Abstract
Contractile force transduction by myosin II derives from its assembly into bipolar filaments. The coiled-coil tail domain of the myosin II heavy chain mediates filament assembly, although the mechanism is poorly understood. Tail domains contain an alternating electrostatic repeat, yet only a small region of the tail (termed the “assembly domain”) is typically required for assembly. Using computational analysis, mutagenesis, and electron microscopy we discovered that the assembly domain does not function through self-interaction as previously thought. Rather, the assembly domain acts as a unique, positively charged interaction surface that can stably contact multiple complementary, negatively charged surfaces in the upstream tail domain. The relative affinities of the assembly domain to each complementary interaction surface sets the characteristic molecular staggers observed in myosin II filaments. Together these results explain the relationship between the charge repeat and assembly domain in stabilizing myosin bipolar filaments.
Keywords: contraction, cytokinesis, macromolecular assembly
Contractile forces are fundamentally important on many scales of biological function, from skeletal muscle movement (1) to cell division and motility (2, 3). On a molecular level, contraction derives from the bipolar structure of the myosin II filament in which the actin-binding motor domains are oriented at opposing filament ends. The assembly of myosin II molecules into force-producing bipolar filaments is dependent on the myosin II heavy chain. The myosin heavy chain is composed of a head region (actin binding, ATPase, force generation), a neck region (light chain binding), and a large coiled-coil tail domain that mediates dimerization of the heavy chains and is sufficient for bipolar filament assembly (4). The sequence of the tail domain reveals a heptad repeat that is characteristic of coiled-coils, and a 28-residue charge repeat of alternating zones of negatively and positively charged residues that somehow mediate electrostatic interactions between tail domains in the filament backbone (5–7). Indeed the widely reported molecular staggers of approximately 14.3 nm and 43 nm found in filaments from various myosin II isoforms correspond to favorable overlaps of this charge repeat (7, 8).
Despite the conserved charge repeat along the entire tail, most tail segments are not competent to form filaments, demonstrating that more information is needed to specify filament assembly. A small region of the tail (termed the assembly domain), usually near the COOH-terminus, mediates tail domain assembly in diverse organisms (9–16). However, the precise features that make an assembly domain unique in its ability to promote filament assembly have yet to be identified, and the role of assembly domains in tail–tail interactions within the bipolar filament is poorly understood.
We examined the mechanism of Drosophila nonmuscle myosin II filament assembly, because we have recently identified its assembly domain (16) and its overall sequence is highly conserved with vertebrate myosin IIs (Fig. S1) (17, 18). To identify elements within myosin II that are critical for filament assembly we combined computational analysis of a tail domain model with comprehensive mutagenesis of the assembly domain. This work has allowed us to identify critical interactions in important binary tail–tail interactions and explain the multiplicity of interactions that occur in a myosin bipolar filament. Based on these studies we propose a model in which multiple stable overlaps of the tail are possible that correspond to the interaction of a specific positively charged assembly domain region with discrete negatively charged clusters.
Results and Discussion
A Strongly Positively Charged Region Within the Assembly Domain Stabilizes Tail–Tail Interactions.
The Drosophila nonmuscle myosin II assembly domain encompasses residues 1849–1940 and was identified as a tail region that is necessary and sufficient for myosin filament assembly (16). The simplest view of the assembly domain is that it possesses a unique ability to self-interact, and this self-interaction is critical for priming the remaining tail for association at a particular register of the charge repeat. Thus, we first sought to determine the favorability of self-interaction as a potential mechanism for assembly domain function in myosin II filament assembly. Although several heuristic methods have been used to examine the contribution of the charge repeat to tail–tail interaction energy (13, 19, 20), we sought to calculate the explicit electrostatic energy associated with all possible parallel and antiparallel configurations. We constructed a three-dimensional structural model of the nonmuscle myosin II heavy chain from Drosophila (zipper) by threading its sequence onto a highly regular coiled-coil [the trigger site of the actin crosslinker cortexillin I (21); PDB ID: 1D7M]. The resulting structural model (Fig. 1A) has a pitch of approximately 1.45 Å per residue, which is consistent with myosin II tail domains observed by electron microscopy (7, 14, 22).
Fig. 1.
Modeling zipper electrostatic tail–tail interactions. (A) Structural model of zipper tail domain (1111–1968) based on a highly regular coiled-coil (the trigger site of the actin crosslinker cortexillin I; Burkhard et al., 2000; PDB ID: 1D7M). Skip residues were removed to keep the heptad repeat in register (see Materials and Methods). Electrostatic potential mapping onto the structure reveals the relative concentration of negative and positive charge along the tail. The scale ranges from -19 (red) to 19 (blue) KbT/ec. (B) Electrostatic interaction energy of two assembly domains (1849–1940) in parallel and antiparallel orientations. These calculations were performed using Coulomb’s law as implemented in Advanced Poisson–Boltzman Solver. Interaction energy is the difference in energy of a particular configuration compared to two isolated tail domains. Asterisks mark the configurations shown in the following panel. (C) Structural models of stable binary assembly domain interactions in parallel and antiparallel orientations. The assembly domain is marked with a black bar. Note that the electrostatic surface maps were calculated for isolated assembly domains. (D) Electrostatic interaction energy of two tail fragments (1744–1968) in parallel and antiparallel orientations. In addition to the wild-type sequence, interaction energies for 4D (basic residues 1880, 1883, 1887, and 1890 mutated to aspartic acid) and 3K (acidic residues 1780, 1781, and 1784 mutated to lysine) mutations are shown. Asterisks mark the configurations shown in the following panel. (E) Structural models of the most stable, binary tail fragment interactions for the 1744–1968 fragment in parallel and antiparallel orientations. Note that the electrostatic surface maps were calculated for isolated assembly domains.
To determine tail–tail electrostatic interaction energetics, we positioned two tail domain coiled-coils adjacent to one another with a center–center spacing of 20 Å (23) and calculated the electrostatic interaction energy for parallel and antiparallel orientations using Coulomb’s law as implemented in Advanced Poisson–Boltzman Solver (24). For each orientation, we calculated the energy for staggers ranging from completely overlapped (zero offset) to completely separated at 5 Å increments. For antiparallel configurations, we also calculated energies at negative staggers.
The electrostatic interaction energy of two assembly domains displays an oscillatory pattern resulting from alternating favorable and nonfavorable overlaps of the 28-residue charge repeat (Fig. 1B). Both parallel and antiparallel assembly domain orientations have energy minima that correspond to particularly stable staggers but also have slightly less favorable alternative staggers (Fig. 1 B and C). However, in the context of a larger tail fragment, 1744–1968, assembly domains do not self-interact in the most stable parallel and antiparallel tail–tail configurations (Fig. 1 D and E). Instead, each assembly domain contacts a conserved, negatively charged flanking region directly NH2-terminal to the assembly domain of its interacting partner. Thus, although the assembly domain is the smallest region of the tail that can form stable interactions, assembly domains may not interact with one another in intact myosin II.
The assembly domain contains a region with the highest concentration of positive charge in the tail. To test the importance of this region in forming stable staggers, we computationally mutated residues within the 1744–1968 tail model and recalculated tail–tail interaction energies. As shown in Fig. 1D, reversing the charge of the positive region (4D: basic residues 1880, 1883, 1887, and 1890 mutated to aspartic acid) leads to a loss of any stable configurations, whereas mutation of other regions does not. Reversing the charge of the negatively charged flanking region (3K: acidic residues 1780, 1781, and 1784 mutated to lysine) results in multiple staggers with stabilities approximately equal to one another but less stable than the primary stagger seen in wild type. This observation suggests that the role of the assembly domain is to provide an essential positively charged region required for tail–tail interactions, whereas the upstream flanking region sets the stagger in the filament by stabilizing one particular stagger relative to others.
Finally, we examined the interactions of the full 858-residue tail domain (Fig. S1). Although the full domain is significantly larger than the 1744–1968 segment, the most stable antiparallel staggers are identical. In addition to the 28-residue oscillation, we observe a longer periodicity of approximately 286 Å in both parallel and antiparallel orientations (Fig. S1). This longer periodicity has been suggested to be important for filament formation (20, 25) and would favor molecular staggers of odd multiples of 143 Å, which have been observed in myosin II filaments and paracrystals (7, 23, 26, 27). Similar to the tail fragment 1744–1968, one particularly favorable antiparallel stagger of approximately 850-Å offset (395-Å overlap of tail domains) is present with other less favorable alternative overlaps (Fig. S1). The 39.5-nm antiparallel tail overlap is close to the widely reported 43–45-nm antiparallel tail overlap for other myosin IIs, and the discrepancy may reflect the absence of the nonhelical tailpiece from our structural model (19, 22, 28–31).
Interestingly, it appears that the interaction of parallel full-length tail domains is generally unfavorable, though energy minima are observed at regular intervals (Fig. S1), which correspond to 14.3-nm and 43-nm staggered tails observed in myosin II filaments (23, 26, 27). In contrast, parallel interactions are energetically favorable in the tail fragment 1744–1968 (Fig. 1D). One possible explanation for this discrepancy is that parallel interactions involving the NH2-terminal portion of the tail domain are less favorable than the COOH-terminal portion. Less favorable NH2-terminal interactions could lead to “flaring” of head regions away from the filament backbone as observed in smooth muscle myosin II (32), which may be a common feature of myosin II filaments.
Given the high sequence conservation of zipper with other metazoan myosin IIs (Fig. S1) and the apparent conservation of molecular staggers between interacting tails, we predict that this model is general for metazoan nonmuscle myosin II. A specific stagger has been proposed for human nonmuscle myosin IIb (19) that corresponds to the most stable zipper stagger as predicted by the electrostatic calculations. We analyzed the energetics of tail–tail interactions of human nonmuscle myosin IIb and found a nearly identical pattern of stable staggers as in zipper (Figs. S1 and S2). Like zipper, the most favorable antiparallel tail overlap is 39.5 nm, and parallel interactions are generally unfavorable, with energy minima at intervals corresponding to 14.3-nm and 43-nm staggered tails (Fig. S2). Taken together, metazoan nonmuscle myosin IIs appear to utilize a conserved filament assembly mechanism. In the following sections we experimentally test specific features of this model, using a combination of mutagenesis, tail association assays, and electron microscopy.
Positively Charged Regions Within the Assembly Domain Are Critical for Filament Assembly.
The model described above predicts that the critical element of the assembly domain is a small, highly positively charged region that can interact with multiple negatively charged regions within the tail domain to yield stable overlaps. To identify regions within the assembly domain that are required for assembly, we made small deletions within the context of a larger fragment, 1744–2011 (the coiled-coil structure becomes unstable for fragments smaller than the assembly domain). We deleted 28 residue segments to preserve the heptad repeat and the 28-residue charge repeat (Fig. 2). We tested these tail domain constructs for their ability to form filaments using a salt-dependent filament assembly assay that exploits the fact that oligomers sediment upon centrifugation, but unassembled coiled-coil dimers remain soluble (16, 25). For the tail fragment 1744–2011 filament formation is highly salt-dependent, only assembling at intermediate salt concentrations (we did not interpret differences in sedimentation when no salt was present, conditions that may promote nonspecific aggregation) (Fig. 2B and Fig. S3). Deletion of the first 1/3 of the assembly domain (Δ1850–1877) does not alter the ability of this fragment to sediment in a salt-dependent manner. Deletion of residues 1878–1905 and 1913–1940, which contains the bulk of positive charge, resulted in solubility throughout the salt concentration range, indicating a loss of filament assembly (Fig. 2B and Fig. S3). Truncation of the tail region COOH-terminal to the assembly domain slightly alters the salt-dependent characteristics of filament assembly but does not significantly alter filament stability (Fig. 2B). Thus, the C-terminal 2/3 of the assembly domain (1878–1940) appears to be critical for filament assembly, whereas the remainder is dispensable. This region contains the positively charged region of the tail domain (Fig. 1) and is conserved with assembly domains discovered in other myosin IIs, suggesting that assembly domain function in the formation of different types of filaments is conserved across myosin II isoforms and species (13, 14, 16, 19).
Fig. 2.
Identification of conserved regions and amino acids within the assembly domain that are important for filament assembly. (A) Structural model of the tail fragment (1744–1968) used for deletion and mutational analyses. Gray bar identifies the assembly domain (1849–1940). Black bar denotes the essential region for filament assembly identified by internal deletion analysis. (B) Quantification of solubility of myosin II tail domains with regions removed. Error bars represent one SD from three independent experiments. (C–E) Quantification of solubility properties of mutation-containing tail fragments organized by location of mutation in distinct tail regions. Error bars represent one SD from three independent measurements.
Basic Residues Within the Assembly Domain Are Required for Tail–Tail Interactions.
Our electrostatic calculations predict that basic residues within the assembly domain are critical for filament assembly. To test this prediction, we comprehensively reversed the charge at individual sites within the assembly domain and tested the proteins for the ability to assemble. Like other coiled-coils, the tail domain features a heptad repeat pattern of residues (Fig. S3, a–g). Residues in the a and d positions of the amphipathic α-helices form a buried hydrophobic seam that constitutes the heavy chain dimer interface, whereas the other positions are generally polar or charged and form the interaction surfaces for filament assembly (33, 34). To identify residues involved in tail–tail interactions we reversed the charge of residues primarily in the b, c, and f positions to potentially disrupt electrostatic interactions between tail fragments. We constructed the mutations in the context of the 1744–2011 tail fragment because it is predicted to form tail–tail interactions with staggers similar to the intact tail domain (Figs. 1 D and E and Fig. S1).
Interestingly, we found that while most residues within the assembly domain are not essential for tail–tail interactions, mutation of specific residues completely disrupts filament stability (Fig. 2 and Fig. S3). Importantly, mutation of these conserved residues in human nonmuscle myosin II also disrupts filament assembly (Fig. S2). Essential residues are concentrated within a 15-residue positively charged region (1880–1894), as predicted by the electrostatic calculations (Fig. 1D). Within this segment, we also found that mutation of an acidic residue (E1891) and an apolar residue (M1894) partially disrupts filament assembly (Fig. 2D). This suggests sequence-specific interactions between tail domains, in addition to gross charge, is important for filament assembly and could add a layer of specificity to preferred tail domain staggers.
Acidic Residues in the Upstream Flanking Region Specify the Molecular Stagger of Tail–Tail Interactions.
Another prediction by the electrostatic calculations is that specific acidic residues within the conserved flanking region are not required for assembly because the positively charged region can interact with multiple negatively charged regions. Thus, alteration of acidic residues within the upstream negatively charged flanking region would lead to a shift in the stagger but not loss of assembly. We found that a tail fragment (1800–2011), which does not contain the complementary, negatively charged flanking region, is competent for filament assembly (Fig. 3A). Furthermore, mutation of either of the acidic residue clusters (1749–1750; 1780–1792) within the flanking region had no effect on filament assembly (Fig. 3A). These results are consistent with the ability of the essential, positively charged region within the assembly domain to interact with multiple negatively charged regions. Interpreting these results in the context of the interaction model leads to the prediction that these mutations cause the molecular stagger to shift to another stable overlap to compensate for the loss of the primary interaction surface.
Fig. 3.
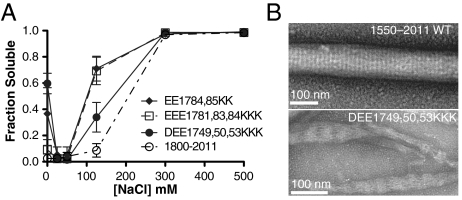
Mutation of negative charge patches lead to shift in stable interaction stagger. (A) Quantification of filament solubility of tail fragments containing deletion or mutation of upstream residues. Error bars represent SD from three independent measurements. (B) Electron micrographs of negatively stained wild-type and mutated tail fragment (1550–2011) assemblies.
To test for shifts in filament stagger, we imaged wild-type and mutant tail fragments using transmission electron microscopy. Myosin II tail fragments that assemble can form ordered aggregates known as paracrystals with repeats of 14.3 nm and 43 nm in low-salt buffers (4, 35). Native tail–tail interactions are important for paracrystal formation and packing (35, 36). The 1744–2011 fragment did not grow large, uniform paracrystals suitable for this analysis. We therefore analyzed mutants in the context of a larger fragment (1550–2011) that forms large, ordered paracrystals with a uniform 14.3-nm periodicity (Fig. 3B). A tail fragment containing mutations in the conserved negatively charged flanking region (DEE1749, 50, 53KKK) formed larger aggregates robustly (Fig. 3A), but the aggregates were not well-ordered like wild type (Fig. 3B). The tangled paracrystalline structures formed by this mutant displayed an altered periodicity of approximately 45 nm, suggesting that the mutations altered the molecular packing of tail fragments, but did not disrupt assembly (Fig. 3). This is consistent with the ability of tail fragments to interact with alternative unnatural staggers when stable native staggers are disrupted.
Tail–Tail Interactions Identified by Filament Assembly Rescue.
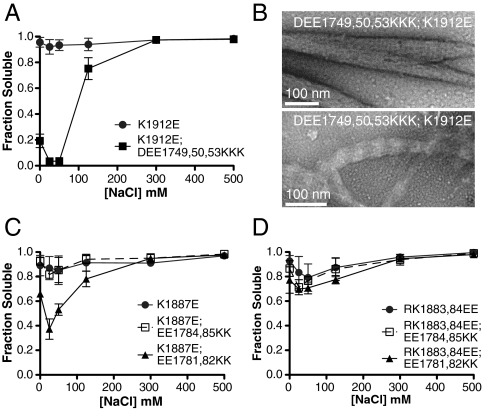
To further test the interactions predicted by the electrostatic calculations, we attempted to complement deleterious mutations with a second set of mutations to restore electrostatically favorable contacts and filament assembly (Fig. 4A). In the most stable overlap, a positively charged region (1880–1894) interacts with an upstream negatively charged region (1780–1792) in both the parallel and antiparallel orientations (Fig. 1E). A second interaction occurs between an essential residue K1912 and acidic residues between 1749 and 1753 in the antiparallel orientation, but this basic residue does not make significant electrostatic contacts in the parallel oriented interaction (Fig. 1E). As described, the basic residues in these putative interactions are necessary for filament assembly as mutation to acidic residues disrupts the interaction. We tested if mutation of the predicted interacting acidic residues to basic residues could complement these deleterious mutations, restoring assembly thereby identifying specific interaction sites. Mutation of acidic residues 1749, 1750, and 1753 to basic residues completely complemented the mutation of K1912 to an acidic residue (Fig. 4A). Because these residues only contact each other in the antiparallel orientation, it is likely that mutating K1912 disrupts antiparallel interactions and filament nucleation (32). Interestingly, two types of paracrystalline structures are formed by this double-mutant tail fragment (Fig. 4B). Both large, well-ordered paracrystals displaying a uniform 14.3-nm periodicity and tangled paracrystals displaying the altered 45-nm periodicity are observed at low salt (Fig. 4B). The multiple forms adopted by this mutant suggest that comutation of these residues can restore wild-type molecular packing but that this packing is less stable, leading to a distribution of molecular arrangements. Consistent with decreased stability of tail–tail packing, mutant paracrystals were sensitive to negative staining, which limited the contrast of electron micrographs (Fig. 4B).
Fig. 4.
Identification of interacting charge patches by filament assembly rescue. (A, C, and D) Quantification of filament solubility of tail fragments containing sets of complementary mutations. Error bars represent SD from three independent measurements. (B) Electron micrographs of two populations of negatively stained tail fragment (1550–2011) assemblies containing a pair of complementary mutations. Double-mutant filaments were less stable than their wild-type counterparts, which necessitated the use of less stain, accounting for the lower contrast in these images.
K1887 makes important contacts in both the parallel (E1784, E1785) and antiparallel (E1781, E1782, and potentially E1784) interactions of tail domains. Mutation of K1887 completely disrupts filament assembly, but comutation of residues involved in the antiparallel interaction restores assembly significantly (Fig. 4C). Comutation of parallel interacting residues (E1784, E1785) with K1887 does not restore filament assembly (Fig. 4C). Interestingly, comutation of upstream residues with a pair of inhibiting mutations (R1883, K1884) does not restore filament assembly (Fig. 4D), suggesting that both parallel and antiparallel interactions must be restored for proper filament assembly. Taken together, these results suggest that we have reasonably predicted the contacts made between two tail fragments in important binary tail–tail interactions.
Conclusions
Our results clarify the role of assembly domains in mediating tail–tail interactions. The assembly domain acts as a versatile interaction surface that promotes filament assembly and organization through interaction with complementary surfaces throughout the tail domain rather than through assembly domain self-interaction. We found that multiple configurations of tail–tail interactions are possible. Energetically favorable tail–tail interactions occur at positions characterized by the association of a highly positively charged region with upstream, negatively charged regions. The locations within the tail domain and relative affinities of these complementary surfaces result in the characteristic molecular staggers observed in myosin II filaments. The full-length tail domain displays one particularly favorable antiparallel interaction that likely serves as the nucleation interaction as well as one of the major tail–tail interactions within the myosin II filament. Less stable alternative antiparallel interactions along with parallel interactions could stabilize the relative shift between antiparallel dimers in the bipolar filament.
In this study we described conserved sequences in myosin II tail domains that mediate tail–tail interactions during myosin filament assembly. However, the nature of myosin II isoform-specific information that sets filament size and geometry remains unknown. One possible explanation is that tail–tail interactions of each myosin II isoform occur at similar molecular staggers but with a unique dependence on axial rotation in the context of the assembling filament. Alternatively, nonelectrostatic interactions may organize interacting tail domains into a particular, isoform-specific structure. We found that mutation of an apolar residue (M1894) partially disrupted filament assembly, suggesting that nonelectrostatic interactions play a significant, yet underappreciated, role in tail–tail interactions (Fig. 2D). Nevertheless, the model presented here provides significant constraints on the structure of the overall filament.
Materials and Methods
Cloning, Overexpression, and Purification.
Zipper regions were subcloned using a plasmid containing the full-length Zipper B sequence that was kindly provided by Dan Kiehart (37). Deletions were created using splicing by overlapping extension PCR. Zipper tail fragments were mutated using QuikChange™ Site Directed Mutagenesis Kit (Stratagene) according to the manufacturer’s instructions. All deletions and mutations were confirmed by DNA sequencing. Zipper regions were expressed in Escherichia coli using the pET-19b derivative pBH vector (38). Recombinant his-tagged fusion proteins were purified using Ni-NTA resin and standard protocols, which yielded > 90% pure protein. Ion-exchange chromatography was used to further purify proteins if necessary. The Ni-NTA purified proteins were dialyzed extensively against salt-free buffer (10 mM Tris, pH 8.0; 1 mM DTT; 1 mM EDTA) at 4 °C. Purity was established using SDS/PAGE. We confirmed that proteins that were unable to assemble into filaments were properly folded into coiled-coils by examining circular dichroism spectra.
Filament Assembly Assay.
The filament assembly assay was performed as previously described(16). Briefly, we incubated zipper tail constructs (10 μM) at a range of salt concentrations in assembly buffer (10 mM Tris-HCl, pH 8.0; 1 mM DTT; 1 mM EDTA) for 30 min at 4 °C followed by centrifugation at 100,000 × g for 30 min. We then separated the soluble and insoluble phases and loaded equal volumes for analysis by SDS/PAGE. We used ImageJ (NIH) to determine relative protein amounts from scanned, coomassie brilliant blue stained gels.
Electron Microscopy.
For paracrystal formation tail fragments (1 μM) were incubated in buffer (10 mM Tris-HCl, pH 8.0; 50 mM NaCl, 1 mM DTT) with the addition of 2 mM or 10 mM MgCl2 or CaCl2. For negative staining 10 μl samples were applied to 300-mesh carbon stabilized copper grids coated with formvar (#01753-F; Ted Pella), that were render hydrophilic by glow discharge in a partial vacuum, for 30 s. The grids were then washed with 6 drops of the same buffer and stained with 1–2% uranyl acetate for 30 s and dried. Electron micrographs were taken on an FEI Titan FEG-TEM electron microscope, at an acceleration voltage of 80 kV, with a Gatan 2K × 2K CCD for image capture.
Construction of Zipper Tail Structural Model and Electrostatic Calculations.
The structural model for the zipper tail domain was created by duplicating and aligning a repeatable coiled-coil subunit to generate a long, straight coiled-coil rod. The repeatable subunit was derived from the structure of the coiled-coil trigger site of the actin crosslinker cortexillin I from Dictyostelium discoideum (21) (1D7M). We then threaded the zipper tail sequence onto this structure with skip residues removed to keep the heptad repeat in register. Skip residues are residues that disrupt the continuity of heptad repeats and are compensated for by localized over- or under-winding of the coiled-coil fold, which is difficult to model. The residue side chains were energy minimized to yield a feasible zipper tail domain structure. An identical method was used to construct the model for human myosin II.
We generated models of parallel or antiparallel myosin rod pairs (with the monomer model described above) using PyMol. The rods were placed 2 nm apart (center to center) at the desired overlap. To avoid steric clashes, we subjected amino acid side chains in the model to 20 cycles of conjugate gradient energy minimization using CNS. Charge states were assigned with pdb2pqr and electrostatic energies were calculated with APBS (24) using a dielectric of 80. The resulting energy profile was normalized against positions of the two tails not interacting in solution to render the excess energy of interaction. Energetic effects of axial rotations were negligible compared to altering tail–tail overlap positions, presumably due to the rotational pseudosymmetry of the coiled-coil and the energy minimization of amino acid side chains. Thus, we discarded this information from the model for simplicity.
Supplementary Material
Acknowledgments.
We thank Brad Nolen for reviewing the manuscript and Natasha Fewkes for help at the inception of the project. This study was supported by National Institutes of Health Grant GM068032 (K.E.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007025107/-/DCSupplemental.
References
- 1.Huxley AF. Cross-bridge action: Present views, prospects, and unknowns. J Biomech. 2000;33:1189–1195. doi: 10.1016/s0021-9290(00)00060-9. [DOI] [PubMed] [Google Scholar]
- 2.Warrick HM, Spudich JA. Myosin structure and function in cell motility. Annu Rev Cell Biol. 1987;3:379–421. doi: 10.1146/annurev.cb.03.110187.002115. [DOI] [PubMed] [Google Scholar]
- 3.Robinson DN, Spudich JA. Mechanics and regulation of cytokinesis. Curr Opin Cell Biol. 2004;16:182–188. doi: 10.1016/j.ceb.2004.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowey S, Slayter HS, Weeds AG, Baker H. Substructure of the myosin molecule. I. Subfragments of myosin by enzymic degradation. J Mol Biol. 1969;42:1–29. doi: 10.1016/0022-2836(69)90483-5. [DOI] [PubMed] [Google Scholar]
- 5.Parry DA. Structure of rabbit skeletal myosin. Analysis of the amino acid sequences of two fragments from the rod region. J Mol Biol. 1981;153:459–464. doi: 10.1016/0022-2836(81)90290-4. [DOI] [PubMed] [Google Scholar]
- 6.McLachlan AD, Karn J. Periodic charge distributions in the myosin rod amino acid sequence match cross-bridge spacings in muscle. Nature. 1982;299:226–231. doi: 10.1038/299226a0. [DOI] [PubMed] [Google Scholar]
- 7.Atkinson SJ, Stewart M. Molecular interactions in myosin assembly. Role of the 28-residue charge repeat in the rod. J Mol Biol. 1992;226:7–13. doi: 10.1016/0022-2836(92)90118-4. [DOI] [PubMed] [Google Scholar]
- 8.Craig R, Woodhead JL. Structure and function of myosin filaments. Curr Opin Struct Biol. 2006;16:204–212. doi: 10.1016/j.sbi.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Nyitray L, et al. The proteolytic substructure of light meromyosin. Localization of a region responsible for the low ionic strength insolubility of myosin. J Biol Chem. 1983;258:13213–13220. [PubMed] [Google Scholar]
- 10.Cross RA, Vandekerckhove J. Solubility-determining domain of smooth muscle myosin rod. FEBS letters. 1986;200:355–360. doi: 10.1016/0014-5793(86)81168-1. [DOI] [PubMed] [Google Scholar]
- 11.O’Halloran TJ, Ravid S, Spudich JA. Expression of Dictyostelium myosin tail segments in Escherichia coli: Domains required for assembly and phosphorylation. J Cell Biol. 1990;110:63–70. doi: 10.1083/jcb.110.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sinard JH, Rimm DL, Pollard TD. Identification of functional regions on the tail of Acanthamoeba myosin-II using recombinant fusion proteins. II. Assembly properties of tails with NH2- and COOH-terminal deletions. J Cell Biol. 1990;111:2417–2426. doi: 10.1083/jcb.111.6.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shoffner JD, De Lozanne A. Sequences in the myosin II tail required for self-association. Biochem Bioph Res Co. 1996;218:860–864. doi: 10.1006/bbrc.1996.0153. [DOI] [PubMed] [Google Scholar]
- 14.Sohn RL, et al. A 29 residue region of the sarcomeric myosin rod is necessary for filament formation. J Mol Biol. 1997;266:317–330. doi: 10.1006/jmbi.1996.0790. [DOI] [PubMed] [Google Scholar]
- 15.Cohen C, Parry DA. A conserved C-terminal assembly region in paramyosin and myosin rods. J Struct Biol. 1998;122:180–187. doi: 10.1006/jsbi.1998.3983. [DOI] [PubMed] [Google Scholar]
- 16.Liu SL, Fewkes N, Ricketson D, Penkert RR, Prehoda KE. Filament-dependent and -independent localization modes of Drosophila non-muscle myosin II. J Biol Chem. 2008;283:380–387. doi: 10.1074/jbc.M703924200. [DOI] [PubMed] [Google Scholar]
- 17.Hodge T, Cope MJ. A myosin family tree. J Cell Sci. 2000;113:3353–3354. doi: 10.1242/jcs.113.19.3353. [DOI] [PubMed] [Google Scholar]
- 18.Berg JS, Powell BC, Cheney RE. A millennial myosin census. Mol Biol Cell. 2001;12:780–794. doi: 10.1091/mbc.12.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakasawa T, et al. Critical regions for assembly of vertebrate nonmuscle myosin II. Biochemistry. 2005;44:174–183. doi: 10.1021/bi048807h. [DOI] [PubMed] [Google Scholar]
- 20.Straussman R, Squire JM, Ben-Ya’acov A, Ravid S. Skip residues and charge interactions in myosin II coiled-coils: Implications for molecular packing. J Mol Biol. 2005;353:613–628. doi: 10.1016/j.jmb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Burkhard P, Kammerer RA, Steinmetz MO, Bourenkov GP, Aebi U. The coiled-coil trigger site of the rod domain of cortexillin I unveils a distinct network of interhelical and intrahelical salt bridges. Structure. 2000;8:223–230. doi: 10.1016/s0969-2126(00)00100-3. [DOI] [PubMed] [Google Scholar]
- 22.Kendrick-Jones J, Szent-Gyorgyi AS, Cohen C. Segments from vertebrate smooth muscle myosin rods. J Mol Biol. 1971;59:527–529. doi: 10.1016/0022-2836(71)90316-0. [DOI] [PubMed] [Google Scholar]
- 23.Niederman R, Pollard TD. Human platelet myosin. II. In vitro assembly and structure of myosin filaments. J Cell Biol. 1975;67:72–92. doi: 10.1083/jcb.67.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc Natl Acad Sci USA. 2001;98:10037–10041. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hostetter D, et al. Dictyostelium myosin bipolar thick filament formation: importance of charge and specific domains of the myosin rod. PLoS Biol. 2004;2:e356. doi: 10.1371/journal.pbio.0020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woodhead JL, et al. Atomic model of a myosin filament in the relaxed state. Nature. 2005;436:1195–1199. doi: 10.1038/nature03920. [DOI] [PubMed] [Google Scholar]
- 27.Zoghbi ME, Woodhead JL, Moss RL, Craig R. Three-dimensional structure of vertebrate cardiac muscle myosin filaments. Proc Natl Acad Sci USA. 2008;105:2386–2390. doi: 10.1073/pnas.0708912105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onishi H, Wakabayashi T. Electron microscopic studies on myosin molecules from chicken gizzard muscle III. Myosin dimers. J Biochem. 1984;95:903–905. doi: 10.1093/oxfordjournals.jbchem.a134686. [DOI] [PubMed] [Google Scholar]
- 29.Trybus KM, Lowey S. Conformational states of smooth muscle myosin. Effects of light chain phosphorylation and ionic strength. J Biol Chem. 1984;259:8564–8571. [PubMed] [Google Scholar]
- 30.Trybus KM, Lowey S. Assembly of smooth muscle myosin minifilaments: Effects of phosphorylation and nucleotide binding. J Cell Biol. 1987;105:3007–3019. doi: 10.1083/jcb.105.6.3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barylko B, Zurek B, Jockusch BM. Brain myosin assembly: Characterization of aggregation-competent fragments by antibodies. Eur J Cell Biol. 1989;50:41–47. [PubMed] [Google Scholar]
- 32.Cross RA, Geeves MA, Kendrick-Jones J. A nucleation—elongation mechanism for the self-assembly of side polar sheets of smooth muscle myosin. EMBO J. 1991;10:747–756. doi: 10.1002/j.1460-2075.1991.tb08006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lupas A. Coiled coils: New structures and new functions. Trends Biochem Sci. 1996;21:375–382. [PubMed] [Google Scholar]
- 34.Burkhard P, Stetefeld J, Strelkov SV. Coiled coils: A highly versatile protein folding motif. Trends Cell Biol. 2001;11:82–88. doi: 10.1016/s0962-8924(00)01898-5. [DOI] [PubMed] [Google Scholar]
- 35.Bennett PM. The structure of spindle-shaped paracrystals of light meromyosin. J Mol Biol. 1981;146:201–221. doi: 10.1016/0022-2836(81)90432-0. [DOI] [PubMed] [Google Scholar]
- 36.Chowrashi PK, Pepe FA. Light meromyosin paracrystal formation. J Cell Biol. 1977;74:136–152. doi: 10.1083/jcb.74.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su Z, Kiehart DP. Protein kinase C phosphorylates nonmuscle myosin-II heavy chain from Drosophila but regulation of myosin function by this enzyme is not required for viability in flies. Biochemistry. 2001;40:3606–3614. doi: 10.1021/bi010082j. [DOI] [PubMed] [Google Scholar]
- 38.Peterson FC, Penkert RR, Volkman BF, Prehoda KE. Cdc42 regulates the Par-6 PDZ domain through an allosteric CRIB-PDZ transition. Mol Cell. 2004;13:665–676. doi: 10.1016/s1097-2765(04)00086-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.