Abstract
Females should be choosier than males about prospective mates because of the high costs of inappropriate mating decisions. Both theoretical and empirical studies have identified factors likely to influence female mate choices. However, male–male social interactions also can affect mating decisions, because information about a potential mate can trigger changes in female reproductive physiology. We asked how social information about a preferred male influenced neural activity in females, using immediate early gene (IEG) expression as a proxy for brain activity. A gravid female cichlid fish (Astatotilapia burtoni) chose between two socially equivalent males and then saw fights between these two males in which her preferred male either won or lost. We measured IEG expression levels in several brain nuclei including those in the vertebrate social behavior network (SBN), a collection of brain nuclei known to be important in social behavior. When the female saw her preferred male win a fight, SBN nuclei associated with reproduction were activated, but when she saw her preferred male lose a fight, the lateral septum, a nucleus associated with anxiety, was activated instead. Thus social information alone, independent of actual social interactions, activates specific brain regions that differ significantly depending on what the female sees. In female brains, reproductive centers are activated when she chooses a winner, and anxiety-like response centers are activated when she chooses a loser. These experiments assessing the role of mate-choice information on the brain using a paradigm of successive presentations of mate information suggest ways to understand the consequences of social information on animals using IEG expression.
Keywords: cellular homologue of fos, early growth response factor 1, preoptic area, egr-1
Females should be choosier about prospective mates than males, because bad mating decisions result in high costs (1). Consequently, studies on female assessment of male characteristics have produced conceptual, theoretical, and empirical information identifying key factors mediating female mate choice (for reviews, see refs. 2 and 3). Perhaps unsurprisingly, there is evidence that females also may use information about male–male social interactions in their mate-choice decisions (4–9), but little is known about how the brain responds to this kind of information.
In many species, social information about potential mates can change female reproductive physiology and gene expression. For example, in zebra finch females (Taeniopygia guttata), expression of immediate early genes (IEGs) in telencephalic auditory areas increase in response to hearing a preferred male's song (10). Similarly, in female European starlings (Sturnus vulgaris), recent social interactions with males influence forebrain gene expression in response to mate-choice cues (11). This has also been shown in female swordtail fish (12). We predicted that social information also could affect nuclei in the social behavior network (SBN) initially identified by Newman (13). These nuclei are reciprocally connected, are implicated in multiple forms of social behavior, and include the extended medial amygdala (Dm), the lateral septum (LS), the preoptic area (POA), the anterior hypothalamus (AH), the ventromedial hypothalamus, and the periaqueductal gray (PAG). Newman (13) proposed the SBN as an integrated neural network implicated in male mating behavior, female sexual behavior, parental behavior, and various forms of aggression. She hypothesized that an animal's social response is comprised of a repertoire of closely interrelated, hormonally regulated behaviors shaped by development and experience but acutely is modulated by proximate signals in the social environment. The SBN hypothesis has been extended to birds (14) and fish (15). In vocalizing fish, patterns of neural activation during vocal communication show remarkable resemblance to patterns of activation in the brain nuclei identified by Newman (12). Brain nuclei in the vertebrate SBN are known to be involved in responses to changes in social conditions, but it is unknown whether they also might respond to important social information.
We used a female mate-choice paradigm in an African cichlid fish, Astatotilapia burtoni, to investigate which brain regions might respond to visual information about chosen mates. A. burtoni is an ideal model system, because prior studies showed that social information provided to males rapidly influences cellular and molecular physiology (e.g., 16, 17). A. burtoni live in a lek-like mating system in Lake Tanganyika where males perform a mating display; females choose among available males and then provide sole care to the offspring (18, 19). Gravid (i.e., reproductively “ready”) females prefer to associate with dominant, reproductively active males, whereas nongravid females prefer to associate with nondominant, nonreproductive males, suggesting a hormonal influence on female choice (20). How does information acquired during female mate choice affect the female brain?
Experimentally, we compared IEG expression in the brains of gravid females who chose a mate and then saw a fight between their male choices. Their preferred males either won or lost the fight, and we reasoned that this comparison might produce salient differences in brain gene expression. To measure differential brain responses, we quantified differences in IEG expression levels in context-relevant processing regions of the brain as a proxy for neural activation (21). From previous behavioral tests (16, 17), we knew that IEG measurements could reveal differences between conditions, because specific IEGs are known to play many roles, including activation of signal transduction cascades through which neurons convert extracellular chemical or electrical signals into genomic activity (22). Two of the most widely expressed IEGs are cellular homologue of fos (c-fos) and early growth response factor 1 (egr-1, also known as zenk, zif268, NGFI-A, and krox24) (21): c-fos reflects immediate neural activity, whereas egr-1, a transcription factor, is hypothesized to indicate up-regulation of later-acting genes, such as gonadotropin-releasing hormone (GnRH1), a primary signal essential for reproduction (23).
To identify brain nuclei specifically responsive to this information about preferred males, we measured expression of egr-1 and c-fos (21, 24). We compared mRNA expression levels for these IEGs in the fish homologs of the six nuclei comprising the SBN: the Dm, LS, POA, ventromedial hypothalamus (Vm), anterior hypothalamus, and periaqueductal gray (PAG), as well as the dorsolateral telencephalon (Dl), the cerebellum (Cce) and the raphe nucleus (R).
A gravid female A. burtoni viewed two males, matched for size and dominance, in a forced-choice paradigm (Fig. 1 Upper) and established a preference for one male over the other that was quantified using previously established procedures (20). She then saw her chosen male either win or lose a fight (Fig. 1 Lower). Both experiments, mate preference and staged male fights, provided only visual information. Brain tissue was collected, and mRNA levels of egr-1 and c-fos were measured using real-time PCR. We hypothesized that females who saw their preferred males win a fight would show increases in IEG expression in nuclei of the brain associated with reproduction (e.g., the POA, Vm, and AH) and that females who saw their preferred males lose a fight would show decreases or no change in IEG expression levels in these same nuclei.
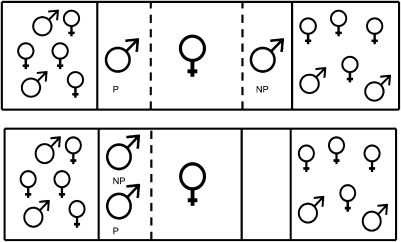
Fig. 1.
Aquaria used for both the female choice part of the experiment (Upper) and for exposing females to their preferred male (P) either winning or losing a fight to their nonpreferred male (NP) (Lower). Dashed lines within the figures represent clear barriers. Solid lines represent both clear and opaque barriers. In these tanks, clear and opaque barriers were used to expose or occlude parts of the tank.
Results
To our surprise, females seeing their chosen males win or lose a fight had dramatically different brain IEG expression patterns. First, there were significantly different levels of IEG expression in different brain areas for both egr-1 and c-fos (overall models repeated-measures ANOVA: egr-1: F1,10 = 8.148, P = 0.02; c-fos: F1,10 = 2.792, P = 0.012) (Fig. 2 A and B). Second, there were significant overall differences between females who had seen their preferred males win a fight and females who had seen their preferred males lose a fight across all brain nuclei (egr-1: F5,6 = 27.001, P = 0.005; c-fos: F5,6 = 7.075, P = 0.017). Importantly, this difference depended critically on the brain nuclei examined (brain nucleus*treatment interaction: egr-1: F5,6 = 24.312, P = 0.0004; c-fos: F5,6 = 27.001, P = 0.005).
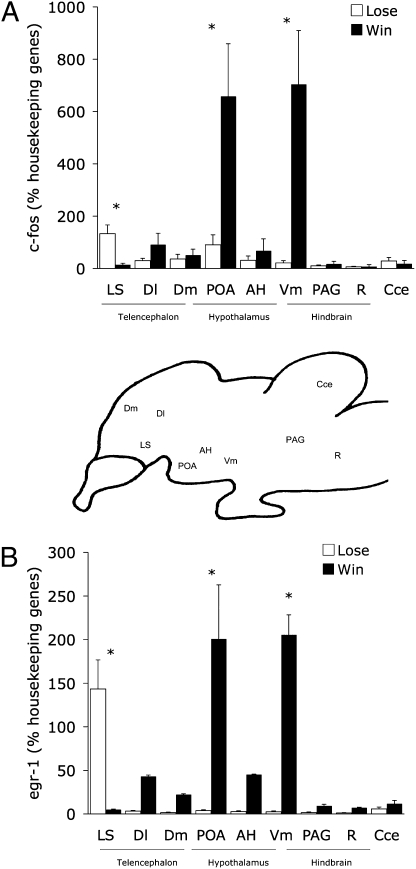
Fig. 2.
Mean (+SE) of relative gene expression of (A) c-fos and (B) egr-1 for each of the six nodes of the social behavior network, plotted as a function of whether females saw their preferred males win (filled bars) or lose (open bars) a fight. Asterisks above pairs of mean values indicate significant differences (t tests, corrected for multiple comparisons). Between panels A and B is a schematic sagittal section of the A. burtoni brain showing the approximate locations of the brain regions sampled. Cce, cerebellum; R, Raphe nucleus. As described in the text, all these brain regions are interconnected, sensitive to steroid hormones, and show high densities of androgen receptors (12, 13).
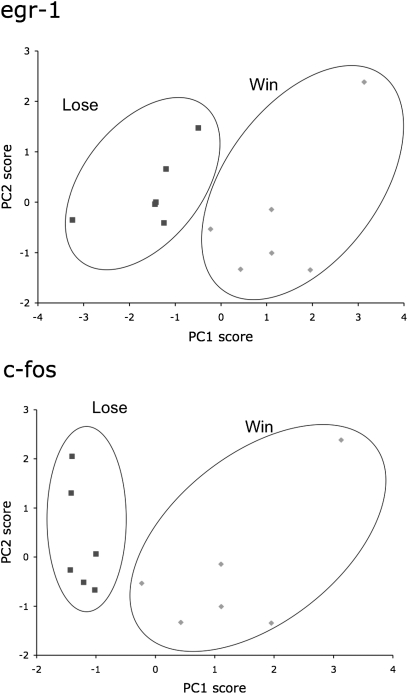
To identify possible functional connectivity among nuclei in the SBN, we assessed the number of uncorrelated variables to understand the internal structure of the data using principle components analysis (PCA). Using the correlations between IEG levels in each nucleus from the entire data set, we found two principal components representing nuclei that covaried. Indeed, IEG expression in individual nuclei of the SBN was highly correlated, as evidenced by the fact that the two principle components accounted for most of the total variance in both egr-1 (77.67%) and c-fos (65.32%). Importantly, the PCA plots (Fig. 3 Upper and Lower) showed that functional associations differed significantly between females who saw their preferred males win a fight and those who saw their preferred males lose a fight. To discover whether overall brain gene expression patterns differed between our two groups, we use discriminant function analysis to reveal dependence of variables and found that overall brain gene expression patterns differ significantly between females viewing their chosen males winning versus losing.
Fig. 3.
Average principal component 1 (PC1) scores of each female as a function of principle component 2 (PC2) scores for egr-1 (Upper) and c-fos (Lower). Females who saw their preferred males win a fight formed a significant cluster distinct from females who saw their preferred males lose a fight. This clustering is based exclusively on PC1 loadings. PC1 loadings suggest a continuum of brain activation from anxiety-like (large positive loading from the LS) to reproductive (large positive loadings from the POA, Vm, and AH), whereas PC2 could suggest a stress axis with large positive loadings from the LS, Dm, and PAG.
Females who saw their preferred males win a fight had higher IEG expression in the POA (egr-1: F1,11 = 11.992, P = 0.005; c-fos: F1,1 = 11.992, P = 0.005) and in the Vm (egr-1: F1,11 = 55.075, P = 0.0001; c-fos: F1,11 = 12.719, P = 0.0034) than females who saw their preferred males lose a fight. Both the POA and Vm are nuclei involved in reproduction and reproductive behaviors. The opposite was true for expression in the LS, where females who saw their preferred males lose a fight had much higher expression of egr-1 and c-fos than females who saw their preferred males win (egr-1: F1,11 = 12.557, P = 0.005; c-fos: F1,11 = 8.931, P = 0.013). In all other brain areas sampled, there were no detectable differences in egr-1 or c-fos between females who had seen their preferred males win or lose a fight (AH: egr-1: F1,11 = 0.656, P = 0.43; c-fos: F1,11 = 0.468, P = 0.506; Dm: egr-1: F1,11 = 1.074, P = 0.32; c-fos: F1,11 = 0.386, P = 0.55; PAG: egr-1: F1,11 = 1.155, P = 0.302; c-fos: F1,11 = 0.913, P = 0.36).
Discussion
Females seeing their chosen males win had dramatically different IEG expression patterns reflecting differential brain activation in key nuclei of the SBN. This result suggests dramatic changes in functional interactions of the SBN solely in response to different visual information about male–male social encounters. Such large differences in SBN functional activity likely reflect changes in the firing rate of presynaptic cells onto target neurons, based on an emerging view of IEG expression (25). Because IEG expression is linked to the activity of excitatory postsynaptic receptors by second messenger cascades (21, 26), IEGs are thought to link membrane depolarization to expression of late-response target genes that directly influence neuronal function (24). This study measuring how mate-choice information alone can influence brain activity extends the SBN concept to include a role for assessing social information and not just influencing motor output in response to social encounters.
Females who saw their preferred males win a fight had high IEG mRNA expression in the POA and the Vm; both brain nuclei are known to be centrally important for reproduction in all vertebrates. The POA contains GnRH1 neurons (27) that regulate the release of luteinizing hormone and follicle-stimulating hormone from the pituitary into the bloodstream, stimulating production of gonadal steroid hormones and gonad growth. Numerous other dopamine -containing nuclei in the POA also are important for sexual behavior, including arginine vasotocin-containing neurons that regulate aspects of social behavior in birds and fish (14, 28, 29). The Vm is implicated in the regulation of sexual behavior in female rats, specifically in the copulatory posture or lordosis reflex (30, 31). Thus, it is likely that this increase in c-fos and egr-1 expression in the POA and Vm in females who have seen their preferred males win is a first step in preparing females to spawn and also could reinforce mating decisions through the reward pathway.
In striking contrast, the LS showed the highest IEG expression when females saw their preferred males lose a fight. The LS, one of the nuclei included in the SBN, is connected with a number of limbic, diencephalic, and midbrain regions and plays a role in regulating the processes related to mood and motivation in vertebrates (32). It also has been implicated in the modulation of anxiety-like behavior via the histaminergic system (33). Females who have just seen their preferred males lose a fight could experience anxiety because they are ripe with eggs and ready to mate, so the LS may be active in regulating this anxiety. The LS also is involved in the reward pathway, as has been shown by microinjections of GABAA receptor agonists directly into the LS that produced an antianxiety-like effect (34), whereas injections of morphine had the opposite effect (35).
Importantly, the salient difference in female brain activation between females seeing a prospective mate win or lose is caused entirely by receiving different visual information. Thus animals modify gene expression patterns in response to what they see and evidently anticipate specific outcomes based on this social information. Such a genetic “early warning system” may allow a more rapid response to the unfolding social world because key social information is transduced appropriately in important brain areas based on perception alone.
The IEGs measured here are the earliest genomic response to a stimulus and require no prior activation by any other gene (21). This response, however, is the tip of the genetic activation iceberg, because the total number of genes that comprise one neuron's inducible genomic response has been estimated from tens to hundreds (36), and the collection of rapidly inducible genes in any particular cell in the brain may be still larger (37); thus the IEG expression measured here is likely a small fraction of total activity. Nonetheless, this glimpse of the genetic response to social information shows not only that females use the information received from watching males interact but also that such information has dramatic effects on their brains in key nuclei rather than causing general arousal. Because social information is necessary for a correct response in social encounters, our data could provide valuable insights to scientists interested in how the brain processes social information.
Methods
All subjects were descendents of wild Astatotilapia burtoni in Lake Tanganyika, Africa (18). Fish were kept in aquaria under seminatural conditions before the experiment (28 °C, pH 8, 12:12 h light:dark cycle) and were fed each morning ad libitum with cichlid pellets and flakes (AquaDine). Procedures for catching and killing the fish were in accordance with Stanford's Administrative Panel for Laboratory Animal Care.
One large aquarium was subdivided into five compartments (Fig. 1). A gravid female was placed in the center compartment to serve as the focal, or test, fish. Females were deemed gravid when their abdomens appeared distended before morning feeding. In addition to visual inspection, gonad size was measured after the experiment to verify reproductive status. Females that were not gravid (gonadal somatic index < 0.1) were excluded from the experiment. One male was housed in a side compartment adjacent to the female, and a size-matched male was housed in a compartment on the other side of the female. The outermost compartments of the tank housed small communities of fish—several males and several females—and a shelter (half of a terra cotta pot). Community tanks were established adjacent to the stimulus males to maintain social activity between observations. Males of this species are highly social, and individually housed males tend to lose their bright coloration and behave differently from males housed in or adjacent to a community. In the hours between behavioral observations, opaque barriers were in place between the female compartment and each of the male compartments so that the focal female interacted with stimulus males only during the testing phase. Immediately before the trial, the opaque barriers between the focal female and the two stimulus males were removed and were placed between the males and their adjacent communities to ensure that male attention would be directed toward the female under investigation and not toward the communities of other fish. The female's behavior was observed for 20 min on 2 consecutive d, and time spent within 4 in of each male was recorded. In this experiment, affiliation time was used as a proxy for mate choice. Immediately following the second 20-min preference test, the test female was exposed to social information about her preferred male. Specifically, she was allowed to observe her preferred male either losing or winning a fight with her nonpreferred male (Fig. 1 Lower). Given that resident males tend to be dominant over intruder males (38–41), we were able to predict the type of social information the female would receive. Fifteen trials were performed. In seven trials females saw their preferred males as the residents and the winners, and in eight trials females saw their preferred males as the intruders and the losers. After observing males fighting for 20 min, females were killed, and whole brains were removed and frozen for later analysis of gene expression. These trials were conducted at similar times of the day to control for any diurnal variations in behavior, hormones, and brain gene expression.
To measure c-fos and egr-1 expression, we used procedures developed previously in our laboratory (23). After females were killed by cervical transection, whole brains were removed, immediately frozen, and mounted in −20 °C optimal cutting temperature medium. Brains were stored at –80 °C until microdissected and analyzed for IEG expression. Brains were coronally sectioned at 300 μm using a cryostat, and sections then were mounted on slides and kept frozen at –80 °C to allow later microdissection of specific areas. A frozen stage (BFS-30MP; Physitemp) mounted to a dissection scope was used during microdissection, which was performed with a modified 27-G needle with an internal diameter of 190 μm. An established protocol was followed to microdissect specific brain regions from the 300-μm thick slices (17, 42–44). Brain atlases from A. burtoni (45, 46) and other fishes (47–49) were used to target the nuclei of the SBN: PAG, AH, Vm, Dm, POA, and LS.
Quantitative RT-PCR (qRT-PCR) was used to quantify mRNA expression in each region of the brain of each female separately. The qRT-PCR protocol used had been implemented successfully before and is described in Burmeister et al. (23). Primers for egr-1, c-fos, and actin and 18s rRNA (housekeeping genes) were designed according to published sequences (c-fos: GenBank accession #HQ232413) (16, 23, 50). The qRT-PCR was performed using 30-μL duplicate reactions with 1X IQ SYBR Greener Supermix (Invitrogen), 0.5 μL of each primer, and 0.5 μL of template cDNA (RNA equivalent). The reactions were run on the iQ5 real-time PCR system (Bio-Rad).
Data Analysis.
Behavioral observations of female subjects were used to determine which male each female preferred during her preference trials. Specifically, the male with whom the female affiliated for more than 50% of THE time on both mate-preference trial days was determined to be her preferred mate. Behavioral observations of male subjects were used to determine which male won the fight in the second part of the experiment. Winners were characterized by higher aggressive behaviors, decreased fleeing (submissive) behaviors, and more time spent defending the substrate and on the bottom half of the tank relative to losers. Losers were characterized by decreased aggressive behaviors, increased fleeing (submissive) behaviors, and more time spent in the top half of the tank away from the substrate.
Original fluorescence readings were analyzed using a curve-fitting real-time PCR algorithm (49). Computed cDNA concentrations of the two housekeeping genes (18s and Actin) were not significantly different from each other and also did not differ between the groups we were comparing, so we used the geometric mean of these genes as a normalized standard for each tissue sample. The relative mRNA levels of the target genes (c-fos and egr-1) were calculated as the percentage of the geometric mean of the housekeeping genes.
Acknowledgments
We thank R. Carpenter and K. Maruska for comments on this manuscript and K. Eaton, H. Cooper, and J. Nam for observations and technical help. J.K.D. was supported by a Natural Sciences and Engineering Research Council of Canada Postdoctoral Fellowship. R.D.F. and J.K.D. were supported by National Science Foundation Grant IOS 0923588.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Trivers RL. Parental investment and sexual selection. In: Campbell B, editor. Sexual Selection and the Descent of Man. Chicago: Aldine; 1972. [Google Scholar]
- 2.Ryan MJ. Female mate choice in a neotropical frog. Science. 1980;209:523–525. doi: 10.1126/science.209.4455.523. [DOI] [PubMed] [Google Scholar]
- 3.Andersson M, Simmons LW. Sexual selection and mate choice. Trends Ecol Evol. 2006;21:296–302. doi: 10.1016/j.tree.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Doutrelant C, McGregor PK. Eavesdropping and mate choice in female fighting fish. Behav. 2000;137:1655–1669. [Google Scholar]
- 5.Otter K, et al. Do female great tits (Parus major) assess males by eavesdroppiong? A field study using interactive song playback. Proc Biol Sci. 1999;266:1305–1309. [Google Scholar]
- 6.Mennill DJ, Ratcliffe LM, Boag PT. Female eavesdropping on male song contests in songbirds. Science. 2002;296:873. doi: 10.1126/science.296.5569.873. [DOI] [PubMed] [Google Scholar]
- 7.Earley RL, Dugatkin LA. Three poeciliid pillars: Fighting, mating and networking. In: McGregor PK, editor. Animal Communication Networks. Cambridge, UK: Cambridge Univ Press; 2005. pp. 84–113. [Google Scholar]
- 8.Oliveira RF. Hormones, social context and animal communication. In: McGregor PK, editor. Animal Communication Networks. Cambridge Univ Press; 2005. pp. 481–520. [Google Scholar]
- 9.Smulders TV, et al. Failure to detect seasonal changes in the song system nuclei of the black-capped chickadee (Poecile atricapillus) J Neurobiol. 2006;66:991–1001. doi: 10.1002/neu.20281. [DOI] [PubMed] [Google Scholar]
- 10.Woolley SC, Doupe AJ. Social context-induced song variation affects female behavior and gene expression. PLoS Biol. 2008;6:e62–e67. doi: 10.1371/journal.pbio.0060062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sockman KW, Gentner TQ, Ball GF. Recent experience modulates forebrain gene-expression in response to mate-choice cues in European starlings. Proc Biol Sci. 2002;269:2479–2485. doi: 10.1098/rspb.2002.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cummings ME, Larkins-Ford J, Reilly CRL, Wong RY, Ramsey M, Hofmann HA. Sexual and social stimuli elicit rapid and contrasting genomic responses. Proc Roy Soc London B Biol Sci. 2008;275:393–402. doi: 10.1098/rspb.2007.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- 14.Goodson JL. The vertebrate social behavior network: Evolutionary themes and variations. Horm Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodson JL, Bass AH. Vocal-acoustic circuitry and descending vocal pathways in teleost fish: Convergence with terrestrial vertebrates reveals conserved traits. J Comp Neurol. 2002;448:298–322. doi: 10.1002/cne.10258. [DOI] [PubMed] [Google Scholar]
- 16.Burmeister SS, Jarvis ED, Fernald RD. Rapid behavioral and genomic responses to social opportunity. PLoS Biol. 2005;3:e363. doi: 10.1371/journal.pbio.0030363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desjardins JK, Fernald RD. What do fish make of mirror images? Biol Lett. 2010 doi: 10.1098/rsbl.2010.0247. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernald RD, Hirata NR. Field study of Haplochromis burtoni: Quantitative behavioural observations. Anim Behav. 1977;25:964–975. [Google Scholar]
- 19.Fernald RD. Quantitative behavioural observations of Haplochromis burtoni under semi-natural conditions. Anim Behav. 1977;25:643–653. [Google Scholar]
- 20.Clement TS, Grens KE, Fernald RD. Female association preference depends on reproductive state in the African cichlid fish, Astatotilapia burtoni. Behav Ecol. 2005;16:83–88. [Google Scholar]
- 21.Clayton DF. The genomic action potential. Neurobiol Learn Mem. 2000;74:185–216. doi: 10.1006/nlme.2000.3967. [DOI] [PubMed] [Google Scholar]
- 22.Morgan JI, Curran T. The immediate early gene response and neuronal death and regeneration. Neurosci. 1995;1:68–75. [Google Scholar]
- 23.Burmeister SS, Fernald RD. Evolutionary conservation of the egr-1 immediate-early gene response in a teleost. J Comp Neurol. 2005;481:220–232. doi: 10.1002/cne.20380. [DOI] [PubMed] [Google Scholar]
- 24.Jarvis ED. Learned birdsong and the neurobiology of human language. Ann N Y Acad Sci. 2004;1016:749–777. doi: 10.1196/annals.1298.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Velho TAF, Pinaud R, Rodrigues PV, Mello CV. Co-induction of activity-dependent genes in songbirds. Eur J Neurosci. 2005;22:1667–1678. doi: 10.1111/j.1460-9568.2005.04369.x. [DOI] [PubMed] [Google Scholar]
- 26.Amano M, Urano A, Aida K. Distribution and function of gonadotropin-releasing hormone (GnRH) in the teleost brain. Zoolog Sci. 1997;14:1–11. doi: 10.2108/zsj.14.1. [DOI] [PubMed] [Google Scholar]
- 27.Foran CM, Bass AH. Preoptic GnRH and AVT: Axes for sexual plasticity in teleost fish. Gen Comp Endocrinol. 1999;116:141–152. doi: 10.1006/gcen.1999.7357. [DOI] [PubMed] [Google Scholar]
- 28.Greenwood A, Wark AR, Fernald RD, Hofmann HA. Expression of arginine vasotocin in distinct preoptic regions is associated with dominant and subordinate behaviour in an African cichlid fish. Proc Roy Soc London B Biol Sci. 2008;275:2393–2402. doi: 10.1098/rspb.2008.0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathews D, Edwards DA. Involvement of the ventromedial and anterior hypothalamic nuclei in the hormonal induction of receptivity in the female rat. Physiol Behav. 1977;19:319–326. doi: 10.1016/0031-9384(77)90345-6. [DOI] [PubMed] [Google Scholar]
- 30.Sakuma Y, Pfaff DW. Mesencephalic mechanisms for integration of female reproductive behavior in the rat. Am J Phys. 1979;237:285–290. doi: 10.1152/ajpregu.1979.237.5.R285. [DOI] [PubMed] [Google Scholar]
- 31.Sheehan TP, Chambers RA, Russell DS. Regulation of affect by the lateral septum: Implications for neuropsychiatry. Brain Res Brain Res Rev. 2004;46:71–117. doi: 10.1016/j.brainresrev.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Zarrindast MR, Babapoor-Farrokhran S, Babapoor-Farrokhran S, Rezayof A. Involvement of opioidergic system of the ventral hippocampus, the nucleus accumbens or the central amygdala in anxiety-related behavior. Life Sci. 2008;82:1175–1181. doi: 10.1016/j.lfs.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 33.Degroot A, Kashluba S, Treit D. Septal GABAergic and hippocampal cholinergic systems modulate anxiety in the plus-maze and shock-probe tests. Pharmacol Biochem Behav. 2001;69:391–399. doi: 10.1016/s0091-3057(01)00541-x. [DOI] [PubMed] [Google Scholar]
- 34.Le Merrer J, Gavello-Baudy S, Galey D, Cazala P. Morphine self administration into the lateral septum depends on dopaminergic mechanisms: Evidence from pharmacology and Fos neuroimaging. Behav Brain Res. 2006;180:203–217. doi: 10.1016/j.bbr.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 35.Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4:477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- 36.Nedivi E, Hevroni D, Naot D, Israeli D, Citri Y. Numerous candidate plasticity-related genes revealed by differential cDNA cloning. Nature. 1993;363:718–722. doi: 10.1038/363718a0. [DOI] [PubMed] [Google Scholar]
- 37.Miczek KA. Effects of L-dopa, d-Amphetamine and cocaine on intruder-evoked aggression in rats and mice. Prog Neuro-pharmacol. 1977;1:271–277. [Google Scholar]
- 38.Blanchard RJ, Blanchard DC. Aggressive behavior in the rat. Behav Biol. 1977;21:197–224. doi: 10.1016/s0091-6773(77)90308-x. [DOI] [PubMed] [Google Scholar]
- 39.Grosenick L, Clement TS, Fernald RD. Fish can infer social rank by observation alone. Nature. 2007;445:429–432. doi: 10.1038/nature05511. [DOI] [PubMed] [Google Scholar]
- 40.Fernández-Espejo E, Mir D. Behavioral study in rats of paired accumbens-lesioned residents and intact intruders. Physiol Behav. 1990;47:941–947. doi: 10.1016/0031-9384(90)90022-v. [DOI] [PubMed] [Google Scholar]
- 41.Korzan WJ, Summers TR, Summers CH. Monoaminergic activities of limbic regions are elevated during aggression: Influence of sympathetic social signaling. Brain Res. 2000;870:170–178. doi: 10.1016/s0006-8993(00)02420-3. [DOI] [PubMed] [Google Scholar]
- 42.Øverli Ø, et al. Behavioral and neuroendocrine correlates of aggression in trout. Horm Behav. 2004;45:324–329. doi: 10.1016/j.yhbeh.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Korzan WJ, Summers CH. Serotonergic response to social stress and artificial social sign stimuli during paired interactions between male Anolis carolinensis. Neuroscience. 2004;123:835–845. doi: 10.1016/j.neuroscience.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 44.Fernald RD, Shelton LC. The organization of the diencephalon and the pretectum in the cichlid fish, Haplochromis burtoni. J Comp Neurol. 1985;238:202–217. doi: 10.1002/cne.902380207. [DOI] [PubMed] [Google Scholar]
- 45.Burmeister SS, Munshi RG, Fernald RD. Cytoarchitecture of a cichlid fish telencephalon. Brain Behav Evol. 2009;74:110–120. doi: 10.1159/000235613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maler L, Sas E, Johnston S, Ellis W. An atlas of the brain of the electric fish Apteronotus leptorhynchus. J Chem Neuroanat. 1991;4:1–38. doi: 10.1016/0891-0618(91)90030-g. [DOI] [PubMed] [Google Scholar]
- 47.Reiner A, Northcutt RG. An immunohistochemical study of the telencephalon of the senegal bichir (Polypterus senegalus) J Comp Neurol. 1992;319:359–386. doi: 10.1002/cne.903190305. [DOI] [PubMed] [Google Scholar]
- 48.Munoz-Cueto JA, Sarasquete C, Zohar Y, Kah O. An Atlas of the Brain of the Gilthead Seabream (Sparus aurata) College Park, MD: Maryland Sea Grant; 2001. [Google Scholar]
- 49.Greenwood AK, et al. Multiple corticosteroid receptors in a teleost fish: Distinct sequences, expression patterns, and transcriptional activities. Endocrinology. 2003;144:4226–4236. doi: 10.1210/en.2003-0566. [DOI] [PubMed] [Google Scholar]
- 50.Zhao S, Fernald RD. Comprehensive algorithm for quantitative real-time polymerase chain reaction. J Comput Biol. 2005;12:1047–1064. doi: 10.1089/cmb.2005.12.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]