Heavy metals and metalloids are toxic for living organisms through oxidative and/or genotoxic mechanisms (1). Among them, the nonessential element arsenic (As) is known to be a poison and also to promote various cancers (skin, lungs, and kidneys) in humans. Exposure to As occurs mainly by drinking contaminated water (2) or eating plant food originating from As-polluted soils. This happens particularly in Asian populations with diets relying mainly on rice (3). As sources in the environment are natural or the result of human activities (Fig. 1). It is worthwhile to note that As-contaminated water of some Asian regions is often associated with reducing conditions occurring in paddy soils, which are known to facilitate As mobilization from ferric oxides. Such a situation can explain As contamination of rice grains, which leads to a serious public health problem (4). Understanding As tolerance in plants is potentially useful knowledge for solving this problem. In this context, the paper by Song et al. (5) in PNAS is of primary importance, because it reports identification of the transporters responsible for arsenite detoxification by plant vacuoles.
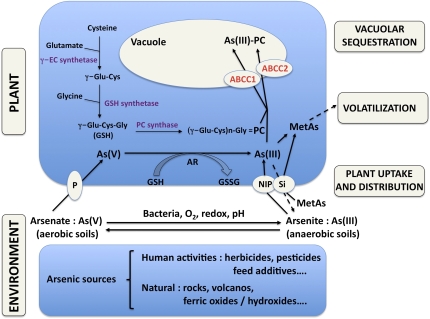
Fig. 1.
Schematic representation of the As cycle in the environment and plants. Equilibrium between arsenate [As(V)] and arsenite [As(III)] in soil solutions is mainly dependent on the redox conditions. Arsenate is taken up by roots by phosphate transporters (P), and arsenite is taken up by a subclass of aquaporins (NIP), some of them also transporting silicon (Si). Methylated forms of As (MetAs) are also taken up by NIP and Si transporters. Inside plants, these types of transporters are also involved in the distribution of As between organs and tissues. As(V) is enzymatically reduced into As(III) in plant cells by arsenate reductase (AR), leading to the conversion of glutathione (GSH) to its oxidized form (GSSG). Arsenite can be effluxed to the environment by a root Si transporter or methylated. A cascade of methylation can then transform As into a gaseous form that is volatilized. Another pathway of detoxification occurs by the synthesis of phytochelatins (PCs) corresponding to a three enzymatic step condensation of three amino acids: cysteine (Cys), glutamate (Glu), and glycine (Gly). PC synthesis and their complexation to As(III) are coordinated to the transport of the PC–As(III) complex to the vacuole subcellular compartment through the activity of two members of a subclass of ATP binding cassette (ABC) transporters: ABCC1 and -2.
As Transport and Detoxification in Plants
Plants play a major role in the entrance of metals and metalloids into the food chain (6). It is important to understand the mechanisms by which they take up As from the soil, distribute it within the plant, and detoxify it. Indeed, this knowledge is a prerequisite for breeding or biotechnological solutions to produce uncontaminated crop staples or use plants for remediation strategies (7, 8).
As can be found in three main forms in the soil: the oxidized form arsenate [As(V)], the reduced form arsenite [As(III)], and various methylated forms (MetAs) originating in part from human contaminants (Fig. 1). As(V) is taken up by roots through the activity of phosphate (Pi) transporters (9), whereas As(III) and MetAs cross cell membranes through the nodulin 26-like intrinsic proteins (NIPs), a subclass of the water channel aquaporins (reviewed in ref. 8). Among the NIPs, some members are silicon transporters (10) able, particularly in rice, to transport MetAs (11), load As(III) into the xylem (12), or secrete As(III) outside of the roots (13). Therefore, Pi transporters and NIPs are the major known As transporters to traffic As in and out plants and distribute this pollutant between organs and tissues. After it is inside plant cells, arsenate is reduced into arsenite by the arsenate reductase, concomitantly oxidizing glutathione (GSH) to its oxidized form GSSG (Fig. 1). Two different pathways then occur in plants to detoxify As. Although MetAs undergoes a series of methylations, ultimately leading to its volatilization, arsenite can be detoxified by complex formation with phytochelatins (PCs) synthesized by the condensation of glutamate, cysteine, and glycine residues through three sequential enzymatic reactions (14) (Fig. 1). The resulting As(III)–PC2 complex is ultimately detoxified by its safe storage within plant vacuoles. The transport of As(III)–PC2 across the vacuolar tonoplast is, therefore, of primary importance to confer tolerance to As. The identification of the long-sought but never reported vacuolar PC transporters by Song et al. (5) constitutes, therefore, a major breakthrough.
Two ATP Binding Cassette Transporters for Shuttling As(III)-PC2 into Plant Vacuoles
ATP binding cassette (ABC) C transporters (15, 16) are a subclass of ABC transporters, two of them being known to detoxify As in humans by transporting GSH–As complexes (17). The 15 members of the Arabidopsis ABCC family, many of which are localized at the tonoplast and some of which transport GSH conjugates, were, therefore, good candidates to be scrutinized for As detoxification. Song et al. (5) obtain homozygous KO lines for all 15 Arabidopsis ABCC genes. Although unaffected in the presence of As(V), the growth of two of them, abcc1 and abcc2, is impaired in the presence of disodium acid methane arsonate, an As herbicide, and their sensitivity is similar to that of cad1-3, an Arabidopsis mutant impaired in PC synthesis (18). At a cellular level, ABCC1 and -2 both localize at the vacuolar membrane. Surprisingly, expression of these plant genes in a Saccharomyces cerevisiae yeast strain lacking ABCC transporters does not confer As tolerance to this strain, suggesting that AtABCC1 and -2 are not efficient GSH–As complex transporters. Budding yeast does not synthesize PC, and the same strain as described above but expressing a wheat PC synthase is not tolerant to As exposure. In contrast, when AtABCC1 and -2 are expressed in this PC-producing yeast strain exposed to As, more PCs are synthesized, more As accumulates, and cells grow better than controls. These heterologous reconstruction experiments indicate that PC synthesis alone is not sufficient to confer As tolerance, but they suggest that As–PC complex transport to the vacuole by ABCC1 and -2 is required.
Song et al. report the identification of the long-sought vacuolar PC transporters.
Indeed, vesicles isolated from yeast cells expressing AtABCC1 and -2 take up efficiently more As(III)–PC2 than apo–PC2 in a time-dependent manner. This transport activity is completely abolished by vanadate but not by dissipating the proton gradient with NH4+, two characteristics of ABC transporters. The concentration-dependent As(III)–PC2 transport by AtABCC1 and -2 does not follow classical saturation kinetics but exhibits an apparent sigmoid curve. This is consistent with the observation that the higher the concentration of As in the plant environment, and the higher the difference in total PC content between WT and atabcc1 atabcc2 plants, the latter accumulating less As than nonmutant plants. All these observations support the conclusion that continuous As-induced synthesis of PC requires the coordinated transport of As(III)–PC2 into the vacuole.
Perspectives and Conclusion
In the perspective of phyroremediation, engineering plants able to take up more As from the soil would require the use of plants also more tolerant to As. Such a goal could greatly benefit from the discovery by Song et al. (5) that AtPCS1 (Arabidopsis PC synthase 1)–AtABCC1 cooverexpression Arabidopsis lines are more tolerant to As exposure than WT plants or plants overexpressing AtPCS1 or AtABCC1 independently. However, it remains to be understood why double mutant plants take up slightly more As but have a reduced transfer of this pollutant from roots to shoots.
Not only does the paper by Song et al. (5) report the identification of the long-sought vacuolar PC transporters, but these authors accomplish a major step forward for our understanding of the As detoxification pathway by showing that AtABCC1 and -2 transporters and PC synthase may function in a concerted way (5).
Footnotes
The author declares no conflict of interest.
See companion article on page 21187.
References
- 1.Briat JF, Lebrun M. Plant responses to metal toxicity. C R Acad Sci III. 1999;322:43–54. doi: 10.1016/s0764-4469(99)80016-x. [DOI] [PubMed] [Google Scholar]
- 2.Nordstrom DK. Public health. Worldwide occurrences of arsenic in ground water. Science. 2002;296:2143–2145. doi: 10.1126/science.1072375. [DOI] [PubMed] [Google Scholar]
- 3.Williams PN, et al. Variation in arsenic speciation and concentration in paddy rice related to dietary exposure. Environ Sci Technol. 2005;39:5531–5540. doi: 10.1021/es0502324. [DOI] [PubMed] [Google Scholar]
- 4.Ng JC, Wang J, Shraim A. A global health problem caused by arsenic from natural sources. Chemosphere. 2003;52:1353–1359. doi: 10.1016/S0045-6535(03)00470-3. [DOI] [PubMed] [Google Scholar]
- 5.Song W-Y, et al. Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters. Proc Natl Acad Sci USA. 2010;107:21187–21192. doi: 10.1073/pnas.1013964107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colangelo EP, Guerinot ML. Put the metal to the petal: Metal uptake and transport throughout plants. Curr Opin Plant Biol. 2006;9:322–330. doi: 10.1016/j.pbi.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Zhu YG, Rosen BP. Perspectives for genetic engineering for the phytoremediation of arsenic-contaminated environments: From imagination to reality? Curr Opin Biotechnol. 2009;20:220–224. doi: 10.1016/j.copbio.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao FJ, McGrath SP, Meharg AA. Arsenic as a food chain contaminant: Mechanisms of plant uptake and metabolism and mitigation strategies. Annu Rev Plant Biol. 2010;61:535–559. doi: 10.1146/annurev-arplant-042809-112152. [DOI] [PubMed] [Google Scholar]
- 9.Catarecha P, et al. A mutant of the Arabidopsis phosphate transporter PHT1;1 displays enhanced arsenic accumulation. Plant Cell. 2007;19:1123–1133. doi: 10.1105/tpc.106.041871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma JF, et al. A silicon transporter in rice. Nature. 2006;440:688–691. doi: 10.1038/nature04590. [DOI] [PubMed] [Google Scholar]
- 11.Li RY, et al. The rice aquaporin Lsi1 mediates uptake of methylated arsenic species. Plant Physiol. 2009;150:2071–2080. doi: 10.1104/pp.109.140350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma JF, et al. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci USA. 2008;105:9931–9935. doi: 10.1073/pnas.0802361105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao FJ, et al. The role of the rice aquaporin Lsi1 in arsenite efflux from roots. New Phytol. 2010;186:392–399. doi: 10.1111/j.1469-8137.2010.03192.x. [DOI] [PubMed] [Google Scholar]
- 14.Pal R, Rai JP. Phytochelatins: Peptides involved in heavy metal detoxification. Appl Biochem Biotechnol. 2010;160:945–963. doi: 10.1007/s12010-009-8565-4. [DOI] [PubMed] [Google Scholar]
- 15.Klein M, Burla B, Martinoia E. The multidrug resistance-associated protein (MRP/ABCC) subfamily of ATP-binding cassette transporters in plants. FEBS Lett. 2006;580:1112–1122. doi: 10.1016/j.febslet.2005.11.056. [DOI] [PubMed] [Google Scholar]
- 16.Wanke D, Uner Kolukisaoglu H. An update on the ABCC transporter family in plants: Many genes, many proteins, but how many functions? Plant Biol (Stuttg) 2010;12(Suppl 1):15–25. doi: 10.1111/j.1438-8677.2010.00380.x. [DOI] [PubMed] [Google Scholar]
- 17.Leslie EM, Haimeur A, Waalkes MP. Arsenic transport by the human multidrug resistance protein 1 (MRP1/ABCC1). Evidence that a tri-glutathione conjugate is required. J Biol Chem. 2004;279:32700–32708. doi: 10.1074/jbc.M404912200. [DOI] [PubMed] [Google Scholar]
- 18.Ha SB, et al. Phytochelatin synthase genes from Arabidopsis and the yeast Schizosaccharomyces pombe. Plant Cell. 1999;11:1153–1164. doi: 10.1105/tpc.11.6.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]