Abstract
Mitogen-activated protein kinase kinase 6 (MKK6) is a member of the mitogen-activated protein kinase (MAPK) kinase (MAP2K) subfamily that specifically phosphorylates and activates the p38 MAPKs. Based on both biochemical and cellular assays, we found that MKK6 was extremely sensitive to oxidation: It was inactivated by oxidation and its kinase activity was fully restored upon treatment with a reducing agent. Detailed mechanistic studies showed that cysteines 109 and 196, two of the six cysteines in MKK6, formed an intramolecular disulfide bond upon oxidation that inactivated MKK6 by inhibiting its ATP binding. This mechanism is distinct from that seen in other redox-sensitive kinases. The two cysteines involved in intramolecular disulfide formation are conserved in all seven members of the MAP2K family. Consistently, we confirmed that other MAP2Ks were also sensitive to oxidation. Our work reveals that MKK6 and other MAP2Ks are a distinct class of cellular redox sensors.
Protein kinases form one of the largest protein families in mammalian proteomes. There are over 500 members in both human and mouse kinomes (1, 2). These kinases function in diverse intracellular signal transduction pathways that control important cellular processes ranging from cell fate determination, proliferation, and differentiation, to apoptosis. Mutations found in some of the kinases are causally linked to a number of human diseases including cancers. A number of effective targeted cancer therapies currently being used in clinics involve drugs (e.g., Gleevec) that selectively target the mutated kinases (3, 4). Therefore, a thorough understanding of the structure–function relationship of various protein kinases as well as their modes of regulation will be greatly beneficial to the development of more effective drugs.
Among all the kinases, the mitogen-activated protein kinases (MAPKs) are one of the most extensively and best studied kinases (5–9). A typical MAPK pathway consists of a three-tier kinase cascade: MAPK, MAP2K, and MAP2K kinase (or MAP3K), with a MAP3K directly phosphorylating and activating a MAP2K, and the latter in turn phosphorylating and activating a MAPK. MAPKs can be further divided into four subgroups: extracellular signal-regulated kinases (ERKs), cJun N-terminal kinases (JNKs), p38 MAPKs, and ERK5. Upstream of MAPKs, there are seven well-characterized MAP2Ks in the human and mouse genomes: with mitogen-activated protein kinase kinase (MKK)1/MKK2 specifically activating ERKs, MKK3/MKK6 specifically activating p38 MAPKs, MKK7 specifically activating JNKs, and MKK5 specifically activating ERK5. An exception is MKK4, which can activate both JNK and p38 MAPK (10). MAP2Ks directly phosphorylate MAPKs on the threonine and tyrosine residues in the conserved “TXY” motif in the kinase subdomain VIII (i.e., activation loop). MAPKs are activated by a wide range of physiological and pathological stimuli that include growth factors, proinflammatory cytokines, and oxidative stress. Oxidative stress accompanies a number of human diseases including cancer, diabetes, and neurodegenerative diseases, as well as cardiovascular diseases (11, 12). Oxidative stress is triggered by an imbalanced cellular redox state that is normally tightly regulated by oxidants including reactive oxygen species as well as reactive nitrogen species, and antioxidants including glutathione and thioredoxin (11). Many cellular proteins are known to be directly regulated by the cellular redox state with the thiol group of cysteine (Cys) being a major target for oxidation-induced chemical modification (13, 14). Depending on the microenvironment surrounding cysteine residues in a particular tertiary structure of a protein, selected cysteines can be preferentially deprotonated to form thiolate ions that can be readily oxidized to form sulfenic acids or undergo S-nitrosylation. Sulfenic acid is generally unstable and can be either further oxidized to form sulfinic acid and sulfonic acid or converted to a disulfide with another thiol from proteins (i.e., intramolecular or intermolecular disulfide) or small thiol-containing molecules (e.g., glutathione) (11, 13–15). In addition, sulfenic acid may also be converted to cyclic sulfenamide through the nucleophilic attack of sulfur by a neighboring backbone amide nitrogen (14, 16, 17). These different forms of oxidation-induced cysteine modification are known to profoundly affect the activity and function of various redox-sensitive proteins.
We demonstrated here that MKK6 as well as other MAP2Ks are a unique class of cellular redox sensors, which could be inactivated by oxidation due to the formation of a specific intramolecular disulfide bond that blocked ATP binding.
Results
Kinase Activity of MKK6 Was Sensitive to Oxidation Both in Vitro and in Mammalian Cells.
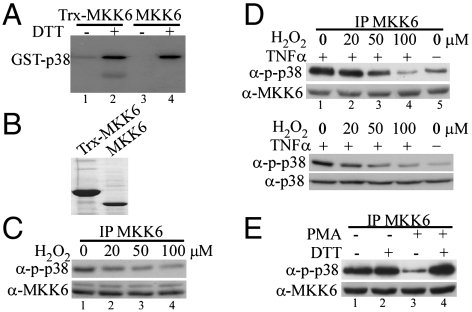
When a recombinant human MKK6 protein with a thioredoxin(Trx)-6xHis-tag was purified from bacteria, dialyzed in the absence of any reducing agent, and subjected to in vitro kinase assays using a purified glutathione S-transferase (GST)-p38α(K82M) (i.e., kinase-dead) as a substrate, unexpectedly, we found that the kinase activity of MKK6 was barely detectable (Fig. 1A, lane 1). However, the addition of 1 mM DTT in the kinase reaction greatly restored the kinase activity of MKK6 (lane 2). To exclude the possibility that the observed effect was due to the presence of the Trx-6xHis tag, we cleaved the fusion protein with thrombin and removed the Trx-6xHis tag by passing the mixtures through a column filled with the nickel-containing resins. The purified MKK6 without the Trx-6xHis tag (Fig. 1B) was then subjected to in vitro kinase assays with or without 1 mM DTT. Consistently, the kinase activity of MKK6 was undetectable without DTT but was fully restored in the presence of 1 mM DTT (Fig. 1A, lanes 3 and 4). To find out whether the endogenous MKK6 in mammalian cells was similarly regulated by oxidation, we first treated HeLa cells with varying doses of hydrogen peroxide (H2O2) which is known to readily permeate into cells to induce cellular oxidation (18). We immunoprecipitated the endogenous MKK6 from cell lysates and measured its kinase activity toward p38 MAPK. Compared to the nontreated control, 20 μM of H2O2 treatment already partially decreased the kinase activity of MKK6 (Fig. 1C, lane 2), whereas 100 μM of H2O2 greatly reduced its activity (lane 4). In a separate experiment, we pretreated HeLa cells with varying doses of H2O2 for 5 min followed by stimulation with 10 ng/mL of tumor necrosis factor α (TNFα), a proinflammatory cytokine known to activate the p38 MAPK pathway (19). We reasoned that H2O2 pretreatment would inactivate the endogenous MKK6, which could prevent subsequent activation of p38 MAPK by a well-established inducer like TNFα. As shown in Fig. 1D, without H2O2 pretreatment, TNFα indeed potently enhanced the kinase activity of MKK6 (Fig. 1D, Top, compare lanes 1 and 5). However, H2O2 pretreatment dose-dependently inactivated MKK6 as measured by the immune-complex kinase assays (Fig. 1D, Top, lanes 2–4). Consistently, the TNFα-induced p38 phosphorylation in the cells was also found to be inhibited by H2O2 pretreatment in a dose-dependent manner (Fig. 1D, Bottom). To find out whether an oxidation-inducing agent other than H2O2 could also have similar effects on MKK6, we treated RAW264.7 cells, a mouse monocyte/macrophage cell line, with 1 μM phorbol 12-myristate 13-acetate (PMA), which was known to trigger potent cellular oxidation (20). To preserve any potential oxidation-induced modification on MKK6, we lysed cells in a reducing agent-free buffer. By immune-complex kinase assays, we found that the endogenous MKK6 was potently inactivated by PMA (Fig. 1E, lane 3). Addition of 1 mM DTT in the kinase reaction restored the kinase activity of MKK6 (lane 4). Collectively, our results above indicated that the kinase activity of MKK6 was sensitive to oxidation both in vitro and in mammalian cells.
Fig. 1.
The kinase activity of MKK6 was sensitive to oxidation both in vitro and in cells. (A) The bacterially expressed human MKK6 with (lanes 1 and 2) or without (lanes 3 and 4) a Trx tag was purified and extensively dialyzed in the absence of any reducing agent. The purified MKK6 was subjected to in vitro kinase assays using GST-p38(KM) as a substrate in the presence or absence of 1 mM DTT. The phosphorylated p38 was detected by autoradiography. (B) The purified MKK6 with or without a Trx tag was electrophoresed on a SDS-polyacrylamide gel and visualized by Coomassie blue staining. (C and D) HeLa cells were either left untreated or treated with increasing doses of H2O2 as indicated. In C, cells were harvested after 5 min of H2O2 treatment. In D, cells were pretreated with H2O2 for 5 min followed by stimulation with 10 ng/mL TNFα for 10 min. (E) Serum-starved RAW264.7 cells were either left untreated or treated with 1 μM PMA for 30 min. The same amount of the endogenous MKK6 from C–E was immunoprecipitated from whole cell extracts (WCE) and subjected to in vitro kinase assays with GST-p38(KM) as a substrate. The phosphorylated p38 (p-p38) was revealed by Western blot analysis. Ten percent of total WCE used in the immunoprecipitation experiments in C–E was analyzed by Western blotting to reveal the amount of MKK6 as well as p-p38 and total p38 (D) in each sample.
The fact that the oxidation-induced inactivation of MKK6 could be readily reversed by DTT suggested the involvement of certain cysteine residues in MKK6. Although cysteine could be oxidized to different forms, the simplest hypothesis to explain the data above was that the oxidized MKK6 likely formed disulfides. There are six cysteines in human MKK6 (i.e., C38, C109, C128, C196, C216, and C294) (Fig. S1A). To quickly check whether the oxidized MKK6 formed intermolecular disulfides, we electrophoresed the oxidized recombinant MKK6 with or without the Trx-6xHis tag on a nonreducing SDS-polyacrylamide gel with or without β-mercaptoethanol preincubation. Should the oxidized MKK6 form intermolecular disulfides, we would expect to detect bands corresponding to the size of dimer or multimers on the gel and a simultaneous reduction in the level of the monomeric MKK6. As shown in Fig. S1B, both the reduced and oxidized MKK6, with or without the Trx-6xHis tag, mainly existed in the monomeric form, suggesting that the disulfides, if present, in the oxidized MKK6 were most likely intramolecular. Consistently, the oxidized MKK6 was found to migrate slightly faster than the reduced form on the nonreducing gel (Fig. S1B), which was a recognized feature of the oxidized proteins with intramolecular disulfides (21, 22).
Cys109 and Cys196 Formed an Intramolecular Disulfide Bond in MKK6 Oxidized in Vitro.
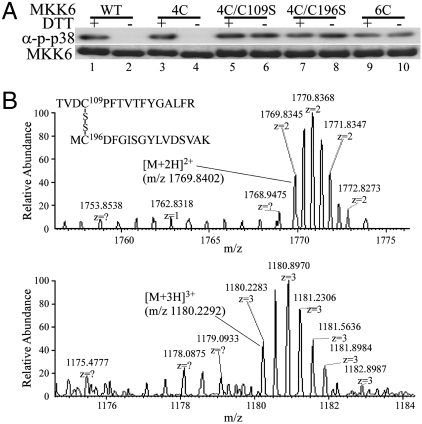
To confirm whether the oxidized MKK6 indeed formed intramolecular disulfides and to identify which of the six cysteines in MKK6 were involved, we first examined the crystal structure of a constitutively active mutant of MKK6 (Protein Data Bank ID code 3FME) (Fig. S1C). Because the small N-terminal fragment (amino acids 1–46) of MKK6 was not included in the crystal structure, the position of Cys38 relative to other residues was not clear. For the remaining five cysteines, Cys128 and Cys294 were buried in two distinct hydrophobic structural cores and thus were unlikely to form disulfide bonds with other cysteine residues (Fig. S1C). The remaining three cysteines (i.e., Cys109, Cys196, and Cys216) were located in relatively flexible loop regions. Cys196 was about 12-Å away from Cys109 (measured between the sulfur of two Cys) in the crystal structure of the active MKK6 mutant (Fig. S1C). Cys109 is located in a loop C-terminal to the αC helix in the N lobe of the kinase core, and this αC helix is known to undergo significant conformational change when protein kinases cycle between their active and inactive forms (23). Thus, it was highly possible that a small conformational change in the αC helix of N lobe could bring Cys109 and Cys196 within the optimal distance (∼8 Å between their Cα carbons) to form a disulfide bond (24). As to Cys216, it is located in the first α-helix of the kinase C-lobe and is far away from any other surface-exposed cysteine in the protein, suggesting that it is unlikely to be involved in the disulfide formation. To experimentally test our hypothesis, we generated a quadruple cysteine mutant of MKK6 (designated as MKK6-4C), with Cys38 and Cys216 being changed to serines (as these two cysteines are solvent exposed), and Cys128 and Cys294 being changed to leucine and methionine, respectively (as they are buried in the hydrophobic cores). In its reduced form, the kinase activity of MKK6-4C was indistinguishable from that of the wild-type kinase (Fig. 2A, Upper, lanes 1 and 3). However, like the wild-type MKK6, MKK6-4C was readily inactivated by air-induced oxidation, suggesting that Cys38, 128, 216, and 294 were not directly involved in disulfide formation (Fig. 2A, Upper, lanes 1–4). In contrast, mutation of the remaining two cysteines (i.e., Cys109 and Cys196) in MKK6-4C to serines, either singly or together, resulted in three MKK6 mutants (designated as MKK6-4C/C109S, MKK6-4C/C196S, and MKK6-6C) that were resistant to oxidation-induced inactivation (Fig. 2A, Upper, lanes 5–10). This result suggested that Cys109 and Cys196 were involved in intramolecular disulfide formation. As a control, we made sure that the wild-type MKK6 and its multicysteine mutants used in the kinase assays were purified in the same batch under identical conditions and that the purity and the amount of the proteins were similar among different samples (Fig. 2A, Lower). Interestingly, when we electrophoresed different MKK6 proteins on a nonreducing SDS-polyacrylamide gel, we found that the oxidized forms of both wild-type MKK6 and MKK6-4C migrated slightly faster than their reduced forms (Fig. 2A, Lower, lanes 1–4), consistent with the hypothesis that they contained intramolecular disulfides. In contrast, no faster migration bands were observed in the oxidized MKK6-4C/C109S, MKK6-4C/C196S, or MKK6-6C (lanes 5–10). To directly reveal the number of thiol groups involved in disulfide formation in the oxidized MKK6, we subjected both the reduced and oxidized MKK6 to Ellman’s reagent-based colorimetric assays (25). As shown in Table 1, in the wild-type MKK6, approximately six and four free thiols were found in the reduced and oxidized forms, respectively, suggesting that two of the six cysteines in the oxidized MKK6 were involved in disulfide formation. For MKK6-4C, two and zero free thiols were found in its reduced and oxidized forms, respectively, consistent with the idea that Cys109 and Cys196 were involved in disulfide formation. As a control, one free thiol was found in both the reduced and oxidized MKK6-4C/C109S and MKK6-4C/C196S, and no free thiol was found in either the reduced or oxidized MKK6-6C mutant.
Fig. 2.
Identification of cysteines involved in intramolecular disulfide bond formation in MKK6 oxidized in vitro. (A) Similar amount of purified WT MKK6, MKK6-4C (4C), MKK6-4C/C109S (4C/C109S), MKK6-4C/C196S (4C/C196S), and MKK6-6C (6C) were subjected to in vitro kinase assays with GST-p38(KM) as a substrate with or without 1 mM DTT (Upper). The phosphorylated p38 (p-p38) was revealed by Western blot analysis. The amount and purity of MKK6 proteins used in the above kinase assays were shown on a Coomassie blue-stained nonreducing gel (Lower). (B) Full MS spectra of the [M + 2H]2+ (m/z 1,769.84) and [M + 2H]3+ (m/z 1,180.23) ions of the disulfide-linked TVDC109PFTVTFYGALFR and MC196DFGISGYLVDSVAK.
Table 1.
Ellman’s reagent-based colorimetric assay
| MKK6 |
Theoretical −SH content |
Measured −SH content |
|
| Reduced | Oxidized | ||
| WT | 6 | 5.95 ± 0.23 | 4.12 ± 0.21 |
| 4C | 2 | 2.23 ± 0.17 | 0.19 ± 0.05 |
| 4C/C109S | 1 | 1.16 ± 0.08 | 0.93 ± 0.15 |
| 4C/C196S | 1 | 1.08 ± 0.12 | 0.88 ± 0.27 |
| 6C | 0 | 0.15 ± 0.09 | 0.08 ± 0.11 |
The bacterially expressed wild-type and mutant MKK6 proteins were purified and subjected to 5′,5′-dithiobis(2-nitrobenzoic acid) (DTNB) assays. The theoretical number of free thiol groups per MKK6 molecule and the number of thiol groups derived from the DTNB assays in either the reduced or oxidized MKK6 are summarized in the table. The symbols for MKK6 and its mutants were the same as those in the legend of Fig. 2A.
To further ascertain that Cys109 and Cys196 were involved in intramolecular disulfide formation, we also subjected purified MKK6 protein to mass spectrometry analysis. In the first experiment, we employed a sequential alkylation method followed by mass spectrometry analysis. Both the reduced and oxidized MKK6 were first reacted with iodoacetamide to alkylate existing free thiol groups in the proteins (mass shift by 57.02). The proteins were then reduced to regenerate the free thiol groups that were previously engaged in disulfide formation, followed by a second alkylation reaction with N-ethylmaleimide (NEM) (mass shift by 125.04 Da). We then subjected the modified MKK6 to tryptic digestion and mass spectrometry analysis. No NEM-modified peptides were identified in the reduced MKK6. In contrast, in the oxidized MKK6, we identified two peptides, each with an expected gain of 125.04 Da in mass, which was indicative of the formation of an NEM adduct. The tandem mass spectra clearly revealed that they were Cys109- and Cys196-containing peptides, respectively (i.e., TVDC109PFTVTFYGALFR and MC196DFGISGYLVDSVAK) (Fig. S2). In the second experiment, we subjected the unmodified recombinant MKK6, in both the reduced and oxidized forms, to tryptic digestion followed by mass spectrometry analysis. In the oxidized sample, we detected a peak with the mass/charge ratio (m/z) corresponding to that of the disulfide-linked peptides consisting of TVDC109PFTVTFYGALFR and MC196DFGISGYLVDSVAK (Fig. 2B). No such disulfide-linked peptides were seen in the reduced MKK6. This experiment ruled out the possibility that Cys109 and Cys196 may form reversible sulfenamides and directly proved that Cys109 and Cys196 formed an intramolecular disulfide bond in MKK6 oxidized in vitro.
Cys109 and Cys196 Were Involved in Disulfide Bond Formation in MKK6 Oxidized in Mammalian Cells.
In test tubes with purified MKK6, we already showed that Cys109 and Cys196 formed an intramolecular disulfide bond upon oxidation. To further prove that Cys109 and Cys196 were also involved in disulfide bond formation in MKK6 oxidized in mammalian cells, we first transfected HeLa cells with vectors encoding either the wild-type MKK6 or MKK6-4C. Cells were then either mock treated (control) or treated with 200 μM H2O2 to induce cellular oxidation. Free thiols in cellular proteins were modified by two different methods. In the first method, cells were lysed in the buffer containing biotin-conjugated iodoacetamide (BIAM) that alkylates free thiols in proteins (26, 27). Equal amount of HA-MKK6 was immunoprecipitated from cell lysates, separated by SDS-PAGE followed by Western blot analysis to detect the biotin-labeled MKK6. As shown in Fig. 3A, in the wild-type HA-MKK6, the extent of biotin labeling was decreased in the sample pretreated with H2O2, presumably due to reduced number of free thiols in MKK6 after H2O2-induced disulfide bond formation (compare lanes 1 and 2). In HA-MKK6-4C from untreated cells, due to the loss of four cysteines, it was not surprising to see that the extent of biotin labeling was greatly reduced compared to that in the wild-type HA-MKK6 (compare lanes 1 and 3). Importantly, HA-MKK6-4C from H2O2-treated cells was completely refractory to BIAM-mediated labeling (compare lanes 3 and 4), consistent with the idea that Cys109 and Cys196 were engaged in disulfide bond formation after H2O2 treatment. In the second method, cells were first lysed in the buffer containing iodoacetic acid (IAA) that irreversibly modified free thiols in proteins (26, 27). The IAA-modified HA-MKK6 was then immunoprecipitated from cell lysates followed by DTT treatment to disrupt any preexisting disulfide bond(s) in MKK6 before IAA treatment. The newly exposed free thiol groups could then be relabeled with BIAM and revealed by Western blot analysis. As shown in Fig. 3B, the wild-type HA-MKK6 and HA-MKK6-4C from mock-treated cells did not contain any preexisting disulfide bond because BIAM failed to label the IAA-treated MKK6 or MKK6-4C (lanes 1 and 3). In contrast, the IAA-treated HA-MKK6 and HA-MKK6-4C from H2O2-treated cells could be relabeled with BIAM, indicating the presence of preexisting disulfide bond(s) (lanes 2 and 4). The fact that the extent of biotin labeling in the wild-type MKK6 and MKK6-4C was comparable in Fig. 3B suggested that the number of disulfide bond(s) formed in both proteins was similar. Because there were only two cysteines (i.e, Cys109 and Cys196) left in MKK6-4C, our IAA–BIAM double-labeling data strongly suggested that these two cysteines were involved in disulfide bond formation in living cells upon H2O2 treatment. Furthermore, we also used PMA to trigger oxidative burst in RAW264.7 cells and probed the endogenous MKK6 with BIAM-mediated labeling. Consistently, we found less biotin labeling in MKK6 from PMA-treated RAW264.7 cells (Fig. 3C).
Fig. 3.
Cys109 and Cys196 were involved in disulfide bond formation in MKK6 oxidized in mammalian cells. (A and B) HeLa cells were transiently transfected with plasmids encoding either the WT HA-MKK6 or HA-MKK6-4C (4C). Twenty-four hours after transfection, cells were either mock treated or treated with 200 μM H2O2 for 10 min before harvest. Cellular proteins were either labeled with 100 μM BIAM alone (A), or double labeled with IAA and BIAM (B). (C) Serum-starved RAW264.7 cells were either mock treated or treated with 1 µM PMA for 30 min. Cells were lysed in the buffer containing BIAM. Similar amount of HA-MKK6 from A and B or the endogenous MKK6 from C was then immunoprecipitated from WCE and the biotin-conjugated MKK6 (b-MKK6) was detected by HRP-streptavidin. Ten percent of total WCE used in the immunoprecipitation experiments in A–C was analyzed by Western blotting to reveal the levels of either the transfected or the endogenous MKK6. (D and E) MKK3/6 double knockout cells were transfected with either an empty vector or expression vectors encoding either the WT HA-MKK6, HA-MKK6-4C (4C), or HA-MKK6-6C (6C). Twenty-four hours after transfection, cells were either mock treated or treated with increasing does of H2O2 for 5 min, followed by treatment with sorbitol (0.4 M) for 10 min. Fifty micrograms of WCE were analyzed by Western blotting.
To examine whether H2O2-induced disulfide bond formation in MKK6 affected its kinase activity in mammalian cells, we first individually transfected the wild-type HA-MKK6, HA-MKK6-4C, and HA-MKK6-6C into mouse embryonic fibroblasts (MEFs) derived from MKK3/MKK6 double knockout mice (28). The employment of these cells would help to simplify our interpretation by avoiding the influence of the endogenous MKK3/MKK6. All three MKK6 constructs were expressed at similar levels in MKK3-/-/MKK6-/- MEFs as determined by Western blot analysis (Fig. 3D). MEFs were either mock treated or pretreated with an increasing concentration of H2O2 (from 50–200 μM) for 5 min followed by treatment with 0.4 M sorbitol for another 10 min. Under such a condition, H2O2 pretreatment alone did not activate p38 MAPK in MEFs. In contrast, sorbitol treatment alone induced potent activation of both the transfected MKK6 and the endogenous p38 MAPK in MEFs (Fig. 3E), which was revealed by specific antibodies recognizing the activated MKK6 and p38 MAPK, respectively. In cells transfected with either the wild-type MKK6 or MKK6-4C, the levels of the activated p38 MAPK gradually decreased in cells pretreated with an increasing concentration of H2O2. Notably, the p38 MAPK completely failed to be activated by sorbitol in cells pretreated with 200 μM of H2O2 (lane 6, top two boxes). This result could be due to H2O2-induced inactivation of MKK6 or signaling molecules acting upstream of MKK6. A close examination of MKK6 showed that H2O2 pretreatment (even at 200 μM) only led to a slight decrease in the levels of the phosphorylated (i.e., activated) MKK6 in cells transfected with either the wild-type MKK6 or MKK6-4C (lanes 3-6, top two boxes), suggesting that the signaling molecule(s) acting upstream of MKK6 was not severely affected by H2O2 under such a condition. Importantly, in MEFs transfected with MKK6-6C that resisted H2O2-induced oxidation (Fig. 2A), the p38 MAPK could still be activated by sorbitol even when cells were pretreated with 200 μM of H2O2 (lane 6, bottom box). As expected, the levels of the phosphorylated MKK6-6C remained relatively constant with or without H2O2 pretreatment, which further proved that the amount of H2O2 used did not significantly affect the signaling molecule(s) acting upstream of MKK6. Collectively, our data above showed that cellular oxidation also promoted intramolecular disulfide bond formation between Cys109 and Cys196 in MKK6 in mammalian cells, which resulted in the loss of its kinase activity.
Formation of the Intramolecular Disulfide Bond in MKK6 Interfered with ATP Binding.
To understand mechanistically how the oxidation-induced disulfide bond formation in MKK6 inhibited its kinase activity, we first checked whether the formation of the intramolecular disulfide bond drastically altered the overall structure of MKK6. CD spectroscopy was performed on two samples of the purified MKK6 protein from the same preparation: one without DTT (i.e., oxidized) and the other with 1 mM DTT (i.e., reduced). As shown in Fig. 4A, the spectrum of the oxidized MKK6 overlapped very well with that of the reduced form, suggesting that the overall structure of MKK6 was not significantly altered by oxidation. Next, we examined whether the substrate-binding ability (i.e., binding to p38 MAPK) of MKK6 was affected by oxidation. The purified MKK6 (i.e., oxidized) was incubated with either a GST-p38α fusion protein or GST in the absence or presence of 1 mM DTT followed by GST-based pull-down assays coupled with Western blot analysis. As a negative control, GST failed to pull down MKK6 with or without DTT (Fig. 4B, lanes 1 and 3). In contrast, GST-p38α specifically pulled down a similar amount of MKK6 no matter whether DTT was present or not (lanes 2 and 4), suggesting that the p38α-binding ability of MKK6 was not greatly compromised in the oxidized MKK6. We then turned our focus on the ATP-binding ability of MKK6. Using 2′-(or-3′)-O-(BODIPY FL)-β:γ-imidoadenosine 5′-triphosphate (BODIPY FL-AMPPNP), a fluorescent, nonhydrolyzable ATP analogue, as a probe, we studied the binding of BODIPY FL-AMPPNP to either the oxidized or the reduced MKK6 by equilibrium fluorescence anisotropy. As shown in Fig. 4C, the binding of BODIPY FL-AMPPNP to the oxidized MKK6 was essentially undetectable. In contrast, the Kd of the reduced MKK6 for BODIPY FL-AMPPNP was determined to be 5.7 μM. Thus, our data clearly showed that the oxidation of MKK6 abolished its ATP-binding capacity, which rendered the kinase inactive.
Fig. 4.
The oxidized MKK6 was defective in ATP binding. (A) The CD spectra for both reduced and oxidized MKK6 were shown. W/O, without. (B) GST (lanes 1 and 3) or GST-p38α (lanes 2 and 4) immobilized on glutathione-Sepharose beads were incubated with purified MKK6 with or without 1 mM DTT in a GST pull-down assay. The bound MKK6 was detected by Western blot analysis. (C) Equilibrium fluorescence anisotropy was measured with 30 nM BODIPY FL-AMPPNP and increasing amount of MKK6 in a binding buffer with or without 1 mM DTT. The binding curve was fitted with the MicroCal Origin software package.
Cysteines Corresponding to Cys109 and Cys196 of MKK6 Were Conserved in Other Members of the MAP2K Family.
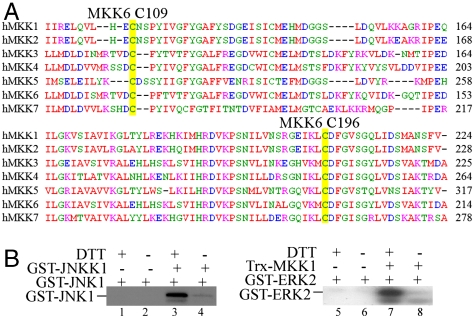
To find out whether cysteines corresponding to Cys109 and Cys196 of MKK6 are conserved in other members of the MAP2K family, we performed sequence alignment for seven MAP2Ks in the human genome. As shown in Fig. 5A, Cys196 and the amino acids surrounding it are highly conserved in all seven human MAP2Ks. As for Cys109, it is also conserved in all seven MAP2Ks even though the sequences surrounding it are not highly conserved. Thus, our sequence analysis suggested that other MAP2Ks may also be sensitive to oxidation. To experimentally test this hypothesis, we selected JNKK(MKK4) and MKK1 as examples. We purified the recombinant JNKK1 and MKK1 from Escherichia coli in the absence of any reducing agent, and subjected them to in vitro kinase assays with or without DTT using GST-JNK1 and GST-ERK2 as a substrate, respectively. As shown in Fig. 5B, in the absence of JNKK1 or MKK1, the autophosphorylation of GST-JNK1 or GST-ERK2 was very low regardless of the presence of DTT (lanes 1, 2, 5, 6). In the absence of DTT, the addition of JNKK1 or MKK1 did not significantly promote the phosphorylation of GST-JNK1 or GST-ERK2 (lanes 4 and 8). In contrast, addition of 1 mM DTT potently enhanced the kinase activity of both JNKK1 and MKK1 as evidenced by greatly enhanced phosphorylation of GST-JNK1 and GST-ERK2, respectively (lanes 3 and 7). Thus, like MKK6, the other members of the MAP2K family were also sensitive to oxidation.
Fig. 5.
The kinase activity of JNKK1 and MKK1 was also sensitive to oxidation. (A) The amino acid sequences (partial) of seven human MAP2Ks were aligned using the Clustal W program. Cysteines corresponding to Cys109 and Cys196 of MKK6 were highlighted in yellow. (B) The bacterially expressed JNKK1 (Left) and MKK1 (Right) were incubated with GST-JNK1 and GST-ERK2, respectively, in the in vitro kinase assays with or without 1 mM DTT. The phosphorylated JNK1 and ERK2 were detected by autoradiography.
Discussion
Although a number of protein kinases have been shown to be regulated by cellular redox, the underlying mechanisms vary from one kinase to another. In general, the redox-sensitive kinases can be divided into two groups. For kinases in group I, oxidation regulates their activities in an indirect manner. For example, apoptosis signal-regulating kinase 1 (ASK1) is normally bound and repressed by Trx in its reduced form. Upon oxidation, two cysteines in the active site of Trx are oxidized, which leads to dissociation of Trx from ASK1 and subsequent activation of ASK1 (29). A recent paper suggests that the mechanism for oxidation-induced ASK1 activation may be more complicated than we originally thought, because a cysteine outside the kinase domain of ASK1 (i.e., Cys250) could also be directly modified by oxidation, which affects its ability to activate JNK and to mediate H2O2-induced apoptosis (30). However, it remains unclear how the redox state of Cys250 dictates the activity of ASK1 toward JNK. Two indirect mechanisms have also been proposed to explain the oxidation-induced JNK activation: the first involves the oxidation-induced dissociation of glutathione S-transferase pi (GSTp) from JNK (31). Similar to the effect of Trx on ASK1, the monomeric GSTp was also found to bind and inhibit JNK. The second indirect mechanism involves the oxidation-induced inactivation of a JNK-specific phosphatase through direct modification of the catalytic cysteine (32). For kinases in group II, oxidation can regulate their activities by directly modifying key cysteines in these kinases. In the case of mitogen-activated protein kinase kinase kinase 1, a member of the MAP3K family, oxidation selectively induces glutathionylation of Cys1238 near the conserved GXGXXG(or S/A) motif (i.e., P loop) in the kinase subdomain I (i.e., GLGAFSSC1238) that is known to directly bind ATP (15). As for Src, a recent paper showed that oxidation induced the formation of a homodimer via an intermolecular disulfide bond between two Cys277 in the middle of the conserved P loop (i.e., GQGC277FG in rat Src) (33). In both cases above, oxidation of a specific cysteine in the kinase domain inactivates the kinases, which is fully reversible upon treatment with a reducing agent. Although ATP binding could be compromised due to the location of the cysteine being modified, no direct experimental evidence was provided in these two studies. We demonstrate here that MKK6 is a distinct cellular redox sensor belonging to the redox-sensitive kinases in group II. MKK6 is inactivated by oxidation both in vitro and in living cells due to the formation of a specific intramolecular disulfide bond between Cys109 and Cys196 in the kinase domain, which directly inhibits ATP binding. In MKK6, Cys196 was immediately followed by an aspartic acid (i.e., D197) in the highly conserved triplet “DFG” in the kinase subdoamin VII (Fig. 5A). D197 is 1 of the 12 invariant or nearly invariant amino acids highly conserved in most members of the mammalian kinome and is known to directly chelate the Mg2+ ion that binds to the β- and γ-phosphates of ATP and facilitates the transfer of the γ-phosphate (23, 34). Therefore, it is likely that oxidation of Cys196 may interfere with the Mg-ATP binding function of D197, leading to the inactivation of the kinase. Similarly, in the case of ERK that also has a cysteine (i.e., Cys166) immediately in front of the DFG triplet, covalent modification of Cys166 by FR148083, a small compound isolated from fermentation culture broth, led to specific inactivation of ERK (35). Both JNK and p38 MAPK were resistant to FR148083 due to the lack of such a cysteine in front of the DFG triplet. In addition to MKK6, the remaining six MAP2Ks all contain equivalents of Cys109 and Cys196 (Fig. 5A). Therefore, one can predict that these MAP2Ks were also subject to similar redox regulation. Indeed, we found that both JNKK1 (MKK4) and MKK1 were sensitive to redox regulation (Fig. 5B). A quick survey of the primary sequence of a number of other protein kinases shows that some of them (e.g., GSK3α, NinaC) also contain cysteines at positions corresponding to Cys109 and Cys196 in MKK6, suggesting that the oxidation-induced inactivation mechanism identified here for MAP2Ks could also apply to those kinases as well.
Although our data here clearly show that oxidation inactivates MAP2Ks, paradoxically, some reports showed that oxidation could activate various MAPKs (29, 36), whereas others claimed that oxidation had minimal effect on the activity of selected MAPKs (37, 38). A survey of the literature suggests that the factors contributing to the confusion include different types and doses of oxidants, different cell types, as well as different treatment time used in various studies. In our own experimental scheme, we did not see obvious activation of the p38 MAPK by H2O2 at the concentration that inhibited the activity of MKK6. Moreover, in our cell-based studies, we employed a dual-treatment scheme (i.e., first treating cells with H2O2 for 5 min followed by stimulation with sorbitol or TNFα for another 10 min) that could minimize some potential complicated issues (e.g., half-life of H2O2 in cells, resynthesis of proteins of interest after their inactivation by oxidation, etc.). As to the paradoxical effect of oxidation on MAPK signaling in the literature, the following mechanisms may provide some explanations. First of all, it is likely that oxidation-induced activation and inactivation of MAP2Ks occur in distinct phases: with activation occurring first followed by inactivation. Such a scenario suggests that inactivation of MAP2Ks could serve as a negative feedback mechanism to turn off the MAPK signaling. Secondly, in addition to the classical MAP3K-MAP2K-MAPK signaling cascade, MAPK may also be activated by a MAP2K-independent manner (39, 40). This mechanism may explain why oxidation sometimes activates MAPK even though MAP2Ks are inactive. Lastly, the oxidation-induced inactivation of MAPK-specific phosphatases, all of which contain a catalytic cysteine in their active sites, could be another mechanism that sustains MAPK activation even when MAP2Ks are inactivated (41–43).
In summary, our studies here define MAP2Ks as a unique class of cellular redox sensor. Our mechanistic studies reveal that formation of a specific intramolecular disulfide bond between Cys109 and Cys196 in the kinase domain inactivates MKK6 by inhibiting ATP binding. Similar phenomenon has not previously been seen in other oxidation-sensitive kinases. Moreover, this mechanism is not unique to MKK6, because we show that other members of MAP2K are similarly regulated by oxidation due to the presence of cysteines at positions equivalent to Cys109 and Cys196 of MKK6. In the future, it would be extremely interesting and informative to investigate the biological effect of an oxidation-resistant MKK6 under various pathophysiological conditions by knocking in the mutant gene into a desired model organism.
Materials and Methods
Detailed information on DNA constructs, cell lines, and chemicals used, as well as standard procedures for a number of assays were described in detail in SI Materials and Methods.
Labeling of Reduced Cysteines by BIAM.
Labeling of reduced cysteines by BIAM was carried out as described previously with minor modification (26).
Equilibrium Fluorescence Anisotropy.
The fluorescence anisotropy was measured using BODIPY FL-AMPPNP (30 nM) and increasing amount of MKK6 proteins in the binding buffer with or without 1 mM DTT. The titration curves were fitted with the MicroCal Origin software package.
Supplementary Material
Acknowledgments.
This work was supported by the Hong Kong Research Grant Council Grant 663308 and Grant HKUST1/06C (to Z.W.) and an Area of Excellence Scheme (AoE/B-15/01) of the University Grants Council.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007225107/-/DCSupplemental.
References
- 1.Caenepeel S, Charydczak G, Sudarsanam S, Hunter T, Manning G. The mouse kinome: Discovery and comparative genomics of all mouse protein kinases. Proc Natl Acad Sci USA. 2004;101:11707–11712. doi: 10.1073/pnas.0306880101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 3.Eglen RM, Reisine T. The current status of drug discovery against the human kinome. Assay Drug Dev Technol. 2009;7:22–43. doi: 10.1089/adt.2008.164. [DOI] [PubMed] [Google Scholar]
- 4.Sherbenou DW, Druker BJ. Applying the discovery of the Philadelphia chromosome. J Clin Invest. 2007;117:2067–2074. doi: 10.1172/JCI31988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 6.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 7.Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–3112. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- 8.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19:142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005;15:11–18. doi: 10.1038/sj.cr.7290257. [DOI] [PubMed] [Google Scholar]
- 10.Wu Z, Wu J, Jacinto E, Karin M. Molecular cloning and characterization of human JNKK2, a novel Jun NH2-terminal kinase-specific kinase. Mol Cell Biol. 1997;17:7407–7416. doi: 10.1128/mcb.17.12.7407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 12.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eaton P. Protein thiol oxidation in health and disease: Techniques for measuring disulfides and related modifications in complex protein mixtures. Free Radical Biol Med. 2006;40:1889–1899. doi: 10.1016/j.freeradbiomed.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 14.Poole LB, Nelson KJ. Discovering mechanisms of signaling-mediated cysteine oxidation. Curr Opin Chem Biol. 2008;12:18–24. doi: 10.1016/j.cbpa.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cross JV, Templeton DJ. Oxidative stress inhibits MEKK1 by site-specific glutathionylation in the ATP-binding domain. Biochem J. 2004;381:675–683. doi: 10.1042/BJ20040591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salmeen A, et al. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature. 2003;423:769–773. doi: 10.1038/nature01680. [DOI] [PubMed] [Google Scholar]
- 17.van Montfort RL, Congreve M, Tisi D, Carr R, Jhoti H. Oxidation state of the active-site cysteine in protein tyrosine phosphatase 1B. Nature. 2003;423:773–777. doi: 10.1038/nature01681. [DOI] [PubMed] [Google Scholar]
- 18.Veal EA, Day AM, Morgan BA. Hydrogen peroxide sensing and signaling. Mol Cell. 2007;26:1–14. doi: 10.1016/j.molcel.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 19.Raingeaud J, et al. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 20.Nathan CF, Root RK. Hydrogen peroxide release from mouse peritoneal macrophages: Dependence on sequential activation and triggering. J Exp Med. 1977;146:1648–1662. doi: 10.1084/jem.146.6.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moxham CP, Malbon CC. Fat cell beta 1-adrenergic receptor: structural evidence for existence of disulfide bridges essential for ligand binding. Biochemistry. 1985;24:6072–6077. doi: 10.1021/bi00343a007. [DOI] [PubMed] [Google Scholar]
- 22.Silva CM, Cidlowski JA. Direct evidence for intra- and intermolecular disulfide bond formation in the human glucocorticoid receptor. Inhibition of DNA binding and identification of a new receptor-associated protein. J Biol Chem. 1989;264:6638–6647. [PubMed] [Google Scholar]
- 23.Huse M, Kuriyan J. The conformational plasticity of protein kinases. Cell. 2002;109:275–282. doi: 10.1016/s0092-8674(02)00741-9. [DOI] [PubMed] [Google Scholar]
- 24.O’Connor BD, Yeates TO. GDAP: A web tool for genome-wide protein disulfide bond prediction. Nucleic Acids Res. 2004;32:W360–364. doi: 10.1093/nar/gkh376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 26.Giannoni E, Buricchi F, Raugei G, Ramponi G, Chiarugi P. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol Cell Biol. 2005;25:6391–6403. doi: 10.1128/MCB.25.15.6391-6403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JR, Yoon HW, Kwon KS, Lee SR, Rhee SG. Identification of proteins containing cysteine residues that are sensitive to oxidation by hydrogen peroxide at neutral pH. Anal Biochem. 2000;283:214–221. doi: 10.1006/abio.2000.4623. [DOI] [PubMed] [Google Scholar]
- 28.Brancho D, et al. Mechanism of p38 MAP kinase activation in vivo. Genes Dev. 2003;17:1969–1978. doi: 10.1101/gad.1107303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuzawa A, Ichijo H. Redox control of cell fate by MAP kinase: physiological roles of ASK1-MAP kinase pathway in stress signaling. Biochim Biophys Acta. 2008;1780:1325–1336. doi: 10.1016/j.bbagen.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Nadeau PJ, Charette SJ, Landry J. REDOX reaction at ASK1-Cys250 is essential for activation of JNK and induction of apoptosis. Mol Biol Cell. 2009;20:3628–3637. doi: 10.1091/mbc.E09-03-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adler V, et al. Regulation of JNK signaling by GSTp. EMBO J. 1999;18:1321–1334. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamata H, et al. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 33.Kemble DJ, Sun G. Direct and specific inactivation of protein tyrosine kinases in the Src and FGFR families by reversible cysteine oxidation. Proc Natl Acad Sci USA. 2009;106:5070–5075. doi: 10.1073/pnas.0806117106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanks SK, Hunter T. Protein kinases 6. The eukaryotic protein kinase superfamily: Kinase (catalytic) domain structure and classification. FASEB J. 1995;9:576–596. [PubMed] [Google Scholar]
- 35.Ohori M, et al. Role of a cysteine residue in the active site of ERK and the MAPKK family. Biochem Biophys Res Commun. 2007;353:633–637. doi: 10.1016/j.bbrc.2006.12.083. [DOI] [PubMed] [Google Scholar]
- 36.McCubrey JA, Lahair MM, Franklin RA. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid Redox Signaling. 2006;8:1775–1789. doi: 10.1089/ars.2006.8.1775. [DOI] [PubMed] [Google Scholar]
- 37.Go YM, Gipp JJ, Mulcahy RT, Jones DP. H2O2-dependent activation of GCLC-ARE4 reporter occurs by mitogen-activated protein kinase pathways without oxidation of cellular glutathione or thioredoxin-1. J Biol Chem. 2004;279:5837–5845. doi: 10.1074/jbc.M307547200. [DOI] [PubMed] [Google Scholar]
- 38.Lee YJ, et al. Oxidative stress-induced apoptosis is mediated by ERK1/2 phosphorylation. Exp Cell Res. 2003;291:251–266. doi: 10.1016/s0014-4827(03)00391-4. [DOI] [PubMed] [Google Scholar]
- 39.Ge B, et al. MAPKK-independent activation of p38alpha mediated by TAB1-dependent autophosphorylation of p38alpha. Science. 2002;295:1291–1294. doi: 10.1126/science.1067289. [DOI] [PubMed] [Google Scholar]
- 40.Kang YJ, Seit-Nebi A, Davis RJ, Han J. Multiple activation mechanisms of p38alpha mitogen-activated protein kinase. J Biol Chem. 2006;281:26225–26234. doi: 10.1074/jbc.M606800200. [DOI] [PubMed] [Google Scholar]
- 41.Keyse SM. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr Opin Cell Biol. 2000;12:186–192. doi: 10.1016/s0955-0674(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 42.Salmeen A, Barford D. Functions and mechanisms of redox regulation of cysteine-based phosphatases. Antioxid Redox Signaling. 2005;7:560–577. doi: 10.1089/ars.2005.7.560. [DOI] [PubMed] [Google Scholar]
- 43.Tonks NK. Redox redux: Revisiting PTPs and the control of cell signaling. Cell. 2005;121:667–670. doi: 10.1016/j.cell.2005.05.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.