lindblom G. Regulation of lipid composition in biological membranes—biophysical studies of lipids and lipid synthesizing enzymes. Colloids Surf B Biointerfaces. 2002;26:112–124. [Google Scholar]
- 19.Lipson DA, Schmidt SK, Monson RK. Carbon availability and temperature control the post-snowmelt decline in alpine soil microbial biomass. Soil Biol Biochem. 2000;32:441–448. [Google Scholar]
- 20.Tilston EL, Sparrman T, Oquist MG. Unfrozen water content moderates temperature dependence of sub-zero microbial respiration. Soil Biol Biochem. 2010;42:1396–1407. [Google Scholar]
- 21.Brooks PD, McKnight D, Elder K. Carbon limitation of soil respiration under winter snowpacks: Potential feedbacks between growing season and winter carbon fluxes. Glob Change Biol. 2005;11:231–238. [Google Scholar]
- 22.Giesler R, et al. Production of dissolved organic carbon and low-molecular weight organic acids in soil solution driven by recent tree photosynthate. Biogeochemistry. 2007;84:1–12. [Google Scholar]
- 23.Scott-Denton LE, Rosenstiel TN, Monson RK. Differential controls by climate and substrate over the heterotrophic and rhizospheric components of soil respiration. Glob Change Biol. 2006;12:205–216. [Google Scholar]
- 24.Oquist MG, Laudon H. Winter soil frost conditions in boreal forests control growing season soil CO2 concentration and its atmospheric exchange. Glob Change Biol. 2008;14:2839–2847. [Google Scholar]
- 25.Morein S, Andersson AS, Rilfors L, Lindblom G. Wild-type Escherichia coli cells regulate the membrane lipid composition in a “window” between gel and non-lamellar structures. J Biol Chem. 1996;271:6801–6809. doi: 10.1074/jbc.271.12.6801. [DOI] [PubMed] [Google Scholar]
- 26.Mathews CK, van Holde KE, Ahern KG. Biochemistry. 3rd Ed. San Francisco: Robin Heyden; 2000. pp. 1–1186. [Google Scholar]
- 27.Breezee J, Cady N, Staley JT. Subfreezing growth of the sea ice bacterium “Psychromonas ingrahamii”. Microb Ecol. 2004;47:300–304. doi: 10.1007/s00248-003-1040-9. [DOI] [PubMed] [Google Scholar]
- 28.Panikov NS, Sizova MV. Growth kinetics of microorganisms isolated from Alaskan soil and permafrost in solid media frozen down to −35 degrees C. FEMS Microbiol Ecol. 2007;59:500–512. doi: 10.1111/j.1574-6941.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- 29.Frostegard A, Baath E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol Fertil Soils. 1996;22:59–65. [Google Scholar]
- 30.Sundh I, Borga P, Nilsson M, Svensson BH. Estimation of cell numbers of methanotrophic bacteria in boreal peatlands based on analysis of specific phospholipid fatty-acids. FEMS Microbiol Ecol. 1995;18:103–112. [Google Scholar]
- 31.White DC, Davis WM, Nickels JS, King JD, Bobbie RJ. Determination of the sedimentary microbial biomass by extractable lipid phosphate. Oecologia. 1979;40:51–62. doi: 10.1007/BF00388810. [DOI] [PubMed] [Google Scholar]
- 32.Brooks PD, Williams MW, Schmidt SK. Microbial activity under alpine snowpacks, Niwot Ridge, Colorado. Biogeochemistry. 1996;32:93–113. [Google Scholar]
- 33.Brooks PD, Schmidt SK, Williams MW. Winter production of CO2 and N2O from alpine tundra: Environmental controls and relationship to inter-system C and N fluxes. Oecologia. 1997;110:403–413. doi: 10.1007/PL00008814. [DOI] [PubMed] [Google Scholar]
- 34.Sommerfeld RA, Mosier AR, Musselman RC. CO2, CH4 and N2O flux through a wyoming snowpack and implications for global budgets. Nature. 1993;361:140–142. [Google Scholar]
- 35.Soil Survey Staff . Keys to Soil Taxonomy. 9th Ed. Blacksburg, VA: US Department of Agriculture, Pocahontas Press; 2003. p. 332. [Google Scholar]
- 36.Nordgren A. A method for determining microbially available N and P in organic soil. Biol Fertil Soils. 1992;13:195–199. [Google Scholar]
- 37.Schnürer Y. In fluence of soil properties and organic pesticides on soil microbial metabolism. Umeå, Sweden: Swedish University of Agricultural Sciences; 2006. PhD thesis. [Google Scholar]
- 38.Ilstedt U, Nordgren A, Malmer A. Optimum soil water for soil respiration before and after amendment with glucose in humid tropical acrisols and a boreal mor layer. Soil Biol Biochem. 2000;32:1591–1599. [Google Scholar]
- 39.Ulrich EL, et al. Biological Magnetic Resonance Data Bank. Nucleic Acids Res. 2007;36:402–408. doi: 10.1093/nar/gkac1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fan TWM, Lane AN. Structure-based profiling of metabolites and isotopomers by NMR. Prog Nucl Magn Reson Spectrosc. 2008;52:69–117. [Google Scholar]
- 41.Neurohr KJ, Gollin G, Neurohr JM, Rothman DL, Shulman RG. Carbon-13 nuclear magnetic resonance studies of myocardial glycogen metabolism in live guinea pigs. Biochemistry. 1984;23:5029–5035. doi: 10.1021/bi00316a031. [DOI] [PubMed] [Google Scholar]
- 42.Sattler M, Schleucher J, Griesinger C. Heteronuclear multidimensional NMR experiments for the structure determination of proteins in solution employing pulsed field gradients. Prog Nucl Magn Reson Spectrosc. 1999;34:93–158. [Google Scholar]
- 43.Strandberg E, Sparrman T, Lindblom G. Phase diagrams of systems with cationic α-helical membrane-spanning model peptides and dioleoylphosphatidylcholine. Adv Colloid Interface Sci. 2001;89-90:239–261. doi: 10.1016/s0001-8686(00)00056-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.