Abstract
Functional magnetic resonance imaging of brain responses to biological motion in children with autism spectrum disorder (ASD), unaffected siblings (US) of children with ASD, and typically developing (TD) children has revealed three types of neural signatures: (i) state activity, related to the state of having ASD that characterizes the nature of disruption in brain circuitry; (ii) trait activity, reflecting shared areas of dysfunction in US and children with ASD, thereby providing a promising neuroendophenotype to facilitate efforts to bridge genomic complexity and disorder heterogeneity; and (iii) compensatory activity, unique to US, suggesting a neural system–level mechanism by which US might compensate for an increased genetic risk for developing ASD. The distinct brain responses to biological motion exhibited by TD children and US are striking given the identical behavioral profile of these two groups. These findings offer far-reaching implications for our understanding of the neural systems underlying autism.
Keywords: endophenotype, functional magnetic resonance imaging
Autism spectrum disorder (ASD) is a strongly genetic, highly prevalent neurodevelopmental disorder characterized by striking social deficits (1, 2). Among the most scientifically challenging features of ASD are its phenotypic heterogeneity and genetic variability, which constrain successful identification of genes underlying the clinical syndrome. Despite these challenges, we hypothesize that the various factors contributing to the expression of ASD might exert their effects through a circumscribed set of neuroanatomical structures (3); that is, it is possible that the simplest and potentially most powerful signature of ASD will be found at the level of brain systems. Such “neural signatures” of ASD may serve as critical endophenotypes to facilitate the study of the pathophysiological mechanisms underlying this devastating and highly prevalent neurodevelopmental disorder.
Originally described in the psychiatric literature by Gottesman and Shields (4), endophenotypes (i.e., phenotypes not obvious to the unaided eye) provide quantifiable characteristics (e.g., behavioral, physiological, neuropsychological) that reflect genetic liability for a disease. Endophenotypes exist midstream between genotypes and clinical phenotypes in both affected individuals and their unaffected relatives (4–6). As such, they offer the potential to bridge gaps between diagnostic categories of complex diseases, such as neurodevelopmental and psychiatric disorders, and genetic mechanisms (4). Endophenotypes reveal more basic components of a complex phenotype and thus are thought to be more closely related to the underlying pathophysiology than the collection of downstream clinical symptoms. Therefore, genetic factors contributing to endophenotypes are considered more easily identifiable, due to the increased proportion of the variance explained at a given genetic locus compared with that explained by the traditional clinical endpoint. Research on the identification of genetically meaningful, clinically informed endophenotypes, such as activity in brain regions supporting key aspects of social perception, is likely to provide important insight into the underlying components of the core features of ASD and facilitate complementary approaches to genetic studies.
As intrinsically social creatures, humans typically exhibit robust visual sensitivity to other people's movements (7). This is well illustrated by the discovery that point-light displays (i.e., videos created by placing lights on the major joints of a person and filming them moving in the dark), although relatively impoverished stimuli, contain sufficient information to identify the kind of motion being produced (e.g., walking, dancing, reaching), as well as the identity of the agent (8). Visual sensitivity to biological motion is an evolutionarily well-conserved and ontogenetically early-emerging mechanism that is fundamental to adaptive social engagement (9); for example, newly hatched chicks recognize biological motion in point-light displays (10), and 2-d-old human infants preferentially attend to biological motion in point-light displays (11). Preferential attention to biological motion is thought to be critical for filial attachment (12) and is seen as a precursor to subsequent social development, including the ability to perceive emotion (13) and to attribute intentions to others (14).
Given the centrality of biological motion perception to social interaction, recent evidence of disrupted biological motion perception in toddlers with autism is particularly noteworthy. Using eye tracking, Klin et al. (9) demonstrated that a group of 2-y-old children with autism failed to orient preferentially toward point-light displays of canonical biological motion. Instead, their viewing behavior was well explained by preferential attention to nonsocial audiovisual contingencies that were ignored by typically developing children and developmentally delayed children without autism. Moreover, disrupted perceptual sensitivity to biological motion has been documented in older children with ASD (15). Neuroimaging studies examining the neural correlates of point-light biological motion perception in adults with and without ASD have consistently implicated the posterior superior temporal sulcus (pSTS) region as an area of dysfunction (16, 17). Given that ASD is a neurodevelopmental disorder, studying the early stages of this atypical neural response to biological motion is critical. Identifying disruptions in the brain mechanisms for biological motion perception in children with ASD might provide insight into an ongoing developmental process whereby early abnormalities in social engagement shape (and are shaped by) the neural processes that support social interactions.
During a 5.5-min functional magnetic resonance imaging (fMRI) scan, 4- to 17-y-old children and adolescents viewed coherent and scrambled point-light animations of biological motion in a blocked design. Three groups of children were studied: (i) children with ASD (n = 25), (ii) unaffected siblings of children with ASD (US; n = 20), and (iii) typically developing children (TD; n = 17). Comparing the activation to biological motion versus scrambled motion among the three groups identified three kinds of brain activity: (i) state activity, reflecting ASD (regions showing reduced differential activation to biological versus scrambled motion in ASD relative to TD and US); (ii) trait activity, reflecting the genetic vulnerability to develop ASD (regions showing reduced differential activation in ASD and US relative to TD); and (iii) compensatory activity (regions showing enhanced differential activation unique to US). State activity characterizes the nature of disruption in brain circuitry in ASD at the neural systems level, whereas trait activity reflects shared areas of dysfunction in US and children with ASD, thereby providing a robust endophenotype for ASD. Compensatory activity refers to activity unique to US, which might reflect the operation of a neural system–level mechanism by which US overcome an increased genetic risk for developing ASD.
As illustrated in Table 1, the three groups of participants were matched on chronological age and were of similar cognitive ability, all within the average range. Cognitive ability was assessed using either the Differential Ability Scale (DAS-II) (18) or the Wechsler Abbreviated Scale of Intelligence (WASI) (19). The children with ASD were diagnosed with autism (n = 9), Asperger's syndrome (n = 7), or pervasive developmental disorder–not otherwise specified (n = 9) using the gold standard Autism Diagnostic Observation Schedule (ADOS) (20), the Autism Diagnostic Interview–Revised (ADI-R) (21), and experienced clinical judgment (Table 2). Notably, our US and TD groups were matched on the Social Responsiveness Scale (SRS) (22) and the Vineland Adaptive Behavior Scales II (23). This rigorous matching ensured that both groups were unaffected by ASD and demonstrated equivalent levels of social responsiveness. In addition, strict exclusion criteria were used for the TD and US groups to rule out other developmental disorders and the “broader autism phenotype” (BAP) (24) in each participant, as well as in first- and second-degree relatives of the US participants; Table 3 provides the complete list of exclusion criteria. We reasoned that by excluding US and TD participants with subtle social and communicative abnormalities, trait activity could then be interpreted as reflecting a vulnerability to develop ASD, as opposed to being epiphenomenal to the presence of subthreshold ASD symptoms. Our samples included proband–sibling pairs (n = 5 pairs) as well as unrelated probands (n = 19) and US participants (n = 14). Because all of the US participants passed screening for ASD or BAP, and only five US participants were related to probands in the ASD group, we minimized an important potential confound: identifying regions reflecting shared genetic variance unrelated to ASD. Had all of our participants been proband–sibling pairs, then this confound might have limited the scope of our conclusions. Written informed consent was obtained from each participant's parent(s), and verbal assent was obtained from each participant. The Yale School of Medicine's Human Investigations Committee approved the study.
Table 1.
Group characterization and matching variables
| Measure | TD | US | ASD |
| Sex, males: females, n | 12:5 | 9:11 | 20:5 |
| Age, years, (range) | 10.9 (3.1) [4.6–16.7] | 11.3 (2.8) [6.6–16.9] | 11.8 (3.6) [4.0–17.7] |
| SRS, total raw score | 23.7 (14.5) | 18.9 (15.3) | 98.7 (23.5) |
| Vineland-II Communication* | 102.5 (15.8) | 102.0 (12.9) | 78.3 (10.5) |
| Receptive | 15.1 (3.4) | 15.2 (2.3) | 10.4 (2.0) |
| Expressive | 15.5 (2.9) | 15.3 (2.1) | 11.1 (2.4) |
| Written | 15.3 (2.7) | 14.7 (3.1) | 12.2 (2.7) |
| Vineland-II Daily Living* | 93.1 (10.4) | 93.6 (10.9) | 78.5 (11.0) |
| Personal | 13.5 (2.6) | 13.2 (3.7) | 10.5 (2.0) |
| Domestic | 13.0 (1.6) | 13.4 (2.6) | 10.9 (3.0) |
| Community | 15.3 (2.3) | 15.5 (2.3) | 12.8 (3.2) |
| Vineland-II Social* | 102.7 (9.0) | 99.6 (11.3) | 73.5 (9.8) |
| Interpersonal Relationships | 15.6 (1.9) | 14.9 (1.9) | 9.6 (1.9) |
| Play and Leisure Time | 15.2 (2) | 14.8 (2.1) | 10.4 (2.6) |
| Coping Skills | 15.3 (1.6) | 14.4 (2.4) | 10.6 (2.3) |
| DAS-II Global Composite Ability† | 114.1 (16.3) | 115.8 (7.9) | 100.2 (19.7) |
| Verbal | 111.7 (14.1) | 118.2 (15.4) | 99.9 (21.4) |
| Specialized Non-Verbal Composite | 110.1 (17.5) | 113.8 (15.1) | 98.2 (17.7) |
| WASI Full-Scale IQ† | 113.6 (9.7) | 112.9 (15.2) | |
| Verbal | 112.5 (12.4) | 108.4 (15.0) | |
| Performance | 112.6 (8.0) | 114.6 (17.2) | |
| Amount of movement, mm‡ | 0.9 (0.7) | 1.4 (0.9) | 1.1 (0.6) |
Unless noted otherwise, values are reported as average score, with SD in parentheses.
*Vineland-II raw scores were not available for four participants (two TD, one US, and one ASD). The Vineland-II Survey Edition was substituted for five ASD participants.
†Either the DAS-II (15 TD, 6 US, and 25 ASD) or WASI (2 TD and 14 US) was administered to establish average cognitive abilities.
‡Average amount of movement in six planes of motion.
Table 2.
ASD group characterization
| Measure | Average (SD) |
| ADOS Calibrated Severity Score (n = 22)* | 7.0 (1.5) |
| ADOS Mod2, Total (n = 3) | 15.0 (5.0) |
| Social | 11.3 (3.8) |
| RRB | 3.7 (2.1) |
| ADOS Mod2, Total (n = 21) | 11.5 (3.4) |
| Social | 8.8 (2.9) |
| RRB | 2.7 (1.7) |
| ADOS Mod4, Total (n = 1) | 15.0 |
| Social | 13 |
| RRB | 2 |
| ADI-R (n = 22)† | |
| Social | 21.9 (3.9) |
| Communication (verbal) | 18.2 (3.0) |
| RRSB | 5.6 (2.5) |
| Age of onset, years | 3.3 (0.9) |
*The ADOS calibrated severity score (40) was computed only for 22 participants age 4–16 y.
†The ADI-R was not available for 3 participants.
Table 3.
Exclusion criteria
| TD and US exclusion criteria |
| 1. Diagnosed, referred, or suspected ASD, schizophrenia, or other developmental or psychiatric disorder. |
| 2. First- or second-degree relative with diagnosed, referred, or suspected ASD (except sibling of proband with ASD in the case of US). |
| 3. An individual education plan for special education services, including speech/language therapy, occupational therapy, and social skills therapy. |
| 4. Low or moderately low score on any domain of the Vineland Adaptive Behavior Scale-II. |
| 5. Total T score >76 (severe range) on the SRS. |
| 6. Clinical impression suggesting ASD, other developmental delay/disorder, or psychiatric disorder by the highly experienced multidisciplinary clinical team at Yale Child Study Center Characterization Core. |
| Additional US exclusion criteria |
| 1. Parents with elevated spousal ratings on the SRS or self-ratings on the BAP-Q (22). |
Results
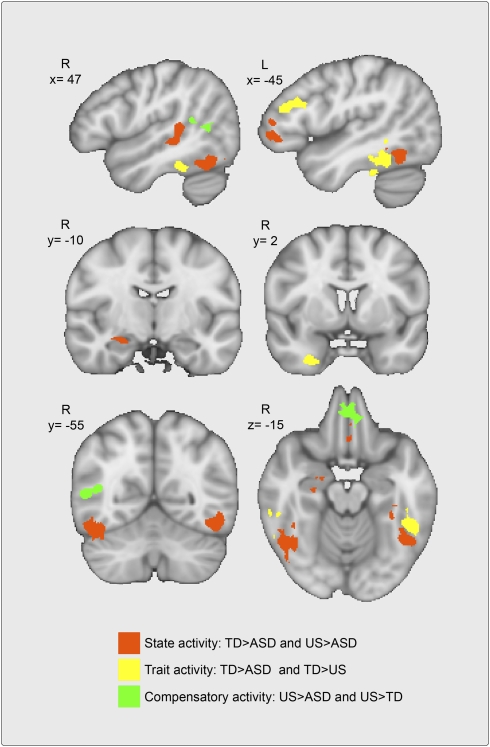
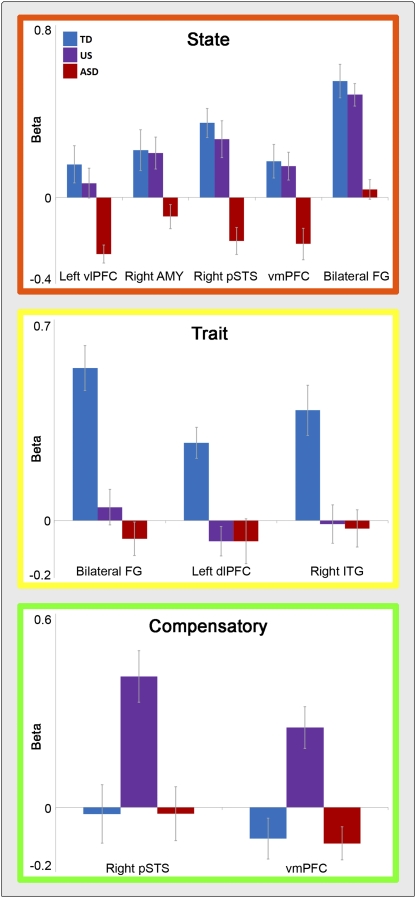
As illustrated in Figs. 1 and 2, conjunction analyses of the biological motion > scrambled motion contrast identified (i) state activity (TD > ASD ∩ US > ASD) localized to the right amygdala, ventromedial prefrontal cortex (vmPFC), left ventrolateral prefrontal cortex (vlPFC), right pSTS, and bilateral fusiform gyri (FG); (ii) trait activity (TD > ASD ∩ TD > US) localized to the left dorsolateral prefrontal cortex (dlPFC), right inferior temporal gyrus (ITG), and bilateral FG (anterior to and nonoverlapping with the portions of the FG identified as state activity); and (iii) compensatory activity (US > TD ∩ US > ASD) localized to the right hemisphere pSTS (caudal to and nonoverlapping with the portion of the right hemisphere pSTS identified as exhibiting state activity) and the vmPFC (anterior and inferior to the portion of vmPFC identified in our state activity analysis). Table 4 lists the weighted centers and the extent of activation for these regions. Region-of-interest analyses excluding the subset of five proband–sibling pairs revealed equivalent patterns of results to those involving the full sample.
Fig. 1.
Localization and response patterns of state, trait, and compensatory activity. Conjunction analyses of the biological motion > scrambled motion contrasts (P < 0.0025, k = 20) identified state activity (orange map) localized to the left ventrolateral prefrontal cortex, right amygdala (AMY), right posterior superior temporal sulcus, ventromedial prefrontal cortex, and bilateral fusiform gyri. Trait activity (yellow map) was localized to the bilateral fusiform gyrus, left dorsolateral prefrontal cortex, and right inferior temporal gyrus. Compensatory activity (green map) was localized to the right posterior superior temporal sulcus and ventromedial prefrontal cortex.
Fig. 2.
Response patterns of state, trait, and compensatory activity. Average beta values (y axis) from the biological > scrambled contrast as a function of region classification. (Top) Beta values from the regions of state-related activity (orange border). (Middle) Beta values from the trait-related activations (yellow border). (Bottom) Beta values from the compensatory activations (green border). Error bars represent the SEM.
Table 4.
Centers and extent of activation for state, trait, and compensatory activity
| Region | x | y | z | Extent* |
| State regions | ||||
| Left ventrolateral prefrontal cortex | −42 | 41 | 2 | 1,196 |
| Ventromedial prefrontal cortex | −4 | 33 | −11 | 533 |
| Right posterior temporal sulcus | 45 | −31 | 4 | 1,184 |
| Right amygdala | 24 | −11 | −13 | 315 |
| Right fusiform gyrus | 43 | −52 | −18 | 2,276 |
| Left fusiform gyrus | −42 | −49 | −12 | 1,943 |
| Trait regions | ||||
| Right inferior temporal gyrus | 27 | 2 | −31 | 648 |
| Left dorsolateral prefrontal gyrus | −43 | 24 | 25 | 860 |
| Right fusiform gyrus | 47 | −36 | −19 | 652 |
| Left fusiform gyrus | −47 | −42 | −15 | 1,519 |
| Compensatory regions | ||||
| Right posterior temporal sulcus | 47 | −52 | 11 | 703 |
| Ventromedial prefrontal cortex | −2 | 41 | −14 | 646 |
*The number of active 1-mm cubic voxels.
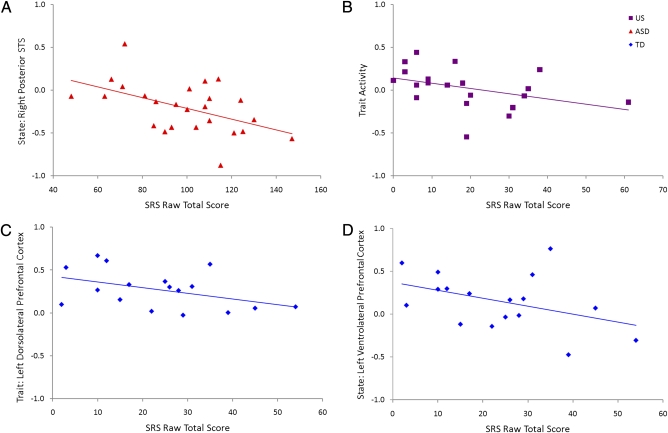
Correlation analyses revealed no significant associations between state activity and cognitive ability in the children with ASD, indicating that differences in cognitive ability likely do not account for the state activity findings. Correlation analyses between SRS scores (an index of the severity of social deficits associated with ASD) and activity from the state, trait, and compensatory regions revealed several significant relationships (Fig. 3). As illustrated in Fig. 3A, in the ASD group, we found a negative correlation between SRS score and activity in the right pSTS region indentified in our analysis of state activity (r = 0.502, P < 0.01). In the US group, we found a negative correlation between SRS score and trait activity (r = −0.403, P < 0.05) (Fig. 3B). As shown in Fig. 3 C and D, in the TD group, we found negative correlations between SRS score and activity in the trait marker region of the left dlPFC (r = −0.433, P < 0.05) and state activity in the left vlPFC (r = −0.420, P < 0.05).
Fig. 3.
Correlations of SRS and state, trait, and compensatory activity. (A) ASD: State-defined right pSTS activity correlates with SRS (r = 0.502, P < 0.01). (B) US: Overall trait activity correlates with SRS (r = −0.403, P < 0.05). (C) TD: State-defined left vlPFC activity correlates with SRS (r = −0.420, P < 0.05). (D) TD: State-defined left dlPFC activity correlates with SRS (r = −0.420, P < 0.05).
Discussion
This study makes key contributions to the understanding of ASD in the identification of state activity, trait activity, and compensatory activity.
State Activity.
The identification of state activity extends previous research implicating the right amygdala, right pSTS, bilateral FG, left vlPFC, and vmPFC in adults with ASD, by showing that dysfunction in these regions is already present in school-age children with ASD (25–29). This is an important advance in the field, given that previous reports of atypical neural response to biological motion included only adult subjects (16, 17). In addition, activity in the state-defined right pSTS was associated with the severity of social deficits in individuals with ASD. Individuals with higher SRS scores exhibited less activation to biological motion within the right pSTS. This finding suggests that activity in the pSTS might serve as a biological marker to subdivide the autism spectrum on the basis of severity. Furthermore, activity in the state-defined region of the left vlPFC was found to reflect the level of social responsiveness of the TD children, indicating a coupling of social behavior and brain mechanisms for social perception. The evidence of dysfunction in brain mechanisms for social perception in young children with ASD explains previous behavioral findings of disrupted biological motion perception (9). Given that social interaction relies on the accurate perception of other people's actions, state activity indicating regions of dysfunction associated with the manifestation of ASD provides a significant step toward more fully characterizing the biological underpinnings of this neurodevelopmental disorder.
Trait Activity.
In accordance with Gottesman and Gould's characterization of endophenotypes (6), trait activations, including those in the left dlPFC, right ITG, and bilateral FG, were shared between affected individuals (ASD group) and first-degree relatives (US group). These findings are particularly noteworthy because we explicitly ruled out the BAP in the US group, in contrast to previous studies (30, 31). This implies that our neuroimaging paradigm offers a remarkable level of sensitivity that transcends clinical evaluation. Although the US group was indistinguishable from the TD group at the behavioral level, the trait activity findings reveal similar neural signatures in the US and ASD groups. Concordant with this interpretation, social responsiveness was associated with overall trait activity in the US group and with trait-defined left dlPFC in the TD group. Furthermore, whereas the state regions could arise as an effect of having ASD, the trait activity cannot be explained in this way; rather, this trait activity likely reflects the genetic vulnerability to develop ASD (32). An alternative explanation—that trait activity merely reflects the experience of living with someone with ASD—is unlikely, because age did not correlate with trait activity, and because our results did not differ as a function of whether the US participant was older or younger than his or her sibling with ASD. Notably, these regions emerged during biological motion perception, emphasizing the link between atypical social perception and a predisposition to ASD.
The key implication of our trait activity findings is that we provide a functional neuroendophenotype that should help bridge the gene–behavior gap and thereby accelerate the search for pathophysiological mechanisms. A central goal in autism research is to map out a mechanistic understanding of the disorder from the gene level to the individual's behavior. The neuroendophenotype described herein represents altered functioning of neural circuits during social perception (a key aspect of dysfunction in ASD) in individuals at increased genetic risk for developing ASD. With this in hand, collaborative work can perhaps use this quantitative endophenotype to conduct genome-wide association studies to identify candidate genetic mechanisms and associated pathophysiological pathways.
Compensatory Activity.
Our US group exhibited unique areas of activation in the vmPFC and the right pSTS, regions previously implicated in aspects of social perception and social cognition (33, 34). These regions might reflect the absence of additional genetic or environmental factors that confer risk for ASD. Alternatively, they could represent a process through which brain function was altered over development to compensate for an increased genetic risk to develop ASD. We found that the activity in these regions did not vary with chronological age. Thus, it is possible that the compensatory regions reflect the outcome of a process occurring earlier in development, during a sensitive period for the development of brain mechanisms for social perception. This might be likely, given that autism is a developmental disorder that emerges during the first years of life, well before age 4 y (the youngest age studied in this sample). Nonetheless, we cannot yet draw firm conclusions regarding the compensatory activity. Indeed, longitudinal research in younger children is critical to better understand the origins of this compensatory activity, which likely has both genetic and environmental influences. Another possibility is that these regions represent protective genetic factors, in which case it would be useful to characterize genes that account for variability in activation levels within these regions. Future studies are needed to compare the activity in these regions in US participants with and without BAP, to determine the function and etiology of this brain response to biological motion. The implication of these findings is that these regions could represent important targets for treatments and provide a measure of the effectiveness of intervention, as well as a better understanding of the mechanisms through which successful treatments function.
Summary.
In this study, we have characterized neural signatures of the state of having ASD, the underlying trait of vulnerability to develop ASD, and regions of compensatory activity that distinguish US from children with those with ASD and TD. Measurement of activity in the pSTS region allows us to subdivide the autism spectrum by severity. Our results identifying trait activity provide a possible neuroendophenotype of ASD and hold promise for future genetic research. This fMRI study features the youngest groups of children with and without ASD studied to date, offering a substantial contribution to characterizing early developmental stages of disruptions in the neural systems associated with ASD. These disruptions in brain function may arise from various genetic and molecular etiologies and are further transformed across development by the experiences and activity of the individual in the world (33). Notably, the presence of state, trait, and compensatory activity, elicited by the viewing of socially relevant biological motion, emphasizes the importance of brain mechanisms in social perception as well as the dysfunction of these mechanisms in this neurodevelopmental disorder.
Materials and Methods
Participants.
A total of 62 children, age 4–17 y, successfully completed the fMRI session with movement parameters <3.5 mm over the scanning session. Three groups of children participated: (i) ASD, children with ASD (n = 25); (ii) US, unrelated siblings of children with ASD (n = 20); and (iii) TD, typically developing children without a sibling or other first- or second-degree relative with ASD (n = 17). To eliminate the potential for confounds arising from group differences in amount of head movement during the scan, an additional 35 children (5 TD, 7 US, and 22 ASD) were excluded due to head motion >3.5 mm. All participants had normal or corrected-to-normal (via MRI-compatible glasses) vision.
Experimental Design.
Children were scanned while viewing coherent and scrambled point-light displays of biological motion created from motion-capture data. The coherent biological motion displays featured an adult male actor performing movements relevant to early childhood experiences, such as playing pat-a-cake (9). The scrambled motion animations were created by randomly selecting 16 points from the biological motion displays and plotting their trajectories on a black background. Thus, the coherent and scrambled displays contained the same local motion information, but only the coherent displays contained the configuration of a person (35).
During the MRI scan, stimuli were presented using E-Prime 2.0 software (Psychological Software Tools). Six biological motion clips and six scrambled motion clips were presented once each in an alternating-block design (time per block, 24 s). The experiment began and ended with a 20-s fixation period (total time, 328 s). The movies were presented without audio. The child was asked to watch the videos and was reminded to remain still and alert.
MRI Data Acquisition.
Scanning was performed on a Siemens MAGNETOM Trio, A Tim System 3T scanner at the Yale Magnetic Resonance Research Center, Yale School of Medicine. T1-weighted anatomical images were acquired using an MPRAGE sequence (repetition time, 2,530 ms; echo time, 3.34 ms; field of view, 25.6 cm; image matrix, 642; 1 × 1 × 1 mm). For each run, 164 whole-brain functional images were acquired using a single-shot, gradient-recalled echo planar pulse sequence (repetition time, 2,000 ms; echo time, 25 ms; flip angle, 60°; field of view, 22 cm; image matrix, 642; voxel size, 3.2 × 3.2 × 3.2 mm; 34 slices) sensitive to blood oxygenation level–dependent contrast.
Data Analyses.
Data were analyzed with Brain Voyager QX version 2.0.8.1480 (Brain Innovation). All 10 volumes before the onset of the first stimulus event were discarded, to allow for T1 equilibrium. Preprocessing of the functional data included interleaved slice time correction using cubic spline interpolation, 3D motion correction using trilinear/sinc interpolation, linear trend removal, and temporal high-pass filtering to remove low-frequency nonlinear drifts of three or fewer cycles per time course (2.8 s). On examination of estimated motion plots and cine loops, participants with >3.5 mm of deviation or rotation from the estimated center of mass in any direction were excluded. Functional data sets were coregistered to the Talairach-transformed (36), within-session, T1-weighted anatomical images. Eight nuisance variables were removed via linear regression, including six motion parameters and two time course signals drawn from 9-mm3 regions of interest in white matter (Talairach coordinate −26, −13, 31) and the left ventricle (Talairach coordinate −19, −35, 15), both of which were verified in each participant's normalized anatomical image.
A multiparticipant statistical analysis was run in which a model predictor was defined by convolving an ideal boxcar response with a double gamma function model of the hemodynamic response (37) across each 24-s trial. Whole-brain random-effects direct group comparisons of the biological motion > scrambled motion contrast were performed in the following four comparisons: TD > ASD, TD > US, US > ASD, and US > TD. Areas of activation were identified at a voxel-wise uncorrected level of P < 0.05. A cluster threshold of k > 20 continguous voxels was used to correct for multiple comparisons (38, 39). This cluster threshold was calculated for each group comparison with a corrected threshold of α < 0.05 using a Brain Voyager QX cluster-level statistical threshold estimator plug-in. After 5,000 iterations of a Monte Carlo simulation, an α value was assigned to each cluster size based on its relative frequency. Restricting the number of active voxels to 20 contiguous voxels within each group comparison resulted in a <5% chance of discovering false-positive voxels in the key analyses. Three separate conjunction analyses were performed to identify regions of state (TD > ASD ∩ US > ASD), trait (TD > ASD ∩ TD > US), and compensatory (US > TD ∩ US > ASD) activity. These regions were identified at a voxel-wise uncorrected level of P < 0.0025 and a cluster threshold of k > 20. Correlation analyses between SRS scores and state, trait, and compensatory activity were conducted at the one-tailed level given our a priori hypotheses regarding the relationship of social function and brain mechanisms for biological motion perception.
Acknowledgments
We thank the children and families who made this research possible. We thank the Yale Magnetic Research Resonance Imaging Center and Yale Child Study Center Autism Program for their support. This work was funded by grants from The Simons Foundation, The John Merck Scholars Fund, Autism Speaks, and the National Institute of Mental Health (to K.A.P.). S.S. was supported by the Natural Sciences and Engineering Research Council of Canada.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- 2.Wing L, Gould J. Severe impairments of social interaction and associated abnormalities in children: Epidemiology and classification. J Autism Dev Disord. 1979;9:11–29. doi: 10.1007/BF01531288. [DOI] [PubMed] [Google Scholar]
- 3.Pelphrey K, Adolphs R, Morris JP. Neuroanatomical substrates of social cognition dysfunction in autism. Ment Retard Dev Disabil Res Rev. 2004;10:259–271. doi: 10.1002/mrdd.20040. [DOI] [PubMed] [Google Scholar]
- 4.Gottesman II, Shields J. Genetic theorizing and schizophrenia. Br J Psychiatry. 1973;122:15–30. doi: 10.1192/bjp.122.1.15. [DOI] [PubMed] [Google Scholar]
- 5.De Geus EJC, Boomsma DI. A genetic neuroscience approach to human cognition. Eur Psychol. 2001;6:241–253. [Google Scholar]
- 6.Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 7.Blake R, Shiffrar M. Perception of human motion. Annu Rev Psychol. 2007;58:47–73. doi: 10.1146/annurev.psych.57.102904.190152. [DOI] [PubMed] [Google Scholar]
- 8.Johansson G. Visual perception of biological motion and a model for its analysis. Percept Psychophys. 1973;14:201–211. [Google Scholar]
- 9.Klin A, Lin DJ, Gorrindo P, Ramsay G, Jones W. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459:257–261. doi: 10.1038/nature07868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vallortigara G, Regolin L, Marconato F. Visually inexperienced chicks exhibit spontaneous preference for biological motion patterns. PLoS Biol. 2005;3:e208. doi: 10.1371/journal.pbio.0030208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simion F, Regolin L, Bulf H. A predisposition for biological motion in the newborn baby. Proc Natl Acad Sci USA. 2008;105:809–813. doi: 10.1073/pnas.0707021105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson MH. Biological motion: A perceptual life detector? Curr Biol. 2006;16:R376–R377. doi: 10.1016/j.cub.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Dittrich WH, Troscianko T, Lea SEG, Morgan D. Perception of emotion from dynamic point-light displays represented in dance. Perception. 1996;25:727–738. doi: 10.1068/p250727. [DOI] [PubMed] [Google Scholar]
- 14.Frith CD, Frith U. Interacting minds—a biological basis. Science. 1999;286:1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- 15.Kaiser MD, Shiffrar M. The visual perception of motion by observers with autism spectrum disorders: A review and synthesis. Psychon Bull Rev. 2009;16:761–777. doi: 10.3758/PBR.16.5.761. [DOI] [PubMed] [Google Scholar]
- 16.Freitag CM, et al. Perception of biological motion in autism spectrum disorders. Neuropsychologia. 2008;46:1480–1494. doi: 10.1016/j.neuropsychologia.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 17.Herrington JD, et al. The role of MT+/V5 during biological motion perception in Asperger syndrome: An fMRI study. Res Autism Spectr Disord. 2007;1:14–27. [Google Scholar]
- 18.Elliott CD. Differential Ability Scales (DAS) San Antonio, TX: Psychological Corp; 1990. [Google Scholar]
- 19.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corp; 1999. [Google Scholar]
- 20.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview–Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 21.Lord C, et al. The Autism Diagnostic Observation Scale–Generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;307:205–223. [PubMed] [Google Scholar]
- 22.Constantino JN, Todd RD. Autistic traits in the general population: A twin study. Arch Gen Psychiatry. 2003;60:524–530. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- 23.Sparrow SS, Cicchetti DV. Diagnostic uses of the Vineland Adaptive Behavior Scales. J Pediatr Psychol. 1985;10:215–225. doi: 10.1093/jpepsy/10.2.215. [DOI] [PubMed] [Google Scholar]
- 24.Hurley RSE, Losh M, Parlier M, Reznick JS, Piven J. The broad autism phenotype questionnaire. J Autism Dev Disord. 2007;37:1679–1690. doi: 10.1007/s10803-006-0299-3. [DOI] [PubMed] [Google Scholar]
- 25.Castelli F, Frith C, Happé F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- 26.Gilbert SJ, Meuwese JD, Towgood KJ, Frith CD, Burgess PW. Abnormal functional specialization within medial prefrontal cortex in high-functioning autism: A multi-voxel similarity analysis. Brain. 2009;132:869–878. doi: 10.1093/brain/awn365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pelphrey KA, Morris JP, McCarthy G, Labar KS. Perception of dynamic changes in facial affect and identity in autism. Soc Cogn Affect Neurosci. 2007;2:140–149. doi: 10.1093/scan/nsm010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinkham AE, Hopfinger JB, Pelphrey KA, Piven J, Penn DL. Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophr Res. 2008;99:164–175. doi: 10.1016/j.schres.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schultz RT, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Arch Gen Psychiatry. 2000;57:331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- 30.Dalton KM, Nacewicz BM, Alexander AL, Davidson RJ. Gaze-fixation, brain activation, and amygdala volume in unaffected siblings of individuals with autism. Biol Psychiatry. 2007;61:512–520. doi: 10.1016/j.biopsych.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 31.Belmonte MK, Gomot M, Baron-Cohen S. Visual attention in autism families: “Unaffected” sibs share atypical frontal activation. J Child Psychol Psychiatry. 2010;51:259–276. doi: 10.1111/j.1469-7610.2009.02153.x. [DOI] [PubMed] [Google Scholar]
- 32.Rutter M. Aetiology of autism: Findings and questions. J Intellect Disabil Res. 2005;49:231–238. doi: 10.1111/j.1365-2788.2005.00676.x. [DOI] [PubMed] [Google Scholar]
- 33.Adolphs R. Social cognition and the human brain. Trends Cogn Sci. 1999;3:469–479. doi: 10.1016/s1364-6613(99)01399-6. [DOI] [PubMed] [Google Scholar]
- 34.Puce A, Perrett D. Electrophysiology and brain imaging of biological motion. Philos Trans R Soc Lond B Biol Sci. 2003;358:435–445. doi: 10.1098/rstb.2002.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bertenthal B, Pinto J. Global processing of biological motions. Psychol Sci. 1994;5:221–224. [Google Scholar]
- 36.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System. An Approach to Cerebral Imaging. New York: Thieme Medical; 1988. [Google Scholar]
- 37.Friston KJ, et al. Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- 38.Forman SD, et al. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 39.Xiong J, Gao JH, Lancaster JL, Fox PT. Cluster pixels analysis for functional MRI activation studies of the human brain. Hum Brain Mapp. 1995;3:287–301. [Google Scholar]
- 40.Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. J Autism Dev Disord. 2009;39:693–705. doi: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]