Abstract
Growing evidence indicates that Wingless-type (Wnt) signaling plays an important role in the maturation of the central nervous system. We report here that Wingless-type family member 5A (Wnt-5a) is expressed early in development and stimulates dendrite spine morphogenesis, inducing de novo formation of spines and increasing the size of the preexisting ones in hippocampal neurons. Wnt-5a increased intracellular calcium concentration in dendritic processes and the amplitude of NMDA spontaneous miniature currents. Acute application of Wnt-5a increased the amplitude of field excitatory postsynaptic potentials (fEPSP) in hippocampal slices, an effect that was prevented by calcium-channel blockers. The physiological relevance of our findings is supported by studies showing that Wnt scavengers decreased spine density, miniature excitatory postsynaptic currents, and fEPSP amplitude. We conclude that Wnt-5a stimulates different aspects of synaptic differentiation and plasticity in the mammalian central nervous system.
The Wingless-type (Wnt) signaling pathway modulates several developmental processes, and it is activated by the interaction of the Wnt ligand with members of the Frizzled (Fz) family of seven transmembrane cell-surface receptors (1). It has been reported that Wnt signaling plays a key role in diverse aspects of neuronal development and connectivity (2), regulating axon guidance and remodeling (3), dendrite development (4), synapse formation, (5) and synaptic plasticity (6, 7). Several components of the Wnt pathway are localized at adult synapses, indicating that the molecular machinery required to transduce Wnt signaling is structurally localized at central synapses (8).
Different pathways have been described downstream of Fz receptors: the canonical Wnt/β–catenin pathway and the noncanonical ones which involve intracellular signaling by Ca2+ (the Wnt/Ca2+ pathway) and the JNK cascade (the Wnt/JNK pathway) (9, 10). Different canonical Wnt ligands have been shown to modulate the presynaptic region. Wnt-7a increases the clustering of synapsin 1 in cerebellar neurons (3) and regulates the trafficking of the α7 nicotinic acetylcholine receptor to presynaptic terminals in hippocampal neurons (11). In addition, double-mutant mice lacking Wnt-7a and Dishevelled 1 show impaired neurotransmitter release at existing synapses, suggesting a role for Wnt signaling in synaptic transmission (5). Wnt-7a and Wnt-3a were shown to induce the recycling and exocytosis of synaptic vesicles in mature hippocampal neurons and to enhance synaptic transmission in adult hippocampal slices (12). Wnt7a/b levels also were increased in CA3 pyramidal neurons by an enriched environment in which the increase in synapse number at the hippocampal stratum lucidum was shown to be mediated by Wnt signaling (13). Wnt-3a is able to modulate presynaptic differentiation (14, 15), and it is released from synapses by an activity-dependent mechanism that facilitates postsynaptic long-term potentiation (6).
Recent studies indicated that a different Wnt ligand, Wnt-5a, inhibits hippocampal synapse formation (15), enhances field excitatory postsynaptic potentials (fEPSP) (12, 16), promotes the clustering of postsynaptic density protein-95 (PSD-95) (16), increases trafficking of GABAA receptors (17), and prevents the toxicity of amyloid-β oligomers at hippocampal synapses (18). These results prompted us to evaluate the role of Wnt-5a in dendrite spine formation and glutamatergic neurotransmission in cultured neurons and in the adult hippocampus. Our results provide definitive evidence indicating that Wnt-5a is a postsynaptic factor acting at the mammalian central nervous system.
Results
Expression and Distribution of Wnt-5a in Cultured Hippocampal Neurons and in the Rat Hippocampus.
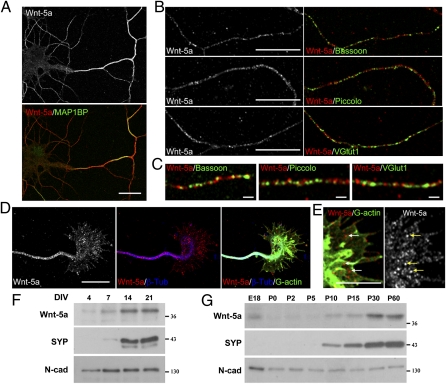
The Wnt signaling pathway plays a role in different aspects of the nervous system development and function (2, 8, 10). In an attempt to understand the role of Wnt ligands in normal synaptic development, we studied the distribution of Wnt-5a in hippocampal neuronal cultures. We observed that during early development, at 4 d in vitro (DIV), Wnt-5a is expressed in neurons and is trafficked mainly to the axon, although Wnt-5a immunoreactivity also is observed in dendrites. At this stage, Wnt-5a was codistributed with phosphorylated MAP1B (Fig. 1A). On DIV 7, Wnt-5a still is present in axons costained with Bassoon and Piccolo, two active-zone cytomatrix proteins associated with a presynaptic precursor transport vesicle, and with the vesicular glutamate transporter 1 (VGlut1), a synaptic vesicle protein (Fig. 1B). Although Wnt-5a is distributed with these axonal proteins, it does not seem to colocalize with Bassoon, Piccolo, or VGlut1 puncta (Fig. 1C). Wnt-5a also was detected on growth cones stained with an anti-β-tubulin antibody to show microtubule distribution and with DNase I to show G-actin (Fig. 1D). Higher magnification shows a punctate staining pattern of Wnt-5a in growth cones (Fig. 1E, arrows).
Fig. 1.
Wnt-5a expression during development in cultured hippocampal neurons and rat hippocampus. (A) Immunodetection of Wnt-5a in hippocampal neurons at DIV 4 shows codistribution of Wnt-5a with phosphorylated MAP1B. (Scale bar: 20 μm.) (B) Wnt-5a immunofluorescence in axons of hippocampal neurons at DIV 7 costained with Bassoon (Top), Piccolo (Middle), and V-Glut1 (Bottom). (Scale bars: 10 μm.) (C) Higher magnification of images shown in B. (Scale bars: 1 μm.) (D) Wnt-5a immunofluorescence in growth cones stained with β-tubulin to show the microtubule distribution and stained with DNase I-FITC to show G-actin in hippocampal neurons at DIV 4. (Scale bar: 10 μm.) (E) Higher magnification of images shown in D. Arrows indicate punctate staining of Wnt-5a. (Scale bar: 5 μm.) (F) Immunoblots of protein extracts from cultured hippocampal neurons through DIV 4–21. (G) Immunoblots of total protein extracts in the developing hippocampus from embryonic day 18 (E18) through postnatal day 50 (P0–P50). The same amount of protein was loaded at different stages. N-cad, N-cadherin; SYP, synaptophysin.
We also analyzed the temporal appearance of Wnt-5a in differentiating hippocampal neurons and observed that Wnt-5a was detected at DIV 4, increasing its expression through DIV 7, reaching a plateau at DIV 14, and maintaining this high level of expression until DIV 21 (Fig. 1F). We also studied the expression of Wnt-5a in the developing hippocampus and observed that Wnt-5a is detectable before birth and starts to increase its expression at postnatal day 10 (P10) reaching its maximum expression level at adult stages (P >30), concomitant with the increase of synaptophysin. N-cadherin, was used as the loading control (19), because it has a constant expression at all ages studied (Fig. 1G).
Wnt-5a Increases Spine Morphogenesis in Cultured Hippocampal Neurons.
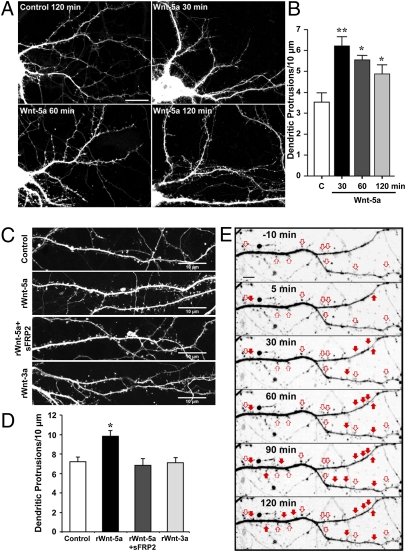
Because Wnt-5a is present in axons, we evaluated its possible effect on postsynaptic neurons. First, we studied the effect of Wnt-5a on dendritic spine density. Hippocampal neurons transfected with EGFP were treated at DIV 12 with control- and Wnt-5a–conditioned media (Fig. 2A). Wnt-5a treatment for 30–120 min induced a transient increase in dendritic protrusions with a maximal effect at 30 min (Fig. 2 A and B). To assess whether the increase in dendritic protrusions produces an increase in dendritic spines, we analyzed the number of protrusions (0.5–3.0 μm in length) containing the postsynaptic scaffold protein PSD-95. As previously reported, Wnt-5a treatment significantly increased PSD-95 clustering (the number of PSD-95–positive spines per neurite length) (16). According to these data, 54% of the induced protrusions are differentiated into dendrite spines that contain the postsynaptic marker, and the ratio of PSD-95–positive/ PSD-95–negative protrusions in a 2-h period increases ≈20%. These results suggest that the effect of Wnt-5a is twofold: It increases dendritic protrusions, and it induces these protrusions to mature into postsynaptic structures.
Fig. 2.
Wnt-5a increases dendritic spine density. (A) Images of dendrites of EGFP-transfected hippocampal neurons treated at DIV 12 with Wnt-5a for 30, 60, and 120 min and with control medium for 120 min. (B) Quantification of the density of dendritic protrusions versus time of treatment with Wnt-5a–conditioned medium and control medium (C). *P < 0.05; **P < 0.01. (C) Images of dendrites of DIV 14 hippocampal neurons transfected with EGFP and treated with rWnt-5a (250 ng/mL), rWnt-5a plus sFRP-2 (1 μg/mL), or rWnt-3a for 120 min. (Scale bars: 10 μm.) (D) Quantification of density of dendritic protrusions with treatments shown in C. Data represent the mean ± SE of three independent experiments; n ≥ 30 dendrite segments. *P < 0.05. ANOVA, Dunnett´s posttest. (E) Wnt-5a induces de novo formation of dendritic spines (filled arrows) and modulates preexisting spine volume (empty arrows). Live-cell time-lapse imaging of an EGFP-transfected neuron dendrite shown before (−10 min) and after 5, 30, 60, 90, and 120 min of treatment with rWnt-5a. (Scale bar: 5 μm.) (An enlarged version of the time lapse is shown in Fig. S1).
The increase in dendritic protrusions also was observed when GFP-transfected neurons at DIV 14 were treated for 2 h with 250 ng/mL recombinant Wnt-5a (rWnt-5a) (Fig. 2C). This effect was blocked by cotreatment with the Wnt antagonist soluble Frizzled-related protein 2 (sFRP-2) (Fig. 2 C and D), which binds to Wnt, preventing interaction with cellular receptors (20). To demonstrate specificity for the Wnt-5a effect, we used recombinant Wnt-3a (rWnt-3a). As indicated in Fig. 2 C and D, no effect on dendrite protrusions was observed by treatment with rWnt-3a. To assess whether the effect of Wnt-5a is limited to de novo formation of spines, we analyzed live-cell time-lapse imaging of the formation of dendrite protrusions in response to rWnt-5a in GFP-transfected hippocampal neurons at DIV 14 (Fig. 2E). Quantification of dendrite protrusions in secondary neurites confirms the de novo formation of dendrite spines in response to rWnt-5a treatment (Fig. 2E and Fig. S1, filled arrows), resulting in a 23 ± 7% increase in the density of dendrite spines. rWnt-5a also was able to induce an increase in the size of spines in 25 ± 8% of preexisting protrusions (Fig. 2E and Fig. S1, empty arrows). These results indicate that Wnt-5a has a positive effect on spine morphogenesis in hippocampal neurons.
To assess the role of endogenous Wnt signaling on spine morphogenesis, DIV 12 hippocampal neurons were cultured for 48 h in the presence of the soluble cysteine-rich domain (CRD) of Fz2, one of the receptors for Wnt-5a (21, 22). Fz receptors have an extracellular N-terminal region that contains a CRD consisting of 120–125 residues with 10 conserved cysteines that is necessary for the binding of Wnt molecules (23). There was a significant decrease in dendritic spine density in neurons treated with 2 μg/mL Fz2-CRD as compared with control neurons (Fig. S2). On the other hand, treatment with the soluble CRD of Fz1, a well-described receptor for Wnt-3a (24, 25), induced only a minor decrease in spine density that was not statistically significant (Fig. S2).
Acute Wnt-5a Application Induces an Increase in fEPSP Amplitude in a Calcium-Dependent Manner in Hippocampal Slices.
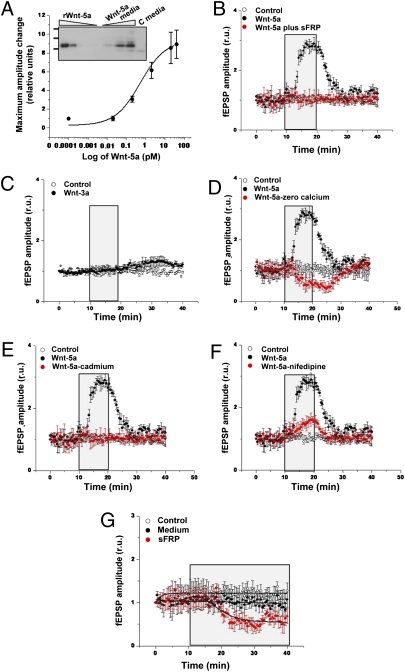
Next, we explored whether the effect of Wnt-5a at the structural postsynaptic level correlates with functional synaptic changes that potentially regulate synaptic plasticity. In previous studies, treatment of hippocampal slices with a concentrated sample of Wnt-5a during long-time exposure resulted in changes in fEPSP without affecting paired pulse facilitation (12, 16). Here we extended these findings and explored more physiological conditions, including short exposure and low concentrations of Wnt-5a in comparison with other trophic factors such as BDNF (26, 27). First we carried out a dose–response experiment in which adult hippocampal slices were exposed to different concentrations of Wnt-5a for 10 min. Under these conditions, we observed a concentration-dependent increase in fEPSP amplitude, as represented by the sigmoid plot shown in Fig. 3A. The Inset shows the immunodetection of Wnt-5a present in the conditioned medium; rWnt-5a was used as control. The calculated EC50 was ≈0.6 pM. These results show that 2.0 pM of Wnt-5a has a clear effect on fEPSP amplitude, and that value was selected for subsequent experiments. The observed effect of Wnt-5a–conditioned medium was completely abolished when slices were coincubated with the Wnt antagonist sFRP-2 (Fig. 3B), and no effect was observed with the canonical ligand Wnt-3a (Fig. 3C), indicating that the increase in fEPSP amplitude is induced specifically by the Wnt-5a ligand.
Fig. 3.
Wnt-5a ligand alters fEPSP in a concentration- and calcium-dependent manner. (A) Hippocampal slices were exposed to different dilutions of Wnt-5a–conditioned medium. Plot of fEPSP amplitude versus the concentrations of Wnt5a in the medium. Inset shows an immunodetection of Wnt-5a in increasing volumes of Wnt-5a–conditioned medium and control-conditioned medium (C media). rWnt-5a was used as control. (B) Plot of fEPSP amplitude changes with treatment with 2 pM Wnt-5a for 10 min (shown in the box), in the absence or presence of sFRP (25 nM). (C) Plot of fEPSP amplitude changes with treatment with Wnt-3a for 10 min. (D–F) Plots of fEPSP amplitude changes with treatment with 2.0 pM Wnt-5a for 10 min in the absence or presence of zero calcium solution (D), 2 mM cadmium (E), or 5 μM nifedipine (F). (G) Changes in fEPSP amplitude with treatment with control-conditioned medium or 25 nM sFRP. Dots and bars represent means ± SE from six different slices.
Because synaptic activity is strongly modulated by calcium, we explored the role of calcium in fESPS amplitude changes induced by Wnt-5a. Incubation of hippocampal slices with Wnt-5a was carried out in the absence of calcium. As indicated in Fig. 3D, this condition completely prevented the effect of Wnt-5a. In addition, cadmium was used as a general blocker of calcium channels. In slices exposed to Wnt-5a in the presence of 2 mM cadmium, no change in the amplitude was observed (Fig. 3E), indicating that calcium entry is responsible for the changes in fEPSP amplitude induced by Wnt-5a. Then hippocampal slices were coincubated with 5 μM nifedipine, an l-type calcium channel blocker, which blocked most of the Wnt-5a effect on fEPSP amplitude (Fig. 3F). Taken together, these experiments indicate that the Wnt-5a effect on fEPSP is a calcium-dependent process, mediated by the entry of calcium through calcium channels.
Finally, to assess the effect of inhibiting endogenous Wnt signaling, hippocampal slices were perfused with sFRP-2 alone, which binds to Wnt and prevents interaction with neuronal receptors. Under this condition, after a delay of 5 min, a clear decrease in the fEPSP amplitude was observed that lasted more than 20 min (Fig. 3G), suggesting that removal of endogenous Wnt-5a modulates fEPSP tone. These results suggest a relationship between the effects of Wnt-5a at the dendritic spine level and synaptic activity.
Wnt-5a Activates the Wnt/Ca2+ Signaling Pathway in Hippocampal Neurons.
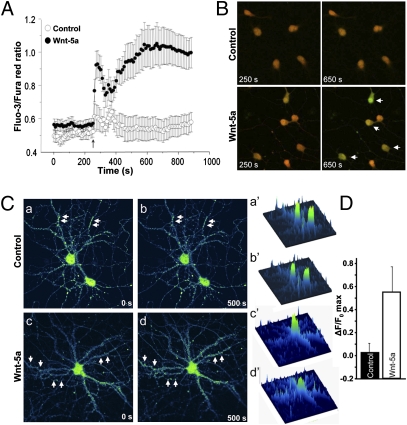
Previous studies in our laboratory indicated that Wnt-5a induces a rapid increase in the calcium-sensitive protein calmodulin-dependent protein kinase II (CaMKII) (16) because of an increase in intracellular calcium, as predicted by the Wnt/Ca2+ pathway (28). Therefore, we studied the role of Wnt-5a in calcium signaling in hippocampal neurons. Intracellular calcium changes were studied using the probes Fluo-3 and Fura Red (29). In developing DIV 3 neurons, Wnt-5a induced a burst of calcium that was not observed in neurons exposed to control medium (Fig. 4A). Fig. 4B shows representative images of control and Wnt-5a–treated neurons just before the addition of the medium (250 s) and after treatment for 400 s (650 s). An increase in the green signal (Fluo-3) concomitant with a decrease in the red signal (Fura Red) was observed in Wnt-5a–treated neurons (Fig. 4B, arrows), indicative of an increase in the intracellular calcium concentration. In mature DIV 14 hippocampal neurons, treatment with recombinant Wnt-5a induced a rapid, transient increase in the intensity of the calcium indicator Fluo-3 contained in the dendritic compartments (Fig. 4C and Movie S1). The maximal neurite intensity of the calcium-sensitive probe was significantly higher in Wnt-5a–treated neurons than in vehicle-treated neurons (Fig. 4D and Movie S2). Interestingly, we have observed previously that the increase of CaMKII activity by Wnt-5a occurs after 5 min of treatment (16). The rapid increase in the intracellular calcium concentration observed here could account for the rapid increase in CaMKII activity in response to Wnt-5a. Taken together, these results indicate that Wnt-5a activates the Wnt/Ca2+ signaling pathway in hippocampal neurons, and this effect probably is linked to the increase observed in dendrite spines and fEPSP amplitude.
Fig. 4.
Wnt-5a activates Ca2+ signaling in hippocampal neurons. (A) Fluo-3/Fura Red fluorescence ratio in DIV 3 cultured hippocampal neurons exposed to control (○) and Wnt-5a–conditioned (●) media. The graph shows the mean of three independent experiments. The arrow indicates the addition of conditioned medium. (B) Representative images of control and Wnt-5a–treated cells just before the addition of the medium (250 s) and after 400 s of treatment (650 s). Arrows indicate cells in which there is increased green staining (Fluo-3) together with decreased red staining (Fura Red), indicative of increased intracellular calcium. (C) Representative pseudocolored images of DIV 14 hippocampal neurons loaded with the calcium indicator fluo-3 and exposed to vehicle (BSA 0.1%) (a, b, a′, b′) or rWnt-5a protein (c, d, c′, d′). Images of fluo-3–loaded neurons (a, b, c, d) and the respective surface intensity distribution (a′, b′, c′, d′) are shown immediately before (a, a’, c, c’) and 500 s after (b, b′, d, d′) the addition of treatments. Arrows indicate the increase in calcium in dendrite spots. (D) Quantification of maximal florescence intensity changes (ΔF/F0 max) in vehicle- and rWnt-5a–treated neurons in neurites.
Wnt-5a Affects Glutamatergic Transmission in Cultured Hippocampal Neurons.
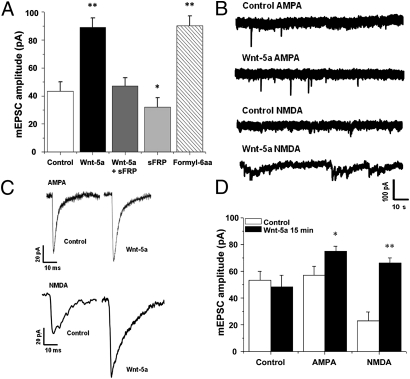
Our studies indicated that Wnt-5a induces changes in spine density and intracellular calcium concentration and increases fEPSP amplitude. Therefore, we evaluated Wnt-5a effects on glutamatergic neurotransmission in cultured hippocampal neurons. Endogenous synaptic activity in hippocampal cultures at DIV 10 are shown in Fig. S3. We observed an increase in the amplitude of miniature excitatory postsynaptic currents (mEPSC) when cultures were exposed to Wnt-5a for 15 min (Fig. 5A). This effect was blocked by coincubation with the Wnt antagonist sFRP-2 and was mimicked by incubation with a formylated hexapeptide (Formyl-6aa) derived from the sequence of Wnt-5a that mimics the action of the full Wnt-5a molecule (Fig. 5A) (16, 30). To evaluate the effect of endogenous Wnt-5a signaling, we incubated neurons with sFRP-2. As indicated in Fig. 5A, treatment with sFRP-2 alone induced a significant decrease in mEPSC amplitude. Then, we used different drugs [TTX, picrotoxin (PTX), 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo(F)quinoxaline (NBQX), and magnesium] to isolate NMDA and AMPA neurotransmission in the presence or absence of Wnt-5a (Fig. 5B). Increased NMDA mEPSC amplitude was observed clearly after 15 min incubation with Wnt-5a, but only a subtle effect was observed for the AMPA current (Fig. 5 C and D). These results indicate that Wnt-5a regulates glutamatergic neurotransmission in cultured hippocampal neurons. These effects indicate that Wnt-5a induces postsynaptic changes and probably has a role in modulating synaptic plasticity in correlation with the calcium entry in dendrite spots and with the structural changes described.
Fig. 5.
Wnt-5a ligand induces changes in glutamatergic neurotransmission. Cultured hippocampal neurons were exposed to Wnt-5a–conditioned medium (2 pM Wnt-5a; 15 min). (A) Plot of total mEPSC amplitude under different conditions: Wnt-5a ligand (2 pM; 15 min) in absence or presence of sFRP (25 nM), sFRP alone (25 nM; 15 min), or Formyl-6aa (10 μM; 15 min). (B and C) Miniature current traces (B) and average traces (C) under control conditions or in the presence of Wnt-5a, isolated by drugs TTX (100 nM), PTX (5 μM), and magnesium (2 mM) for AMPA neurotransmission and TTX (100 nM), PTX (5 μM) and NBQX (1 μM) for NMDA neurotransmission. (D) Plot of the amplitude of miniature currents for particular neurotransmitters in the absence or presence of Wnt-5a ligand. Bars indicate mean ± SE from 12 different cells. *P < 0.05; **P < 0.01.
Discussion
In the last few years there has been wide interest in the role of Wnt signaling in synapse formation and function. In particular, the role of Wnt-7a in presynaptic differentiation and function has been described (3, 5, 11, 12), and more recently a role for Wnt-5a in PSD-95 clustering and GABAA receptor recycling has been established (16, 17). Here we aimed to study the expression and distribution of Wnt-5a in hippocampal neurons and to characterize further the role of this ligand in the postsynaptic structure and function. We found that the Wnt-5a ligand is expressed in hippocampal neurons and is distributed mainly in the axon during early development, showing clustered staining in axons and growth cones. In the Drosophila larval neuromuscular junction, Wingless (Drosophila Wnt homolog) is released on exosome-like vesicles containing the Wnt-binding protein Evenness Interrupted/Wntless/Sprinter (Evi/Wls/Srt) (31). Evi is required for trafficking Wingless from the cell body to the presynaptic terminals and for the secretion of Wingless, which is transported across synapses by Evi-containing vesicles (31). Whether Wnt-5a is secreted via the same mechanism in mammals remains to be determined.
We observed that Wnt-5a expression increases during differentiation of hippocampal neurons and in the hippocampus, showing constant high levels in adult stages. This expression pattern suggests that this ligand may have a role not only during neural differentiation but also in synaptic maintenance and function in adult nervous system (8). A role in synaptic maintenance is supported further by the presence of many Wnt components in adult brain (6, 12, 24, 32, 33). These components have been detected in synaptosomal fractions (5, 14), indicating that the Wnt machinery is present at the synapse, and therefore a local activation of the adult Wnt pathway is plausible. We evaluated the potential role of Wnt-5a in spinogenesis and showed that Wnt-5a induced a transient formation of dendrite protrusions that resulted in a net increase of mature dendrite spines containing the postsynaptic marker PSD-95. Videomicroscopy revealed that Wnt-5a induced de novo dendrite spine formation and also increased the size of preexisting spines, thus implicating a Wnt ligand in dendrite spine morphogenesis in mammals. The effect on dendrite spines, suggests that Wnt-5a has an effect on synapse structure and function. In addition, we observed a strong effect in fEPSP amplitude in response to a short application of Wnt-5a, an effect dependent on calcium entry through cadmium-sensitive, voltage-dependent calcium channels and with the contribution of l-type calcium channels. The precise mechanism by which Wnt-5a induces calcium influx has not been determined.
Calcium is critically involved in synaptic activity, and intracellular calcium changes are classic hallmarks of plasticity in neurons. We determined that Wnt-5a induced a rapid increase in the intracellular calcium concentration, an increase that was observed particularly in dendrite puncta that could be related to synaptic events, suggesting that Wnt-5a may activate the Wnt/Ca2+ signaling pathway. The Wnt/Ca2+ pathway involves an increase in intracellular calcium concentration that modulates the calcium-sensitive proteins CaMKII and PKC (28, 34). We have shown previously that in neurons Wnt-5a induces a rapid increase in CaMKII phosphorylation (16) and that the activation of CaMKII by Wnt-5a induces the recycling of functional GABAA receptors on mature hippocampal neurons (17). Taken together, our findings suggest that Wnt-5a signaling through the Wnt/Ca2+ pathway modulates spine morphogenesis and synaptic function. A recent study indicated that Wnt-3a is able to induce an increase both in the intracellular calcium concentration and in the frequency of mEPSC (35). These changes probably are related to the presynaptic release of neurotransmitter, a result consistent with our earlier studies showing that Wnt-3a modulates the recycling of synaptic vesicles in hippocampal synapses (12). In the present study, we determined that Wnt-3a was not able to increase dendrite spine density and fEPSP amplitude, as Wnt-5a does, suggesting that the effects of Wnt-5a occur at the postsynaptic level. Previous studies from our group described different effects of the Wnt pathway at the synapse. The canonical Wnt-7a ligand acts as a stimulator of the presynaptic activity (11, 12), whereas Wnt-5a ligand could act on the postsynaptic region by changing PSD-95 clustering and GABAA receptor trafficking (16, 17). The different locations of the receptors might contribute to the different effects of the various Wnt ligands.
Concerning the postsynaptic receptors for Wnt-5a, it is noteworthy that different postsynaptic effects of the ligand have been observed, some of which, such as spinogenesis, the enhancement of glutamatergic transmission, and the modulation of GABAA receptor recycling, are related to the Wnt/Ca2+ signaling pathway (17). On the other hand, PSD-95 clustering is induced by the Wnt/JNK pathway (16), also known as the “Wnt/planar cell polarity pathway” (34). Therefore Wnt-5a may interact with at least two different postsynaptic receptors, one of the Fz type, such as Fz2 (21), probably involved in the Wnt/Ca2+ pathway, and another, the receptor tyrosine kinase-like orphan receptor 2 (Ror2) involved in the Wnt-5a/JNK pathway (36), localized in the somatodendritic compartment where it might interact directly with Wnt-5a (37, 38). An alternative Wnt receptor might be the receptor-like tyrosine kinase (Ryk), which is required for Wnt-5a–mediated axon guidance in the corpus callosum (39).
The results presented in this study indicate an acute effect of Wnt-5a on synaptic activity in cultured hippocampal neurons. When acutely applied, Wnt-5a increased the activity, in particular the amplitude of the events. This increase may be related to the effects on calcium entry. In addition, Wnt-5a had a functional effect on glutamatergic neurotransmission, increasing the amplitude of miniature currents of NMDA. It is likely that the effect on glutamatergic neurotransmission is concomitant with the increase in spine density, calcium changes in spot location, and synaptic PSD-95 clustering induced by Wnt-5a in cultured hippocampal neurons.
To address the physiological relevance of the effects of Wnt-5a, we first challenged the effect of this ligand with the soluble version of the Fz2 receptor (Fz2-CRD), one of the receptors described for Wnt-5a (21, 22). Under such conditions, a decreased in dendrite spine density was observed. Second, incubation with sFRP-2 alone decreased mEPSC amplitude in cultured neurons. Third, fEPSP amplitude was reduced steadily by sFRP-2 perfusions to levels below the base line registered using hippocampal slices under control conditions. All these results, obtained under different experimental conditions, strongly suggest that a basal tone of Wnt-5a activity was affected by the treatment with the soluble Wnt receptors and support the idea that endogenous Wnt-5a signaling plays a relevant role in the normal synaptic structure and function of mammalian hippocampus.
In other regions of the nervous system, it has been shown that Wnt-5a ligand regulates ventral midbrain morphogenesis and dopaminergic progenitor cell division and differentiation in vivo (40, 41). Wnt-5a also has been shown to be a downstream effector of nerve growth factor in mediating axonal branching and growth in developing sympathetic neurons (42) and simultaneously increasing cortical axon outgrowth and inducing repulsive turning (43). We have determined that in the hippocampus Wnt-5a is expressed in neurons during development, modulates the formation and maturation of dendritic spines, increases intracellular calcium concentration, stimulates glutamatergic transmission, and therefore might play a role in synaptic plasticity. Overall, these findings indicate that Wnt-5a normally is expressed in the hippocampus, where it plays a trophic role in neuronal differentiation and modulation of synaptic activity.
Methods
Primary Culture of Rat Hippocampal Neurons and Neuronal Transfection.
Rat hippocampal cultures were prepared from Sprague–Dawley rats at embryonic day 18 as described previously (12, 16, 44).
Immunofluorescence.
Immunofluorescence was performed as previously described (14). Primary antibodies used were goat anti-Wnt-5a (R&D Systems), monoclonal anti-Bassoon antibody (Assay Designs), monoclonal anti-MAP1BP antibody (Sternberger Monoclonals), rabbit anti-β-tubulin (Santa Cruz Biotechnology), and rabbit anti-Piccolo (Synaptic Systems). The monoclonal antibody anti-VGlut1 was developed by and obtained from the University of California, Davis/National Institutes of Health (NIH) NeuroMab Facility. Images were captured with a Zeiss LSM 5 Pascal confocal microscope and analyzed using NIH ImageJ software. Live-cell imaging of dendritic spine morphogenesis is described in SI Methods.
Generation of Control- and Wnt-5a–Conditioned Media.
Control- and Wnt-5a–conditioned media were prepared from L cells (ATCC CRL-2648) and L Wnt-5a (ATTC CRL-2814) cells. rWnt-5a, rWnt-3a, and sFRP-2 were purchased from R&D Systems.
Calcium Imaging.
For live-cell calcium imaging, hippocampal neurons were plated at a density of 5 × 104 cells in 60-mm cover slips. Hippocampal cells were loaded with the calcium-sensitive dyes Fluo-3 (5 μM) and Fura Red (15 μM) in DMEM high-glucose medium plus pluronic acid (0.02%) (Molecular Probes) for 30 min at room temperature, washed three times, and placed in a open-bath imaging chamber. Cells were imaged with a Zeiss LSM 5 Pascal confocal microscope with excitation at 488 nm, and emissions were detected at 505–530 nm for Fluo-3 and with an LP650 filter for Fura Red. For experiments with neurons at DIV 3, a 2:1 volume of Wnt-5a–conditioned medium or control-conditioned medium was added with a micropipette to the cells at 250 s and cells were imaged every 10 s for 10 min. Fluo-3/Fura Red intensity ratios of cell bodies were analyzed using ImageJ imaging software. For the experiments in neurons at DIV 14, vehicle (BSA 0.1%) or rWnt-5a (1 μg/mL) was added to the cells loaded with Fluo-3 after 2 min of basal imaging, and maximal fluorescence changes (ΔF/F0 max) were quantified in neuritic regions of interest using the ImageJ software.
Slice Preparation and Electrophysiology.
Hippocampal slices were prepared according to standard procedures from 22- to 30-d-old C57BL/6 mice. Transverse slices (250–300 μm) from the dorsal hippocampus were cut under cold artificial cerebrospinal fluid and recorded as previously described (18). Recordings are detailed in SI Methods.
Whole-Cell Patch Clamp.
The culture medium in the plate was replaced with an external solution containing (in mM): 150 NaCl, 5.4 KCl, 2.0 CaCl2, 1.0 MgCl2, 10 glucose, and 10 Hepes (pH 7.4). The internal solution contained (in mM) 120 KCl, 2.0 MgCl2, 2 ATP-Na2, 10 BAPTA, 0.5 GTP, and 10 Hepes (pH 7.4). The patch-clamp technique was carried out according to Hamill et al. (45). The recordings were obtained in pClamp 10, and responses were analyzed off-line, using the analysis software pClampfit (Axon Instruments, Inc.) and Mini Analysis 6 (Synaptosft, Inc.), which allowed visual detection of events, computing only those events that exceeded an arbitrary threshold calculated based on rms obtained in the computing software. A value fivefold over the rms measured was used in the miniature current analysis.
Supplementary Material
Acknowledgments
We thank Dr. Randall T. Moon (Department of Pharmacology, University of Washington, Seattle, WA) for the kind gift of the Wnt-5a construct. This work was supported by Grant PFB12/2007 from the Basal Center for Excellence in Science and Technology (to N.C.I) and by Grant 79090027 from the Comisión Nacional de Investigación Científica y Tecnológica, Insertion Project (to L.V.-N). I.E.A. is the recipient of a predoctoral fellowship from Comisión Nacional de Investigación Científica y Tecnológica.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1010011107/-/DCSupplemental.
References
- 1.Gordon MD, Nusse R. Wnt signaling: Multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- 2.Salinas PC, Zou Y. Wnt signaling in neural circuit assembly. Annu Rev Neurosci. 2008;31:339–358. doi: 10.1146/annurev.neuro.31.060407.125649. [DOI] [PubMed] [Google Scholar]
- 3.Hall AC, Lucas FR, Salinas PC. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell. 2000;100:525–535. doi: 10.1016/s0092-8674(00)80689-3. [DOI] [PubMed] [Google Scholar]
- 4.Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC. Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat Neurosci. 2005;8:34–42. doi: 10.1038/nn1374. [DOI] [PubMed] [Google Scholar]
- 5.Ahmad-Annuar A, et al. Signaling across the synapse: A role for Wnt and Dishevelled in presynaptic assembly and neurotransmitter release. J Cell Biol. 2006;174:127–139. doi: 10.1083/jcb.200511054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Park CS, Tang SJ. Activity-dependent synaptic Wnt release regulates hippocampal long term potentiation. J Biol Chem. 2006;281:11910–11916. doi: 10.1074/jbc.M511920200. [DOI] [PubMed] [Google Scholar]
- 7.Lim BK, Cho SJ, Sumbre G, Poo MM. Region-specific contribution of ephrin-B and Wnt signaling to receptive field plasticity in developing optic tectum. Neuron. 2010;65:899–911. doi: 10.1016/j.neuron.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Inestrosa NC, Arenas E. Emerging roles of Wnts in the adult nervous system. Nat Rev Neurosci. 2010;11:77–86. doi: 10.1038/nrn2755. [DOI] [PubMed] [Google Scholar]
- 9.Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10:468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- 10.Toledo EM, Colombres M, Inestrosa NC. Wnt signaling in neuroprotection and stem cell differentiation. Prog Neurobiol. 2008;86:281–296. doi: 10.1016/j.pneurobio.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Farías GG, et al. Wnt-7a induces presynaptic colocalization of alpha 7-nicotinic acetylcholine receptors and adenomatous polyposis coli in hippocampal neurons. J Neurosci. 2007;27:5313–5325. doi: 10.1523/JNEUROSCI.3934-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cerpa W, et al. Wnt-7a modulates the synaptic vesicle cycle and synaptic transmission in hippocampal neurons. J Biol Chem. 2008;283:5918–5927. doi: 10.1074/jbc.M705943200. [DOI] [PubMed] [Google Scholar]
- 13.Gogolla N, Galimberti I, Deguchi Y, Caroni P. Wnt signaling mediates experience-related regulation of synapse numbers and mossy fiber connectivities in the adult hippocampus. Neuron. 2009;62:510–525. doi: 10.1016/j.neuron.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 14.Varela-Nallar L, Grabowski CP, Alfaro IE, Alvarez AR, Inestrosa NC. Role of the Wnt receptor Frizzled-1 in presynaptic differentiation and function. Neural Develop. 2009;4:41. doi: 10.1186/1749-8104-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis EK, Zou Y, Ghosh A. Wnts acting through canonical and noncanonical signaling pathways exert opposite effects on hippocampal synapse formation. Neural Develop. 2008;3:32. doi: 10.1186/1749-8104-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farías GG, et al. Wnt-5a/JNK signaling promotes the clustering of PSD-95 in hippocampal neurons. J Biol Chem. 2009;284:15857–15866. doi: 10.1074/jbc.M808986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuitino L, et al. Wnt-5a modulates recycling of functional GABAA receptors on hippocampal neurons. J Neurosci. 2010;30:8411–8420. doi: 10.1523/JNEUROSCI.5736-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cerpa W, et al. Wnt-5a occludes Abeta oligomer-induced depression of glutamatergic transmission in hippocampal neurons. Mol Neurodegener. 2010;5:3. doi: 10.1186/1750-1326-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petralia RS, Sans N, Wang YX, Wenthold RJ. Ontogeny of postsynaptic density proteins at glutamatergic synapses. Mol Cell Neurosci. 2005;29:436–452. doi: 10.1016/j.mcn.2005.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rattner A, et al. A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc Natl Acad Sci USA. 1997;94:2859–2863. doi: 10.1073/pnas.94.7.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slusarski DC, Corces VG, Moon RT. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature. 1997;390:410–413. doi: 10.1038/37138. [DOI] [PubMed] [Google Scholar]
- 22.Sheldahl LC, Park M, Malbon CC, Moon RT. Protein kinase C is differentially stimulated by Wnt and Frizzled homologs in a G-protein-dependent manner. Curr Biol. 1999;9:695–698. doi: 10.1016/s0960-9822(99)80310-8. [DOI] [PubMed] [Google Scholar]
- 23.Dann CE, et al. Insights into Wnt binding and signalling from the structures of two Frizzled cysteine-rich domains. Nature. 2001;412:86–90. doi: 10.1038/35083601. [DOI] [PubMed] [Google Scholar]
- 24.Chacón MA, Varela-Nallar L, Inestrosa NC. Frizzled-1 is involved in the neuroprotective effect of Wnt3a against Abeta oligomers. J Cell Physiol. 2008;217:215–227. doi: 10.1002/jcp.21497. [DOI] [PubMed] [Google Scholar]
- 25.Gazit A, et al. Human frizzled 1 interacts with transforming Wnts to transduce a TCF dependent transcriptional response. Oncogene. 1999;18:5959–5966. doi: 10.1038/sj.onc.1202985. [DOI] [PubMed] [Google Scholar]
- 26.Sallert M, et al. Brain-derived neurotrophic factor controls activity-dependent maturation of CA1 synapses by downregulating tonic activation of presynaptic kainate receptors. J Neurosci. 2009;29:11294–11303. doi: 10.1523/JNEUROSCI.0560-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson-Venkatesh EM, Umemori H. Secreted factors as synaptic organizers. Eur J Neurosci. 2010;32:181–190. doi: 10.1111/j.1460-9568.2010.07338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohn AD, Moon RT. Wnt and calcium signaling: Beta-catenin-independent pathways. Cell Calcium. 2005;38:439–446. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 29.Lipp P, Niggli E. Ratiometric confocal Ca(2+)-measurements with visible wavelength indicators in isolated cardiac myocytes. Cell Calcium. 1993;14:359–372. doi: 10.1016/0143-4160(93)90040-d. [DOI] [PubMed] [Google Scholar]
- 30.Säfholm A, et al. A formylated hexapeptide ligand mimics the ability of Wnt-5a to impair migration of human breast epithelial cells. J Biol Chem. 2006;281:2740–2749. doi: 10.1074/jbc.M508386200. [DOI] [PubMed] [Google Scholar]
- 31.Korkut C, et al. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell. 2009;139:393–404. doi: 10.1016/j.cell.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimogori T, VanSant J, Paik E, Grove EA. Members of the Wnt, Fz, and Frp gene families expressed in postnatal mouse cerebral cortex. J Comp Neurol. 2004;473:496–510. doi: 10.1002/cne.20135. [DOI] [PubMed] [Google Scholar]
- 33.Lein ES, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 34.Montcouquiol M, Crenshaw EB, 3rd, Kelley MW. Noncanonical Wnt signaling and neural polarity. Annu Rev Neurosci. 2006;29:363–386. doi: 10.1146/annurev.neuro.29.051605.112933. [DOI] [PubMed] [Google Scholar]
- 35.Avila ME, et al. Canonical Wnt3a modulates intracellular calcium and enhances excitatory neurotransmission in hippocampal neurons. J Biol Chem. 2010;285:18939–18947. doi: 10.1074/jbc.M110.103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oishi I, et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- 37.Paganoni S, Ferreira A. Expression and subcellular localization of Ror tyrosine kinase receptors are developmentally regulated in cultured hippocampal neurons. J Neurosci Res. 2003;73:429–440. doi: 10.1002/jnr.10674. [DOI] [PubMed] [Google Scholar]
- 38.Paganoni S, Bernstein J, Ferreira A. Ror1-Ror2 complexes modulate synapse formation in hippocampal neurons. Neuroscience. 2010;165:1261–1274. doi: 10.1016/j.neuroscience.2009.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keeble TR, et al. The Wnt receptor Ryk is required for Wnt5a-mediated axon guidance on the contralateral side of the corpus callosum. J Neurosci. 2006;26:5840–5848. doi: 10.1523/JNEUROSCI.1175-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andersson ER, et al. Wnt5a regulates ventral midbrain morphogenesis and the development of A9-A10 dopaminergic cells in vivo. PLoS ONE. 2008;3:e3517. doi: 10.1371/journal.pone.0003517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castelo-Branco G, et al. Differential regulation of midbrain dopaminergic neuron development by Wnt-1, Wnt-3a, and Wnt-5a. Proc Natl Acad Sci USA. 2003;100:12747–12752. doi: 10.1073/pnas.1534900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bodmer D, Levine-Wilkinson S, Richmond A, Hirsh S, Kuruvilla R. Wnt5a mediates nerve growth factor-dependent axonal branching and growth in developing sympathetic neurons. J Neurosci. 2009;29:7569–7581. doi: 10.1523/JNEUROSCI.1445-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li L, Hutchins BI, Kalil K. Wnt5a induces simultaneous cortical axon outgrowth and repulsive axon guidance through distinct signaling mechanisms. J Neurosci. 2009;29:5873–5883. doi: 10.1523/JNEUROSCI.0183-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caceres A, Banker G, Steward O, Binder L, Payne M. MAP2 is localized to the dendrites of hippocampal neurons which develop in culture. Brain Res. 1984;315:314–318. doi: 10.1016/0165-3806(84)90167-6. [DOI] [PubMed] [Google Scholar]
- 45.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.