Abstract
Platelets constitutively express class B scavenger receptors CD36 and SR-BI, two closely related pattern recognition receptors best known for their roles in lipoprotein and lipid metabolism. The biological role of scavenger receptors in platelets is poorly understood. However, in vitro and in vivo data suggest that class B scavenger receptors modulate platelet function and contribute significantly to thrombosis by sensing pathological or physiological ligands, inducing prothrombotic signaling, and increasing platelet reactivity. Platelet CD36 recognizes a novel family of endogenous oxidized choline phospholipids that accumulate in plasma of hyperlipidemic mice and in plasma of subjects with low HDL levels. This interaction leads to the activation of specific signaling pathways and promotes platelet activation and thrombosis. Platelet SR-BI, on the other hand, plays a critical role in the induction of platelet hyper-reactivity and accelerated thrombosis in conditions associated with increased platelet cholesterol content. Intriguingly, oxidized HDL, aSR-BI ligand, can suppress platelet function. These recent findings demonstrate that platelet class B scavenger receptors play roles in thrombosis in dyslipidemia and may contribute to acute cardiovascular events in vivo in hypercholesterolemia.
Introduction
Scavenger receptors (SR) are a group of structurally heterologous cell surface receptors that share an ability to recognize chemically modified or oxidized forms of LDL. SR belong to a wider family of pattern recognition receptors that mediate the innate immune host response which includes Toll-like receptors. Platelets express several SR including class B scavenger receptors CD36 and SR-BI, two closely related multiligand receptors, best known for their roles in lipoprotein and lipid metabolism1. Other platelet SR include LOX-1 and CD68 1. Expression of class B SR in platelets is mainly constitutive while other receptors are rapidly exposed upon platelet activation. Ligands for these receptors can be roughly divided into three groups, physiological ligands, pathological endogenous (changed self) ligands, and pathological exogenous ligands. Pathological endogenous ligands for platelet SR may be present in circulation in a number of pathophysiological states related to dyslipidemia and oxidative stress. Pathological exogenous ligands may be present in cases of infections. The biological role of SR in platelets is not understood yet. However, evidence is accumulating that SR contribute significantly to thrombosis by sensing pathological or physiological ligands, inducing prothrombotic signaling, and increasing platelet reactivity. This in turn may lead to thrombosis in the presence of threshold concentrations of agonists. Platelet hyper-reactivity or increased platelet response to agonists is associated with augmented platelet adhesion, integrin activation, and aggregation 2–4. Subjects with increased measures of platelet reactivity are at increased prospective risk for coronary events and death 3–7. The pathophysiological significance of prothrombotic effects of platelet-hyper-reactivity may be very significant 8. The mechanisms responsible for enhancing platelet reactivity in vivo during dyslipidemia are gradually emerging, particularly due to the availability of novel murine knockout models, but these mechanisms are still poorly understood. The data demonstrating that class B scavenger receptors modulate platelet reactivity in conditions of hyperlipidemia and oxidative stress and contribute to cardiovascular events are reviewed below.
Platelet CD36, oxidized phospholipids, and thrombosis
CD36 is a multifunctional cellular receptor with broad ligand specificity that is expressed on macrophages, platelets, microvascular endothelial cells, and other cells9. It is structurally comprised of two transmembrane and two cytoplasmic domains, as well as a large heavily glycosylated extracellular domain. CD36 regulates cellular adhesion and angiogenesis, serving as a receptor for thrombospondin. It serves as a scavenger receptor in macrophages, mediating uptake of apoptotic cells and modified lipoproteins, and participates in carbohydrate and lipid metabolism, modulating insulin resistance and long chain fatty acid transport10–15. CD36 has been implicated in a variety of pathologic conditions, including atherosclerosis, diabetes and cardiomyopathy10, 13, 16–17.
Even though multiple lines of evidence suggest that CD36 may play a role in platelet activation, earlier studies of platelet function isolated from CD36 deficient patients did not demonstrate a significant role for platelet CD36 in physiological conditions18–22. Since then, a number of various physiological and pathological ligands for CD36 have been identified raising a possibility that CD36 may play a role in platelet activation by pathological ligands. The incomplete list of CD36 ligands includes ghrelin, diacylated bacterial lipopeptide, lipoteichoic acid, phosphatydylserine, beta-amyloid, serum amyloid A, and specific oxidized phospholipids (oxPCCD36) 23–29. oxPCCD36 are generated when LDL or cellular phospholipids containing polyunsaturated fatty acids at the sn-2 position undergo oxidative attack. In general, oxidized phospholipids that are generated via mild physiologically relevant oxidative processes have been shown to possess multiple biological activities and are associated with an increased risk of cardiovascular events 30–33. oxPCCD36 represent a small fraction of total oxidized phospholipids that possess high affinity for CD36 29, 34. Increased concentrations of oxPCCD36 were detected at sites of oxidative stress such as human and animal atherosclerotic lesions 34–35. oxPCCD36 also accumulate in significant concentrations in plasma of hyperlipidemic apoE deficient and LDL receptor deficient mice 36. In humans a subset of patients with low HDL levels has plasma oxPCCD36 levels comparable to that of hyperlipidemic animals36. In macrophages, oxPCCD36 mediate uptake of oxidized LDL (oxLDL) via CD36 and, subsequently, foam cell formation. The roles of oxPCCD36 and CD36 in atherosclerosis were extensively reviewed recently9, 31. Since oxPCCD36 are present in plasma, a hypothesis has been proposed that the interaction of oxPCCD36 with platelet CD36 could modulate platelet function, potentially inducing pro-thrombotic signals associated with hyperlipidemia36.
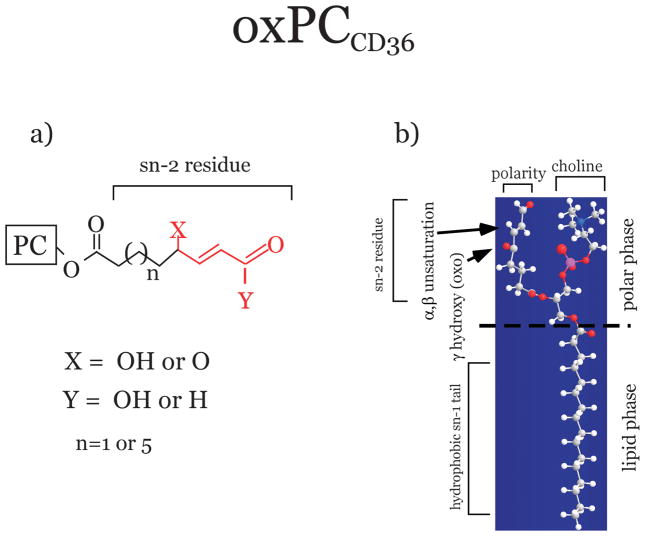
A defining feature of oxPCCD36 is an sn-2 acyl group that incorporates a terminal gamma-hydroxy (or oxo)-alpha, beta-unsaturated carbonyl 29 (Figure 1a). Recent studies of the conformation of oxPCCD36 in the membranehave revealed that due to its polarity the oxidized sn-2 fatty acid moiety of oxPCCD36 instead of being buried within the membrane, protrudes into the aqueous phase, rendering it accessible for the receptors (Figure 1b) 37. Studies using a series of model oxidized phospholipids demonstrated that a terminal negatively charged carboxylate at the sn-2 position of oxPC suffices to generate high binding affinity to both class B scavenger receptors, CD36 and SR-BI 38. The minimal binding domain of CD36 for oxPCCD36, has been identified recently 39. It contains two conserved lysine residues that are indispensable for the binding activity. Subsequently, interaction via an electrostatic mechanism of negatively charged sn-2 moiety of oxidized phosphatidylcholine, exposed on the phospholipid surface, with positively charged residues in CD36 has been shown to play a key role in high affinity binding 39. It should be mentioned that in order to be a ligand for CD36, oxidized sn-2 residues should be part of the otherwise intact PC, containing the sn-1 hydrophobic chain and the sn-3 hydrophilic phosphocholine or phosphatidic acid group38. Additional factors such as polarity, rigidity, optimal chain length of sn-2 and sn-3 positions, and a negative charge at the sn-3 position of phospholipids, further modulate the binding affinity 38. Studies with model oxPC suggested that, in addition to oxPCCD36, other oxidized phospholipids observed in vivo, possessing some but not all features of oxPCCD36, represent weak ligands for class B scavenger receptors 38.
Figure 1. Structure and conformation of oxidized phospholipids specific ligands for CD36 (oxPCCD36).
Truncated oxidized fatty acid esterified to the sn-2 position of oxidized phosphatidylcholine (PC) is shown. It contains a terminal aldehyde or carboxylic acid, a double bond in the βposition, and a ketone or alcohol in the γposition. The core structural motif conserved amongst the various oxidized PC species that are high affinity ligands for CD36 is shown in red. b) Unusual conformation of oxPCCD36 in the phospholipid membrane. The truncated polar sn-2 fatty acid moiety of oxPCCD36 protrudes into the aqueous phase, rendering it accessible for the receptors such as class B scavenger receptors.
In vitro studies have shown that oxPCCD36 bind to platelets via CD36, activate platelets, and sensitize platelets to other agonists at pathophysiological concentrations 36. In vivo relevance of these observations came from experiments with hyperlipidemic mice. A number of groups using several thrombosis models have demonstrated that hyperlipidemic apoE deficient mice or LDLR deficient mice do have a prothrombotic phenotype 36, 40–41. The platelet contribution to this phenotype has been established only recently. In vitro studies demonstrated that platelets of hyperlipidemic apoE deficient mice are hyper-reactive to physiological agonists 36, similar to platelets from hypercholesterolemic patients and other species of hypercholesterolemic animals 2, 42–44. It has been demonstrated that a factorconferring hyper-reactivity resides in hyperlipidemic plasma and identified as oxPCCD36 36. Genetic deletion of CD36 protected mice from hyperlipidemia-associated enhanced platelet reactivity and the pro-thrombotic phenotype. Moreover, studies in vitro have demonstrated that endogenous oxPCCD36 in human plasma modulate the platelet response to agonists via CD36 36. oxPCCD36 binding peptides significantly inhibited thrombosis in vivo in hyperlipidemic apoE−/− mice, and had only a weak effect in normolipidemic mice (S. Panigrahi and E. Podrez, unpublished data). Similar results were obtained using apoA-I mimetic peptide L-4F, capable of removing oxidized phospholipids from the plasma through direct binding 45. Interestingly, recent findings demonstrated that amyloids appearing as a result of protein misfolding induces platelet aggregation via an activation pathway mediated by CD36, p38 (MAPK), and thromboxane A2 46. It has been established that oxidized LDL contains amyloid-like structures suggesting that this may be another CD36 dependent pathway activated by oxidized LDL in platelets 47.
In resting platelets, membrane CD36 is palmitoylated and localized in caveolin negative cholesterol enriched lipid rafts which also contain several Src family tyrosine kinases 48–49. There are also data indicating that in these lipid rafts CD36 exists as a multiprotein complex containing, in addition to Src-family kinases, tetraspanin proteinCD9 and fibrinogen receptor integrin αIIbβ3 or laminin receptor integrin α6β1 48, 50–51. The CD36 enriched fraction of platelet membranes is also enriched in GPIb, another major platelet glycoprotein essential for normal platelet adhesion and clot formation at sites of vascular injury49. The functional significance of the association of CD36 with platelet glycoproteins is not clear even though it has been shown that signaling events induced via CD36 may lead to activation of αIIbβ3 36. Src-family kinases associated with CD36 in platelets include Fyn, Lyn, and c-Yes 48. Lyn and CD36 do not interact directly, but do so through a lipid mediator52, further pointing to the importance of raft-associated lipids in CD36-mediated signal transduction. It has been proposed that in resting platelets CD36-anchored Lyn is negatively regulated by Csk homologous kinase (CHK)53. Upon platelet activation CD36-anchored Lyn is released from suppression through the rapid translocation of CHK and subsequent dephosphorylation of its C-terminal negative regulatory tyrosine residue53.
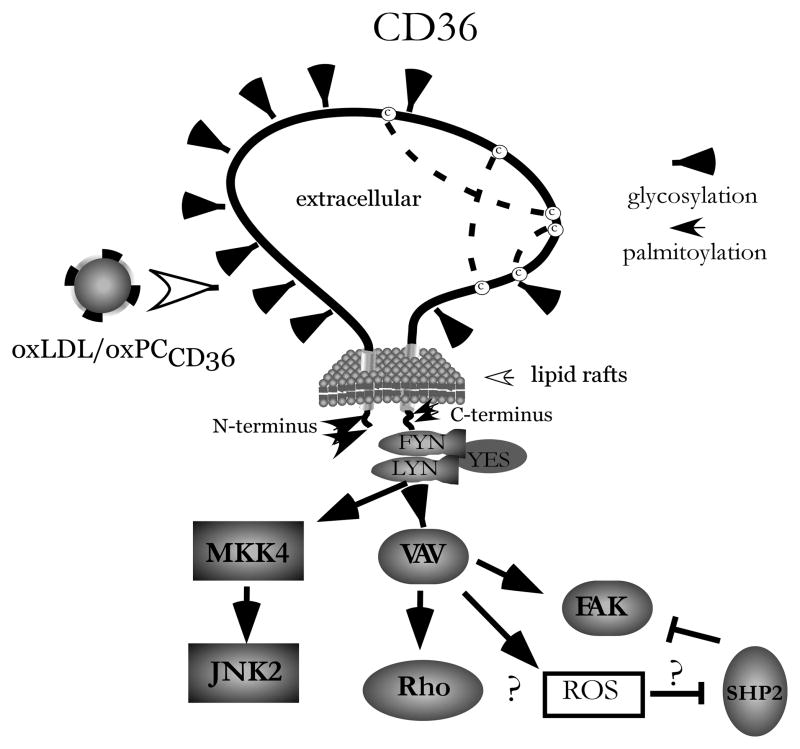
It has been long established that oxidized LDL induces platelet activation and adhesion and enhances platelet aggregation 54–55. Signaling pathways induced by oxLDL in platelets include activation of Rho/Rho kinase 56 and Src-family and Syk tyrosine kinases 57–58. A number of recent studies provided evidence of the signaling events initiated by oxLDL in platelets and mediated by CD36. One of the earlier events in this activation is the recruitment and activation of Lyn and Fyn to CD36 59. This is followed by the downstream activation of the protein kinases MKK4 and JNK2 60 (Figure 2). The significance of this pathway is supported by the observation that pharmacologic inhibition of JNK prolonged thrombosis times in wild type mice, but not in CD36 deficient mice 59. The downstream targets of Lyn and Fyn include the Vav family of proteins. Vavs are rapidly phosphorylated in platelets in a CD36-dependent manner 59. Silverstein et al reported that genetic deletion of Vav family members rescued the high-fat diet induced prothrombotic phenotype in mice in a way similar to the rescue by CD36 knockout 59. Downstream of CD36 is also p38 MAPK which, according to a recent report, requires the scavenger receptor, class A for maximal activation by oxLDL 61. Mechanisms described in other cell types may be relevant to platelet activation by oxLDL but require further studies. In macrophages, the generation of reactive oxygen species by oxLDL takes place through the formation of a complex between CD36 and a heterodimer of toll-like receptors (TLR4 and TLR6)62. Also in macrophages, oxLDL induces the phosphorylation of FAK and the inactivation of the phosphatase SHP2 that are responsible for cytoskeletal changes63. Even though oxPCCD36 is only one biologically active component in oxidized LDL we have observed that oxLDL and oxPCCD36 share activation pathways. Platelet activation induced by both oxLDL60 and oxPCCD36 can be prevented by Src-kinase inhibitors (A. Zimman and E. Podrez, unpublished data). Also, both oxLDL and oxPCCD36 activate PKC and induce the phosphorylation of Syk 57 (A. Zimman and E. Podrez unpublished data). However, it should be mentioned that a complex ligand such as oxidized LDL contains multiple biologically active components that can affect platelet function in a CD36 independent manner. Examples include oxidized lipids that increase intracellular Ca2+ through the PAF receptor64, lysophosphatidylcholine65, oxysterols and TBARS58, lysophosphatydic acid66 and amyloid-like structures 46.
Figure 2. Signal transduction pathways induced by oxLDL and mediated by CD36 in platelets.
oxPCCD36 in oxLDL induces specific signaling cascades after binding to the scavenger receptor CD36. (?) indicates pathways identified in macrophages only.
SR-BI, dyslipidemia, oxidative stress, and platelet function
SR-BI shares 30% sequence homology with CD36, has similar structural organization, and shares with CD36 several common ligands including oxidized lipoproteins and oxidized phospholipids 67–69. Recently the presence of this receptor has been demonstrated on the surface of human and murine platelets 70–71. The major physiological function of SR-BI is to serve as a receptor for HDL and to promote selectiveuptake of HDL cholesteryl esters in steroidogenic tissues and liver 72. In addition, SR-BI stimulates the bidirectional flux of free cholesterol between cells and lipoproteins, modifies membrane cholesterol distribution, and induces signaling events73. Since the antithrombotic function of HDL is well established, it seems logical that HDL interaction with platelet SR-BI may inhibit platelet function. However, previous reports on the effect of HDL on platelets have been contradictory 74–78. One explanation for the inconsistency may be the inadvertent oxidation of HDL during the isolation procedure. When extreme care was taken to prevent HDL oxidation during isolation, HDL or HDL subfractions HDL2 and HDL3 had no significant effect on the activation and aggregation responses of isolated platelets 71. This result looks particularly surprising, since HDL induces cholesterol efflux from cells, and in turn, the removal of cholesterol from platelets using cholesterol chelator MβCD is associated with a strong inhibition of platelet activation 79. Recently, Calkin et al showed that reconstituted HDL, but not HDL, does inhibit platelet activation in vivo 80. These authors suggested the reason for the difference is that reconstituted HDL has a higher capacity for cholesterol efflux than native HDL. Intriguingly, HDL oxidized by various pathways potently inhibits platelet activation and aggregation induced by physiological agonists 71. Studies using murine CD36−/− or SR-BI−/− platelets demonstrated that the anti-thrombotic activity of oxHDL requires binding to platelet SR-BI but not to CD36 and that oxidized phospholipids in oxHDL are likely to play a role in this activity 71. Studies involving SR-BI in platelets are very limited for now. In endothelial cells, one of the most important downstream targets of SR-BI is the activation of eNOS upon HDL binding to this receptor 81. c-Src and PDZK1 form a complex with SR-BI in this cell type. Our preliminary studies have shown the association of SR-BI to c-Src in platelets (Podrez et al, unpublished data). However, the effect of oxHDL on platelets is mediated by a pathway different from the eNOS/Akt pathway operating in endothelial cells71. A global survey of changes in protein phosphorylation by mass spectrometry 82 revealed that oxHDL induces multiple changes in the phosphorylation of platelet proteins, including a group of proteins associated with focal adhesion and tight junctions (A.Zimman and E.Podrez, unpublished data). Taken together this surprising finding suggests that lipid oxidation and oxidative stress appear to trigger not only prothrombotic effects mediated by CD36 but also to produce a previously unrecognized defense mechanism against thrombosis mediated by SR-BI.
The role of SR-BI deficiency and, specifically, platelet SR-BI deficiency in platelet function and thrombosis in vivo has been studied directly using SR-BI−/− mice. It should be taken into account that complete SR-BI deficiency is characterized by severe dyslipoproteinemia associated with platelet abnormalities, includinghigh unesterified cholesterol content, morphological changes, and thrombocytopenia. Platelets of SR-BI−/− mice display either reduced or normal responses to selected physiological agonists 83. This phenotype is particularly surprising taking into account that platelet enrichment in unesterified cholesterol has previously been directly linked to increased platelet reactivity 42, 84–86. To separate the effects of SR-BI dependent dyslipidemia and platelet specific SR-BI deficiency in platelet function, a bone marrow transplantation approach has been used. SR-BI−/− platelets isolated from normolipidemic wild type mice chimeras have normal platelet cholesterol content, normal morphology and show mild reduction in response to physiological agonists that was mostly evident at high concentrations of agonists87. In vivo studies demonstrated that wild type recipient mice with SR-BI deficient bone marrow have delayed thrombosis in ferric chloride induced carotid artery thrombosis assay, demonstrating that this seemingly mild defect still contributes significantly to thrombosis87. Wild type platelets isolated from dyslipidemic SR-BI−/− chimeric mice had cholesterol content increased by 60% and exhibited augmentation of activation responses to physiological agonists, in agreement with the published data on the impact of cholesterol on platelets 85. Results from this study suggest that the unusual platelet phenotype observed in SR-BI deficient mice is a reflection of the opposite effects of platelet SR-BI deficiency and platelet cholesterol overload on platelet reactivity. Most interestingly, in vitro studies revealed that SR-BI deficient platelets are remarkably resistant to hyper-reactivity induced by cholesterol overload, suggesting that platelet SR-BI may play a particularly prominent role in thrombosis in conditions of hyper-cholesterolemia. Indeed, in vivo studies confirmed that platelet SR-BI expression is prothrombotic in vivo in dyslipidemia associated with either apoE or SR-BI deficiency. These data strongly suggest that platelet SR-BI plays an important role in the widely known induction of platelet hyper-reactivity by high platelet cholesterol content 42, 84–86. It should be mentioned that the relevance of these studies to humans is not clear yet. No SR-BI deficient patients have been reported so far. However, a single nucleotide polymorphism of the SCARB1 gene was associated with changes in SR-BI protein levels, which in turn were inversely associated with HDL cholesterol levels and HDL particle size 88, resembling HDL changes in SR-BI−/− mice. SCARBI genetic polymorphisms in humans are associated with variations in plasma lipoproteins and coronary artery disease 89–91. Whether platelet reactivity contributes or not to variations in coronary artery disease is not known. However, SCARB1 polymorphisms show associations with the risk for peripheral artery disease, a condition where platelet dependent thrombosis plays a prominent role 92.
The molecular mechanism of SR-BI involvement in the regulation of platelet function is currently unknown. The function of SR-BI demonstrated in platelets is related to platelet unesterified cholesterol content. Thus it is of particular interest that the C-terminal transmembrane domain of SR-BI directly binds cholesterol 93. This cholesterol binding property is unique to SR-BI among three class B scavenger receptor family members. It has been proposed recently that SR-BI senses alterations in the cholesterol environment in the plasma 93, leading to changes in association of SR-BI with partner protein(s) critical to downstream signaling. We observed that the sensitivity of platelets to physiological agonists is upregulated with increasing platelet cholesterol content if platelets express SR-BI. In the absence of SR-BI expression, responses to ADP and convulxin are oblivious to platelet cholesterol content, and the increase in response to thrombin is significantly blunted87. One possibility is that enrichment in plasma membrane cholesterol leads to conformational changes in SR-BI, and upregulates SR-BI-dependent signaling pathways in platelets. On the other hand, membrane depletion of cholesterol inhibits the SR-BI dependent component of platelet signaling, leaving only a “basal” level of signaling. It should be noted, however, that SR BI expression in other cell types is associated with significant changes in the organization of cholesterol in cell membranes 94–95. Cell membrane cholesterol and, particularly, cholesterol in specialized membrane microdomains is important in the assembly of signaling complexes and plays an important role in signaling events in platelets96. SR-BI is highly enriched in caveolae in several cell types 97. Thus, it may affect signaling events in platelets in a less specific way, by changing membrane structure. In our unpublished studies we observed that cholesterol flux to HDL is reduced by ~50% in SR-BI deficient platelets compared to wild type platelets, potentially reflecting changes in cholesterol organization.
In conclusion
In the context of dyslipidemia and enhanced oxidative stress, platelet CD36 interaction with a small subset of endogenous oxidized phospholipids leads to pro-thrombotic signaling and accelerated thrombosis. The effects of other CD36 ligands on platelet function and thrombosis need further investigation. The role of SR-BI in platelet function is more complex. Liver deficiency of SR-BI leads to platelet hyper-reactivity and accelerated thrombosis due to high cholesterol content in platelets. Platelet SR-BI plays a critical role in the induction of platelet reactivity by high platelet cholesterol content. On the other hand, specific ligands that are generated in oxidative stress and interact with platelet SR-BI may induce suppression of platelet function. It is tempting to speculate that platelet class B scavenger receptors might be a specific target for antiplatelet therapies specifically aimed at platelet hyper-reactivity in dyslipidemia.
Acknowledgments
We thank Detao Gao for technical assistance.
Sources of Funding
This work was supported in part by National Institutes of Health grants HL077213, HL077213-05S1, 5P01HL073311-06, and HL053315.
References
- 1.Valiyaveettil M, Podrez EA. Platelet hyperreactivity, scavenger receptors and atherothrombosis. J Thromb Haemost. 2009;7 (Suppl 1):218–221. doi: 10.1111/j.1538-7836.2009.03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cadroy Y, Lemozy S, Diquelou A, Ferrieres J, Douste-Blazy P, Boneu B, Sakariassen KS. Human type ii hyperlipoproteinemia enhances platelet-collagen adhesion in flowing nonanticoagulated blood. Arterioscler Thromb. 1993;13:1650–1653. doi: 10.1161/01.atv.13.11.1650. [DOI] [PubMed] [Google Scholar]
- 3.Trip MD, Cats VM, van Capelle FJ, Vreeken J. Platelet hyperreactivity and prognosis in survivors of myocardial infarction. N Engl J Med. 1990;322:1549–1554. doi: 10.1056/NEJM199005313222201. [DOI] [PubMed] [Google Scholar]
- 4.Elwood PC, Beswick AD, Sharp DS, Yarnell JW, Rogers S, Renaud S. Whole blood impedance platelet aggregometry and ischemic heart disease. The caerphilly collaborative heart disease study. Arteriosclerosis. 1990;10:1032–1036. doi: 10.1161/01.atv.10.6.1032. [DOI] [PubMed] [Google Scholar]
- 5.Martin JF, Bath PM, Burr ML. Influence of platelet size on outcome after myocardial infarction. Lancet. 1991;338:1409–1411. doi: 10.1016/0140-6736(91)92719-i. [DOI] [PubMed] [Google Scholar]
- 6.Kabbani SS, Watkins MW, Ashikaga T, Terrien EF, Sobel BE, Schneider DJ. Usefulness of platelet reactivity before percutaneous coronary intervention in determining cardiac risk one year later. Am J Cardiol. 2003;91:876–878. doi: 10.1016/s0002-9149(03)00025-0. [DOI] [PubMed] [Google Scholar]
- 7.Vanschoonbeek K, Feijge MA, Keuren JF, Coenraad Hemker H, Lodder JJ, Hamulyak K, van Pampus EC, Heemskerk JW. Thrombin-induced hyperactivity of platelets of young stroke patients: Involvement of thrombin receptors in the subject-dependent variability in ca2+ signal generation. Thromb Haemost. 2002;88:931–937. [PubMed] [Google Scholar]
- 8.Lacoste L, Lam JY, Hung J, Letchacovski G, Solymoss CB, Waters D. Hyperlipidemia and coronary disease. Correction of the increased thrombogenic potential with cholesterol reduction. Circulation. 1995;92:3172–3177. doi: 10.1161/01.cir.92.11.3172. [DOI] [PubMed] [Google Scholar]
- 9.Silverstein RL, Febbraio M. Cd36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal. 2009;2:re3. doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nozaki S, Kashiwagi H, Yamashita S, Nakagawa T, Kostner B, Tomiyama Y, Nakata A, Ishigami M, Miyagawa J, Kameda-Takemura K. Reduced uptake of oxidized low density lipoproteins in monocyte-derived macrophages fromcd36-deficient subjects. J Clin Invest. 1995;96:1859–1865. doi: 10.1172/JCI118231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawson DW, Pearce SF, Zhong R, Silverstein RL, Frazier WA, Bouck NP. Cd36 mediates the in vitro inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol. 1997;138:707–717. doi: 10.1083/jcb.138.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aitman TJ, Glazier AM, Wallace CA, Cooper LD, Norsworthy PJ, Wahid FN, Al-Majali KM, Trembling PM, Mann CJ, Shoulders CC, Graf D, St Lezin E, Kurtz TW, Kren V, Pravenec M, Ibrahimi A, Abumrad NA, Stanton LW, Scott J. Identification of cd36 (fat) as an insulin-resistance gene causing defective fatty acid and glucose metabolism in hypertensive rats. Nat Genet. 1999;21:76–83. doi: 10.1038/5013. [DOI] [PubMed] [Google Scholar]
- 13.Febbraio M, Abumrad NA, Hajjar DP, Sharma K, Cheng W, Pearce SF, Silverstein RL. A null mutation in murine cd36 reveals an importantrole in fatty acid and lipoprotein metabolism. J Biol Chem. 1999;274:19055–19062. doi: 10.1074/jbc.274.27.19055. [DOI] [PubMed] [Google Scholar]
- 14.Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. NatMed. 2000;6:41–48. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- 15.Miyaoka K, Kuwasako T, Hirano K, Nozaki S, Yamashita S, Matsuzawa Y. Cd36 deficiency associated with insulin resistance. Lancet. 2001;357:686–687. doi: 10.1016/s0140-6736(00)04138-6. [DOI] [PubMed] [Google Scholar]
- 16.Febbraio M, Podrez EA, Smith JD, Hajjar DP, Hazen SL, Hoff HF, Sharma K, Silverstein RL. Targeted disruption of the class b scavenger receptor cd36 protects against atherosclerotic lesion development in mice. J Clin Invest. 2000;105:1049–1056. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Podrez EA, Febbraio M, Sheibani N, Schmitt D, Silverstein RL, Hajjar DP, Cohen PA, Frazier WA, Hoff HF, Hazen SL. Macrophage scavenger receptor cd36 is the major receptor for ldl modified by monocyte-generated reactive nitrogen species. J Clin Invest. 2000;105:1095–1108. doi: 10.1172/JCI8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tandon NN, Ockenhouse CF, Greco NJ, Jamieson GA. Adhesive functions of platelets lacking glycoprotein iv (cd36) Blood. 1991;78:2809–2813. [PubMed] [Google Scholar]
- 19.Yamamoto N, Akamatsu N, Yamazaki H, Tanoue K. Normal aggregations of glycoprotein iv (cd36)-deficient platelets from seven healthy japanese donors. Br J Haematol. 1992;81:86–92. doi: 10.1111/j.1365-2141.1992.tb08177.x. [DOI] [PubMed] [Google Scholar]
- 20.McKeown L, Vail M, Williams S, Kramer W, Hansmann K, Gralnick H. Platelet adhesion to collagen in individuals lacking glycoprotein iv. Blood. 1994;83:2866–2871. [PubMed] [Google Scholar]
- 21.Arai M, Yamamoto N, Moroi M, Akamatsu N, Fukutake K, Tanoue K. Platelets with 10% of the normal amount of glycoprotein vi have an impaired response to collagen that results in a mild bleeding tendency. Br J Haematol. 1995;89:124–130. doi: 10.1111/j.1365-2141.1995.tb08900.x. [DOI] [PubMed] [Google Scholar]
- 22.Diaz-Ricart M, Tandon NN, Gomez-Ortiz G, Carretero M, Escolar G, Ordinas A, Jamieson GA. Antibodies to cd36 (gpiv) inhibit platelet adhesion to subendothelial surfaces under flow conditions. Arterioscler Thromb Vasc Biol. 1996;16:883–888. doi: 10.1161/01.atv.16.7.883. [DOI] [PubMed] [Google Scholar]
- 23.Bodart V, Febbraio M, Demers A, McNicoll N, Pohankova P, Perreault A, Sejlitz T, Escher E, Silverstein RL, Lamontagne D, Ong H. Cd36 mediates the cardiovascular action of growth hormone-releasing peptides in the heart. Circ Res. 2002;90:844–849. doi: 10.1161/01.res.0000016164.02525.b4. [DOI] [PubMed] [Google Scholar]
- 24.Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, Crozat K, Sovath S, Shamel L, Hartung T, Zahringer U, Beutler B. Cd36 is a sensor of diacylglycerides. Nature. 2005;433:523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 25.Stuart LM, Deng J, Silver JM, Takahashi K, Tseng AA, Hennessy EJ, Ezekowitz RA, Moore KJ. Response to staphylococcus aureus requires cd36-mediated phagocytosis triggered by the cooh-terminal cytoplasmic domain. J Cell Biol. 2005;170:477–485. doi: 10.1083/jcb.200501113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rigotti A, Acton SL, Krieger M. The class b scavenger receptors sr-bi and cd36 are receptors for anionic phospholipids. J Biol Chem. 1995;270:16221–16224. doi: 10.1074/jbc.270.27.16221. [DOI] [PubMed] [Google Scholar]
- 27.El Khoury JB, Moore KJ, Means TK, Leung J, Terada K, Toft M, Freeman MW, Luster AD. Cd36 mediates the innate host response to beta-amyloid. J Exp Med. 2003;197:1657–1666. doi: 10.1084/jem.20021546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baranova IN, Bocharov AV, Vishnyakova TG, Kurlander R, Chen Z, Fu D, Arias IM, Csako G, Patterson AP, Eggerman TL. Cd36 is a novel serum amyloida (saa) receptor mediating saa binding and saa-induced signaling in human and rodent cells. J Biol Chem. 285:8492–8506. doi: 10.1074/jbc.M109.007526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Podrez EA, Poliakov E, Shen Z, Zhang R, Deng Y, Sun M, Finton PJ, Shan L, Gugiu B, Fox PL, Hoff HF, Salomon RG, Hazen SL. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor cd36. J Biol Chem. 2002;277:38503–38516. doi: 10.1074/jbc.M203318200. [DOI] [PubMed] [Google Scholar]
- 30.Kiechl S, Willeit J, Mayr M, Viehweider B, Oberhollenzer M, Kronenberg F, Wiedermann CJ, Oberthaler S, Xu Q, Witztum JL, Tsimikas S. Oxidized phospholipids, lipoprotein(a), lipoprotein-associated phospholipase a2 activity, and 10-year cardiovascular outcomes: Prospective results from the bruneck study. Arterioscler Thromb Vasc Biol. 2007;27:1788–1795. doi: 10.1161/ATVBAHA.107.145805. [DOI] [PubMed] [Google Scholar]
- 31.Berliner JA, Leitinger N, Tsimikas S. The role of oxidized phospholipids in atherosclerosis. J Lipid Res. 2009;50 (Suppl):S207–212. doi: 10.1194/jlr.R800074-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bochkov VN, Oskolkova OV, Birukov KG, Levonen AL, Binder CJ, Stockl J. Generation and biological activities of oxidized phospholipids. Antioxid Redox Signal. 2010;12:1009–1059. doi: 10.1089/ars.2009.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deigner HP, Hermetter A. Oxidized phospholipids: Emerging lipid mediators in pathophysiology. Curr Opin Lipidol. 2008;19:289–294. doi: 10.1097/MOL.0b013e3282fe1d0e. [DOI] [PubMed] [Google Scholar]
- 34.Podrez EA, Poliakov E, Shen Z, Zhang R, Deng Y, Sun M, Finton PJ, Shan L, Febbraio M, Hajjar DP, Silverstein RL, Hoff HF, Salomon RG, Hazen SL. A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor cd36 and is enriched in atherosclerotic lesions. J Biol Chem. 2002;277:38517–38523. doi: 10.1074/jbc.M205924200. [DOI] [PubMed] [Google Scholar]
- 35.Hoff HF, O’Neil J, Wu Z, Hoppe G, Salomon RL. Phospholipid hydroxyalkenals: Biological and chemical properties of specific oxidized lipids present in atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2003;23:275–282. doi: 10.1161/01.atv.0000051407.42536.73. [DOI] [PubMed] [Google Scholar]
- 36.Podrez EA, Byzova TV, Febbraio M, Salomon RG, Ma Y, Valiyaveettil M, Poliakov E, Sun M, Finton PJ, Curtis BR, Chen J, Zhang R, Silverstein RL, Hazen SL. Platelet cd36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nat Med. 2007;13:1086–1095. doi: 10.1038/nm1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenberg ME, Li XM, Gugiu BG, Gu X, Qin J, Salomon RG, Hazen SL. The lipid whisker model of the structure of oxidized cell membranes. J Biol Chem. 2008;283:2385–2396. doi: 10.1074/jbc.M707348200. [DOI] [PubMed] [Google Scholar]
- 38.Gao D, Ashraf MZ, Kar NS, Lin D, Sayre LM, Podrez EA. Structural basis for the recognition of oxidized phospholipids in oxidized low density lipoproteins by class b scavenger receptors cd36 and sr-bi. J Biol Chem. 2010;285:4447–4454. doi: 10.1074/jbc.M109.082800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kar NS, Ashraf MZ, Valiyaveettil M, Podrez EA. Mapping and characterization of the binding site for specific oxidized phospholipids and oxidized low density lipoprotein of scavenger receptor cd36. J Biol Chem. 2008;283:8765–8771. doi: 10.1074/jbc.M709195200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eitzman DT, Westrick RJ, Xu Z, Tyson J, Ginsburg D. Hyperlipidemia promotes thrombosis after injury to atherosclerotic vessels in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2000;20:1831–1834. doi: 10.1161/01.atv.20.7.1831. [DOI] [PubMed] [Google Scholar]
- 41.Schafer K, Muller K, Hecke A, Mounier E, Goebel J, Loskutoff DJ, Konstantinides S. Enhanced thrombosis in atherosclerosis-prone mice is associated with increased arterial expression of plasminogen activator inhibitor-1. Arterioscler Thromb Vasc Biol. 2003;23:2097–2103. doi: 10.1161/01.ATV.0000097766.36623.DF. [DOI] [PubMed] [Google Scholar]
- 42.Carvalho AC, Colman RW, Lees RS. Platelet function in hyperlipoproteinemia. N Engl J Med. 1974;290:434–438. doi: 10.1056/NEJM197402212900805. [DOI] [PubMed] [Google Scholar]
- 43.Gross PL, Rand ML, Barrow DV, Packham MA. Platelet hypersensitivity in cholesterol-fed rabbits: Enhancement of thromboxane a2-dependent and thrombin-induced, thromboxane a2-independent platelet responses. Atherosclerosis. 1991;88:77–86. doi: 10.1016/0021-9150(91)90259-6. [DOI] [PubMed] [Google Scholar]
- 44.Maeda J, Tsuboi T, Fujitani B, Kadokawa T, Shimizu M. enhancement of platelet function in cholesterol-fed guinea-pigs. Nippon Yakurigaku Zasshi. 1982;80:105–111. [PubMed] [Google Scholar]
- 45.Buga GM, Navab M, Imaizumi S, Reddy ST, Yekta B, Hough G, Chanslor S, Anantharamaiah GM, Fogelman AM. L-4f alters hyperlipidemic (but not healthy) mouse plasma to reduce platelet aggregation. Arterioscler Thromb Vasc Biol. 2010;30:283–289. doi: 10.1161/ATVBAHA.109.200162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herczenik E, Bouma B, Korporaal SJ, Strangi R, Zeng Q, Gros P, Van Eck M, Van Berkel TJ, Gebbink MF, Akkerman JW. Activation of human platelets by misfolded proteins. Arterioscler Thromb Vasc Biol. 2007;27:1657–1665. doi: 10.1161/ATVBAHA.107.143479. [DOI] [PubMed] [Google Scholar]
- 47.Stewart CR, Tseng AA, Mok YF, Staples MK, Schiesser CH, Lawrence LJ, Varghese JN, Moore KJ, Howlett GJ. Oxidation of low-density lipoproteins induces amyloid-like structures that are recognized by macrophages. Biochemistry. 2005;44:9108–9116. doi: 10.1021/bi050497v. [DOI] [PubMed] [Google Scholar]
- 48.Huang MM, Bolen JB, Barnwell JW, Shattil SJ, Brugge JS. Membrane glycoprotein iv (cd36) is physically associated with the fyn, lyn, and yes protein-tyrosine kinases in human platelets. Proc Natl Acad Sci U S A. 1991;88:7844–7848. doi: 10.1073/pnas.88.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dorahy DJ, Lincz LF, Meldrum CJ, Burns GF. Biochemical isolation of a membrane microdomain from resting platelets highly enriched in the plasma membrane glycoprotein cd36. Biochem J. 1996;319 (Pt 1):67–72. doi: 10.1042/bj3190067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miao WM, Vasile E, Lane WS, Lawler J. Cd36 associates with cd9 and integrins on human blood platelets. Blood. 2001;97:1689–1696. doi: 10.1182/blood.v97.6.1689. [DOI] [PubMed] [Google Scholar]
- 51.Dorahy DJ, Berndt MC, Shafren DR, Burns GF. Cd36 is spatially associated with glycoprotein iib-iiia (alpha iib beta 3) on the surface of resting platelets. Biochem Biophys Res Commun. 1996;218:575–581. doi: 10.1006/bbrc.1996.0102. [DOI] [PubMed] [Google Scholar]
- 52.Thorne RF, Law EG, Elith CA, Ralston KJ, Bates RC, Burns GF. The association between cd36 and lyn protein tyrosine kinase is mediated by lipid. Biochem Biophys Res Commun. 2006;351:51–56. doi: 10.1016/j.bbrc.2006.09.156. [DOI] [PubMed] [Google Scholar]
- 53.Hirao A, Hamaguchi I, Suda T, Yamaguchi N. Translocation of the csk homologous kinase (chk/hyl) controls activity of cd36-anchored lyn tyrosine kinase in thrombin-stimulated platelets. EMBO J. 1997;16:2342–2351. doi: 10.1093/emboj/16.9.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ardlie NG, Selley ML, Simons LA. Platelet activation by oxidatively modified low density lipoproteins. Atherosclerosis. 1989;76:117–124. doi: 10.1016/0021-9150(89)90094-4. [DOI] [PubMed] [Google Scholar]
- 55.Zhao B, Rickert CH, Filler TJ, Liu B, Verhallen PF, Dierichs R. Adhesion of washed blood platelets in vitro is advanced, accelerated, and enlarged byoxidized low-density lipoprotein. Am J Hematol. 1995;49:177–182. doi: 10.1002/ajh.2830490302. [DOI] [PubMed] [Google Scholar]
- 56.Retzer M, Siess W, Essler M. Mildly oxidised low density lipoprotein induces platelet shape change via rho-kinase-dependent phosphorylation of myosin light chain and moesin. FEBS Lett. 2000;466:70–74. doi: 10.1016/s0014-5793(99)01762-7. [DOI] [PubMed] [Google Scholar]
- 57.Maschberger P, Bauer M, Baumann-Siemons J, Zangl KJ, Negrescu EV, Reininger AJ, Siess W. Mildly oxidized low density lipoprotein rapidly stimulates via activation of the lysophosphatidic acid receptor src family and syk tyrosine kinases and ca2+ influx in human platelets. J Biol Chem. 2000;275:19159–19166. doi: 10.1074/jbc.M910257199. [DOI] [PubMed] [Google Scholar]
- 58.Mahfouz MM, Kummerow FA. Oxysterols and tbars are among the ldl oxidation products which enhance thromboxane a2 synthesis by platelets. Prostaglandins Other Lipid Mediat. 1998;56:197–217. doi: 10.1016/s0090-6980(98)00056-2. [DOI] [PubMed] [Google Scholar]
- 59.Silverstein RL, Li W, Park YM, Rahaman SO. Mechanisms of cell signaling by the scavenger receptor cd36: Implications in atherosclerosis and thrombosis. Trans Am Clin Climatol Assoc. 121:206–220. [PMC free article] [PubMed] [Google Scholar]
- 60.Chen K, Febbraio M, Li W, Silverstein RL. A specificcd36-dependent signaling pathway is required for platelet activation by oxidized low-density lipoprotein. Circ Res. 2008;102:1512–1519. doi: 10.1161/CIRCRESAHA.108.172064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Korporaal SJ, Van Eck M, Adelmeijer J, Ijsseldijk M, Out R, Lisman T, Lenting PJ, Van Berkel TJ, Akkerman JW. Platelet activation by oxidized low density lipoprotein is mediated by cd36 and scavenger receptor-a. Arterioscler Thromb Vasc Biol. 2007;27:2476–2483. doi: 10.1161/ATVBAHA.107.150698. [DOI] [PubMed] [Google Scholar]
- 62.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, Moore KJ. Cd36 ligands promote sterile inflammation through assembly of a toll-like receptor 4 and 6 heterodimer. Nat Immunol. 11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park YM, Febbraio M, Silverstein RL. Cd36 modulates migration of mouse and human macrophages in response to oxidized ldl and may contribute to macrophage trapping in the arterial intima. J Clin Invest. 2009;119:136–145. doi: 10.1172/JCI35535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen R, Chen X, Salomon RG, McIntyre TM. Platelet activation by low concentrations of intact oxidized ldlparticles involves the paf receptor. Arterioscler Thromb Vasc Biol. 2009;29:363–371. doi: 10.1161/ATVBAHA.108.178731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murohara T, Scalia R, Lefer AM. Lysophosphatidylcholine promotes p-selectin expression in platelets and endothelial cells. Possible involvement of protein kinase c activation and its inhibition by nitric oxide donors. Circ Res. 1996;78:780–789. doi: 10.1161/01.res.78.5.780. [DOI] [PubMed] [Google Scholar]
- 66.Siess W, Zangl KJ, Essler M, Bauer M, Brandl R, Corrinth C, Bittman R, Tigyi G, Aepfelbacher M. Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc Natl Acad Sci U S A. 1999;96:6931–6936. doi: 10.1073/pnas.96.12.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Acton SL, Scherer PE, Lodish HF, Krieger M. Expression cloning of sr-bi, a cd36-related class b scavenger receptor. J Biol Chem. 1994;269:21003–21009. [PubMed] [Google Scholar]
- 68.Rigotti A, Miettinen HE, Krieger M. The role of the high-density lipoprotein receptor sr-bi in the lipid metabolism of endocrine and other tissues. Endocr Rev. 2003;24:357–387. doi: 10.1210/er.2001-0037. [DOI] [PubMed] [Google Scholar]
- 69.Ashraf MZ, Kar NS, Chen X, Choi J, Salomon RG, Febbraio M, Podrez EA. Specific oxidized phospholipids inhibit scavenger receptor bi-mediated selective uptake of cholesteryl esters. J Biol Chem. 2008;283:10408–10414. doi: 10.1074/jbc.M710474200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Imachi H, Murao K, Cao W, Tada S, Taminato T, Wong NC, Takahara J, Ishida T. Expression of human scavenger receptor b1 on and in human platelets. Arterioscler Thromb Vasc Biol. 2003;23:898–904. doi: 10.1161/01.ATV.0000067429.46333.7B. [DOI] [PubMed] [Google Scholar]
- 71.Valiyaveettil M, Kar N, Ashraf MZ, Byzova TV, Febbraio M, Podrez EA. Oxidized high-density lipoprotein inhibits platelet activation and aggregation via scavenger receptor bi. Blood. 2008;111:1962–1971. doi: 10.1182/blood-2007-08-107813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Krieger M. Scavenger receptor class b type i is a multiligand hdl receptor that influences diverse physiologic systems. J Clin Invest. 2001;108:793–797. doi: 10.1172/JCI14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yancey PG, Bortnick AE, Kellner-Weibel G, de la Llera-Moya M, Phillips MC, Rothblat GH. Importance of different pathways of cellular cholesterol efflux. Arterioscler Thromb Vasc Biol. 2003;23:712–719. doi: 10.1161/01.ATV.0000057572.97137.DD. [DOI] [PubMed] [Google Scholar]
- 74.Hassall DG, Owen JS, Bruckdorfer KR. The aggregation of isolated human platelets in the presence of lipoproteins and prostacyclin. Biochem J. 1983;216:43–49. doi: 10.1042/bj2160043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Naqvi TZ, Shah PK, Ivey PA, Molloy MD, Thomas AM, Panicker S, Ahmed A, Cercek B, Kaul S. Evidence that high-density lipoprotein cholesterol is an independent predictor of acute platelet-dependent thrombus formation. Am J Cardiol. 1999;84:1011–1017. doi: 10.1016/s0002-9149(99)00489-0. [DOI] [PubMed] [Google Scholar]
- 76.Korporaal SJ, Akkerman JW. Platelet activation by low density lipoprotein and high density lipoprotein. Pathophysiol Haemost Thromb. 2006;35:270–280. doi: 10.1159/000093220. [DOI] [PubMed] [Google Scholar]
- 77.Takahashi Y, Chiba H, Matsuno K, Akita H, Hui SP, Nagasaka H, Nakamura H, Kobayashi K, Tandon NN, Jamieson GA. Native lipoproteins inhibit platelet activation induced by oxidized lipoproteins. Biochem Biophys Res Commun. 1996;222:453–459. doi: 10.1006/bbrc.1996.0765. [DOI] [PubMed] [Google Scholar]
- 78.Nofer JR, Walter M, Kehrel B, Wierwille S, Tepel M, Seedorf U, Assmann G. Hdl3-mediated inhibition of thrombin-induced platelet aggregation and fibrinogen binding occurs via decreased production of phosphoinositide-derived second messengers 1,2-diacylglycerol and inositol 1,4,5-tris-phosphate. Arterioscler Thromb Vasc Biol. 1998;18:861–869. doi: 10.1161/01.atv.18.6.861. [DOI] [PubMed] [Google Scholar]
- 79.van Lier M, Verhoef S, Cauwenberghs S, Heemskerk JW, Akkerman JW, Heijnen HF. Role of membrane cholesterol in platelet calcium signalling in response to vwf and collagen under stasis and flow. Thromb Haemost. 2008;99:1068–1078. doi: 10.1160/TH07-08-0528. [DOI] [PubMed] [Google Scholar]
- 80.Calkin AC, Drew BG, Ono A, Duffy SJ, Gordon MV, Schoenwaelder SM, Sviridov D, Cooper ME, Kingwell BA, Jackson SP. Reconstituted high-density lipoprotein attenuates platelet function in individuals with type 2 diabetes mellitus by promoting cholesterol efflux. Circulation. 2009;120:2095–2104. doi: 10.1161/CIRCULATIONAHA.109.870709. [DOI] [PubMed] [Google Scholar]
- 81.Saddar S, Mineo C, Shaul PW. Signaling by the high-affinity hdl receptor scavenger receptor b type i. Arterioscler Thromb Vasc Biol. 2010;30:144–150. doi: 10.1161/ATVBAHA.109.196170. [DOI] [PubMed] [Google Scholar]
- 82.Zimman A, Chen SS, Komisopoulou E, Titz B, Martinez-Pinna R, Kafi A, Berliner JA, Graeber TG. Activation of aortic endothelial cells by oxidized phospholipids: A phosphoproteomic analysis. J Proteome Res. 2010;9:2812–2824. doi: 10.1021/pr901194x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dole VS, Matuskova J, Vasile E, Yesilaltay A, Bergmeier W, Bernimoulin M, Wagner DD, Krieger M. Thrombocytopenia and platelet abnormalities in high-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2008 doi: 10.1161/ATVBAHA.108.162347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heemskerk JW, Feijge MA, Simonis MA, Hornstra G. Effects of dietary fatty acids onsignal transduction and membrane cholesterol content in rat platelets. Biochim Biophys Acta. 1995;1255:87–97. doi: 10.1016/0005-2760(94)00225-n. [DOI] [PubMed] [Google Scholar]
- 85.Shattil SJ, Anaya-Galindo R, Bennett J, Colman RW, Cooper RA. Platelet hypersensitivity induced by cholesterol incorporation. J Clin Invest. 1975;55:636–643. doi: 10.1172/JCI107971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stuart MJ, Gerrard JM, White JG. Effect of cholesterol on production of thromboxane b2 by platelets in vitro. N Engl J Med. 1980;302:6–10. doi: 10.1056/NEJM198001033020102. [DOI] [PubMed] [Google Scholar]
- 87.Ma Y, Ashraf MZ, Podrez EA. Scavenger receptor bi modulates platelet reactivity and thrombosis in dyslipidemia. Blood. 2010;116:1932–1941. doi: 10.1182/blood-2010-02-268508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.West M, Greason E, Kolmakova A, Jahangiri A, Asztalos B, Pollin TI, Rodriguez A. Scavenger receptor class b type i protein as an independent predictor of high-density lipoprotein cholesterol levels in subjectswith hyperalphalipoproteinemia. J Clin Endocrinol Metab. 2009;94:1451–1457. doi: 10.1210/jc.2008-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Osgood D, Corella D, Demissie S, Cupples LA, Wilson PW, Meigs JB, Schaefer EJ, Coltell O, Ordovas JM. Genetic variation at the scavenger receptor class b type i gene locus determines plasma lipoprotein concentrations and particle size and interacts with type 2 diabetes: The framingham study. J Clin Endocrinol Metab. 2003;88:2869–2879. doi: 10.1210/jc.2002-021664. [DOI] [PubMed] [Google Scholar]
- 90.Ritsch A, Sonderegger G, Sandhofer A, Stanzl U, Tancevski I, Eller P, Schgoer W, Wehinger A, Mueller T, Haltmayer M, Patsch JR. Scavenger receptor class b type i polymorphisms and peripheral arterial disease. Metabolism. 2007;56:1135–1141. doi: 10.1016/j.metabol.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 91.Morabia A, Ross BM, Costanza MC, Cayanis E, Flaherty MS, Alvin GB, Das K, James R, Yang AS, Evagrafov O, Gilliam TC. Population-based study of sr-bi genetic variation and lipid profile. Atherosclerosis. 2004;175:159–168. doi: 10.1016/j.atherosclerosis.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 92.Aronow WS. Antiplatelet therapy in peripheral arterial disease. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4:265–267. doi: 10.2174/1568006043336104. [DOI] [PubMed] [Google Scholar]
- 93.Assanasen C, Mineo C, Seetharam D, Yuhanna IS, Marcel YL, Connelly MA, Williams DL, de la Llera-Moya M, Shaul PW, Silver DL. Cholesterol binding, efflux, and a pdz-interacting domain of scavenger receptor-bi mediate hdl-initiated signaling. J Clin Invest. 2005;115:969–977. doi: 10.1172/JCI200523858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huang ZH, Gu D, Lange Y, Mazzone T. Expression of scavenger receptor bi facilitates sterol movement between the plasma membrane and the endoplasmic reticulum in macrophages. Biochemistry. 2003;42:3949–3955. doi: 10.1021/bi0269207. [DOI] [PubMed] [Google Scholar]
- 95.Kellner-Weibel G, de La Llera-Moya M, Connelly MA, Stoudt G, Christian AE, Haynes MP, Williams DL, Rothblat GH. Expression of scavenger receptor bi in cos-7 cells alters cholesterol content and distribution. Biochemistry. 2000;39:221–229. doi: 10.1021/bi991666c. [DOI] [PubMed] [Google Scholar]
- 96.Lopez JA, del Conde I, Shrimpton CN. Receptors, rafts, and microvesicles in thrombosis and inflammation. J Thromb Haemost. 2005;3:1737–1744. doi: 10.1111/j.1538-7836.2005.01463.x. [DOI] [PubMed] [Google Scholar]
- 97.Fielding CJ, Fielding PE. Cholesterol and caveolae: Structural and functional relationships. Biochim Biophys Acta. 2000;1529:210–222. doi: 10.1016/s1388-1981(00)00150-5. [DOI] [PubMed] [Google Scholar]