Abstract
Intestinal stem cells (ISCs) in the Drosophila adult midgut are essential for maintaining tissue homeostasis and replenishing lost cells in response to tissue damage. Here we demonstrate that the Hippo (Hpo) signaling pathway, an evolutionarily conserved pathway implicated in organ size control and tumorigenesis, plays an essential role in regulating ISC proliferation. Loss of Hpo signaling in either midgut precursor cells or epithelial cells stimulates ISC proliferation. We provide evidence that loss of Hpo signaling in epithelial cells increases the production of cytokines of the Upd family and multiple EGFR ligands that activate JAK-STAT and EGFR signaling pathways in ISCs to stimulate their proliferation, thus revealing a unique non–cell-autonomous role of Hpo signaling in blocking ISC proliferation. Finally, we show that the Hpo pathway mediator Yorkie (Yki) is also required in precursor cells for injury-induced ISC proliferation in response to tissue-damaging reagent DSS.
Keywords: Yap, Wts, regeneration
Adult stem cells play critical roles in maintaining tissue homeostasis throughout adult life. Proliferation of adult stem cells and differentiation of their progenies are under tight control to achieve the normal balance between removing dead cells and producing new cells, and disruption of the regulatory mechanisms could result in excessive proliferation of stem cells/progenitor cells, which eventually leads to tumor growth.
The Drosophila adult midgut has emerged as an attractive model to investigate how stem cell proliferation and differentiation are regulated not only because the cell lineage in this tissue is simple and well characterized but also because it bears similarities to the mammalian intestine (1). The Drosophila adult midgut contains intestine stem cells (ISCs) that are located adjacent to the basement membrane of the midgut epithelium (2, 3). The asymmetric division of an ISC produces a renewed ISC and an enteroblast (EB) that can undergo differentiation to become either absorptive enterocyte (EC) or secretory enteroendocrine cell (EE) (2, 3). Several conserved signaling pathways including Notch, Wingless (Wg)/Wnt, and JAK-STAT pathways regulate the maintenance, proliferation, and differentiation of ISCs (3–8). Moreover, tissue damage can induce ISC proliferation and differentiation to replenish damaged cells, and the JAK-STAT and Insulin pathways are the critical mediators of damage-induced ISC proliferation (6, 9–12). However, our understanding of ISC regulation is still incomplete, and it is likely that additional pathways may participate in the regulation of ISC proliferation and differentiation.
Initially discovered in Drosophila, the Hippo (Hpo) signaling pathway has emerged as an evolutionarily conserved pathway that controls cell growth, proliferation, and survival, and its abnormal activity has been linked to several types of cancer (13–15). The Hpo pathway acts through a kinase cascade consisting of an upstream kinase Hpo and a downstream kinase Warts (Wts) to restrict the activity of the transcriptional coactivator Yorkie (Yki) (13, 14, 16, 17). In the absence of Hpo signaling activity, Yki enters the nucleus and forms a complex with the TEAD family of transcription factor Scalloped (Sd) to activate genes involved in cell proliferation, cell growth, and apoptosis (18–20).
Although the Hpo pathway has been extensively studied in the Drosophila appendage development at the larval stage, its role in stem cell proliferation and adult tissue homeostasis has not been investigated. In this study, we demonstrate that the Hpo pathway restricts ISC proliferation in the adult midgut. We uncover a non–cell-autonomous mechanism by which the Hpo pathway negatively regulates stem cell proliferation by restricting the production of cytokines and mitogens that activate the JAK-STAT and EGFR pathways. In addition, we demonstrate that Yki is required in the precursor cells for dextran sulfate sodium (DSS)-induced ISC proliferation.
Results
Loss of Hpo Signaling in Precursor Cells Stimulates ISC Proliferation.
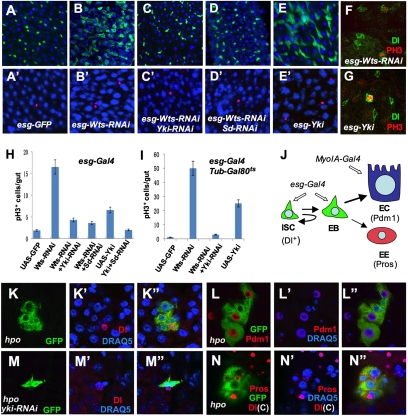
As an initial step to explore the role of Hpo signaling in midgut homeostasis, we expressed a Wts transgenic RNAi line (UAS-Wts-RNAi) along with UAS-GFP using esg-Gal4, which is specifically expressed in adult ISCs and EBs (collectively referred to as precursor cells; Fig. 1J). Wts knockdown clearly increased the number of esg-GFP+ cells (compare Fig. 1B with Fig. 1A), suggesting an expansion of the precursor cell population. Coexpression of UAS-Yki-RNAi or UAS-Sd-RNAi transgene with UAS-Wts-RNAi suppressed the increase in the number of esg-GFP+ cells (Fig.1 C and D), suggesting that Wts restricts the precursor cell population through inhibiting Yki/Sd. Immunostaining with an antibody against Phospho-Histone3 (PH3), a specific marker for mitotic cells, indicates that Wts RNAi guts contained increased PH3+ cells, and such increase was suppressed by knockdown of Yki or Sd (Fig. 1 B′–D′ and H). Overexpression of Yki also led to an increase in the number of esg-GFP+ and PH3+ cells (Fig. 1 E, E′, and H), which was suppressed by Sd RNAi (Fig. 1H). Previous studies indicate that ISCs are the only cells that can undergo cell division in the midgut (2, 3). Double labeling with Dl antibody, which specifically marks the ISCs (21), confirmed that PH3+ cells in Wts RNAi or Yki overexpression guts are ISCs (Fig. 1 F and G). Taken together, these results demonstrate that Wts restricts ISC proliferation by limiting the activity of Yki/Sd in precursor cells.
Fig. 1.
Hpo signaling restricts ISC proliferation by inhibiting Yki/Sd. (A–E′) Adult fly midguts, 3–5 d old, expressing esg-Gal4/UAS-GFP without (A and A′) or with UAS-Wts-RNAi (B and B′), UAS-Wts-RNAi + UAS-Yki-RNAi (C and C′), UAS-Wts-RNAi + UAS-Sd-RNAi (D and D′), or UAS-Yki (E and E′) were immunostained with GFP (green) and PH3 (red) antibodies, and a nuclear dye DRAQ5 (blue). (F and G). Adult midguts expressing esg-Wts-RNAi (F) or esg -Yki (G) were immunostained with Dl (green) and PH3 (red) antibodies. (H and I) Quantification of PH3+ cells in midguts from adults of the indicated genotypes (mean ± SD, n ≥ 15 for each genotype). (J) ISC lineage in Drosophila adult midguts. EB, enteroblast; EC, enterocyte; EE, enteroendocrine cell; ISC, intestine stem cell. (K–N′′) Adult midguts containing GFP-labeled hpo clones (K–L′′ and N–N′′) or hpo clones expressing Yki-RNAi (M-M′′) were immunostained to show the expression of GFP (green), Dl (cytoplasmic red in K–K′′ and M–N′′), Pdm1 (red in L–L′′), Pros (nuclear red in N–N′′), and DRAQ5 (blue). Guts were dissected out from adult flies grown at 18 °C for 5 d after clone induction.
esg-Gal4 is also expressed in adult midgut progenitor cells (AMPs) that proliferate during larval development (22). To confirm that perturbation of Hpo signaling at the adult stage is sufficient to affect ISC proliferation, we used tublin-Gal80ts to temporarily control esg-Gal4 target gene expression (esgts) (23). After shifting adult flies to 29 °C for 14 d, esgts-Wts-RNAi and esgts-Yki adult midguts exhibited a dramatic increase in the number of esg-GFP+ and PH3+ cells (Fig. 1I and Fig. S1). Furthermore, Yki RNAi reversed the phenotypes caused by Wts RNAi (Fig. 1I and Fig. S1). Taken together, these results suggest that Hpo signaling is required in the adult midgut precursor cells to inhibit ISC proliferation.
Hpo Pathway Mutant Clones Exhibit Elevated Proliferation and Produce Differentiated Cells.
To further explore the role of Hpo signaling in adult midguts, we generated GFP positively marked clones mutant for various hpo pathway components in the ISC cell lineage using the mosaic analysis with a repressible cell marker (MARCM) system (24). We then compared the clone size (indicated by the number of GFP+ cells per clone) between the control clones and mutant clones for hpo pathway components. The adult flies were grown at 18 °C for 5 d after clone induction (Experimental Procedures). Under such condition, the control clones contained 2–3 cells per clone (Fig. S2). In contrast, hpo or wts mutant clones contained 5–7 cells per clone (Fig. 1 K–K′′ and Fig. S2). GFP+ clones mutant for fat (ft), which encodes a protocadherin that functions as a Hpo pathway receptor (25–27), also contained 5–7 cells per clone under the same condition (Fig. S2). Expressing Yki-RNAi in Hpo pathway mutant clones reduced their clone size (Fig. 1 M–M′′ and Fig. S2). The increased clone size associated with Hpo pathway mutant clones suggests that Hpo signaling is required to restrict ISC growth and proliferation during adult midgut homeostasis.
To determine whether the basal Yki activity is essential for ISC proliferation, we generated GFP positively marked yki mutant clones. Twenty days after clone induction, the clone size for yki mutant clones was comparable to that of control clones (Fig. S3). Thus, the basal Yki activity is not critical for ISC proliferation under normal gut homeostasis.
Dl staining revealed that isolated hpo, wts, or ft mutant clones often contained one Dl+ cell per clone (Fig. 1K–K′′ and Fig. S2), implying that Hpo pathway mutant ISCs can self-renew and produce differentiated cells. Immunostaining with anti-Pdm1 and anti-Prospero (Pro) antibodies, which label EC and EE, respectively (Fig. 1J) (2–4), confirmed that hpo, wts, or ft mutant clones contained differentiated EC and EE cells (Fig. 1 L–L′′ and N–N′′ and Fig. S4). Thus, although loss of Hpo signaling activity increases ISC proliferation, it does not block ISC lineage differentiation.
hpo Mutant Clones Can Induce ISC Proliferation Nonautonomously.
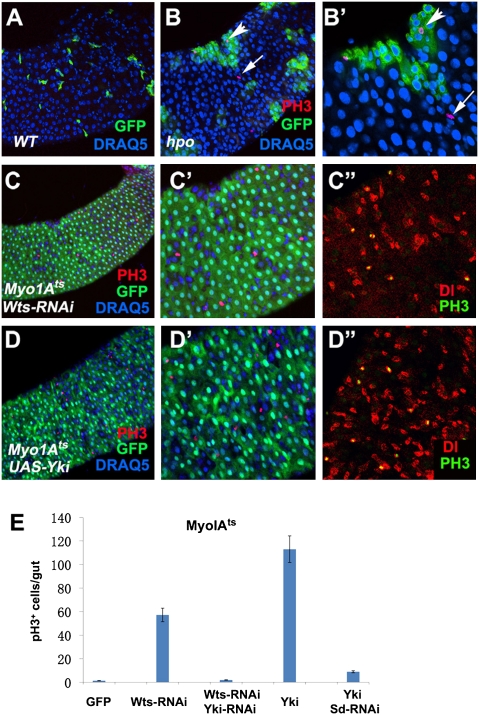
PH3 staining of Drosophila midguts carrying control or hpo mutant clones revealed an increase in the number of mitotic cells within the hpo mutant clones compared with control clones (Fig. 2 A, B, and B′; quantification in Fig. 4F), which is expected if Hpo signaling acts cell autonomously to restrict ISC proliferation. Unexpectedly, we also observed an increase in the number of PH3+ cells located outside of the hpo mutant clones (arrows in Fig. 2 B and B′). These observations imply that hpo mutant cells may produce a secreted factor(s) that acts in a paracrine fashion to stimulate the proliferation of neighboring WT ISCs.
Fig. 2.
Loss of Hpo signaling can induce ISC proliferation nonautonomously. (A–B′) Adult guts containing WT (WT) control clones (A) or hpoBF33 clones (B and B′; B′ is an enlarged view of B) were immunostained to show the expression of GFP (green), PH3 (red), and DRAQ5 (blue). Control and mutant clones were generated using the MARCM system and marked by GFP expression. hpo Mutant clones stimulated cell division of neighboring WT ISCs (arrows in B and B′). Arrowheads indicate dividing cells within hpo mutant clones. (C–D′′) Adult midguts that expressed UAS-Wts-RNAi (C–C′′) or UAS-Yki (D–D′′) together with UAS-GFP using MyoIAts were immunostained to show the expression of PH3 (red in C and C′ and D and D′; green in C′′ and D′′), Dl (red in C′′ and D′′), GFP (green in C, C′, D, and D′) and DRAQ5 (blue). GFP marked ECs. Guts were dissected from adult flies grown at 29 °C for 5 d. (E) Quantification of PH3+ cells in midguts of the indicated genotypes (mean ± SD, n ≥ 15).
Fig. 4.
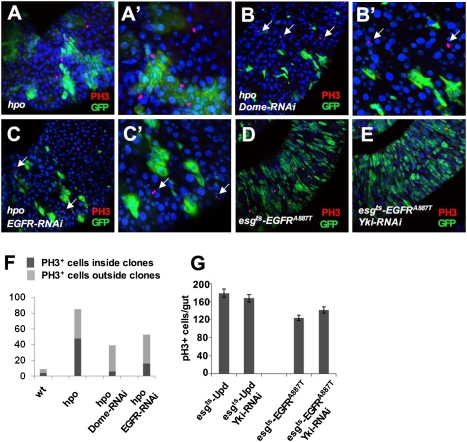
Inactivation of JAK-STAT or EGFR signaling in hpo mutant ISCs suppressed their proliferation. (A–C′) Low-magnification (A–C) and high-magnification (A′–C′) images of adult midguts carrying hpo mutant clones (A and A′), hpo mutant clones expressing Dome-RNAi (B and B′), or hpo mutant clones expressing EGFR-RNAi (C and C′) and stained with anti-PH3 (red) and anti-GFP (green) antibodies. Mutant clones were generated by the MARCM system and the guts were dissected out from flies grown at 25 °C for 5 d after clone induction. hpo Mutant clones expressing Dome-RNAi or EGFR-RNAi exhibited reduced clone size and contained less PH3+ cells within the clones; however, ectopic PH3 signals can be readily detected in WT cells near the mutant clones (arrows in B–C′). (D and E) esg-GFP (green) and PH3 (red) expression in adult midguts expressing an active form of EGFR, EGFRA887T, in the absence (D) or presence (E) of Yki-RNAi transgene using the esgts system. Adult flies were shifted to 29 °C for 6 d before dissection. (F) Quantification of PH3 positive cells inside or outside the control or mutant clones of the indicated genotypes. A total of 12 guts were counted for each genotype. (G) Quantification of PH3 positive cells from guts of the indicated genotypes (mean ± SD, n ≥ 5).
The implicated secreted factor(s) associated with hpo mutant clones could be produced by precursor cells, differentiated cells or both. To determine whether loss of Hpo signaling in differentiated cells could also stimulate ISC proliferation, we used an EC specific Gal4 driver, Myo1A-Gal4, in conjunction with tub-Gal80ts (Myo1Ats) to express Wts-RNAi or UAS-Yki in adult midgut epithelia. Strikingly, knockdown of Wts or overexpression of Yki in ECs induced a dramatic increase in ISC proliferation, as indicated by the increased number of PH3+ cells that are also Dl positive (Fig. 2 C, D′′, and E). Coexpression of Yki-RNAi with Wts-RNAi or SD-RNAi with UAS-Yki suppressed the stimulated effect on ISC proliferation (Fig. 2E). Taken together, these results suggest that the Hpo-Yki pathway may act in the ECs to regulate the production of a secreted factor(s) that controls ISC proliferation in a paracrine fashion.
Inactivation of Hpo Signaling Increases Production of Upds and EGFR Ligands.
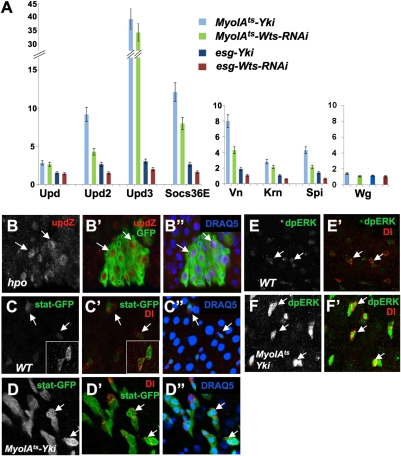
To identify the secreted factor(s) deregulated by loss of Hpo signaling, we examined the expression of ligands for pathways implicated in ISC proliferation, including the Wg/Wnt, JAK-STAT, and EGFR pathways (5, 6, 11, 12). We expressed Wts-RNAi or UAS-Yki in either ECs or ISC/EBs and measured the expression of Wg, the JAK-STAT pathway ligands: Unpaired (Upd), Upd2, and Upd3 (28), and the EGFR ligands: Vein (Vn), Keren (Krn), and Spitz (Spi) (29), by reverse transcriptase quantitative PCR (RT-qPCR). Wts RNAi or Yki overexpression in ECs or ISC/EBs did not cause a significant increase in wg mRNA levels (Fig. 3A). On the other hand, Wts RNAi or Yki overexpression in ECs up-regulated the mRNA levels of the three Upd genes as well as the three EGFR ligands, with Upd3 up-regulated by ∼40-fold and Vn by ∼8-fold with Yki overexpression (Fig. 3A). However, Wts RNAi or Yki overexpression in ISCs/EBs only slightly increased the expression of Upds and EGFR ligands (Fig. 3A). In midguts containing GFP-labeled hpo mutant clones, the expression of an upd reporter gene, upd-lacZ, was increased in hpo mutant cells, most notably in mutant ECs (arrows in Fig. 3 B–B′′). Taken together, these results reveal that loss of Hpo signaling, mainly in ECs, results in ectopic expression of multiple ligands of the JAK-STAT and EGFR pathways.
Fig. 3.
Hpo signaling regulates the JAK-STAT and EGFR pathways. (A) Relative mRNA levels of Upds, Socs36E, EGFR ligands, and Wg in whole midguts of the indicated genotypes measured by RT-qPCR. Numbers indicate fold of activation over the control guts. Wts RNAi or Yki overexpression in ECs (MyoIAts) and, to a much lesser extent, in precursor cells (esg-Gal4), induced elevated mRNA levels of the JAK-STAT and EGFR pathway ligands as well as the JAK-STAT pathway target Socs36E. (B–B′′) Expression of upd-lacZ in midguts carrying hpo mutant clones induced by the MARCM system. upd-lacZ was ectopically expressed in hpo mutant clones as well as some WT cells adjacent to mutant clones. (C–D′′) Expression of the JAK-STAT reporter, 10XSTAT-dGFP (STAT-GFP) in WT (C–C′′) or MyoIAts-Yki (D–D′′) guts. Insets in C and C′ show images obtained by scanning at increased gain. 10XSTAT-dGFP is expressed in the precursors with lower levels in ISCs. Yki overexpression in ECs induced increased expression of 10XSTAT-dGFP in ISCs (arrows in D and D′′) as well as associated EBs and differentiating cells. (E and F′) Expression of phosphorylated ERK (dpERK) in WT (E and E′) and MyoIAts-Yki (F and F′′) guts. MyoIAts-Yki flies were shifted to 29 °C for 1 d before dissection. Low levels of dpERK signal were detected in ISCs and associated EBs in control guts (E and E′). Yki overexpression in ECs increased the levels of the dpERK signal in ISCs (arrows) and associated EBs (F and F′).
Excessive Yki Activity in ECs Activates JAK-STAT and EGFR Signaling in ISCs.
The increased expression of Upds and EGFR ligands predicted that loss of Hpo signaling or excessive Yki activity might result in elevated JAK-STAT and EGFR signaling activity. Indeed, RT-qPCR revealed that Wts RNAi or Yki overexpression in ECs induced a ∼10-fold increase in the expression of Socs36E, a JAK-STAT pathway target gene, whereas inactivation of Wts or overexpression of Yki in ISCs/EBs induced a much lesser increase (<3-fold) (Fig. 3A). To monitor the JAK-STAT pathway activity more closely, we examined the expression of a pathway reporter gene, 10XSTAT-dGFP (30). In line with previous studies (6–8), 10XSTAT-dGFP was expressed in midgut precursor cells, with ISCs exhibiting weaker expression than EBs (arrows in Fig. 3 C–C′′). We found that overexpression of Yki in ECs but not in ISCs/EBs resulted in a clear increase in the level of 10XSTAT-dGFP expression in precursor cells as well as ectopic expression in large differentiating cells (arrows in Fig. 3 D–D′′ and Fig. S5A).
To monitor the EGFR pathway activity, we examined the expression of phosphorylated Drosophila ERK (dpERK, an EGFR pathway activity readout) by immunostaining with a phospho-specific antibody (31). In control guts, dpERK signals were weakly detected in ISCs and EBs (arrows in Fig. 3 E and E′). Acute activation of Yki in ECs (1 d after temperature shift of MyoIAts-Yki guts) resulted in a clear increase in the levels of dpERK signal in both ISCs and associated EBs (arrows Fig. 3 F and F′); however, prolonged Yki activation in ECs (3 d after temperature shift of MyoIAts-Yki guts) induced ectopic dpERK signals mainly in large differentiated cells (Fig. S6). In contrast, overexpression of Yki in ISCs/EBs induced little, if any, increase in the levels of dpERK signal (Fig. S5B). Taken together, these results demonstrate that excessive Yki activation in midgut epithelia increased the production of Upds and EGFR ligands, which in turn activate JAK-STAT and EGFR signal pathways in ISCs.
JAK-STAT and EGFR Pathway Activities Are Required for Elevated ISC Proliferation Induced by Loss of Hpo Signaling.
To determine whether JAK-STAT and EGFR pathway activities are required for the elevated ISC proliferation due to deregulation of Hpo signaling, we carried out genetic epistasis experiments. We generated hpo mutant clones in which JAK-STAT or EGFR signaling was inactivated by knockdown of Dome (Dome-RNAi), the receptor for the JAK-STAT pathway (28), or EGFR/TOP (EGFR-RNAi), the receptor for the EGFR pathway (29), using the MACRM system (Experimental Procedures). Compared with hpo mutant clones, hpo mutant clones expressing Dome-RNAi (referred to as hpo−Dome−) or EGFR-RNAi (referred to as hpo−EGFR−) exhibited reduced clone size (Fig. 4 A–C′). Furthermore, PH3 staining indicated that Dome or EGFR RNAi suppressed the elevated mitosis within but not outside the expression domain of the MARCM clones (Fig. 4 A–C′ and F). Thus, blocking the JAK-STAT or EGFR pathway suppressed ISC proliferation induced by loss of Hpo signaling. The sustained, elevated mitosis outside hpo−Dome− or hpo− EGFR− clones suggests that these mutant clones may still produce excessive ligands for the JAK-STAT/EGFR pathways that can stimulate the proliferation of neighboring WT ISC in a paracrine fashion.
To further probe the relationship between the Hpo and the JAK-STAT/EGFR pathways in the regulation of ISC proliferation, we ectopically activated JAK-STAT or EGFR signaling by overexpressing Upd or an activated EGFR (EGFRA887T) (32) in ISCs/EBs and simultaneously inactivated Yki by coexpressing Yki-RNAi using the esgts system. Consistent with JAK-STAT and EGFR pathways acting downstream of the Hpo pathway to control ISCs proliferation, we found that inactivation of Yki did not affect ISC proliferation induced by Upd overexpression or EGFR activation (Fig. 4 D, E, and G and Fig. S7).
Yki Is Required in Precursor Cells for DSS-Induced ISC Proliferation.
Tissue damage caused by feeding flies with DSS or bleomycin, or by bacterial infection (such as Pseudomonas entomophila or PE) stimulates ISC proliferation (6, 9–12). We found that bleomycin and PE but not DSS activated the JAK/STAT pathway in ISCs (Fig. S8), suggesting that different tissue damaging reagents may stimulate ISC proliferation via distinct mechanisms. To determine whether the Hpo pathway participates in the regulation of tissue damage-induced ISC proliferation, flies expressing Yki-RNAi in either ISCs/EBs or ECs were treated with various tissue damaging reagents. Yki RNAi in ISCs/EBs but not in ECs suppressed DSS-induced ISC proliferation, as indicated by the reduction in the number of esg-GFP+ and PH3+ cells (Fig. 5 A–C). These observations suggest that Yki is required in ISCs/EBs for DSS-stimulated ISC proliferation. In contrast, bleomycin or PE-induced ISC proliferation was not significantly affected by Yki inactivation in either ISCs/EBs or ECs (Fig. 5 A–C).
Fig. 5.
Yki is required in the precursor cells for DSS-stimulated ISC proliferation. (A) Adult flies expressing esg-Gal4/UAS-GFP (Top) or esg-Gal4/UAS-GFP + UAS-Yki-RNAi (Bottom) were treated with sucrose (SC), DSS, bleomycin (BN), or PE for 3 d before midguts were dissected out and immunostained with GFP (green) and PH3 (red) antibodies and DRAQ5 (blue). (B–E) Quantification of PH3+ cells in control adult midguts or adult midguts expressing esg-Yki-RNAi (B), MyoIAts-Yki-RNAi (C), esg-Wts-RNAi (D), or MyoIAts-Wts-RNAi (E) treated with sucrose, DSS, bleomycin, or PE (mean ± SD, n ≥ 10 for each genotype).
If DSS stimulates proliferation through the Hpo pathway in ISCs, it should not further stimulate cell proliferation when Hpo signaling is inactivated in ISCs. Indeed, DSS treatment did not further increase ISC proliferation in esg-Wts-RNAi guts compared with mock treatment (Fig. 5D). However, DSS treatment further increased ISC proliferation in Myo1Ats-Wts-RNAi guts (Fig. 5E), suggesting that DSS acts through an Yki-dependent mechanism in ISCs that parallels the non–cell-autonomous mechanism elicited by loss of Hpo signaling in ECs. Both bleomycin treatment and PE infection had an additive effect with Wts RNAi either in ISCs/EBs or ECs to drive ISC proliferation (Fig. 5 D and E). Taken together, these observations suggest that DSS stimulates ISC proliferation likely through a cell autonomous role of Yki in the progenitors, whereas bleomycin and PE can stimulate ISC proliferation through an Yki-independent mechanism.
Although Yki RNAi did not significantly affect bleomycin or PE-induced ISC proliferation, it did not rule out the possibility that bleomycin or PE-induced tissue damage could induce Yki activation. Indeed, treatment with bleomycin and PE but not DSS increased the expression of an Hpo pathway responsive gene (ex-lacZ) in ECs of the posterior midguts (Fig. S9) (33). Recently, Staley et al. showed that a more complete inactivation of Yki in ECs by the combined expression of UAS-Yki-RNAi and UAS-dicer 2 partially blocked bleomycin-induced ISC proliferation (33). Thus, it is possible that bleomycin and PE may stimulate ISC proliferation through both Yki-dependent and Yki-independent mechanisms.
Discussion
The Hpo signaling pathway controls organ size in both Drosophila and mammals (13, 14, 34, 35); however, its role in stem cell regulation has not been explored until very recently. Using the Drosophila adult midgut as a model, we provide evidence that Hpo signaling plays a critical role in restricting adult stem cell proliferation. In addition, we provide evidence that Hpo signaling can regulate cell proliferation through a non–cell-autonomous mechanism.
The prevailing view regarding the mechanism of Hpo action is that Hpo signaling acts cell autonomously to control cell growth, proliferation, and survival by regulating genes involved in these processes (13, 14). Our finding that loss of Hpo signaling or overexpression of Yki in the precursor cells can stimulate ISC proliferation is in line with this view. Unexpectedly, however, we found that hpo mutant clones can stimulate the proliferation of neighboring WT ISCs. Strikingly, we found that loss of Hpo signaling or overexpression of Yki in enterocytes also stimulated ISC proliferation, thus demonstrating a non–cell-autonomous mechanism. Furthermore, we provided evidence that loss of Hpo signaling in enterocytes leads to increased production of cytokines and mitogens that activate the JAK-STAT and EGFR pathways in ISCs to drive their proliferation. The nonautonomous mechanism demonstrated here is likely to be conserved in mammals, as a recent study showed that activation of the mammalian ortholog of Yki, Yap, in cultured breast epithelial cells activates an EGFR ligand that can drive cell proliferation nonautonomously in vitro (36).
While our manuscript was under review, Stanley et al. reported that Wts inactivation or Yki overexpression in ECs induced ISC proliferation nonautonomously (33), which is in line with our findings here. However, they did not observe a cell-autonomous role for Hpo signaling in the regulation of ISC proliferation. The reason for such a discrepancy is not clear but could be due to difference in experimental conditions used by different laboratories.
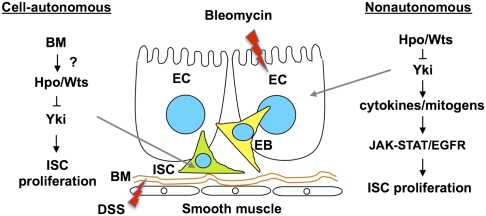
We found that Wts RNAi or Yki overexpression with esg-Gal4 did not significantly increase either the JAK-STAT or EGFR pathway activity in ISCs. Thus, the increased ISC proliferation observed in esg-Wts-RNAi and esg-Yki midguts may reflect a cell-autonomous role of Hpo signaling in the regulation of ISC proliferation. Interestingly, Yki is specifically required in ISCs/EBs for DSS to stimulate ISC proliferation. Furthermore, loss of Hpo signaling in ECs but not in ISCs/EBs can cooperate with DSS to stimulate ISC proliferation. A likely explanation is that DSS deregulates Hpo signaling in ISCs and promotes the cell-autonomous function of Yki to drive ISC proliferation (Fig. 6). The mechanism by which DSS regulates Hpo signaling is not clear, but our previous study reveals that DSS disrupts the basement membrane organization whereas bleomycin causes damage of midgut epithelia (12). As ISCs contact the basement membrane, our finding thus raises an interesting possibility that the Hpo pathway may sense ISC interaction with the basement membrane to restrict ISC proliferation, and that disruption of basement membrane organization may alleviate Hpo pathway-mediated contact inhibition of ISC proliferation.
Fig. 6.
Hpo signaling regulates ISC proliferation through both cell-autonomous and non–cell-autonomous mechanisms. Hpo/Wts restricts the activity of Yki in the precursor cells to inhibit ISC proliferation. This cell-autonomous mechanism could be regulated by contact between ISC and basement membrane (BM), which is disrupted by DSS. Hpo signaling also acts in the ECs to restrict the production of ligands for the JAK-STAT and EGFR pathways, thereby inhibiting ISC proliferation by limiting the activities of these two pathways. Bleomycin and possibly PE cause damage of ECs and induce ISC proliferation through Yki-dependent and Yki-independent mechanisms. Of note, each arrow does not necessarily mean direct regulation.
Our finding has important implications regarding tumorigenesis. Deregulation of Hpo signaling has been implicated in several types of human cancer (37–40). Indeed, liver specific overexpression of Yap or knockout of MST1/2 (the mammalian ortholog of Hpo) caused hepatocellular carcinomas (34, 41–44). Furthermore, MST1/2 mutant livers contain proliferative oval cells that are facultative stem cells contributing to both the hepatocyte and biliary lineages, implying that Hpo signaling may repress adult liver stem cell activation (42). Thus, it would be important to determine whether Hpo signaling is more widely involved in adult stem cell regulation and whether Hpo pathway activity is deregulated in response to tissue injury that is thought to contribute to tumorigenesis (45). Our finding that Hpo signaling can regulate stem cell proliferation through non–cell-autonomous mechanisms implies that deregulation of Hpo signaling in tumor microenvironments may also contribute to tumorigenesis.
Experimental Procedures
Drosophila Stocks and Genetics.
The following fly stains were used for this study: hpoBF33 (46); wtsX1 (47); ykiB5 (16); ftG-rv (48); esg-Gal4 (2); MyoIA-Gal4, UAS-Dome-RNAi, UAS-Upd, and upd-lacZ (6); UAS-Wts-RNAi (VDRC #106174); UAS-Yki-RNAi, UAS-Sd-RNAi, and UAS-Yki (18); UAS-EGFR-RNAi (VDRC #43267); UAS-EGFRA887T (BL #9534). Mutant clones were generated using the MARCM system (24). Fly stocks were crossed and cultured at 18 °C. F1 adult flies, 5 d old, with the appropriate genotypes were subjected to heat shock at 37 °C for 1 h. After clone induction, flies were raised at 18 °C or 25 °C for the indicated period before dissection. For experiments involving tubGal80ts, crosses were set up and cultured at 18 °C to restrict Gal4 activity. F1 adult flies were then shifted to 29 °C to inactivate Gal80ts. The genotypes for making mutant clones are described in SI Experimental Procedures.
Feeding Experiments.
Female adult flies, 5–10 d old, were used for feeding experiments. Flies were cultured in an empty vial containing a piece of 2.5 × 3.75-cm chromatography paper (Fisher) wet with 5% sucrose solution as feeding medium. Flies were fed with 3% of dextran sulfate sodium (MP Biomedicals) or 25 μg/mL bleomycin (Sigma) dissolved in 5% sucrose for 3 d at 29 °C. PE infection was carried out as previously described (6).
RT-qPCR.
RNA was extracted from 10 female midguts using RNAeasy Mini kit (Qiagen), and cDNA was synthesized using the iScript cDNA synthesis kit (Bio-Rad). RT-qPCR was performed using iQ5 System (Bio-Rad). Primer sequences are listed in SI Experimental Procedures. RT-qPCR was performed in duplicate on each of three independent biological replicates. RpL11 was used as a normalization control.
Immunostaining.
Female flies were used for gut immunostaining in all experiments. The entire gastrointestinal tract was taken and fixed in 1 X PBS plus 4% EM grade formaldehyde (Polysciences) for 3 h, except for Delta staining, for which the fixation time was 30 min. Samples were washed and incubated with primary and secondary antibodies in a solution containing 1 X PBS, 0.5% BSA, and 0.1% Triton X-100. The following primary antibodies were used: mouse anti-Delta (DSHB), 1:100; mouse anti-Prospero (DSHB), 1:50; rabbit anti-PH3 (Upstate Biotechnology), 1:2,000; rabbit anti-GFP (Santa Cruz), 1:500; rabbit anti-Pdm1 (gift from Xiaohang Yang, Institute of Molecular and Cell Biology, Singapore), 1:2,000; 1:1,000; rabbit anti-dpERK (Cell Signaling Technology), and 1:500; DRAQ5 (Cell Signaling Technology).
Supplementary Material
Acknowledgments
We thank Drs. Jessica Treisman (Skirball Institute, NY), Huaqi Jiang (The Fred Hutchinson Cancer Research Center, Seattle), and Xiaohang Yang, DSHB, VDRC, and Bloomington Stock Center for reagents. We thank Dr. Huaqi Jiang for discussion and comments. J.J. is supported by the National Institutes of Health Grant GM061269, the Welch Foundation (I-1603) and CPRIT (RP100561), and is a Eugene McDermott Endowed Scholar of Biomedical Science at University of Texas Southwestern. Y.T.I. is supported by National Institutes of Health Grants R21DK75545 and R01DK83450 and is a member of the UMass Diabetes Endocrinology Research Center (DK32520).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1012759107/-/DCSupplemental.
References
- 1.Casali A, Batlle E. Intestinal stem cells in mammals and Drosophila. Cell Stem Cell. 2009;4:124–127. doi: 10.1016/j.stem.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- 3.Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- 4.Lee WC, Beebe K, Sudmeier L, Micchelli CA. Adenomatous polyposis coli regulates Drosophila intestinal stem cell proliferation. Development. 2009;136:2255–2264. doi: 10.1242/dev.035196. [DOI] [PubMed] [Google Scholar]
- 5.Lin G, Xu N, Xi R. Paracrine Wingless signalling controls self-renewal of Drosophila intestinal stem cells. Nature. 2008;455:1119–1123. doi: 10.1038/nature07329. [DOI] [PubMed] [Google Scholar]
- 6.Jiang H, et al. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu W, Singh SR, Hou SX. JAK-STAT is restrained by Notch to control cell proliferation of the Drosophila intestinal stem cells. J Cell Biochem. 2010;109:992–999. doi: 10.1002/jcb.22482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beebe K, Lee WC, Micchelli CA. JAK/STAT signaling coordinates stem cell proliferation and multilineage differentiation in the Drosophila intestinal stem cell lineage. Dev Biol. 2010;338:28–37. doi: 10.1016/j.ydbio.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 9.Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell. 2009;4:49–61. doi: 10.1016/j.stem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchon N, Broderick NA, Chakrabarti S, Lemaitre B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 2009;23:2333–2344. doi: 10.1101/gad.1827009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection: Activation of host defense and stem cell proliferation. Cell Host Microbe. 2009;5:200–211. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Apidianakis Y, Pitsouli C, Perrimon N, Rahme L. Synergy between bacterial infection and genetic predisposition in intestinal dysplasia. Proc Natl Acad Sci U S A. 2009;106:20883–20888. doi: 10.1073/pnas.0911797106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, Yue T, Jiang J. Hippo signaling pathway and organ size control. Fly (Austin) 2009;3:68–73. doi: 10.4161/fly.3.1.7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan D. Hippo signaling in organ size control. Genes Dev. 2007;21:886–897. doi: 10.1101/gad.1536007. [DOI] [PubMed] [Google Scholar]
- 15.Zhao B, Li L, Lei Q, Guan KL. The Hippo-YAP pathway in organ size control and tumorigenesis: An updated version. Genes Dev. 2010;24:862–874. doi: 10.1101/gad.1909210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Zeng Q, Hong W. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell. 2008;13:188–192. doi: 10.1016/j.ccr.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, et al. The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev Cell. 2008;14:377–387. doi: 10.1016/j.devcel.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu S, Liu Y, Zheng Y, Dong J, Pan D. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev Cell. 2008;14:388–398. doi: 10.1016/j.devcel.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Goulev Y, et al. SCALLOPED interacts with YORKIE, the nuclear effector of the hippo tumor-suppressor pathway in Drosophila. Curr Biol. 2008;18:435–441. doi: 10.1016/j.cub.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 21.Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- 22.Jiang H, Edgar BA. EGFR signaling regulates the proliferation of Drosophila adult midgut progenitors. Development. 2009;136:483–493. doi: 10.1242/dev.026955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGuire SE, Mao Z, Davis RL. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci STKE. 2004;2004:pl6. doi: 10.1126/stke.2202004pl6. [DOI] [PubMed] [Google Scholar]
- 24.Lee T, Luo L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 2001;24:251–254. doi: 10.1016/s0166-2236(00)01791-4. [DOI] [PubMed] [Google Scholar]
- 25.Bennett FC, Harvey KF. Fat cadherin modulates organ size in Drosophila via the Salvador/Warts/Hippo signaling pathway. Curr Biol. 2006;16:2101–2110. doi: 10.1016/j.cub.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 26.Cho E, et al. Delineation of a Fat tumor suppressor pathway. Nat Genet. 2006;38:1142–1150. doi: 10.1038/ng1887. [DOI] [PubMed] [Google Scholar]
- 27.Silva E, Tsatskis Y, Gardano L, Tapon N, McNeill H. The tumor-suppressor gene fat controls tissue growth upstream of expanded in the hippo signaling pathway. Curr Biol. 2006;16:2081–2089. doi: 10.1016/j.cub.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Arbouzova NI, Zeidler MP. JAK/STAT signalling in Drosophila: Insights into conserved regulatory and cellular functions. Development. 2006;133:2605–2616. doi: 10.1242/dev.02411. [DOI] [PubMed] [Google Scholar]
- 29.Shilo BZ. Regulating the dynamics of EGF receptor signaling in space and time. Development. 2005;132:4017–4027. doi: 10.1242/dev.02006. [DOI] [PubMed] [Google Scholar]
- 30.Bach EA, et al. GFP reporters detect the activation of the Drosophila JAK/STAT pathway in vivo. Gene Expr Patterns. 2007;7:323–331. doi: 10.1016/j.modgep.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Gabay L, Seger R, Shilo BZ. In situ activation pattern of Drosophila EGF receptor pathway during development. Science. 1997;277:1103–1106. doi: 10.1126/science.277.5329.1103. [DOI] [PubMed] [Google Scholar]
- 32.Lesokhin AM, Yu SY, Katz J, Baker NE. Several levels of EGF receptor signaling during photoreceptor specification in wild-type, Ellipse, and null mutant Drosophila. Dev Biol. 1999;205:129–144. doi: 10.1006/dbio.1998.9121. [DOI] [PubMed] [Google Scholar]
- 33.Staley BK, Irvine KD. Warts and Yorkie mediate intestinal regeneration by influencing stem cell proliferation. Curr Biol. 2010;20:1580–1587. doi: 10.1016/j.cub.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song H, et al. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc Natl Acad Sci USA. 2010;107:1431–1436. doi: 10.1073/pnas.0911409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lian I, et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010;24:1106–1118. doi: 10.1101/gad.1903310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J, et al. YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Nat Cell Biol. 2009;11:1444–1450. doi: 10.1038/ncb1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zender L, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–1267. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Overholtzer M, et al. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci USA. 2006;103:12405–12410. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steinhardt AA, et al. Expression of Yes-associated protein in common solid tumors. Hum Pathol. 2008;39:1582–1589. doi: 10.1016/j.humpath.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McClatchey AI, Giovannini M. Membrane organization and tumorigenesis—the NF2 tumor suppressor, Merlin. Genes Dev. 2005;19:2265–2277. doi: 10.1101/gad.1335605. [DOI] [PubMed] [Google Scholar]
- 41.Dong J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu L, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci USA. 2010;107:1437–1442. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou D, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16:425–438. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang N, et al. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell. 2010;19:27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–331. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- 46.Jia J, Zhang W, Wang B, Trinko R, Jiang J. The Drosophila Ste20 family kinase dMST functions as a tumor suppressor by restricting cell proliferation and promoting apoptosis. Genes Dev. 2003;17:2514–2519. doi: 10.1101/gad.1134003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: The Drosophila lats gene encodes a putative protein kinase. Development. 1995;121:1053–1063. doi: 10.1242/dev.121.4.1053. [DOI] [PubMed] [Google Scholar]
- 48.Willecke M, et al. The fat cadherin acts through the hippo tumor-suppressor pathway to regulate tissue size. Curr Biol. 2006;16:2090–2100. doi: 10.1016/j.cub.2006.09.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.