Abstract
G protein-coupled receptors (GPCRs), the largest family of signaling receptors expressed in the CNS, mediate the neuropsychiatric effects of a diverse range of clinically relevant drugs. It is increasingly clear that GPCRs can activate distinct G protein-dependent and -independent transduction pathway(s), and that certain drugs differ in the ability to regulate distinct signaling mechanisms linked to the same receptors. A fundamental question in neuropharmacology is whether such “biased agonism” occurs in physiologically relevant neurons and with endogenous receptors. Here we show that propranolol and carvedilol, two β-blocker drugs that inhibit β-adrenergic signaling via heterotrimeric G proteins, function in hippocampal pyramidal neurons as potent and selective activators of an alternate receptor-linked calcium signaling pathway mediated by β-arrestin-2 and ERK1/2. Our results support the emerging view of β-arrestin–biased agonism as a significant mechanism of drug action and do so in CNS-derived neurons expressing only native receptors.
Keywords: functional selectivity, total internal reflection microscopy, non-canonical signaling
Norepinephrine signaling through β-adrenergic receptors mediates endogenous regulation of complex CNS processes, including attention, arousal, learning, and memory (1). The multiple therapeutic actions of β-blocker drugs, classified according to their shared ability to function as antagonists or inverse agonists of norepinephrine-induced signaling by these receptors, highlights the importance of β-adrenergic signaling in the brain (2). β-Blockers can alleviate performance anxiety and akathisia, manage migraines, and possibly prevent posttraumatic stress disorder by reducing the consolidation of emotional memories (3, 4). However, administration of β-blockers can also produce unwanted neuropsychiatric effects, including depression, sedation, fatigue, and, occasionally, psychosis (2). Structurally distinct β-blockers differ markedly in their tendency to produce particular neuropsychiatric effects (2, 4, 5), but at the cellular level, the only known mechanism underlying the CNS actions of all of these drugs is norepinephrine antagonism.
β-Adrenergic receptors are members of the largest family of seven-transmembrane signaling receptors, often called G protein–coupled receptors (GPCRs) because they mediate ligand-dependent activation of the heterotrimeric G proteins Gs and Golf (6). These G proteins stimulate adenylyl cyclase and subsequently the cAMP-dependent protein kinase (PKA), thereby controlling a large number of downstream effectors. Some β-blocker drugs, instead of acting as antagonists (or inverse agonists) of canonical G protein–dependent signaling, have been shown to function as agonists of β-arrestin–dependent signaling (7–14). These observations emerged from studies of the effects of heterologous receptor expression and were recently found to apply to endogenous receptors in HEK-293 cells as well (12). Might such β-arrestin–biased agonism contribute to the effects of β-blocker drugs on normal CNS neurons? Here we describe a pathway of neural calcium signaling selectively elicited by a subset of β-blocker drugs. This signaling is mediated via a noncanonical β-arrestin–dependent mechanism linked to endogenous β-adrenergic receptors. These results, by providing the first demonstration of β-arrestin–biased agonism in native CNS neurons, have fundamental implications for neuropharmacology and provide a rational basis for understanding the diverse behavioral effects of β-blocker drugs.
Results
To analyze β-adrenergic regulation of intracellular calcium near the plasma membrane, we used the calcium indicator Fluo-4 and total internal reflection fluorescence microscopy (TIRFM). By selectively illuminating a shallow region extending ∼100 nm from the plasma membrane (15), TIRFM provides information on local fluorescence with an increased signal-to-noise ratio and reduced photodamage compared with standard imaging techniques (16–18). Dissociated hippocampal neurons (DIV12–21) were loaded with Fluo-4, and standard epifluorescence microscopy was used to identify individual cells with soma and proximal dendrites located within the TIRFM illumination field (Fig. S1). Sequential frames (10 Hz) were then acquired by TIRFM at 37 °C. Average fluorescence within a defined area enclosing the soma and proximal dendrites was measured and plotted versus time (Fig. S1A).
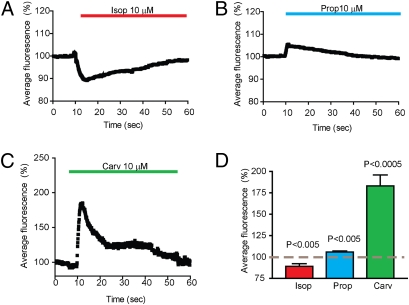
As a first step in investigating the regulation of surface-localized cytoplasmic free calcium by β-adrenergic receptors, we examined the effect of bath application of the β-adrenergic agonist isoproterenol (10 μM). To avoid any network activity in our cultures, we made all measurements in the presence of the sodium channel blocker TTX (1 μM). Isoproterenol, a specific β-adrenergic agonist, induced a rapid decrease in the fluorescence signal (Fig. 1 A and E) that desensitized to a new steady-state level in the continuous presence of isoproterenol (Fig. 1B). Unlike isoproterenol, application of propranolol, a nonselective β-adrenergic blocker, induced an opposite increase in fluorescence that did not desensitize to any detectable degree during the course of our experiments (60 s) (Fig. 1 C and E).
Fig. 1.
Bidirectional regulation of cytoplasmic free calcium concentration near the neuronal plasma membrane by β-adrenergic receptors. (A) Dissociated rat hippocampal neurons (DIV12–21) were loaded with Fluo-4 and imaged sequentially every 100 ms by TIRFM. Fluorescence intensity is displayed in pseudocolor (scale palette on the left) before (5 s) and after (15 s) bath application of 10 μM isoproterenol (added at t = 10 s relative to the time stamp in the lower right corner). (B) Time course of the calcium indicator fluorescence intensity measured in the cell body of 10 neurons and plotted versus time. The black line indicates mean intensity, and gray shading represents SEM. The red line represents the presence of isoproterenol during the indicated times. (C) Average fluorescence intensity measured before and after the addition of 10 μM propranolol. The blue line indicates the presence of propranolol (n = 7). (D) The presence of 10 μM alprenolol, a specific β-blocker, prevented the effect of isoproterenol (red line) on calcium indicator fluorescence (n = 6). (E) Analysis across independent experiments of the effect of norepinephrine (NE; 10 μM) and isoproterenol (Isop; 10 μM) on calcium indicator fluorescence measured at 5 s after bath application. Preincubation of neurons (1 min) with 10 μM alprenolol blocked the norepinephrine-, isoproterenol-, and propranolol-induced changes in calcium indicator fluorescence. (F) Preincubation with 50 μM CGP-20712, a concentration that effectively blocks β1- and β2-adrenergic receptors, also prevented the calcium-signaling effects of norepinephrine, isoproterenol, and propranolol. Preincubation with 50 nM CGP-20712 (a concentration that selectively blocks β1-adrenergic receptors) did not prevent isoproterenol- or propranolol-induced changes in calcium indicator fluorescence. (G) Dose–response curve of the isoproterenol-induced decrease in calcium indicator fluorescence measured at 5 s after bath application, normalized to the maximum response. (H) Dose–response curve of the propranolol-induced increase in calcium indicator fluorescence normalized to the maximum response.
We repeated the foregoing experiments in the presence of alprenolol, a potent and specific β-blocker drug that by itself did not produce any detectable change in local cytoplasmic calcium concentration (Fig. S1B). In the continuous presence of alprenolol, application of isoproterenol, propranolol, or norepinephrine did not significantly alter calcium indicator fluorescence, indicating that the observed calcium-signaling effects depend on the activation of β-adrenergic receptors (Fig. 1 D and E). This is consistent with previous evidence indicating that pyramidal neurons in the hippocampus express primarily β2-adrenergic receptors, whereas α1-adrenergic receptors are restricted to interneurons (19). To determine whether these distinct effects were mediated by the same β-adrenergic receptor subtype (like both β1- and β2-adrenergic receptors are expressed in the hippocampus), we preincubated neurons with the selective β1 antagonist CGP 20712 (20) and tested the ability of subsequent addition of norepinephrine, isoproterenol, or propranolol to rapidly change indicator fluorescence (Fig. 1F). Preincubation of neurons with 50 μM CGP, a relatively high concentration that blocks both β1- and β2-adrenergic receptors, prevented the calcium-signaling effects produced by norepinephrine, isoproterenol, and propranolol. In contrast, preincubation with 50 nM CGP, which selectively blocks β1-adrenergic receptors (20), did not. We also verified that the opposite effects of isoproterenol and propranolol both exhibited a dose–response relationship consistent with direct action on β-adrenergic receptors (Fig. 1 G and H). Taken together, these results indicate that the divergent calcium-signaling effects observed were indeed elicited by binding of the respective ligands to β-adrenergic receptors, primarily by the β2-adrenergic receptor subtype.
To verify that isoproterenol-induced changes in neuronal calcium levels occur via regulation of plasma membrane calcium channels, we preincubated neurons with the nonselective calcium channel blocker cadmium (100 μM). The isoproterenol-induced decrease in local cytoplasmic calcium also eliminated the selective L-type channel blocker nifedipine (5 μM) (Fig. S1 C and D). Remarkably, the same manipulations also prevented the propranolol-induced increase in cytoplasmic calcium (Fig. S1E). Taken together, these results suggest that isoproterenol and propranolol regulate local cytoplasmic calcium through the same β-adrenergic receptors and produce opposite signaling effects via the same type of calcium channel.
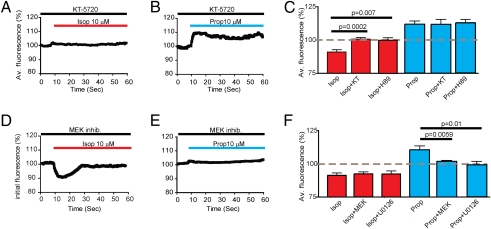
We hypothesized that the isoproterenol-induced decrease of local cytoplasmic calcium was mediated by receptor-dependent activation of the canonical Gs–PKA signaling pathway, and that the propranolol-induced increase likely resulted from inhibition of the same pathway. The latter hypothesis is based on previous studies of nonneural cell types in which β-blockers, such as propranolol, were shown to function as inverse agonists of β-adrenergic receptors by reducing receptor constitutive activity (20, 21). To test these hypotheses, we repeated calcium imaging experiments in the presence of the PKA inhibitor KT-5720. KT-5720 prevented the isoproterenol-induced decrease in cytoplasmic free calcium concentration (Fig. 2 A and C), confirming that this effect was mediated by the canonical Gs–PKA signaling pathway. H-89, a structurally different PKA inhibitor, also blocked the response (Fig. 2C). Surprisingly, KT-5720 and H-89 did not prevent the converse increase in local calcium elicited by propranolol (Fig. 2 B and C). These data, although verifying isoproterenol-induced regulation of neuronal calcium via Gs–PKA signaling, suggest that the functionally opposite regulation elicited by propranolol is mediated by a distinct, PKA-independent mechanism (Fig. 2C).
Fig. 2.
β-Adrenergic receptor–mediated calcium-signaling effects are distinguishable by their dependence on PKA or the MEK/ERK cascade. (A) Average calcium indicator fluorescence intensity was measured in pyramidal neurons preincubated with the specific PKA inhibitor KT-5720 (1 μM; black line) before and after bath application of isoproterenol (10 μM; red line). (B) Fluorescence intensity measured similarly in response to 10 μM propranolol (blue line). (C) Analysis across multiple experiments of fluorescence intensities measured at 5 s after the addition of the indicated adrenergic ligand, verifying that the isoproterenol-induced decrease in calcium fluorescence (Isop) was prevented by KT-5720 (Isop + KT), as well as by the chemically distinct PKA inhibitor H89 (Isop + H89) (n = 6 and n = 7, respectively). The propranolol-induced increase in calcium indicator fluorescence (Prop) was not significantly different in the presence of either KT-5720 (Prop + KT) or H89 (Prop + H89). Each bar represents the mean ± SEM of average fluorescence changes (relative to basal levels) across between six and eight experiments. (D and E) Effect of the MEK/ERK inhibitor mixture on the ability of isoproterenol (D) and propranolol (E) to change calcium indicator fluorescence, using the same experimental design as in A and B. (F) Analysis across multiple experiments of calcium indicator fluorescence measured at 5 s after adrenergic ligand addition, verifying that the MEK/ERK inhibitor mixture (Isop + MEK) and the selective MEK inhibitor U0126 (Isop + U0126) did not affect the isoproterenol-induced decrease of calcium indicator fluorescence, but did inhibit the propranolol-induced increase (n = 6–8 experiments for each condition).
There is extensive evidence showing that in heterologous systems, β-adrenergic receptors can elicit PKA-independent signaling via activation of MAP kinase modules (8). Of particular interest, inverse agonists, such as propranolol, have been shown to activate ERK1/2 in heterologous cells (8, 12). Thus, we considered the possibility that such alternate signaling might contribute to the observed PKA-independent regulation of local cytoplasmic calcium. To test this, we preincubated hippocampal neurons with a MEK/ERK inhibitor mixture (1 μM each of PD 98059, SL 327, and U0126). As expected, these inhibitors did not detectably affect the isoproterenol-induced decrease in local cytoplasmic calcium measured by TIRFM (Fig. 2D). In marked contrast, the presence of MEK/ERK inhibitors blocked the propranolol-induced increase in local cytoplasmic calcium (Fig. 2E). Analysis across multiple experiments verified that the presence of MEK/ERK inhibitor mixture, as well as the specific MEK inhibitor U0126 by itself, prevented the propranolol-induced increase in calcium fluorescence without blocking the converse decrease in local cytoplasmic calcium produced by isoproterenol (Fig. 2F). These observations verify the ability of propranolol to activate a PKA-independent calcium-signaling mechanism and further indicate that this alternate signaling mechanism requires activation of the MEK-ERK MAP kinase cascade.
To further probe the effects of β-blockers on neural calcium signaling, and to examine the possibility of still more diversity among individual β-blocker drugs, we investigated the actions of carvedilol. Carvedilol is an inverse agonist of the Gs–PKA signaling pathway and has been observed to activate ERK1/2 with greater efficacy than propranolol in heterologous cell models (9, 10). Application of carvedilol to neurons led to a large but transient increase in the local Fluo-4 signal that exceeded the increase produced by propranolol, followed by a prolonged elevation of local free calcium comparable in magnitude to that produced by propranolol (Fig. 3 A and B). Despite the markedly different kinetic profile of neuronal calcium signaling elicited by these distinct β-blocker drugs, both the early phase (measured at 5 s after carvedilol addition) and the late phase (measured at 50 s after carvedilol addition) of the response were dependent on β-adrenergic receptors, as indicated by the blockade by alprenolol (Fig. 3C). Furthermore, chemical inhibition of the MAPK pathway with either the MEK/ERK inhibitor mixture or the specific MEK inhibitor U0126 blocked both kinetic components of the carvedilol effect (Fig. 3 D and E). The dose–response properties of the later (prolonged) effect were consistent with typical β-adrenergic signaling. The dose–response curve of the early (transient) phase was right-shifted, possibly reflecting kinetic effect(s) of carvedilol binding to β-adrenergic receptors (Fig. 3 F and G).
Fig. 3.
Carvedilol produces a biphasic calcium-signaling effect mediated by β-adrenergic receptors and the MEK/ERK cascade. (A) Calcium indicator fluorescence intensity, depicted as pseudocolor images before (5 s) and after (15 s) the addition of 10 μM carvedilol to the culture medium. Carvedilol was added at t = 10 s relative to the time stamp in the lower right corner of each image. (B) Time course showing average fluorescence changes measured by live TIRFM imaging on application of carvedilol to the incubation media. Sequential images were recorded from isolated neurons during a 1-min image series, with carvedilol added at t = 10 s (n = 7). (C) Alprenolol did not alter calcium indicator fluorescence and effectively blocked both the early (measured 5 s after carvedilol addition) and late (measured 50 s after carvedilol addition) effects (n = 7). (D and E) Analysis across independent experiments (n = 7) indicating that both early (D) and late (E) phases of carvedilol's calcium signaling effect were inhibited in the presence of the MEK/ERK inhibitor mixture (Carv + MEK) or the specific MEK inhibitor U0126 (Carv + U0126). (F and G) Dose–response curves of the carvedilol-induced increase in calcium indicator fluorescence were plotted for the early (5 s) and late (50 s) phases, normalized to the maximum response measured in each time interval.
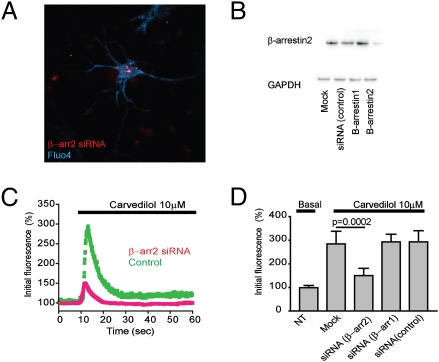
Through what mechanisms do these distinct β-blockers mediate ERK-dependent signaling? Extensive studies of heterologous cell models have shown that β-adrenergic receptors, along with coupling to Gs, can activate MAP kinase modules via the distinct trimeric G protein Gi or by G protein–independent coupling to β-arrestins (8, 9, 12, 22). Overnight incubation of neuronal cultures with pertussis toxin did not detectably inhibit the increase in local calcium produced by β-blockers, arguing against Gi coupling as the mechanism of alternate neuronal calcium signaling. To examine whether β-arrestins mediate the observed increase in cytoplasmic free calcium, we used small interfering RNA (siRNA) technology to specifically deplete the endogenous proteins. Hippocampal neurons were transfected with fluorescently labeled siRNA oligonucleotides (Fig. 4A), and effective knockdown was verified by immunoblot analysis of culture extracts (Fig. 4B). Quantification across multiple experiments indicated an ∼70% depletion of β-arrestin-2/arrestin-3 (Fig. 4). The carvedilol-induced increase in local cytoplasmic calcium was significantly reduced by this knockdown (Fig. 4C). In contrast, we found no detectable inhibition by the siRNA duplex targeting β-arrestin-1/arrestin-2 or by a control (scrambled) RNA duplex (Fig. 4D). These results suggest that the alternate PKA-independent pathway of neuronal calcium signaling elicited by β-blockers is mediated by β-arrestin-2–dependent activation of ERK.
Fig. 4.
The carvedilol-induced changes in cytoplasmic free calcium are β-arrestin-2 dependent. Hippocampal neurons were transfected with siRNA duplexes targeting β-arrestin-1 (arrestin 2), β-arrestin-2 (arrestin 3), or control (nonsilencing) duplex at 48–72 h before analysis. (A) Cells were loaded with Fluo-4 and identified for siRNA transfection by wide-field imaging using standard epifluorescence illumination. (B) Whole-cell lysates from siRNA-transfected primary hippocampal cultures were immunobloted for β-arrestin-2. GAPDH immunoblot analysis was used to verify equivalent loading. Quantification across multiple experiments (n = 4) by scanning densitometry indicated that β-arrestin-2 siRNA effectively reduced the level of endogenous protein to 29 ± 14% relative to mock transfection. (C) Effect of β-arrestin-2 siRNA (red curve) compared with control duplex (green curve) on carvedilol-induced changes in calcium indicator fluorescence measured by TIRFM. Each curve represents the mean from independent imaging of five neurons. (D) Analysis across independent experiments of siRNA effects on the carvedilol response measured within 5 s after bath application (carvedilol, 185 ± 45% relative to basal levels, n = 5; β-arrestin-2 siRNA, 50.1 ± 24% relative to basal levels, n = 5; β-arrestin-1 siRNA, 193 ± 32% relative to basal levels, n = 6; control siRNA, 187 ± 39% relative to basal levels, n = 5).
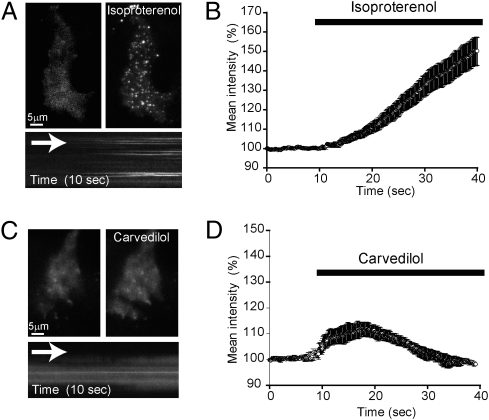
Activation of the β2-adrenergic receptor by classical agonists promotes redistribution of β-arrestins from the cytoplasm to the plasma membrane (14). We visualized this process by TIRFM in HEK-293 cells expressing β2-adrenergic receptors at moderate levels (∼1 pmol/mg), using β-arrestin-2–GFP and taking advantage of the shallow field of illumination to selectively detect changes in labeled protein concentration near the cell surface. The addition of isoproterenol to the incubation media induced a robust increase in local β-arrestin-2–GFP fluorescence and resulted in visible concentration of the tagged arrestin in discrete puncta (Fig. 5 A and B), shown previously by this method to represent clathrin-coated pits in the plasma membrane (23). Carvedilol induced a more rapid and transient increase in surface recruitment of β-arrestin-2–GFP (Fig. 5 C and D). Unlike isoproterenol, carvedilol did not cause visible concentration of β-arrestin-2–GFP in coated pits, but instead induced recruitment of β-arrestin-2–GFP to a largely diffuse membrane distribution. These results confirm that certain β-blockers induce β-arrestin translocation to the cell surface and establish that these β-blockers produce a different spatiotemporal pattern of β-arrestin recruitment compared with a classical agonist.
Fig. 5.
Isoproterenol and carvedilol induce distinct spatiotemporal patterns of β-arrestin recruitment to the plasma membrane. HEK 293 cells stably expressing flag-tagged β2-adrenergic receptors (∼1 pmol/mg) were transfected with β-arrestin-2–GFP and imaged by TIRFM to visualize arrestin translocation and surface distribution. (A) The addition of 10 μM isoproterenol to the incubation media induced an increase in total average fluorescence and the appearance of discrete puncta. (Lower) A kymograph from the same cell indicating the appearance of arrestin-labeled puncta. (B) Analysis of the effect of isoproterenol on mean intensity of β-arrestin-2–GFP fluorescence measured before and after bath application (n = 12 cells). (C) Application of 10 μM carvedilol to the incubation media induced an increase in surface fluorescence, indicating translocation of β-arrestin-2–GFP to the plasma membrane. Unlike isoproterenol, addition of carvedilol produced a largely diffuse surface distribution, as also shown in the kymograph. (D) Analysis of the effect of carvedilol on the mean intensity of β-arrestin-2–GFP fluorescence measured before and after bath application (n = 20 cells).
We next asked whether the alternate calcium-signaling mechanism elicited by β-blocker drugs is sufficient to mediate a functionally relevant calcium-dependent response. To investigate this, we examined a known effector of neuronal calcium signaling and compared the absolute magnitude of effector regulation produced by β-blocker drugs to that elicited by isoproterenol. We focused on hippocalcin, a calcium-binding protein implicated in hippocampal long-term depression (24) and in the gating of potassium conductance in pyramidal neurons (25, 26). In addition to these specific neural-signaling functions, hippocalcin is representative of a large family of neural calcium sensor proteins that are expressed in various neurons and function as downstream mediators or effectors by undergoing calcium-dependent recruitment to the neural plasma membrane via a conserved myristoyl-switch domain (27).
EGFP-tagged hippocalcin (hippocalcin-EGFP) was expressed in hippocampal neurons by virus-mediated gene transfer, and TIRFM imaging was used to detect the recruitment of hippocalcin-EGFP to the neural plasma membrane. As expected, isoproterenol produced a rapid reduction in surface recruitment of hippocalcin-EGFP (Fig. 6A) that paralleled the decrease in local cytoplasmic calcium measured in independent experiments using Fluo-4 (compare Fig. 6D and Fig. 1F), suggesting a tight relationship between the plasma membrane recruitment of this calcium-signaling effector and calcium regulation elicited by a classical agonist. In contrast, the addition of propranolol or carvedilol to the culture medium produced an increase in the recruitment of hippocalcin-EGFP to the plasma membrane, with different kinetic profiles (Fig. 6 B and C) resembling the respective effects of these drugs on local calcium concentration. Furthermore, the absolute magnitude of increased surface recruitment of this calcium-signaling effector elicited by the β-blocker drugs was similar to (with propranolol) or greater than (with carvedilol) the decrease produced by isoproterenol when applied at saturating concentrations. Thus, the β-arrestin-2–ERK mechanism, which is potently activated by certain β-blocker drugs, produces distinct regulation of neuronal calcium signaling that is comparable (or greater) in degree relative to the Gs–PKA mechanism activated by classical agonists.
Fig. 6.
Distinct effects of β-blocker drugs on plasma membrane recruitment of hippocalcin. Hippocampal neurons were infected with lentiviral vector encoding hippocalcin-EGFP for 24–48 h before TIRFM imaging. (A) Application of 10 μM norephinephrine decreased hippocalcin fluorescence levels to 89.1 ± 3% relative to basal levels within 5 s after drug addition (n = 6). (B) Application of 10 μM propranolol increased surface hippocalcin fluorescence to 106.5 ± 1.8% of basal levels within 5 s after drug addition (n = 6). (C) Application of 10 μM carvedilol increased surface hippocalcin-EGFP fluorescence to a maximum of 183 ± 12.7% relative to basal levels within 5 s after bath application, and therafter produced a sustained phase of recruitment lasting ∼1 min (n = 6). (D) Bar graph analysis of surface hippocalcin-EGFP fluorescence intensity effects measured across multiple neurons within 5 s after bath application (isoproterenol, 89.1 ± 3%, n = 6; propranolol, 106.5 ± 1.8%, n = 6; carvedilol, 183 ± 12.7%, n = 6). All percentages represent mean fluorescence determinations relative to basal levels.
Discussion
The present results identify a distinct signaling mechanism through which β-blocker drugs rapidly modulate local cytoplasmic free calcium, and establish the occurrence of β-arrestin–biased agonism in CNS neurons expressing only endogenous receptors. We have defined biochemically distinct pathways through which β-blockers and classical β-adrenergic agonists mediate essentially opposite regulation of local calcium, and demonstrated that the absolute magnitude of this opposite effect on plasma membrane recruitment of a representative calcium effector is similar (with propranolol) or greater (with carvedilol) than that of a natural catecholamine agonist or isoproterenol (Fig. 6). Thus, the present results suggest that this alternate signaling mechanism is significant to the neuropharmacologic actions of β-blockers.
Our findings confirm and extend the emerging view that certain β-blocker drugs, including propranolol and carvedilol, can activate MAP kinase modules via β-arrestin (8, 12). This view is consistent with the general concept of biased agonism or functional selectivity that has been proposed based on previous studies of various GPCRs, including β-adrenergic receptors (7). Our results provide specific experimental support for this hypothesis in CNS neurons expressing only native receptors, and establish a role of the β-arrestin–ERK axis in neuronal calcium signaling. Our results also contribute more broadly to elaborating the remarkable breadth of cellular responses elicited by seven-transmembrane receptors, as well as to identifying additional pathway-selective effects of clinically relevant drugs (7, 28–31). The ability of certain β-blocker drugs to mediate neuronal calcium signaling via a distinct mechanism and in an opposite direction relative to conventional agonists emphasizes the remarkable selectivity that can be achieved by such biased agonism in a native neuronal context.
β-Blockers have established utility in treating cardiovascular disease and movement disorders and are being increasingly used in neuropsychiatry (2, 3). Despite widespread application and benefits, β-blockers can produce diverse and drug-selective adverse effects on CNS function, the etiologies of which remain incompletely understood (5). We have identified a biochemically and functionally distinct mechanism of neuronal calcium signaling that is elicited by a subset of β-blocker drugs. This discrete signaling action might explain some of the diverse CNS effects of β-blocker drugs in current use. It also might facilitate the future development of therapies with fewer CNS side effects or with enhanced utility for treating complex brain diseases.
Materials and Methods
Materials and Reagents.
The MEK/ERK inhibitor mixture was purchased from Tocris. Other drugs, kinase inhibitors, and general reagents were obtained from Sigma-Aldrich unless specified otherwise.
Neuronal Culture and Transfection.
Hippocampi dissected from E18/E19 rats were dissociated and cultured as described previously (18). In brief, hippocampi were dissociated by papain digestion, followed by brief mechanical trituration. Cells were plated on poly-d-lysine–treated (Sigma-Aldrich) 35-mm glass-bottomed dishes (Matek) and maintained in Neurobasal media supplemented with B27, penicillin, streptomycin, and l-glutamine (all from Invitrogen).
Fluo-4 Loading.
Hippocampal neurons were loaded with 5 μM Fluo-4 AM (Invitrogen) in 0.02% (vol/vol) pluronic acid F-127 (Sigma-Aldrich) for 20 min at room temperature in extracellular media [125 mM NaCl, 2 mM KCl, 2 mM CaCl2, 10 mM d-glucose, 25 mM Hepes (pH 7.4); 305 mOsm]. After loading, cells were thoroughly washed with fresh extracellular media (three times, with a 10-min incubation) to remove any dye not specifically associated with the cells.
Live Cell Imaging and Analysis.
Imaging was performed using a Nikon TE-2000E inverted microscope with perfect focus using a 100 × 1.49 numerical aperture. A 488-nm single-line argon ion laser (Melles Griot) was used as a light source for both wide-field and TIRFM illumination modes. Neurons were imaged in 125 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 10 mM d-glucose, and 25 mM Hepes (pH 7.4) in the presence of 1 mM TTX and α-adrenergic receptor blockers unless specified otherwise. Time-lapse sequences were acquired at a continuous rate of 10 frames per second using a Cascade II EMCCD camera (Photometrics) controlled by NIS-Elements Advanced Research software (Nikon). Image analysis was performed using NIS-Elements (Nikon) and Metamorph software (Molecular Devices). Regions of interest were defined to include the cell body and proximal dendrites. Average pixel intensity was analyzed after background subtraction from raw data sequences. Statistical analysis and curves were obtained using Prism 3.0 (Graph Pad Software). Values in all summary graphs are mean ± SEM. Statistical analysis of differences was performed using the Student t test for single comparisons and one-way ANOVA for multiple groups, calculated using Prism software. A P value < 0.05 was considered statistically significant. Hippocampal pyramidal neurons were selected by morphological criteria (in wide field) before imaging in the TIRFM mode (18).
Arrestin Translocation.
A total of 293 cells stably expressing Flag-tagged β2-adrenergic receptors were plated onto glass-bottomed dishes and transfected with β-arrestin-2–GFP (a gift from Marc Caron, Duke University) using Lipofectamine 2000 (Invitrogen), according to the manufacturer's protocol. Imaging was performed at 96 h after transfection.
siRNA Arrestin Labeling and Knockdown.
Oligonucleotides targeting rat β-arrestin-1 (NM_012910; Qiagen catalog no. SI00254072) and rat β-arrestin-2 (NM_012911; Qiagen catalog nos. SI00254093 and SI00254114) were transfected 3–4 d before imaging. Neurons were transfected using Lipofectamine RNAiMAX (Invitrogen) using 1–50 nmol siRNA for a 35-mm dish. siRNA labeling was performed following the protocol of the Ambion Silencer siRNA Labeling Kit. Immunoblot analysis of neuronal extracts to verify knockdown was performed using a commercially available antibody specifically recognizing β-arrestin-2 (H-9; Santa Cruz Biotechnology). Quantification of endogenous protein depletion was done by enzyme-linked chemiluminescence (Amersham ECL Plus; GE Life Sciences) and scanning densitometry (Alpha Innotech) in the linear range of detection.
Virus-Mediated Gene Transfer.
Hippocalcin-EGFP was subcloned into a pSCA1 vector, and Semliki Forest viral particles were generated by transfecting pSCA1-hippocalcin-EGFP and pHelper into HEK293T cells (26). At 48–72 h after transfection, supernatants were harvested, aliquoted, and stored at −80 °C. Before infection, the viral particles were activated with chymotrypsin. For infection, viral solutions were added to rat-dissociated neurons 24–48 h before imaging.
Supplementary Material
Acknowledgments
We thank Roger Nicoll (University of California San Francisco) for reagents and valuable discussion and Kirsten Bjorgan for technical assistance. Some of the experiments described here were performed in the University of California San Francisco/Nikon Imaging Facility under the direction of Kurt Thorn. This work was supported by National Institutes of Health Grants DA012864 (to M.v.Z.), K99-DA023444 (to G.A.Y.), and NS060890 (to A.V.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1004169107/-/DCSupplemental.
References
- 1.Winder DG, et al. ERK plays a regulatory role in induction of LTP by theta frequency stimulation and its modulation by beta-adrenergic receptors. Neuron. 1999;24:715–726. doi: 10.1016/s0896-6273(00)81124-1. [DOI] [PubMed] [Google Scholar]
- 2.Huffman JC, Stern TA. Neuropsychiatric consequences of cardiovascular medications. Dialogues Clin Neurosci. 2007;9:29–45. doi: 10.31887/DCNS.2007.9.1/jchuffman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunet A, et al. Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J Psychiatr Res. 2008;42:503–506. doi: 10.1016/j.jpsychires.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Kindt M, Soeter M, Vervliet B. Beyond extinction: Erasing human fear responses and preventing the return of fear. Nat Neurosci. 2009;12:256–258. doi: 10.1038/nn.2271. [DOI] [PubMed] [Google Scholar]
- 5.McAinsh J, Cruickshank JM. Beta-blockers and central nervous system side effects. Pharmacol Ther. 1990;46:163–197. doi: 10.1016/0163-7258(90)90092-g. [DOI] [PubMed] [Google Scholar]
- 6.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 7.Violin JD, Lefkowitz RJ. Beta-arrestin–biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci. 2007;28:416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Azzi M, et al. Beta-arrestin–mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein–coupled receptors. Proc Natl Acad Sci USA. 2003;100:11406–11411. doi: 10.1073/pnas.1936664100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wisler JW, et al. A unique mechanism of beta-blocker action: Carvedilol stimulates beta-arrestin signaling. Proc Natl Acad Sci USA. 2007;104:16657–16662. doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galandrin S, Bouvier M. Distinct signaling profiles of beta1 and beta2 adrenergic receptor ligands toward adenylyl cyclase and mitogen-activated protein kinase reveals the pluridimensionality of efficacy. Mol Pharmacol. 2006;70:1575–1584. doi: 10.1124/mol.106.026716. [DOI] [PubMed] [Google Scholar]
- 11.Kenakin T. Collateral efficacy in drug discovery: Taking advantage of the good (allosteric) nature of 7TM receptors. Trends Pharmacol Sci. 2007;28:407–415. doi: 10.1016/j.tips.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Drake MT, et al. Beta-arrestin–biased agonism at the beta2-adrenergic receptor. J Biol Chem. 2008;283:5669–5676. doi: 10.1074/jbc.M708118200. [DOI] [PubMed] [Google Scholar]
- 13.Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- 14.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 15.Steyer JA, Almers W. A real-time view of life within 100 nm of the plasma membrane. Nat Rev Mol Cell Biol. 2001;2:268–275. doi: 10.1038/35067069. [DOI] [PubMed] [Google Scholar]
- 16.Demuro A, Parker I. Imaging single-channel calcium microdomains by total internal reflection microscopy. Biol Res. 2004;37:675–679. doi: 10.4067/s0716-97602004000400025. [DOI] [PubMed] [Google Scholar]
- 17.Zenisek D, Davila V, Wan L, Almers W. Imaging calcium entry sites and ribbon structures in two presynaptic cells. J Neurosci. 2003;23:2538–2548. doi: 10.1523/JNEUROSCI.23-07-02538.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yudowski GA, Puthenveedu MA, von Zastrow M. Distinct modes of regulated receptor insertion to the somatodendritic plasma membrane. Nat Neurosci. 2006;9:622–627. doi: 10.1038/nn1679. [DOI] [PubMed] [Google Scholar]
- 19.Hillman KL, Doze VA, Porter JE. Functional characterization of the beta-adrenergic receptor subtypes expressed by CA1 pyramidal cells in the rat hippocampus. J Pharmacol Exp Ther. 2005;314:561–567. doi: 10.1124/jpet.105.084947. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann C, Leitz MR, Oberdorf-Maass S, Lohse MJ, Klotz KN. Comparative pharmacology of human beta-adrenergic receptor subtypes: Characterization of stably transfected receptors in CHO cells. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:151–159. doi: 10.1007/s00210-003-0860-y. [DOI] [PubMed] [Google Scholar]
- 21.Chidiac P, Hebert TE, Valiquette M, Dennis M, Bouvier M. Inverse agonist activity of beta-adrenergic antagonists. Mol Pharmacol. 1994;45:490–499. [PubMed] [Google Scholar]
- 22.Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- 23.Puthenveedu MA, von Zastrow M. Cargo regulates clathrin-coated pit dynamics. Cell. 2006;127:113–124. doi: 10.1016/j.cell.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 24.Palmer CL, et al. Hippocalcin functions as a calcium sensor in hippocampal LTD. Neuron. 2005;47:487–494. doi: 10.1016/j.neuron.2005.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braunewell K-H. The darker side of Ca2+ signaling by neuronal Ca2+-sensor proteins: From Alzheimer's disease to cancer. Trends Pharmacol Sci. 2005;26:345–351. doi: 10.1016/j.tips.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Tzingounis AV, Kobayashi M, Takamatsu K, Nicoll RA. Hippocalcin gates the calcium activation of the slow after-hyperpolarization in hippocampal pyramidal cells. Neuron. 2007;53:487–493. doi: 10.1016/j.neuron.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Callaghan DW, Tepikin AV, Burgoyne RD. Dynamics and calcium sensitivity of the Ca2+/myristoyl switch protein hippocalcin in living cells. J Cell Biol. 2003;163:715–721. doi: 10.1083/jcb.200306042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beaulieu JM, Gainetdinov RR, Caron MG. Akt/GSK3 signaling in the action of psychotropic drugs. Annu Rev Pharmacol Toxicol. 2009;49:327–347. doi: 10.1146/annurev.pharmtox.011008.145634. [DOI] [PubMed] [Google Scholar]
- 29.Galandrin S, Oligny-Longpré G, Bouvier M. The evasive nature of drug efficacy: Implications for drug discovery. Trends Pharmacol Sci. 2007;28:423–430. doi: 10.1016/j.tips.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Kenakin T. New concepts in drug discovery: Collateral efficacy and permissive antagonism. Nat Rev Drug Discov. 2005;4:919–927. doi: 10.1038/nrd1875. [DOI] [PubMed] [Google Scholar]
- 31.Urban JD, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.