Abstract
Inositol pyrophosphates have been implicated in numerous biological processes. Inositol hexakisphosphate kinase-2 (IP6K2), which generates the inositol pyrophosphate, diphosphoinositol pentakisphosphate (IP7), influences apoptotic cell death. The tumor suppressor p53 responds to genotoxic stress by engaging a transcriptional program leading to cell-cycle arrest or apoptosis. We demonstrate that IP6K2 is required for p53-mediated apoptosis and modulates the outcome of the p53 response. Gene disruption of IP6K2 in colorectal cancer cells selectively impairs p53-mediated apoptosis, instead favoring cell-cycle arrest. IP6K2 acts by binding directly to p53 and decreasing expression of proarrest gene targets such as the cyclin-dependent kinase inhibitor p21.
Among the inositol phosphates, inositol 1,4,5-trisphosphate is best known for its release of intracellular calcium (1). The inositol pyrophosphates, synthesized by inositol hexakisphosphate kinases (IP6Ks), regulate numerous processes including chemotaxis (2), telomere length (3, 4), endocytic trafficking (5), exocytosis (6), and apoptosis (7, 8). The principal inositol pyrophosphate, diphosphoinositol pentakisphosphate (5-PP-IP5), here designated IP7, is generated by three IP6 kinases that are the products of three separate genes (9). Another isomer of IP7, 3-PP-IP5, is synthesized by a distinct enzyme, Vip1, and regulates cell shape, growth, and phosphate disposition of yeast (10, 11). IP6K1 has been directly implicated in vesicular trafficking and tissue growth, because IP6K1-deleted mice manifest diminished insulin release, slowed growth, and defects in spermiogenesis (12). IP6K2 selectively impacts cell death, because its overexpression sensitizes cells to diverse apoptotic stimuli such as DNA damage, hypoxia, hydrogen peroxide, and interferon-β; knockdown of IP6K2 but not IP6K1 or IP6K3 diminishes sensitivity to such stimuli (7, 8, 13, 14). Recently, Lindner and coworkers (15) developed IP6K2 knockout mice that are predisposed to invasive aerodigestive tract carcinoma driven by chemical carcinogenesis, and fibroblasts from the mice resist γ-irradiation. The mechanism by which IP6K2 regulates cell death has not been established. We now show that IP6K2 is required for p53-mediated apoptosis and acts by binding p53 and selectively diminishing expression of its pro-cell-cycle arrest targets.
Results
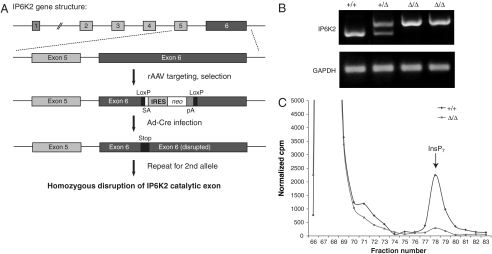
To assess apoptotic functions of IP6K2 in human cells, we created a cell line lacking IP6K2 activity. We utilized a recombinant adeno-associated virus (rAAV) -based somatic cell gene disruption technique in HCT116 cells (Fig. 1A). We targeted exon 6 of IP6K2 to disrupt the gene prior to the coding region for essential residues required for ATP coordination (Fig. S1A). We confirmed targeting of the IP6K2 locus by RT-PCR (Fig. 1B) and Western blot (Fig. S1B). The IP6K2-disrupted cells display an almost total loss of IP7 generation from [3H]inositol in vivo (Fig. 1C). Thus, in these cells IP6K2 appears to have been the dominant source of IP7.
Fig. 1.
Gene disruption of IP6K2 in HCT116 cells. (A) Targeting scheme for disruption of IP6K2 gene in HCT116 cells. rAAV was employed to disrupt exon 6 of IP6K2 by inserting a cassette containing a splice acceptor (SA) followed by an internal ribosome entry sequence (IRES), the coding sequence of neomycin transferase (neo), and a polyadenylation signal (pA), flanked by recognition sites (LoxP) for Cre recombinase. Cre-expressing adenovirus (Ad-Cre) was used to excise this cassette, facilitating targeting of the second allele. The second round of rAAV targeting and Ad-Cre excision results in two disrupted IP6K2 alleles. (B) Confirmation of IP6K2 targeting by RT-PCR. (C) Gene disruption of IP6K2 reduces intracellular IP7 levels. Parental and IP6K2 Δ/Δ cells were labeled with [3H]myo-inositol, and intracellular inositol phosphates were resolved by HPLC.
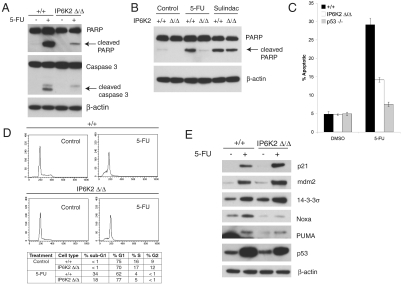
p53 is a major tumor suppressor that kills by apoptosis. The prominent anticancer drug 5-fluorouracil (5-FU) kills cells selectively through the p53 pathway (16). Cells lacking IP6K2 display profoundly reduced 5-FU–mediated apoptosis with markedly decreased cleavage of poly(ADP-ribose) polymerase (PARP) and caspase-3 (Fig. 2A). Sulindac is a cyclooxygenase inhibitor that induces Bax-dependent, but p53-independent, apoptosis in colorectal cancer cells (17). Disruption of IP6K2 does not alter sulindac-induced apoptotic PARP cleavage, indicating that IP6K2 is selectively required for p53-dependent death (Fig. 2B). Cell death elicited by 5-FU is markedly impaired in IP6K2-disrupted cells and virtually abolished in cells lacking p53 (Fig. 2C). Cell-cycle analysis reveals that 5-FU treatment of IP6K2-disrupted cells elicits a smaller sub-G1 population but a larger G1-arrested population than in parental HCT116 cells (Fig. 2D), suggesting a shift in the p53 response from apoptosis to cell-cycle arrest. p53 causes apoptosis by transactivation of proapoptotic genes such as PUMA and NOXA, which encode BH3-only proteins of the Bcl-2 family and activate the mitochondrial death cascade (18, 19). p53 mediates cell-cycle arrest by activating a distinct set of target genes, most notably the cyclin-dependent kinase inhibitor p21, which results in G1 arrest and also has antiapoptotic functions (20, 21), and 14-3-3σ, which is required for G2/M arrest (22). Mdm2, an E3 ubiquitin ligase that degrades p53, is itself induced by p53 and, hence, is a negative regulator of p53 (23). Treatment with 5-FU induces expression of all these cell death and cell arrest targets in WT cells. Expression of p21, 14-3-3σ, and mdm2 is increased in 5-FU–treated IP6K2-deleted cells as compared to 5-FU–treated WT cells. By contrast, no such augmentation is evident for NOXA or PUMA (Fig. 2E). The augmentation of p21 protein in IP6K2-deleted cells treated with 5-FU reflects enhanced transcription, because chromatin immunoprecipitation reveals increased p53 binding to the p21 promoter (Fig. S2A), and RT-PCR reveals an increase in p21 mRNA in 5-FU–treated IP6K2-disrupted cells (Fig. S2B). These findings indicate that IP6K2 physiologically suppresses the expression of proarrest p53 targets, thus shifting the p53 response toward apoptosis.
Fig. 2.
IP6K2 is required for p53-mediated apoptosis. (A) Disruption of IP6K2 blocks 5-FU–induced apoptosis. HCT116 IP6K2 +/+ and Δ/Δ cells were treated with 5-FU 400 μM or DMSO control for 18 h. Immunoblotting for cleaved PARP and caspase 3 provides a measure of apoptosis, with β-actin as a loading control. (B) IP6K2 is required for p53-dependent, but not p53-independent, apoptosis. HCT116 IP6K2 +/+ and Δ/Δ cells were treated with 5-FU (400 μM), sulindac sulfide (120 μM), or DMSO control for 18 h and then immunoblotted for cleaved PARP. (C) Disruption of IP6K2 blocks 5-FU–induced apoptosis. HCT116 IP6K2 +/+ and Δ/Δ cells and p53 −/− cells were treated with 5-FU (400 μM) or DMSO control for 18 h. Annexin V/propidium iodide staining followed by flow cytometry was used to measure the apoptotic population. Bars represent means ± SD of two independent experiments, with results representative of results obtained with a second clone of IP6K2-disrupted cells. (D) Disruption of IP6K2 favors G1 cell-cycle arrest over apoptosis in response to 5-FU. HCT116 IP6K2 +/+ and Δ/Δ cells were treated with 5-FU (400 μM) or DMSO control for 18 h, fixed, stained with propidium iodide, and subjected to cell-cycle analysis by flow cytometry. (E) Disruption of IP6K2 selectively increases expression of pro-cell-cycle arrest p53 targets. HCT116 IP6K2 +/+ and Δ/Δ cells were treated with 5-FU (400 μM) or DMSO control for 18 h and immunoblotted for p53 targets as indicated.
We assessed whether loss of IP6K2 elicits similarly increased p21 induction in response to additional stressors. WT or IP6K2-null cells were treated with etoposide, which induces DNA damage via topoisomerase inhibition, or novobiocin, a C-terminal Hsp90 inhibitor previously shown to cause cell death in an IP6K2-dependent manner (14). In IP6K2-null cells, etoposide- or novobiocin-induced p21 induction is increased relative to WT cells, whereas apoptotic PARP cleavage is reduced (Fig. S2C).
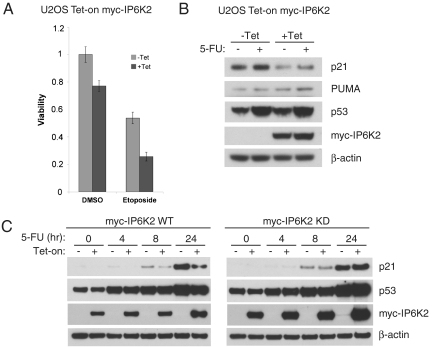
We confirmed an apoptotic role for IP6K2 by monitoring its tetracycline-inducible overexpression in U2OS cells, which markedly enhances cytotoxic effects of etoposide (Fig. 3A), similar to previous findings in other cell types (8, 13). We evaluated the effect of IP6K2 on expression of p53 targets in response to 5-FU. IP6K2 overexpression substantially decreases the induction of p21 but not PUMA, consistent with a selective inhibition of cell arrest (Fig. 3B). The action of IP6K2 reflects its IP7-generating activity, because the reduction of p21 expression is not evident in cells expressing kinase-dead IP6K2 (Fig. 3C).
Fig. 3.
IP6K2 down-regulates p21 expression in a kinase activity-dependent manner. (A) Tetracycline-inducible IP6K2 expression in U2OS cells sensitizes to etoposide-induced cytotoxicity. Cells were induced with tetracycline (1 μM) for 18 h and then treated with etoposide (20 μM) for 42 h, followed by MTT viability assay. Bars represent means ± SD of four independent experiments. (B) Tetracycline-inducible IP6K2 expression in U2OS cells leads to down-regulation of 5-FU–induced p21, but not PUMA, expression. Cells were induced with tetracycline (1 μM) for 18 h and then treated with 5-FU (400 μM) for 8 h. (C) Kinase activity of IP6K2 is required for p21 down-regulation. U2OS Tet-inducible cell lines expressing either myc-IP6K2 WT or kinase dead (KD) were induced with tetracycline (1 μM) for 18 h as indicated and then treated with 5-FU (400 μM) for 0, 4, 8, and 24 h.
We assessed the influence of IP6K2 on p53 function in other cell lines. The human embryonic kidney cell line HEK293 expresses WT p53 and has previously been used to study p53-mediated transcription and apoptosis (24). Overexpression of IP6K2 sensitizes HEK293 cells to etoposide-induced apoptotic PARP cleavage and suppresses induction of p21 but not PUMA (Fig. S3A). The caspase-3 null breast carcinoma cell line MCF-7 harbors wild-type p53 and has been widely used to characterize the p53 transcriptional response. As expected, overexpression of IP6K2 in these cells suppresses p21 but not PUMA expression following etoposide treatment (Fig. S3B).
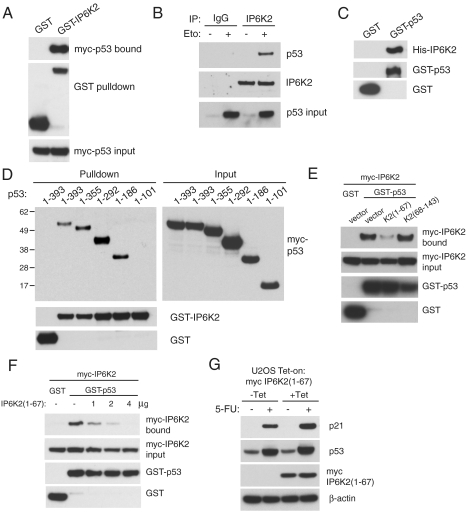
By what molecular mechanism might IP6K2 regulate p53? Because apoptotic stimuli elicit nuclear translocation of IP6K2 (25) as well as p53, we explored possible binding of the two proteins. In p53-null HCT116 cells, overexpressed IP6K2 binds overexpressed p53 (Fig. 4A). We observe binding of endogenous IP6K2 and p53 in U2OS cells following etoposide treatment (Fig. 4B). The interaction between the two proteins is direct as is evident from the binding of purified IP6K2 and p53 (Fig. 4C). The interaction of IP6K2 and p53 does not depend on the catalytic activity of IP6K2, because kinase-dead IP6K2 also binds p53 (Fig. S4). We mapped sites on p53 responsible for binding to IP6K2 by utilizing a series of carboxyl-terminal deletions of p53. The critical region for binding IP6K2 resides in amino acids 101–186 within the DNA binding core domain of p53 (Fig. 4D). Furthermore, we showed that IP6K2 and p53 colocalize in the nucleus following genotoxic stress (Fig. S5).
Fig. 4.
IP6K2 directly binds p53 via the DNA-binding domain. (A) Binding of IP6K2 and p53 in a cooverexpression system. GST or GST-IP6K2 were coexpressed with myc-p53 in HCT116 p53 −/− cells. Glutathione-sepharose pulldown reveals binding of myc-p53 to GST-IP6K2 but not GST control. (B) Endogenous coimmunoprecipitation of p53 and IP6K2 from etoposide-treated U2OS cells. U2OS cells were treated with 20 μM etoposide or DMSO control for 18 h and then immunoprecipitated with control rabbit IgG or anti-IP6K2 polyclonal antibody. (C) Direct in vitro binding of IP6K2 and p53. Recombinant His-IP6K2, GST-p53, and GST control were purified from Escherichia coli and subjected to an in vitro binding reaction followed by glutathione-sepharose pulldown. (D) Mapping of the IP6K2 binding site on p53. Myc-tagged C-terminally truncated p53 constructs were coexpressed with GST or GST-IP6K2 in HCT116 p53 −/− cells, followed by glutathione-sepharose pulldown. p53 1–101 failed to bind IP6K2, whereas 1–186 was sufficient for binding. (E) Identification of dominant-negative fragments for IP6K2-p53 binding. In HCT116 p53 −/− cells, GST or GST-p53, myc-IP6K2, and myc-tagged IP6K2 fragment 1–67 or 68–143 were coexpressed as indicated. Fragment K2(1–67) but not K2(68–143) had a dominant-negative effect on the binding of full-length proteins. (F) IP6K2 fragment (1–67) disrupts full-length IP6K2-p53 binding. Increasing amounts of myc-IP6K2(1–67) DNA (0, 1, 2, 4 μg) were cotransfected with GST or GST-p53 and myc-IP6K2. IP6K2(1–67) disrupted p53-IP6K2 binding in a concentration-dependent manner. (G) Expression of dominant-negative fragment IP6K2(1–67) increases p21 induction by 5-FU. U2OS cells inducibly expressing the dominant-negative fragment myc-IP6K2(1–67) were induced with tetracycline (1 μM) for 24 h and then treated with 5-FU (400 μM) for 18 h.
To develop a dominant-negative construct of IP6K2, we overexpressed various fragments on the basis of IP6K2 exons and monitored IP6K2-p53 binding. The fragment IP6K2(1–67) abolishes binding, whereas IP6K2(68–143) has no effect (Fig. 4E). IP6K2(1–67) disrupts binding of full-length p53 and IP6K2 in a concentration-dependent manner (Fig. 4F). To determine whether the direct binding of IP6K2 to p53 underlies the regulatory influences of IP6K2 upon p53 signaling, we examined the effect of IP6K2(1–67) overexpression upon the response of U2OS cells to 5-FU (Fig. 4G). Disruption of IP6K2-p53 binding by expression of IP6K2(1–67) leads to increased p21 expression in response to 5-FU.
Discussion
Our findings establish that IP6K2 is required for p53-mediated apoptosis. The molecular mechanism underlying this action involves direct binding to p53 of IP6K2, whose catalytic activity inhibits expression of pro-cell-cycle arrest targets by p53, thus leading to augmented apoptosis. IP6K2 appears to enhance p53-dependent apoptosis by selectively decreasing expression of proarrest proteins such as p21. Cells deficient in p21 display augmented apoptosis, in part because their failure to arrest the cell cycle in the face of genotoxic stress augments the apoptotic program (26, 27) and in part because p21 has direct antiapoptotic properties (21). Several other proteins can alter the balance between the proarrest and proapoptotic targets of p53 (28). Like IP6K2, some of these directly interact with the core domain of p53. For instance, the proapoptotic proteins ASPP1 and ASPP2 directly interact with p53 via the core domain and promote transcription of proapoptotic gene targets such as Bax, PUMA, and PIG3 (29). Hzf, another p53 core domain-binding protein and itself a p53 transcriptional target, selectively increases levels of proarrest targets such as p21 and 14-3-3σ and diminishes levels of proapoptotic targets such as Bax, PUMA, and NOXA (30). Although IP6K2 also regulates p53 by direct binding, its catalytic activity generating IP7 is essential for its influence on p53 signaling.
IP6K2 knockout mice were recently described (15). The mice exhibit substantially increased tumorigenesis in response to 4-nitroquinoline-1-oxide, a UV-mimetic carcinogen. Fibroblasts from these mice are resistant to γ-irradiation and exhibit enhanced DNA repair. These observations fit with a modulation of the p53 response by IP6K2, because p53-mediated apoptosis is required for γ-irradiation-induced death (31, 32), whereas p53-mediated growth arrest engages responses that contribute to DNA repair (33). Unlike p53 knockouts, the IP6K2 mutants do not develop spontaneous tumors, which may reflect IP6K2 influencing only one of p53’s actions, its differential effects on proarrest versus proapoptotic functions. Similarly, mice with deletion of selectively proapoptotic p53 effector genes PUMA and NOXA display a phenotype more closely resembling IP6K2 than p53 knockouts (34, 35).
Our findings may have therapeutic relevance. Agents that activate IP6K2 may facilitate the treatment of cancers whose growth is dependent upon impaired p53 function. Aside from its role as a tumor suppressor, p53 regulates death of multiple cell types including neurons (36). p53 has an integral role in neuronal death due to ischemia or excitotoxicity and has been implicated in the pathology of Huntington disease and other neurodegenerative conditions (36, 37). Agents that prevent neuronal cell death may be useful therapies for stroke and neurodegenerative diseases. IP6K2 is highly expressed in the brain (38). Thus, IP6K2 inhibitors may benefit these neurologic conditions.
Materials and Methods
Detailed methods for generation and characterization of IP6K2 d/d HCT116 cells, cell lines and culture conditions, immunoblotting, immunoprecipitation, and in vitro binding experiments, and viability, apoptosis, and cell-cycle analysis are available in SI Materials and Methods.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Bert Vogelstein, Fred Bunz, Stuart Schreiber, and Paul Nghiem for kindly providing experimental tools and Rashna Bhandari, Gary Ho, and members of the Snyder laboratory for helpful comments. This work was supported by US Public Health Service Grant DA-000266 and Research Scientist Award DA00074 (to S.H.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015671107/-/DCSupplemental.
References
- 1.Irvine RF, Schell MJ. Back in the water: The return of the inositol phosphates. Nat Rev Mol Cell Biol. 2001;2:327–338. doi: 10.1038/35073015. [DOI] [PubMed] [Google Scholar]
- 2.Luo HR, et al. Inositol pyrophosphates mediate chemotaxis in Dictyostelium via pleckstrin homology domain-PtdIns(3,4,5)P3 interactions. Cell. 2003;114:559–572. doi: 10.1016/s0092-8674(03)00640-8. [DOI] [PubMed] [Google Scholar]
- 3.Saiardi A, Resnick AC, Snowman AM, Wendland B, Snyder SH. Inositol pyrophosphates regulate cell death and telomere length through phosphoinositide 3-kinase-related protein kinases. Proc Natl Acad Sci USA. 2005;102:1911–1914. doi: 10.1073/pnas.0409322102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.York SJ, Armbruster BN, Greenwell P, Petes TD, York JD. Inositol diphosphate signaling regulates telomere length. J Biol Chem. 2005;280:4264–4269. doi: 10.1074/jbc.M412070200. [DOI] [PubMed] [Google Scholar]
- 5.Saiardi A, Sciambi C, McCaffery JM, Wendland B, Snyder SH. Inositol pyrophosphates regulate endocytic trafficking. Proc Natl Acad Sci USA. 2002;99:14206–14211. doi: 10.1073/pnas.212527899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Illies C, et al. Requirement of inositol pyrophosphates for full exocytotic capacity in pancreatic beta cells. Science. 2007;318:1299–1302. doi: 10.1126/science.1146824. [DOI] [PubMed] [Google Scholar]
- 7.Morrison BH, Bauer JA, Kalvakolanu DV, Lindner DJ. Inositol hexakisphosphate kinase 2 mediates growth suppressive and apoptotic effects of interferon-beta in ovarian carcinoma cells. J Biol Chem. 2001;276:24965–24970. doi: 10.1074/jbc.M101161200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagata E, et al. Inositol hexakisphosphate kinase-2, a physiologic mediator of cell death. J Biol Chem. 2005;280:1634–1640. doi: 10.1074/jbc.M409416200. [DOI] [PubMed] [Google Scholar]
- 9.Shears SB. Understanding the biological significance of diphosphoinositol polyphosphates (‘inositol pyrophosphates’) Biochem Soc Symp. 2007;74:211–221. doi: 10.1042/BSS0740211. [DOI] [PubMed] [Google Scholar]
- 10.Mulugu S, et al. A conserved family of enzymes that phosphorylate inositol hexakisphosphate. Science. 2007;316:106–109. doi: 10.1126/science.1139099. [DOI] [PubMed] [Google Scholar]
- 11.Lee YS, Mulugu S, York JD, O’Shea EK. Regulation of a cyclin-CDK-CDK inhibitor complex by inositol pyrophosphates. Science. 2007;316:109–112. doi: 10.1126/science.1139080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhandari R, Juluri KR, Resnick AC, Snyder SH. Gene deletion of inositol hexakisphosphate kinase 1 reveals inositol pyrophosphate regulation of insulin secretion, growth, and spermiogenesis. Proc Natl Acad Sci USA. 2008;105:2349–2353. doi: 10.1073/pnas.0712227105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morrison BH, et al. Inositol hexakisphosphate kinase 2 sensitizes ovarian carcinoma cells to multiple cancer therapeutics. Oncogene. 2002;21:1882–1889. doi: 10.1038/sj/onc/1205265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chakraborty A, et al. HSP90 regulates cell survival via inositol hexakisphosphate kinase-2. Proc Natl Acad Sci USA. 2008;105:1134–1139. doi: 10.1073/pnas.0711168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrison BH, et al. Gene deletion of inositol hexakisphosphate kinase 2 predisposes to aerodigestive tract carcinoma. Oncogene. 2009;28:2383–2392. doi: 10.1038/onc.2009.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bunz F, et al. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J Clin Invest. 1999;104:263–269. doi: 10.1172/JCI6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B. Role of BAX in the apoptotic response to anticancer agents. Science. 2000;290:989–992. doi: 10.1126/science.290.5493.989. [DOI] [PubMed] [Google Scholar]
- 18.Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 19.Villunger A, et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 20.Waldman T, Kinzler KW, Vogelstein B. p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res. 1995;55:5187–5190. [PubMed] [Google Scholar]
- 21.Jänicke RU, Sohn D, Essmann F, Schulze-Osthoff K. The multiple battles fought by anti-apoptotic p21. Cell Cycle. 2007;6:407–413. doi: 10.4161/cc.6.4.3855. [DOI] [PubMed] [Google Scholar]
- 22.Bunz F, et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 23.Kruse JP, Gu W. Modes of p53 regulation. Cell. 2009;137:609–622. doi: 10.1016/j.cell.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ofek P, Ben-Meir D, Kariv-Inbal Z, Oren M, Lavi S. Cell cycle regulation and p53 activation by protein phosphatase 2C alpha. J Biol Chem. 2003;278:14299–14305. doi: 10.1074/jbc.M211699200. [DOI] [PubMed] [Google Scholar]
- 25.Morrison BH, Tang Z, Jacobs BS, Bauer JA, Lindner DJ. Apo2L/TRAIL induction and nuclear translocation of inositol hexakisphosphate kinase 2 during IFN-beta-induced apoptosis in ovarian carcinoma. Biochem J. 2005;385:595–603. doi: 10.1042/BJ20040971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu J, Wang Z, Kinzler KW, Vogelstein B, Zhang L. PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc Natl Acad Sci USA. 2003;100:1931–1936. doi: 10.1073/pnas.2627984100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seoane J, Le HV, Massague J. Myc suppression of the p21(Cip1) Cdk inhibitor influences the outcome of the p53 response to DNA damage. Nature. 2002;419:729–734. doi: 10.1038/nature01119. [DOI] [PubMed] [Google Scholar]
- 28.Vousden KH, Prives C. Blinded by the light: The growing complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 29.Samuels-Lev Y, et al. ASPP proteins specifically stimulate the apoptotic function of p53. Mol Cell. 2001;8:781–794. doi: 10.1016/s1097-2765(01)00367-7. [DOI] [PubMed] [Google Scholar]
- 30.Das S, et al. Hzf determines cell survival upon genotoxic stress by modulating p53 transactivation. Cell. 2007;130:624–637. doi: 10.1016/j.cell.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993;362:847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- 32.Lowe SW, Ruley HE, Jacks T, Housman DE. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993;74:957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- 33.Gatz SA, Wiesmuller L. p53 in recombination and repair. Cell Death Differ. 2006;13:1003–1016. doi: 10.1038/sj.cdd.4401903. [DOI] [PubMed] [Google Scholar]
- 34.Jeffers JR, et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell. 2003;4:321–328. doi: 10.1016/s1535-6108(03)00244-7. [DOI] [PubMed] [Google Scholar]
- 35.Shibue T, et al. Integral role of Noxa in p53-mediated apoptotic response. Genes Dev. 2003;17:2233–2238. doi: 10.1101/gad.1103603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacobs WB, Kaplan DR, Miller FD. The p53 family in nervous system development and disease. J Neurochem. 2006;97:1571–1584. doi: 10.1111/j.1471-4159.2006.03980.x. [DOI] [PubMed] [Google Scholar]
- 37.Bae BI, et al. p53 mediates cellular dysfunction and behavioral abnormalities in Huntington’s disease. Neuron. 2005;47:29–41. doi: 10.1016/j.neuron.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Saiardi A, Erdjument-Bromage H, Snowman AM, Tempst P, Snyder SH. Synthesis of diphosphoinositol pentakisphosphate by a newly identified family of higher inositol polyphosphate kinases. Curr Biol. 1999;9:1323–1326. doi: 10.1016/s0960-9822(00)80055-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.