Abstract
Secreted frizzled related protein 2 (Sfrp2) is known as an inhibitor for the Wnt signaling. In recent studies, Sfrp2 has been reported to inhibit the activity of Xenopus homolog of mammalian Tolloid-like 1 metalloproteinase. Bone morphogenic protein 1 (Bmp1)/Tolloid-like metalloproteinase plays a key role in the regulation of collagen biosynthesis and maturation after tissue injury. Here, we showed both endogenous Sfrp2 and Bmp1 protein expressions were up-regulated in rat heart after myocardial infarction (MI). We hypothesize that Sfrp2 could inhibit mammalian Bmp1 activity and, hence, the exogenous administration of Sfrp2 after MI would inhibit the deposition of mature collagen and improve heart function. Using recombinant proteins, we demonstrated that Sfrp2, but not Sfrp1 or Sfrp3, inhibited Bmp1 activity in vitro as measured by a fluorogenic peptide based procollagen C-proteinase activity assay. We also demonstrated that Sfrp2 at high concentration inhibited human and rat type I procollagen processing by Bmp1 in vitro. We further showed that exogenously added Sfrp2 inhibited type I procollagen maturation in primary cardiac fibroblasts. Two days after direct injection into the rat infarcted myocardium, Sfrp2 inhibited MI-induced type I collagen deposition. As early as 2 wk after injection, Sfrp2 significantly reduced left ventricular (LV) fibrosis as shown by trichrome staining. Four weeks after injection, Sfrp2 prevented the anterior wall thinning and significantly improved cardiac function as revealed by histological analysis and echocardiographic measurement. Our study demonstrates Sfrp2 at therapeutic doses can inhibit fibrosis and improve LV function at a later stage after MI.
Myocardial infarction and postinfarction heart failure are the major cause of mortality and morbidity in the United States. Recently, stem- and progenitor-based cell therapy has shown promise in the treatment of myocardial infarction, yet the underlying mechanism remains elusive. We reported that intracardiac implantation of genetically modified mesenchymal stem cell overexpressing Akt (Akt-MSC) dramatically reduced infarct size and restored cardiac function in rodent hearts after coronary artery ligation (1). We postulated that the beneficial effects of Akt-MSC are paracrine in nature (2, 3) and identified Sfrp2 as a key factor released by Akt-MSC–mediating myocardial survival and repair (4).
Sfrps are secreted proteins that structurally resemble the Wnt frizzled receptors and serve as modulators of Wnt signaling (5). Recent studies demonstrated that the Secreted Frizzled (Sizzled) protein (sfrp related protein in Xenopus and zebrafish) played an important role in dorsal-ventral patterning by stabilizing Chordin through the inhibition of the Tolloid-family metalloproteinase in Xenopus (Xolloid-related, Xlr) (6) and Zebrafish (Tolloid-like 1, Tll1) (7). Interestingly, Lee et al. (6) showed that recombinant mammalian Sfrp2 could also inhibit Chordin cleavage by inhibiting Xlr, a Xenopus homolog of mammalian Tolloid (mTLD)-like 1. These studies raised the possibility that Sfrps might have important biological functions other than regulation of Wnt signaling (8).
Bmp1/ TLD-like metalloproteinase belongs to a subgroup of astacin family and plays a key role in the regulation of ECM formation and activation of transforming growth factor β (TGF-β) (9). Four mammalian Bmp1/TLD-like proteases have been identified: Bmp-1, mammalian Tolloid like, mTll-1, and mTll-2. These proteases have procollagen C-proteinase (PCP) activities that are responsible for the cleavage of C-propeptides from procollagen precursors to produce mature collagen fibrils. In this work, we showed that Sfrp2, but not Sfrp1 or Sfrp3, inhibited recombinant Bmp1 activity in vitro. Exogenous Sfrp2 inhibited the type I procollagen maturation in primary cardiac fibroblast culture medium. Most importantly, we demonstrated that injection of Sfrp2 protein into the infarct area of rat left ventricle 2 d after permanent coronary artery ligation inhibited MI-induced fibrosis measured as early as 2 wk after injection. Injection of Sfrp2 also prevented anterior wall thinning and significantly improved cardiac function 4 wk later when remodeling was complete. Taken together, we showed that Sfrp2 at therapeutic dosage has a strong antifibrotic effect. It can prevent post-MI collagen deposition and improve cardiac function.
Results
Up-Regulation of Bmp1, Collagens, and TGF-β in the Rat Left Ventricle After MI.
Type I and III collagens are the major forms of collagen fibrils deposited in scar tissues after acute myocardial infarction (10). We first examined the type I and III collagen expression in rat left ventricles at the different time points after acute MI (Fig. 1 A and B). Newly synthesized type I and type III collagens were strongly up-regulated in infarcted area at day 3 and maintained high levels of expression thereafter. These fibrillar collagens are synthesized by myofibroblasts as procollagen precursors, with N and C propeptides that must be removed before the triple helical part of the mature monomers can assemble into the fibrils. We next examined the protein levels of Bmp1 metalloproteinase, which is responsible for the removal of C-propeptide. Consistent with its role in procollagen maturation, Bmp1 was strongly up-regulated in infarcted area at day 3 after MI (Fig. 1C).
Fig. 1.
Bmp1, type I collagen, and TGF-β expression after permanent ligation of rat LAD. Tissue lysates from rat left ventricles were collected at different time points after LAD ligation. Protein samples were subjected to Western blot analysis and probed with rabbit anti-type I collagen antibody (A), rabbit anti-type III antibody (B), mouse anti-Bmp1 antibody (C), and mouse anti-TGF-β antibody (D). Data shown are representative results from two independent experiments. See Fig. S1 for quantified data.
A previous study reported that Bmp1 was also involved in collagen biosynthesis other than maturation through the activation of TGF-β (11). Indeed, mature TGFβ was significantly up-regulated at day 3 in infarct area and maintained at a high expression level thereafter (Fig. 1D). Mature TGF-β was also up-regulated in the noninfarct area at the later stages after MI.
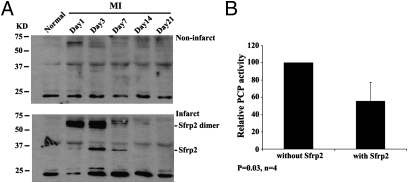
Up-Regulation of Sfrp2 Protein in Infarct Area After MI and Inhibition of PCP Activity by Recombinant Sfrp2.
A previous study showed that Sfrp2 inhibited the activity of Xenopus homolog of mammalian Tolloid-like 1 (Tll1) metalloproteinase (6). To explore the role of Sfrp2 in the regulation of mammalian Bmp1 function after MI, we first examined the endogenous Sfrp2 protein level in left ventricular (LV) tissues. Similar to Bmp1, Sfrp2 expression was significantly up-regulated in infarct area after MI peaking at day 3. However, its expression decreased afterward and became undetectable 14 d after MI (Fig. 2A). It is believed that Bmp1, its alternative spliced form mTLD, and mTll1 were responsible for the majority of PCP activities in animal tissues. Indeed, we found that Bmp1 protein expression was up-regulated 3 d after MI, a time point when Type I procollagen expression were dramatically up-regulated. To test whether Sfrp2 has any effects on PCP activity, we assayed the PCP activities in tissue extracts from left ventricles 3 d after MI with the addition of recombinant Sfrp2. We found that Sfrp2 had strong inhibitory effects on the PCP activities (Fig. 2B). These observations raised the possibility that the inhibitory effect of exogenous Sfrp2 on the PCP activity in MI tissues extracts was due to its inhibition of Bmp1 protein activity.
Fig. 2.
Sfrp2 expression after MI and inhibition of PCP activity in LV tissue lysates. (A) Tissue lysates from rat left ventricles were collected at different time points after LAD ligation. Protein samples were subjected to Western blot analysis and probed with goat anti-Sfrp2 antibody. Data shown are representative results from two independent experiments. See Fig. S2 for quantified data. (B) Rat hearts were subjected to permanent ligation, and proteins were extracted from left ventricles 3 d after MI with 10 vol of extraction buffer (25 mM Hepes and 0.01% Brij35 at pH 7.5). PCP activities were assayed with or without the incubation with recombinant Sfrp2 protein (100 nM). Sfrp2 inhibited the PCP activity in the left ventricle extracts. Values are mean ± SD (n = 4).
Inhibition of Bmp1 Activity in Vitro by Recombinant Sfrp2.
To further prove the direct inhibition of Bmp1 activity by Sfrp2, we performed the PCP activity assay by using recombinant human Bmp1 proteins and a synthetic flurogenic peptide as substrate (7). As shown in Fig. 3A, Sfrp2 exhibited dose-dependent inhibition on Bmp1 activity. Moreover, to test the specificity of this inhibitory effect, we compared the effect of Sfrp1, Sfrp2, and Sfrp3 on Bmp1 activity. Sfrp1 and Sfrp2 protein are phylogenetically and closely related, whereas Sfrp3 belongs to a separate subgroup. Only Sfrp2 significantly inhibited Bmp1 activity, whereas Sfrp1 and Sfrp3 showed no effects or even slightly enhanced Bmp1 activity (Fig. 3B). Bmp1 has multiple substrates in vivo that are involved in collagen maturation and biosynthesis. To test the effects of Sfrp2 on Bmp1 activity with its native substrate, we used purified human type I procollagen to perform the in vitro Bmp1 cleavage assay. We found that at low concentration (10–20 nM) Sfrp2 enhanced Bmp1 activity as revealed by increasing the formation of mature α1 and α2 subunits of type I collagen (Fig. 3 C–E).
Fig. 3.
Sfrp2 inhibits Bmp1 activity in vitro. (A) Bmp1 activity assay was performed by using recombinant Bmp1 protein (10 ng/μL) together with different amounts of recombinant Sfrp2 protein. Sfrp2 can dose-dependently inhibit Bmp1 activity. Values are mean ± SD (n = 3). (B) Bmp1 activity assays were performed by using recombinant Bmp1 protein (10 ng/μL) together with recombinant Sfrp1, 2, and 3 proteins (100 nM). Values are mean ± SD (n = 3). Only Sfrp2 showed inhibitory effect on Bmp1 activity (P < 0.01). (C) Bmp1 cleavage assay was performed with purified human type I procollagen as substrate. Four hundred nanograms of procollagen was incubated with 15 ng Bmp1 with or without different concentration of Sfrp2. At low concentrations, (10–20nM) Sfrp2 enhanced Bmp1 activity, whereas at high concentrations (100–200 nM), Sfrp2 inhibited Bmp1 activity. (D and E) show the quantitation of intensity of α1 (I) and α2 (I) band in C. Similar results were obtained from three independent experiments. (F) Physical interaction between Bmp1 and Sfrp2. HEK 293 cells were transfected with Sfrp2-FLAG expression vectors. Cell lysates were collected and incubated with anti-FLAG–conjugated agarose beads. After washing, beads were incubated with different amounts of recombinant Bmp1 protein followed by extraction with SDS sample buffer. Supernatants after centrifugation were subjected to SDS/PAGE and Western blots were probed with an anti-Bmp1 or anti-FLAG antibody. Results showed the Sfrp2-FLAG can pull down Bmp1 protein.
However, at high concentration (100–200 nM) Sfrp2 inhibited Bmp1 activity, mainly on the formation of α1 subunit of type I collagen. Previous studies demonstrated that Sizzled protein can interact with zebrafish Bmp1a and Tll1 or Xenopus Xlr (6, 7). To test whether Sfrp2 can directly interact with mammalian Bmp1, we performed a coimmunoprecipitation assay. Recombinant human Bmp1 protein could be pulled down by anti-FLAG agarose beads binding with Sfrp2-FLAG proteins (Fig. 3F). These data suggested that Sfrp2 can specifically regulate mammalian Bmp1 activity through direct interaction with Bmp1 protein.
Inhibition of Procollagen Maturation by Sfrp2 in Conditioned Medium from Primary Culture of Cardiac Fibroblasts.
Cardiac fibroblasts are the major source of collagen deposition and play a key role in the scar formation after MI (12). Bmp1 expression has been detected in primary culture of cardiac fibroblast, and PCP activity has been shown to increase in response to collagen-inducing reagents (13). To test whether Sfrp2 has any effects on procollagen maturation in cardiac fibroblasts, we first used conditioned medium from these cells as substrate to perform in vitro Bmp1 cleavage assay. Again, Sfrp2 increased the levels of Bmp1-induced mature collagen α1 at low concentrations, whereas it showed inhibitory effects on Bmp1 activity at high concentrations (100 nM) (Fig. 4A). We next added Sfrp2 directly into the culture medium of cardiac fibroblasts to mimic the in vivo situation. We demonstrated that exogenous Sfrp2 increased the ascorbic acid induced soluble type I procollagen content in the culture medium in a dose-dependent manner, whereas it had no effects on type III procollagen maturation (Fig. 4B). Interestingly, Sfrp2 did not show any effects on the total type I collagen level in cytosol fraction (Fig. 4C), suggesting that Sfrp2 did not directly interfere with the synthesis or secretion of collagen. These results further demonstrated that Sfrp2 at high concentration acted as a Bmp1 inhibitor.
Fig. 4.
Inhibition of procollagen processing by Sfrp2 in the culture medium of primary cardiac fibroblast cells. (A) Cardiac fibroblasts were isolated from rat left ventricles and cultured in 35-mm culture dishes. Cells were stimulated with ascorbic acid for 48 h, and culture medium was collected. Conditioned medium (40 μL) was incubated with 15 ng of Bmp1 with and without different concentration of Sfrp2 protein. Samples were subjected to SDS/PAGE followed by Western blot analysis. Blots were probed with an anti-collagen type I antibody. Sfrp2 increased α1 level at low concentrations (20 nM) but showed the inhibitory effects on Bmp1 activity at high concentrations (100 nM). (B) Cardiac fibroblasts were stimulated with ascorbic acid together with different concentration of exogenous Sfrp2. Forty-eight hours later, condition medium and total cell lysates (C) were collected and samples were subjected to SDS/PAGE followed by Western blot analysis. Sfrp2 could increase the soluble precollagen level in the culture medium in a dose-dependent manner and had no effects on the total collagen level in cytosol.
Inhibition of MI-Induced Fibrosis by Sfrp2 in Vivo.
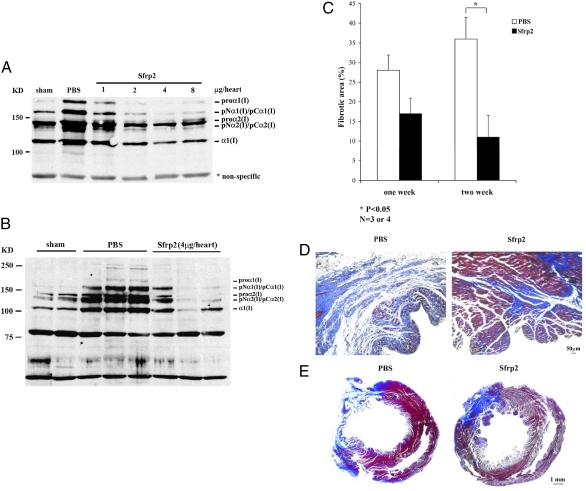
Because we already showed that Sfrp2, Bmp1, and type I collagen expression were significantly up-regulated in infarct areas 3 d after MI and Sfrp2 could inhibit Bmp1 activity and collagen maturation in vitro, we decided to test whether exogenously added Sfrp2 can interfere with collagen maturation in vivo. Two days after permanent left anterior descending (LAD) ligation, recombinant Sfrp2 proteins were injected directly into the infarct areas. Two days after injection, left ventricle heart tissues were collected and type I collagen expressions were assayed by Western blot analysis. We demonstrated that Sfrp2 showed strong inhibition of type I collagen deposition in infarct tissues in a dose-dependent manner (Fig. 5A). The inhibitory effects were highly reproducible when Sfrp2 was injected at a single dose of 4 μg of protein per heart (Fig. 5B). Exogenous Sfrp2 (with a C-terminal 6×His tag) was still detectable in these tissue extracts 6 d after acute MI (Fig. S3). In addition, we performed trichrome staining on heart sections and analyzed collagen fibril deposition and area of fibrosis 1 and 2 wk after Sfrp2 injection. There was a trend toward less area of fibrosis in the Sfrp2-treated animals 1 wk after treatment (≈35% reduction), as compared with PBS controls, but the differences did not reach statistical significance (Fig. 5C). However, significant differences in area of fibrosis between PBS- and Sfrp2-treated animals were observed 2 wk after intramyocardial injection (Fig. 5 C–E; ≈66% reduction).
Fig. 5.
Sfrp2 Inhibited collagen maturation and fibrosis in vivo. Recombinant Sfrp2 protein was injected to the infarct areas in rat left ventricles 2 d after permanent ligation of LAD. Two days after injection, protein extracts from left ventricles were subjected to SDS/PAGE, and Western blots were probed with an anti-collagen type I antibody. Intramyocardial injection of Sfrp2 could reduce the type I collagen processing in a dose-dependant manner (A), and reproducible results were obtained at the dose of 4 μg per heart (B; n = 3). See Fig. S2 for quantified data. One and 2 wk after injection of 4 μg per heart Sfrp2, heart tissues were collected and paraffin-embedded sections were subjected to trichrome staining. Area of fibrosis within the left ventricles from rats injected with Sfrp2 was reduced compared with PBS-injected tissues 2 wk after injection (C; mean ± SE, P < 0.05, n = 3 or 4). Representative left ventricle sections were shown in D and E.
Prevention of Anterior Wall Thinning and Improvement of LV Function After MI by Sfrp2.
Fibrosis and remodeling usually are complete 1 mo after acute MI in rodent model. To test the long-term effects of Sfrp2 on the LV remodeling and function after MI, we performed permanent LAD ligation and injected 4 μg per heart of Sfrp2 into the infarct area 2 d after surgery. Three and four weeks after acute MI, we performed echocardiographic analysis followed by trichrome staining on heart sections from the same animals. Consistent with 2-wk data on fibrosis, injection of Sfrp2 protein significantly reduced area of fibrosis in LV in these animals (Fig. 6A; ≈50% reduction). Interestingly, the ratio of the anterior to posterior wall thickness was significantly decreased 4 wk after MI in PBS-treated animals but not in Sfrp2-treated animals (Fig. 6B). Consistent with this observation, LV function was improved 4 wk after Sfrp2 treatment as revealed by increasing fraction shortening (FS) and mean velocity of fiber shortening (mVcfc) corrected by heart rate (Table S1). These data demonstrated Sfrp2 at therapeutic dose acted as an inhibitor of fibrosis and remodeling and improved LV function after MI.
Fig. 6.
Sfrp2 prevented anterior wall thinning after MI. Recombinant Sfrp2 protein was injected to the infarct areas in rat left ventricles 2 d after permanent ligation of LAD. Three and 4 wk after injection, heart tissues were collected and paraffin-embedded sections were subjected to trichrome staining. Area of fibrosis within the left ventricles from rats injected with Sfrp2 was reduced compared with PBS-injected tissues (A; values are mean ± SD). The radio of the anterior to posterior wall thickness (AWT/PWT) was significantly decreased in PBS-treated animals 4 wk after MI (B; values are mean ± SD).
Discussion
Fibroblasts are the major contributors to the interstitial fibrosis that control the production and degradation of ECM components, especially collagen networks. Up-regulated interstitial collagens account for the reduction of capillary density and oxygen diffusion, reduced contractility of myocytes, and increased structural rigidity of the left ventricle (14). Bmp1/TLD-like metalloproteinases are obviously the interesting therapeutic targets for the modulation of fibrosis, because they play key roles in collagen maturation and deposition. However, only a few physiological inhibitors or specific chemical antagonists of these proteinases have been described so far (9), and no such agents were reported to prevent fibrosis in the process of cardiac remodeling in vivo.
Recent studies in Zebrafish and Xenopus revealed the unexpected role of Sfrps in the regulation of Bmp1/TLD-like proteinase activity. Among these Sfrps, Sfrp2 was shown to inhibit Chordin cleavage by inhibiting Xlr, a Xenopus homolog mTll-1. Interestingly, our recent study identified the Sfrp2 as the key factor released by Akt-MSC mediating cardiac protection after MI. Based on this information, we decided to explore the putative modulatory effects of Sfrp2 on the mammalian Bmp1 activity. We hypothesize that (i) Bmp1/TLD-like proteinase is involved in the cardiac fibrosis after MI; (ii) Sfrp2 can inhibit Bmp1 activity and, as a result, administration of Sfrp2 can inhibit after MI collagen maturation and fibrosis. Indeed, our data demonstrated that the endogenous expression of Bmp1/TLD-like proteinase (Fig. 1) was significantly up-regulated at day 3 after MI, the same time point when type I and type III procollagen and TGF-β were also up-regulated. This observation is consistent with the role of Bmp1 as a key player in procollagen biosynthesis and maturation. We also examined endogenous Sfrp2 protein expression in response to acute MI. Unlike Bmp1 and TGF-β, Sfrp2 protein expression level was strongly up-regulated at the early stage of MI (day 1 through day 3 after acute MI) and became undetectable at the later stage when procollagen matured. To test whether Sfrp2 can be an inhibitor of Bmp1, we performed a series of in vitro and in vivo experiments. (i) Indeed, we found that Sfrp2 at high concentration (100 nM) specifically inhibited PCP activity, Bmp1 activity, and collagen maturation in vitro (Figs. 2 and 3), and the underlying mechanism might be the competitive inhibition of the binding of collagen substrate with Bmp1 protein (Fig. 3). (ii) Using conditioned medium from primary cardiac fibroblasts as substrate, we showed that Sfrp2 inhibited procollagen maturation in an in vitro Bmp1 cleavage assay (Fig. 4). We also demonstrated that exogenous Sfrp2 increased the soluble type I procollagen accumulation in the culture medium in a dose-dependant manner but had no effect on collagen synthesis in the total cell lysates (Fig. 4). Because Bmp1 was expressed in cultured cardiac fibroblasts (13), the effects of Sfrp2 on collagen maturation were most likely through inhibiting Bmp1 activity. (iii) We provided in vivo evidences showing that intramyocardial injection of Sfrp2 inhibited MI-induced fibrosis in a dose-dependent manner (Fig. 5). We observed an overall reduction of type I collagen deposition in Sfrp2-injected tissues. Interstitial fibrosis was reduced in the infarct area starting from 2 wk after Sfrp2 injection. Most importantly, we demonstrated significant reduction of LV wall thinning and improvement of LV function 1 mo after therapeutic administration of Sfrp2 (Fig. 6 and Table S1). Taken together, our results strongly suggested that the antifibrosis effect of exogenously administrated Sfrp2 is due to its inhibition of Bmp1 activity. However, as Sfrp2 has multiple physiological functions, our data did not prove the specificity of Sfrp2 in Bmp1 inhibition in vivo.
Early apoptotic events after acute cardiac MI have significant impact on the late fibrosis process. Our previous study on Akt-MSC mediating myocardial survival and repair suggested that Sfrp2 may protect cardiomyocytes from apoptosis after acute MI via the inhibition of Wnt signaling (15). Indeed, endogenous Sfrp2 was strongly up-regulated 1 d after acute MI, consistent with its role as an antiapoptotic factor in the early stage of MI. In this study, to distinguish the antiapoptotic and antifibrotic functions of Sfrp2, we chose to inject Sfrp2 into the infarct myocardium 2 d after MI that was beyond the acute phase of cardiomyocyte apoptosis (at this stage TUNEL-positive cells are localized mainly at the border zone but not in the infarct area; Fig. S4) so that we could examine more directly its in vivo effect on fibrosis. The positive results from Sfrp2 injection on the reduction of fibrosis and restore of LV function suggest Sfrp2 exerts its antifibrotic effects independent from its antiapoptotic action on cardiomyocytes.
We observed exogenous Sfrp2 increased the accumulation of procollagen in the culture medium of cardiac fibroblasts, whereas Sfrp2 injection resulted in an overall reduction of collagens in the myocardium in vivo. One possible explanation is that a high dose of Sfrp2 may inhibit the proliferation or activation of cardiac fibroblasts, thereby reducing the overall level of collagen production. However, we find that Sfrp2 at high doses can actually stimulate the proliferation of cardiac fibroblasts in vitro and does not up-regulate the expression of alpha smooth muscle actin, a marker of myofibroblast (Fig. S5). These observations suggest that reduction of collagen in Sfrp2-treated heart tissues is not the result of a decreased number of activated cardiac fibroblasts. Another explanation for the in vivo Sfrp2 effects might be that Sfrp2 regulates overall level of collagen production through the regulation of Bmp1 activity. Bmp1 has multifaceted biological functions. Besides its role in procollagen C-peptide removal, Bmp1 is also involved in cross-linking of collagen fibrils via Lysyl oxidase (LOX) maturation and collagen biosynthesis via TGF-β activation. Bmp1 can activate the LOX by removing the N-terminal peptide from the preenzyme of LOX. Accordingly, inhibition of Bmp1 by Sfrp2 may also reduce the collagen fibril formation through the direct inhibition of collagen cross-linking. Bmp1 could also indirectly activate TGF-β (11), which plays a key role in the development of cardiac remodeling (16). Because TGF-β is a strong inducer of collagen biosynthesis, inhibition of its activity will reduce the overall collagen deposition in infarct area.
Recently Kobayashi et al. published a study on the role of Sfrp2 in fibrosis associated with MI (17). In Sfrp2-null mice subjected to MI, they observed reduced fibrosis as compared with wild-type mice. In contrast to Kobayashi's observation and consistent with our results, Alfaro et al. recently demonstrated that peri-infarct intramyocardial injection of genetically modified MSC overexpressing Sfrp2 resulted in enhanced engraftment, vascular density, reduced infarct size and remodeling, and increased cardiac function after myocardial injury in mice (18). The discrepancy between the results of Kobayashi et al. vs. those of Alfaro et al. and our laboratory may be explained by the apparent biphasic effect of Sfrp2 on Bmp1. Kobayashi et al. showed that at physiologic doses (10–20 nM), Sfrp2 increased Bmp1 activity as measured by an in vitro procollagen cleavage assay. By using their recently published protocol to assay Bmp1 activity (19), we were able to demonstrate that at a low concentration (10–20 nM), Sfrp2 enhanced Bmp1 activity, whereas in concentrations of 100–200 nM, Sfrp2 inhibited Bmp1 activity. Similar effects were observed by using conditioned medium from cardiac fibroblasts as substrate. Endogenous Sfrp2 was up-regulated at an early stage after MI [>80-fold increase according to Kobayashi et al. (17)] and became undetectable at later stage. If the physiologic concentration of Sfrp2 is ≈10–20nM, then the concentration of Sfrp2 at early stage after MI should be much higher than 100–200 nM. Based on our data, Sfrp2 should inhibit Bmp1 activity at this concentration range. Therefore, effects of Sfrp2 at the early stage after MI might be explained by the integration of both its antiapoptosis and antifibrosis function. Deletion of Sfrp2 will enhance the tissue apoptosis, thereby offset the potential fibrosis consequence. At a later stage after MI, expression level of Sfrp2 dramatically decreased and most likely returned to its physiologic concentration, at which Sfrp2 will enhance Bmp1 activity. Deletion of Sfrp2 will only reduce fibrosis at this stage because apoptosis is no longer a major event. Our hypothesis on the biphasic function of Sfrp2 in vivo seems to be supported by Kobayshi et al.’s data on Sfrp2- null mice. Reduced fibrosis and functional recovery in these mice after acute MI can only be observed at later stage (2 wk) but not at early stage (1 wk) after surgery. At the molecular level, we detected a high level of type I procollagen at the early stage of MI when Sfrp2 expression was high, whereas a low level of procollagen was observed at a later stage when Sfrp2 was undetectable. The beneficial effects of Akt-MSC and Sfrp2- MSC on MI hearts can also be explained by the inhibitory effects of overexpressing Sfrp2 on Bmp1. MSCs overexpressing either Akt or Sfrp2 can secrete high concentrations of Sfrp2 (100-fold increase in Akt-MSC cells; ref. 4), reaching a level that can inhibit Bmp1 activity.
In summary, our findings shed light on the biological function of Sfrp2 and describe a physiologically active agent can directly target the collagen maturation in the infarct heart tissue. Sfrp2 is the key paracrine factor secreted by Akt-MSC. Elucidation of biological function of Sfrp2 will enable the further understanding of the stem cell biology in the treatment of heart failure. Clinically, Sfrp2 has the intriguing potential to be a novel antifibrosis agent for the prevention of heart failure.
Materials and Methods
See SI Materials and Methods for details.
Rat Acute MI Model and Protein Injection.
Ligation of the LAD coronary artery was performed in 6-wk-old Sprague–Dawley male rats as described (3). Average initial MI size is ≈50%. For protein injection, animals were randomized into three groups: sham-operated animals, control animals that received PBS injection, and animals that received recombinant Sfrp2 proteins (R&D Systems). Two days after acute MI, different doses of proteins suspended in PBS or an equivalent volume of PBS alone were injected in five different sites at the infarct area.
Primary Culture of Cardiac Fibroblasts.
Adult rat cardiac fibroblasts were isolated by collagenase-trypsin digestion of rat heart. For the collagen assay, cells were treated with ascorbic acid (10 μg/mL; Sigma) for 48 h with or without the addition of different concentrations of recombinant Sfrp2. Culture mediums were collected and cells were lysed with RIPA buffer. Proteins were separated by SDS/PAGE followed by Western blot analysis probed with a rabbit anti-type I collagen antibody (Abcam).
In Vitro PCP Activity Assay.
A fluorogenic peptide substrate-based (Mca-Tyr-Val-Ala-Asp-Ala-Pro-Lys-(Dnp)-OH) in vitro PCP activity assay was performed according to the instructions by the manufacturer (R&D Systems).
Purification of Type I Procollagen and Assay for Bmp1 Cleavage Activity.
Human type I procollagen was purified as described (20), and procollagen cleavage assay was performed as described in Huang et al. (19). In some of the cleavage experiments, condition medium from primary human dermal or rat cardiac fibroblasts culture were used instead of purified procollagen.
Histological Analysis of Myocardial Fibrosis and Remodeling.
One, 2, 3, and 4 wk after protein injection, rat heart sections were stained with Masson's trichrome stain (Sigma) for the visualization of interstitial collagen deposition. Collagen-positive areas were calculated by using Image J software (NIH). Percent fibrosis was expressed as the ratio of fibrotic area to total LV area. The wall-thinning index was calculated as the ratio of anterior-to-posterior wall thickness.
Echocardiography.
Transthoracic 2-dimensional and M-mode echocardiography was performed 3 and 4 wk after MI in anesthetized rats.
Statistical Analysis.
Results are shown as means ± SD. Unpaired t test was used to compare the differences in fibrosis and LV function in left ventricles between the PBS- and Sfrp2-treated animals. P < 0.05 was considered to be statistical significance.
Supplementary Material
Acknowledgments
We thank Hui Mu for isolating adult cardiac fibroblasts and Dr. Howard A. Rockman for providing echocardiographic support in the study. This work was supported by National Heart, Lung, and Blood Institute Grants R01 HL35610, HL58516, HL72010, and HL73219 and grants from the Edna and Fred L. Mandel, Jr. Foundation and the Leducq Foundation (to V.J.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1004708107/-/DCSupplemental.
References
- 1.Mangi AA, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 2.Gnecchi M, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 3.Gnecchi M, et al. Evidence supporting paracrine hypothesis for Akt- modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 4.Mirotsou M, et al. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci USA. 2007;104:1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones SE, Jomary C. Secreted Frizzled-related proteins: Searching for relationships and patterns. Bioessays. 2002;24:811–820. doi: 10.1002/bies.10136. [DOI] [PubMed] [Google Scholar]
- 6.Lee HX, Ambrosio AL, Reversade B, De Robertis EM. Embryonic dorsal-ventral signaling: Secreted frizzled-related proteins as inhibitors of tolloid proteinases. Cell. 2006;124:147–159. doi: 10.1016/j.cell.2005.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muraoka O, et al. Sizzled controls dorso-ventral polarity by repressing cleavage of the Chordin protein. Nat Cell Biol. 2006;8:329–338. doi: 10.1038/ncb1379. [DOI] [PubMed] [Google Scholar]
- 8.Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. Beyond Wnt inhibition: New functions of secreted Frizzled-related proteins in development and disease. J Cell Sci. 2008;121:737–746. doi: 10.1242/jcs.026096. [DOI] [PubMed] [Google Scholar]
- 9.Hopkins DR, Keles S, Greenspan DS. The bone morphogenetic protein 1/Tolloid-like metalloproteinases. Matrix Biol. 2007;26:508–523. doi: 10.1016/j.matbio.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cleutjens JP, Verluyten MJ, Smiths JF, Daemen MJ. Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol. 1995;147:325–338. [PMC free article] [PubMed] [Google Scholar]
- 11.Ge G, Greenspan DS. BMP1 controls TGFbeta1 activation via cleavage of latent TGFbeta-binding protein. J Cell Biol. 2006;175:111–120. doi: 10.1083/jcb.200606058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibroblast: Therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol. 2005;45:657–687. doi: 10.1146/annurev.pharmtox.45.120403.095802. [DOI] [PubMed] [Google Scholar]
- 13.Shalitin N, Schlesinger H, Levy MJ, Kessler E, Kessler-Icekson G. Expression of procollagen C-proteinase enhancer in cultured rat heart fibroblasts: Evidence for co-regulation with type I collagen. J Cell Biochem. 2003;90:397–407. doi: 10.1002/jcb.10646. [DOI] [PubMed] [Google Scholar]
- 14.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: Pathophysiology and therapy. Circulation. 2000;101:2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Z, et al. Secreted frizzled related protein 2 protects cells from apoptosis by blocking the effect of canonical Wnt3a. J Mol Cell Cardiol. 2009;46:370–377. doi: 10.1016/j.yjmcc.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bujak M, Frangogiannis NG. The role of TGF-β signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007;74:184–195. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi K, et al. Secreted Frizzled-related protein 2 is a procollagen C proteinase enhancer with a role in fibrosis associated with myocardial infarction. Nat Cell Biol. 2009;11:46–55. doi: 10.1038/ncb1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alfaro MP, et al. The Wnt modulator Sfrp2 enhances mesenchymal stem cell engraftment, granulation tissue formation and myocardial repair. Proc Natl Acad Sci USA. 2008;105:18366–18371. doi: 10.1073/pnas.0803437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang G, et al. Fibronectin binds and enhances the activity of bone morphogenetic protein 1. J Biol Chem. 2009;284:25879–25888. doi: 10.1074/jbc.M109.024125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imamura Y, Steiglitz BM, Greenspan DS. Bone morphogenetic protein-1 processes the NH2-terminal propeptide, and a furin-like proprotein convertase processes the COOH-terminal propeptide of pro-alpha1(V) collagen. J Biol Chem. 1998;273:27511–27517. doi: 10.1074/jbc.273.42.27511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.