Abstract
At suprathreshold levels, detection and awareness of visual stimuli are typically synonymous in nonclinical populations. But following postgeniculate lesions, some patients may perform above chance in forced-choice detection paradigms, while reporting not to see the visual events presented within their blind field. This phenomenon, termed “blindsight,” is intriguing because it demonstrates a dissociation between detection and perception. It is possible, however, for a blindsight patient to have some “feeling” of the occurrence of an event without seeing per se. This is termed blindsight type II to distinguish it from the type I, defined as discrimination capability in the total absence of any acknowledged awareness. Here we report on a well-studied patient, D.B., whose blindsight capabilities have been previously documented. We have found that D.B. is capable of detecting visual patterns defined by changes in luminance (first-order gratings) and those defined by contrast modulation of textured patterns (textured gratings; second-order stimuli) while being aware of the former but reporting no awareness of the latter. We have systematically investigated the parameters that could lead to visual awareness of the patterns and show that mechanisms underlying the subjective reports of visual awareness rely primarily on low spatial frequency, first-order spatial components of the image.
Since the early reports in the 1970s of brain-damaged human subjects who are able to discriminate visual events in the absence of self-reported conscious awareness (1, 2), an obvious but difficult question has arisen as to what stimulus properties would drive visual awareness. In nonclinical populations, a number of paradigms have been developed to examine the prerequisites. For example, in attentional-blink paradigms (3), rapid serial visual presentation demonstrates that letters or words presented within a short temporal window following a task-relevant target may be degraded. As is well known, manipulating the functional significance of targets by using the participant's name (4) or emotional stimuli (5) modulates reported perception and discrimination of stimuli. More recently, the flash suppression technique in binocular rivalry, where one eye is presented with a rapid random sequence of abstract shapes while a specific stimulus is presented to the other eye (6), has been used to demonstrate that some classes of stimuli can break through and elicit awareness (7, 8). Common among tasks presented to nonclinical subjects is the assumption that awareness of the stimuli and their discrimination, if not identical, cannot be dissociated from each other.
The blindsight phenomenon in human subjects is unique: following visual cortical lesions, the detection and discrimination of stimulus features can occur in the absence of subjective awareness. For this reason, the research relies on “heroic” approaches such as forced-choice guessing (9). The reported awareness is then recorded using a commentary key paradigm on either a binary (10) or a multipoint scale (11), yielding comparable results. When there is awareness of certain types of visual events presented to the blind field, such awareness is reported to be different from that of the sighted field, because it lacks form and content, and is more akin to a feeling that something occurred. This has been designated as blindsight type 2 or aware mode to distinguish it both from normal vision and type 1 blindsight or unaware mode, defined as above-chance discrimination in the absence of any conscious awareness (12).
We have previously reported that a well-studied blindsight subject, G.Y., was able to perform equally well both with and without conscious experience of a moving dot target, depending on the stimulus parameters (13). A functional brain imaging study showed contrasting patterns of brain activity under the two modes of processing (14), with a shift in the unaware mode toward subcortical activations with a more cortical and especially frontal network being activated in the aware mode.
D.B. was the first blindsight subject studied extensively by Weiskrantz and colleagues (2, 10), and more recently has been shown to have more sensitive detection of first-order gratings in his blind field than in his sighted field or that of aged-matched healthy subjects (15). (By first order we mean gratings formed by a sine-wave profile of luminous changes of specified cycles per degree in which the luminance of the bright and dark bars is matched in overall flux of the background.) He also reports awareness of high-contrast first-order gratings. Therefore it was very surprising to discover that he remained unaware of the presentation of high-contrast texture modulated gratings—i.e., second-order gratings—while nevertheless being able to perform at well above-chance levels in detecting their presence and orientation. (By second order we mean gratings formed by the imposition of a sine-wave envelope of contrast changes on a field of randomly arranged granules or spots.) This pattern of findings has remained stable in several multiple-testing sessions over a 5-y period. This provides a unique opportunity to chart the prerequisites of D.B.’s visual awareness systematically. The result is a series of experiments in which first- and second-order gratings were studied with systematic manipulation of spatial frequency and other stimulus properties to determine their effects on detection and reported awareness. These findings suggest that, though D.B. is able to detect first- and second-order stimuli across a range of spatial frequencies, his awareness of them is critically dependent on the presence of low-frequency sine-wave luminance information, i.e., on first-order information.
Results
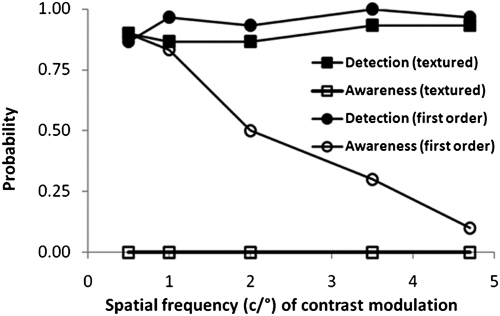
The early observations were made in August 2004. Using a temporal two-alternative forced-choice paradigm, where one interval contained a first-order Gabor patch at 27.5% contrast and the remaining interval a blank (a Gabor is a sine-wave grating, contrast modulated by a Gaussian envelope to smooth and blend the stimulus edges to the background, avoiding sharp spatial transients at the boundaries), D.B. performed well above chance at detection of a range of spatial frequencies (Fig. 1; 30 presentations per frequency), confirming the previous findings (15). His reported awareness was frequent at low spatial frequencies and decreased monotonically with increasing spatial frequency. For fine-textured second-order stimuli at 50% contrast, although equally good detection was found, he did not report any awareness throughout the session.
Fig. 1.
Probability of detection and awareness for first- and second-order stimuli as a function of spatial frequency.
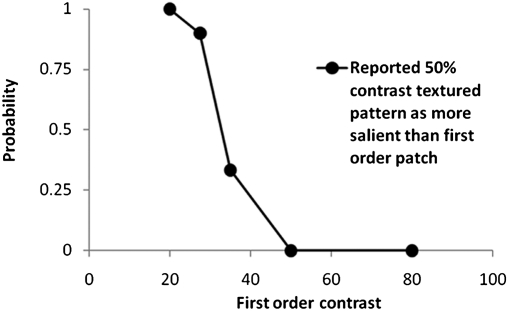
One likely explanation of differing reported awareness for first-order and textured patterns is that the awareness response may be based on the relative saliency of a target. For equal contrasts, a first-order grating appears to be more salient, i.e., more striking or noticeable, than a textured grating within the sighted field. It is not possible to conduct the perceived salience match in the blind field. However, a similar investigation in the sighted field was conducted. Comparison of a reference textured grating (50% contrast) vs. a range of first-order Gabor patches showed that in the sighted field, the textured grating was more salient (in 9 of 10 presentations) than a 27.5% contrast first-order Gabor (Fig. 2).
Fig. 2.
Probability of reporting a 50% contrast-modulated textured grating as more salient than a first-order grating as a function of the Michelson contrast of the latter.
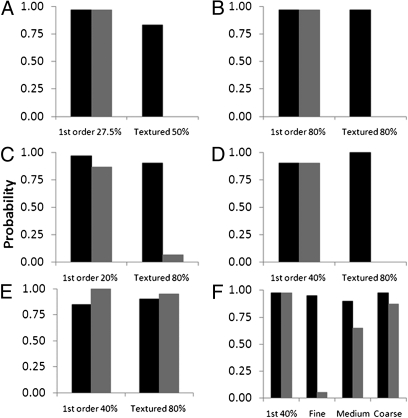
In a further two-alternative forced-choice (2AFC) experiment, we randomly interleaved (30 trials each) the presentation of a first-order (27.5% contrast) and a textured grating (50% contrast) that was reported to be more salient in the sighted field vs. a blank. The same pattern of results was observed. Even though in the normal field the textured stimulus at 50% contrast appeared more salient than a 27.5% contrast first-order grating, there were no reports of awareness (but excellent detection) for the textured stimulus in D.B.'s blind field (Fig. 3A).
Fig. 3.
Probability of detection (dark bars) and awareness (gray bars) of first- and second-order stimuli under a variety of conditions. (A) 2004 results for Gabor stimuli at 27.5% (first order) and 50% (second order). (B) 2005 results for stimuli with contrast increased to 80%. (C) 2008 results for 20% contrast first-order stimuli and 80% contrast second-order stimuli. (D) Square-wave stimuli (40% contrast first order; 80% contrast second order). (E) Coarse-textured patterns. (F) 80% contrast second-order stimuli of varying texture cell sizes.
March 2005.
On a separate visit with D.B. in March of 2005, we increased the salience of the textured grating by increasing the contrast to 80%. The findings replicated the earlier observation of matched discrimination to that of the first-order target, with no awareness of textured gratings (Fig. 3B). We subsequently investigated whether D.B. could perform orientation discrimination (vertical vs. oblique) for both stimulus types. Previously, D.B. was reported to be able to discriminate a 10° orientation difference of bars and gratings (9). Therefore, we instructed D.B. to report (by guessing) vertical or oblique after each single-stimulus presentation in a forced-response paradigm. In separate blocks of the first-order or textured gratings, randomly interleaved vertical and oblique targets were presented (2-s duration). D.B. could detect an orientation difference of 10° for first-order stimuli [d′ = 2.4 (±0.5)] and was aware of all stimulus presentations. Similarly, significant correct discrimination was found for the textured gratings with an orientation difference of 10° [d′ = 1.7 (±0.4)], again with no reported awareness on any of the trials. Therefore, not only could the textured gratings be detected, but the orientation of the contrast modulation was also processed in the absence of awareness.
October 2008.
After an interval of more than 3.5 y, we revisited with D.B. the same issues of detection and awareness. In a temporal 2AFC paradigm, where one interval contained a blank and another either a 20% contrast first-order or an 80% contrast texture-defined grating (30 trials each), we found results similar to previous visits (Fig. 3C). The findings again confirmed high detection ability in forced-choice guessing with a high level of awareness (26/30) for the first-order stimuli, and very little awareness for the textured defined pattern (2/30).
To investigate the effect of spatial edges, we changed the waveform for contrast modulation from sine wave to square wave and removed the spatial Gaussian envelope of the Gabor for both stimulus types. Results, shown in Fig. 3D, show that the pattern of findings again remained unchanged, with high detection scores for both stimuli and a differential level of awareness for the two stimulus types. To probe what determines whether a textured pattern leads to awareness, we increased the cell size of the textured pattern from 2.1 (fine) to 10.5 arc min (coarse).
Effect of Textured Coarseness.
Fig. 3E shows the detection and awareness for presentation of either a first-order or a textured grating with coarse cell size (10.5 arc min, 20 trials each). This time D.B. reported a high level of awareness for presentation of coarse-textured gratings. Subsequently, we systematically investigated the effect of cell size by randomly interleaving first-order stimuli and textured gratings with fine, medium, and coarse (2.1, 6.3, and 10.5 arc min) cell sizes. All stimuli were spatially contrast modulated by a square waveform (40 trials of each). Results plotted in Fig. 3F shows that awareness increases monotonically with cell size, and detection remains high across all stimulus conditions.
The question arises of what changes are introduced with increasing texture cell size, that in turn give rise to awareness of textured gratings. There are two changes to the stimuli that may be important: their Fourier amplitude spectra (i.e., the mathematical analysis of the stimuli into its sine-wave luminance components) and the presence of extended contours formed by the edges of the larger cells.
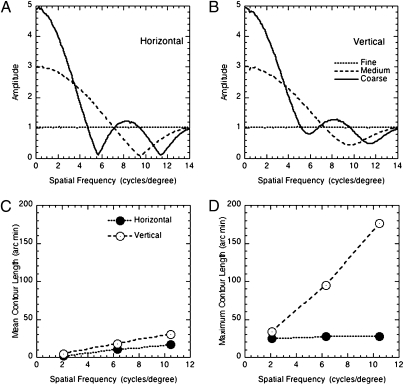
The effects of changes in cell size of the Fourier amplitude spectrum are presented in Fig. 4, which shows the average Fourier amplitude spectrum (for horizontal and vertical orientations) for 10,000 sample images. As cell size increases, amplitude is progressively concentrated at low spatial frequencies. The fact that awareness increases with increasing cell size is consistent with the association of awareness, with information carried by low spatial frequency first-order components in the stimuli.
Fig. 4.
Fourier amplitude spectra for (A) horizontal and (B) vertical components for the texture-grating stimuli with the three cell sizes used in the experiment. (C) Mean and (D) maximum length of horizontal and vertical contours, as a function of cell size. All results are based on 10,000-example stimuli.
Another possible explanation is that it is the lengths of contours in the images, rather than their spatial frequency content, that is responsible for the increased awareness. This will increase with increasing cell size, and will be lower for horizontal than for vertical contours. This is simply because the maximum length of a horizontal edge is determined by the width of the bars. Fig. 4 C and D show the variations in both the mean and maximum length of horizontal and vertical contours as a function of cell size (calculated from 10,000 samples). Both parameters increase with increasing cell size. One interpretation of these results is that awareness is associated with the presence of extended contours in the images. However, this would not account for the effects of spatial frequency on awareness for first-order stimuli. The length of contours in these stimuli is not affected by spatial frequency, and the density of contours increases with spatial frequency. The presence and length of extended contours cannot therefore account for the fact that awareness decreases with increasing spatial frequency of first-order stimuli. It is also important to note that D.B. was consistently unaware of second-order stimuli with a fine texture, even when the spatial frequency of contrast modulation was low.
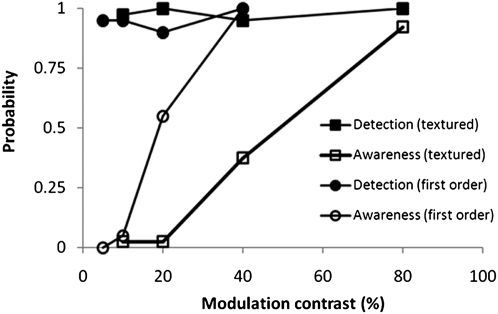
If we systematically increase the contrast of a course-textured pattern, the amplitude of all components, including low spatial frequency components, will increase, whereas contour lengths will remain unchanged. This means that if contour length is the significant factor, then D.B.’s awareness of coarse-textured patterns should remain high, irrespective of large variations in pattern contrast. Fig. 5 shows the result of the exact experiment. Similar to previous methods, detection and awareness were measured using a temporal 2AFC task for the first-order (20 trials per data point) and contrast-modulated coarse-textured pattern (40 trials per point). Despite identical detection performance, the awareness curves are not identical, indicating that contour information is not sufficient to signal awareness. Therefore, it is likely that the increased local contrast signal at low spatial frequencies is triggering the increased reported awareness.
Fig. 5.
Probability of detection and awareness of first- and second-order stimuli as a function of contrast.
Discussion
In summary, we have shown that both luminance-defined gratings (first-order stimuli) and textured-defined contrast-modulated gratings (second-order stimuli) presented within the blind field can be detected well above chance by D.B. This conclusion holds across a wide range of variations in contrast, spatial frequency, and alterations in the texture of second-order stimuli. In contradistinction, awareness of first-order stimuli decreased as their spatial frequency increased or their contrast decreased. D.B. reported no awareness of second-order stimuli with a fine texture, regardless of their spatial frequency or contrast modulation. Awareness did, however, increase with an increase in the texture cell size. For these latter stimuli, awareness dropped with decreasing contrast, despite the fact that good detection performance was maintained across the range of contrasts presented. We propose that reported awareness is primarily driven by mechanisms responsive to low spatial frequency first-order components of the image. Increasing these components in either first-order patterns by varying the spatial frequency, or in second-order patterns by varying the cell size, augments reported awareness.
Neuropsychological studies have established a double dissociation between impaired first- and second-order processing as a result of brain damage. Patient F.D., who has damage to extrastriate tissue just dorsal to V5, performed normally on first-order motion tests, but was impaired in tests of second-order motion (16); whereas patient R.A., who has damage to V2 and V3, showed normal second-order but impaired first-order processing on a range of motion tasks. Despite these dissociations, the detection of second-order patterns appears to depend on input from mechanisms that process first-order information (17, 18).
The thrust of earlier work has been on establishing the limits for detection of first- and second-order stimuli, and determining the similarities and differences between mechanisms involved in extracting such information. The findings we have reported here relating to luminance-defined and textured-defined stimuli are importantly different because they do not relate to detection, but focus on the differences in the reported awareness of the stimuli. Therefore, some further discussion of what is meant by awareness, its relationship with detection, and possible neural mechanisms is pertinent.
It is important to emphasize, however, that we do not advocate a dichotomous division of conscious awareness of events. It is more likely that awareness would fall on a continuum rather than a discrete scale. Also, the situation is not static. Previous research on a number of cases with blindness after brain injury shows that repeated exposure to first-order stimuli over a period, using an experimental paradigm similar to those reported here, can lift performance from chance to well-above-chance levels, but without awareness (type I blindsight). Further systematic exposure to first-order stimuli over extended periods of time can result in type II performance (19, 20). It would be of interest to investigate whether transition from type I to type II would be less likely with repeated stimulation with second-order stimuli. The findings reported here would stipulate such an outcome.
For our experimental purposes, it was beneficial to instruct a patient to report “aware” if he had any awareness whatsoever, and “unaware” otherwise. Using such methodology, we found that the awareness can be dissociated but detection can be the same for the two stimulus types. Though the detection of first and second order could be maintained over a range of variations in contrast and spatial frequency content, the presence of low-frequency first-order information was critical to support awareness. Awareness of textured stimuli thus depends critically on the presence of appropriate first-order information. These conclusions only apply to the presentation of static visual stimuli. Acknowledged awareness of visual events in the blind field can also be triggered by temporal oscillation and movement, especially if these are abrupt and rapid (e.g., see refs. 13 and 21). These characteristics will tend to produce strong responses in the magnocellular layers of the lateral geniculate nucleus (22). Such magnocellular responses might then support awareness through projections to extrastriate visual areas via the superior colliculus (23).
The question of the possible neural circuitry, correlates, and bases of awareness in blindsight has been considered in some detail elsewhere (12). It is clear, for example, that non-V1 cortical areas play an essential role because removal of all cortex except V1 in primates renders them visually unresponsive (24). It is also the case that lesions of the frontal eye field (FEF; the anatomical limits of which remain unsharp) in the monkey in some ways simulate that of visual cortex damage itself because the animal appears to be unresponsive to visual events, and the effects of different subtotal lesions can be mapped specifically in a “monkey perimeter” (25). It is striking that the foci active in awareness in a blindsight subject led to the identification of frontal area 46 (14) with reported awareness, which itself has strong connections with FEF. There is also striking concordance with results from normal subjects participating in a metacontrast masking paradigm, which allows for the comparison between one temporal interval associated with awareness and another temporal interval associated with unawareness, again with discrimination performance of the two intervals matched. Here, too, it was area 46 that was associated with the awareness reports (26). The convergence of findings with normal subjects and a blindsight subject increases the confidence in the importance of that particular location.
It is important to stress a precondition for a valid comparison of performance with and without accompanying awareness must be that the two performance levels are matched, and these are the only two studies (14, 24) in which this condition is met, so far as we are aware. The importance of frontal areas may lie in the routes over which signals can reach them in the lesioned or masked inactivation of V1, and awareness may also depend on their feedback into posterior visual areas. It is of interest to note that in the monkey, the dorsal and ventral streams leaving the visual cortex eventually converge onto frontal areas 46, as well as frontal area 7 and the frontal eye field, and these areas also connect back to posterior visual areas (27). Whether a region such as area 46 and its immediate interconnections are involved in production of the “commentary,” and whether the commentary itself plays any causal role in the generation of awareness, must be left open for future research and theoretical analyses. A strong position is that the commentary actually endows one with the experience of awareness, rather than awareness simply enabling a commentary to be made (10). Such a position also maps onto a philosophical theoretical view of higher-order thoughts and awareness, e.g., by Rosenthal (28).
The question naturally arises whether frontal lesions of area 46 and allied frontal foci would reduce conscious awareness of visual signals—i.e., that such areas are necessary if not sufficient. But it cannot be assumed that area 46 and allied frontal foci will play a general critical role for all forms of conscious awareness, and that therefore we should seek in cases of bilateral frontal cortical damage the loss of all forms of conscious awareness. The evidence we have cited was found only when the visual input is impeded at an early stage of input to visual cortical regions, either because of lesions of V1, as in the patient D.B., or impeded because of metacontrast backward visual masking, also assumed to degrade masked input signals in V1. If a patient were unfortunate enough to suffer loss of V1 and also of the frontal foci, it might be found that type II blindsight would disappear; we know of no such patient. However, a prediction can be ventured that subjects with lesions restricted to frontal damage would show an altered metacontrast function for visual awareness. Indeed, a temporary simulation of this condition by transcranial magnetic stimulation recently yielded just this result (29). It could be that other foci, perhaps also elsewhere in frontal cortex, would play a comparable role for each of the impairments of other sensory systems, as in “numbsight” and “deaf hearing.”
Given the good detection performance for second-order stimuli in blindsight, it remains to be determined by functional imaging and other anatomical methods, such as diffusion tensor imaging (DTI), whether such processing can be carried without any cortical contribution. Evidence of blindsight exists even when all cortex is removed unilaterally in cases of hemispherectomy (as assessed by the redundant target effect) (30, 31). There is DTI evidence of projections from the superior colliculus, the presumed major initial visual target in cases of blindsight, to contralateral cortical regions, as well as evidence that there is increased visually evoked electrical activity in the cortex contralateral to that of a visual cortex lesion, without reported awareness of the stimuli (32). It seems likely, therefore, that second-order grating detection also requires further cortical processing beyond the initial collicular input. However, it remains a possibility that type 1 second-order processing, lacking an adequate low spatial frequency component, could be carried out entirely subcortically. This, like a myriad of other questions, remains a target for further research.
Materials and Methods
Participant.
D.B. was born in 1940 and developed a benign tumor in his right occipital cortex that was surgically removed in 1970. As a result, he developed a left homonymous hemianopia that has persisted. Surgical notes state that the right striate cortex had been removed (see ref. 9 for history and details). D.B. gave informed consent before testing. The experiments were approved by the University of Aberdeen Ethics Committee and performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Apparatus and Setup.
All stimuli were generated using a specialized graphics card (VSG2/5; Cambridge Research Systems) and were presented on a 21-inch CRT monitor (Sony). Subject viewed the monitor through a viewing tunnel, covered in black felt to avoid light scatter, at a viewing distance of 760 mm. A chin/headrest was used to fix and stabilize the head position. The monitor was gamma corrected using a Lumcal device (Cambridge Research Systems) at 256 linear steps, and the screen output was cross-checked using a separate luminance meter (Minolta LS100). A modified pupillometer (ASL5000; Applied Science Laboratories) was used to monitor the fixation throughout the experiments. The apparatus was not able to give absolute eye position, but because the magnified image of the pupil, illuminated by infrared, was presented on a separate 9-inch monitor, a fixation change of 1° could be identified. In practice, because D.B. is an experienced observer, none of the trials reported here had to be discarded due to unwanted eye movements.
First-Order Stimuli.
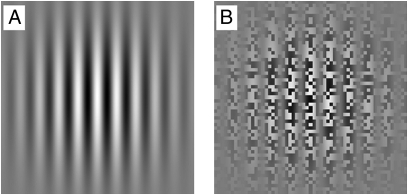
All Gabor patches were limited to ±2σs (where the spatial SD σs = 2.5, i.e., 10° in diameter) (Fig. 6A). The stimulus onset and offset were also smoothed by a temporal Gaussian envelope, with presentation time being limited to ±2σt (where the temporal SD σt = 500 ms, i.e., 2 s in duration).
Fig. 6.
Examples of (A) a sine-wave luminance-defined first-order Gabor patch and (B) a second-order contrast-defined stimulus.
Textured Gratings.
The carrier for the textured patch was binary noise, and the contrast was spatially modulated by a sine-wave function (Fig. 6B). In addition, the edges were smoothed using a Gaussian spatial envelope. Therefore, the luminance of the contrast-modulated textured patch in space and time can be formally expressed as
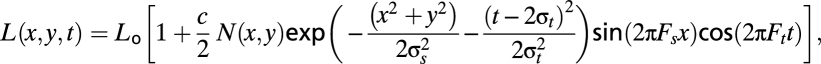 |
where L0 is the background luminance, N(x,y) is the binary noise with mean 0 and amplitude 1; c is the stimulus modulation contrast; Fs is the grating spatial frequency; Ft is the grating temporal frequency; and σs and σt are the SDs of the spatial and temporal Gaussian envelopes, respectively. Ft was set to 0.5 Hz and Fs to 1 c/°. As before, σs was set to 2.5° and σt to 500 ms. Apart from the orientation experiments reported below, in all conditions, we kept the orientation of gratings vertical. The noise pattern consisted of dark and light cells. The cell size for fine-, medium-, or coarse-textured patterns had dimensions of 2.1, 6.3, or 10.5 arc min, respectively. In the later experiments, the Gaussian spatial envelope was removed, and the spatial waveform for contrast modulation was changed from a sine wave to a square wave, as detailed in Results.
Acknowledgments
We are grateful to D.B. for participating in long testing sessions and his continued enthusiasm, and James Urquhart for technical assistance. L.W. was supported by a travel grant from Oxford McDonnell Centre for Cognitive Neuroscience.
Footnotes
The authors declare no conflict of interest.
References
- 1.Pöppel E, Held R, Frost D. Leter: Residual visual function after brain wounds involving the central visual pathways in man. Nature. 1973;243:295–296. doi: 10.1038/243295a0. [DOI] [PubMed] [Google Scholar]
- 2.Weiskrantz L, Warrington EK, Sanders MD, Marshall J. Visual capacity in the hemianopic field following a restricted occipital ablation. Brain. 1974;97:709–728. doi: 10.1093/brain/97.1.709. [DOI] [PubMed] [Google Scholar]
- 3.Raymond JE, Shapiro KL, Arnell KM. Temporary suppression of visual processing in an RSVP task: An attentional blink? J Exp Psychol Hum Percept Perform. 1992;18:849–860. doi: 10.1037//0096-1523.18.3.849. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro KL, Caldwell J, Sorensen RE. Personal names and the attentional blink: A visual “cocktail party” effect. J Exp Psychol Hum Percept Perform. 1997;23:504–514. doi: 10.1037//0096-1523.23.2.504. [DOI] [PubMed] [Google Scholar]
- 5.Milders M, Sahraie A, Logan S, Donnellon N. Awareness of faces is modulated by their emotional meaning. Emotion. 2006;6:10–17. doi: 10.1037/1528-3542.6.1.10. [DOI] [PubMed] [Google Scholar]
- 6.Wolfe JM. Reversing ocular dominance and suppression in a single flash. Vision Res. 1984;24:471–478. doi: 10.1016/0042-6989(84)90044-0. [DOI] [PubMed] [Google Scholar]
- 7.Yang E, Zald DH, Blake R. Fearful expressions gain preferential access to awareness during continuous flash suppression. Emotion. 2007;7:882–886. doi: 10.1037/1528-3542.7.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almeida J, Mahon BZ, Nakayama K, Caramazza A. Unconscious processing dissociates along categorical lines. Proc Natl Acad Sci USA. 2008;105:15214–15218. doi: 10.1073/pnas.0805867105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiskrantz L. Blindsight: A Case Study and Implications. Oxford: Oxford Univ Press; 1986. [Google Scholar]
- 10.Weiskrantz L. Percepts, Brain Imaging and the Certainty Principle: A Triangular Approach to the Scientific Basis of Consciousness. 1999. Herbert H. Reynolds Lectureship in the History and Philosophy of Science, Baylor University. Available at http://www.baylor.edu/content/services/document.php/30844.pdf. Accessed August 20, 2010.
- 11.Sahraie A, Weiskrantz L, Barbur JL. Awareness and confidence ratings in motion perception without geniculo-striate projection. Behav Brain Res. 1998;96:71–77. doi: 10.1016/s0166-4328(97)00194-0. [DOI] [PubMed] [Google Scholar]
- 12.Weiskrantz L. Consciousness Lost and Found: A Neuropsychological Exploration. Oxford: Oxford Univ Press; 1997. [Google Scholar]
- 13.Weiskrantz L, Barbur JL, Sahraie A. Parameters affecting conscious versus unconscious visual discrimination with damage to the visual cortex (V1) Proc Natl Acad Sci USA. 1995;92:6122–6126. doi: 10.1073/pnas.92.13.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahraie A, et al. Pattern of neuronal activity associated with conscious and unconscious processing of visual signals. Proc Natl Acad Sci USA. 1997;94:9406–9411. doi: 10.1073/pnas.94.17.9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trevethan CT, Sahraie A, Weiskrantz L. Can blindsight be superior to ‘sighted-sight’? Cognition. 2007;103:491–501. doi: 10.1016/j.cognition.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Vaina LM, Cowey A. Impairment of the perception of second order motion but not first order motion in a patient with unilateral focal brain damage. Proc Biol Sci. 1996;263:1225–1232. doi: 10.1098/rspb.1996.0180. [DOI] [PubMed] [Google Scholar]
- 17.Langley K, Fleet DJ, Hibbard PB. Linear filtering precedes nonlinear processing in early vision. Curr Biol. 1996;6:891–896. doi: 10.1016/s0960-9822(02)00613-9. [DOI] [PubMed] [Google Scholar]
- 18.Dakin SC, Mareschal I. Sensitivity to contrast modulation depends on carrier spatial frequency and orientation. Vision Res. 2000;40:311–329. doi: 10.1016/s0042-6989(99)00179-0. [DOI] [PubMed] [Google Scholar]
- 19.Sahraie A, et al. Increased sensitivity following repeated stimulation in blindsight. Proc Natl Acad Sci USA. 2006;103:14971–14976. doi: 10.1073/pnas.0607073103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahraie A, et al. Improved detection following Neuro-Eye Therapy in patients with post-geniculate brain damage. Exp Brain Res. 2010;206:25–34. doi: 10.1007/s00221-010-2395-z. [DOI] [PubMed] [Google Scholar]
- 21.Sahraie A, Trevethan CT, MacLeod M-J. Temporal properties of spatial channel of processing in hemianopia. Neuropsychologia. 2008;46:879–885. doi: 10.1016/j.neuropsychologia.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Butler PD, et al. Reply: A few remarks on assessing magnocellular sensitivity in patients with schizophrenia. Brain. 2007;130:e84. doi: 10.1093/brain/awm153. 10.1093/brain/awm154. [DOI] [PubMed] [Google Scholar]
- 23.Schiller PH, Malpeli JG, Schein SJ. Composition of geniculostriate input ot superior colliculus of the rhesus monkey. J Neurophysiol. 1979;42:1124–1133. doi: 10.1152/jn.1979.42.4.1124. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura RK, Mishkin M. Chronic ‘blindness’ following lesions of nonvisual cortex in the monkey. Exp Brain Res. 1986;63:173–184. doi: 10.1007/BF00235661. [DOI] [PubMed] [Google Scholar]
- 25.Latto R, Cowey A. Visual field defects after frontal eye-field lesions in monkeys. Brain Res. 1971;30:1–24. doi: 10.1016/0006-8993(71)90002-3. [DOI] [PubMed] [Google Scholar]
- 26.Lau HC, Passingham RE. Relative blindsight in normal observers and the neural correlate of visual consciousness. Proc Natl Acad Sci USA. 2006;103:18763–18768. doi: 10.1073/pnas.0607716103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young MP. Objective analysis of the topological organization of the primate cortical visual system. Nature. 1992;358:152–155. doi: 10.1038/358152a0. [DOI] [PubMed] [Google Scholar]
- 28.Rosenthal DM. Higher-order thoughts and the appendage theory of consciousness. Philos Psychol. 1993;6:155–166. [Google Scholar]
- 29.Rounis E, Manischalco B, Rothwell J, Passinghham RE, Lau H. Theta burst transcranial magnetic stimulation to the prefrontal cortex impairs metacognitive visual awareness. Cogn. Neurosci. 2010;1:165–175. doi: 10.1080/17588921003632529. [DOI] [PubMed] [Google Scholar]
- 30.Tomaiuolo F, Ptito M, Marzi CA, Paus T, Ptito A. Blindsight in hemispherectomized patients as revealed by spatial summation across the vertical meridian. Brain. 1997;120:795–803. doi: 10.1093/brain/120.5.795. [DOI] [PubMed] [Google Scholar]
- 31.Leh SE, Johansen-Berg H, Ptito A. Unconscious vision: New insights into the neuronal correlate of blindsight using diffusion tractography. Brain. 2006;129:1822–1832. doi: 10.1093/brain/awl111. [DOI] [PubMed] [Google Scholar]
- 32.Goebel R, Muckli L, Zanella FE, Singer W, Stoerig P. Sustained extrastriate cortical activation without visual awareness revealed by fMRI studies of hemianopic patients. Vision Res. 2001;41:1459–1474. doi: 10.1016/s0042-6989(01)00069-4. [DOI] [PubMed] [Google Scholar]