Abstract
Normal lung development follows a series of orchestrated events. Premature birth interrupts normal in utero lung development, which results in significant alterations in lung function and physiology. Increasingly, there are reports documenting the broad range of complications experienced by infants aged 34 to 36 weeks' gestational age (GA). Our objective was to summarize the evidence demonstrating respiratory system vulnerability in infants aged 34 to 36 weeks' GA and to review the developmental and physiologic principles that underlie this vulnerability. A comprehensive search for studies that reported epidemiologic data and respiratory morbidity was conducted on the PubMed, Medline, Ovid Biosis, and Embase databases from 2000 to 2009 by using medical subject headings “morbidity in late preterm infants,” “preterm infants and lung development,” “prematurity and morbidity,” and “prematurity and lung development.” Because the number of studies exclusive to infants aged 34 to 36 weeks' GA was limited, selected studies also included infants aged 32 to 36 weeks' GA. Of the 24 studies identified, 16 were retrospective population-based cohort studies; 8 studies were observational. These studies consistently revealed that infants born at 32 to 36 weeks' GA, including infants of 34 to 36 weeks' GA, experience substantial respiratory morbidity compared with term infants. Levels of morbidity were, at times, comparable to those observed in very preterm infants. The developmental and physiologic mechanisms that underlie the increased morbidity rate and alterations in respiratory function are discussed. We also present evidence to demonstrate that the immaturity of the respiratory system of infants 34 to 36 weeks' GA at birth results in increased morbidity in infancy and leads to deficits in lung function that may persist into adulthood.
Keywords: airway conductance, airway patency, bronchopulmonary dysplasia, functional residual capacity, late preterm, respiratory morbidity, tethering
The frequency of late-preterm (– weeks' gestational age [GA]) births has generally increased in the United States over the past 2 decades, with a slight decline in 2007 (10.61% in 1990, 12.80% in 2006, and 12.66% in 2007).1 Infants of 34 to 36 weeks' GA comprise ~71% of all preterm births (9.03% overall in 2007 [~390 000 live births]).1 Late-preterm infants are often considered to be as physiologically and metabolically mature as term infants and, therefore, at low risk for morbidity and mortality.2,3
A modest but growing body of literature has documented the broad range of complications that late-preterm infants may experience. Compared with term infants, late-preterm infants are at higher risk of developing medical complications and have higher rates of mortality during infancy.4–8 Despite significant declines in the infant mortality rate over the last decade, the infant mortality rate for late-preterm infants has remained three- to fivefold higher than that for term infants.4–7,9 Each weekly reduction in estimated GA at birth was found to be associated with an increased risk of death.10 Late-preterm infants also face increased morbidity before hospital discharge4,11,12 and higher rates of hospital readmission in the first months of life.5,11–13 They are 4 times more likely than term infants to have ≥1 medical condition diagnosed and 3.5 times more likely to have ≥2 conditions diagnosed.13,14 Late-preterm infants are often viewed as normal and are discharged early (<2-night hospital stay), which may be a factor in the increased overall risk of rehospitalization.12,15
In addition to numerous neonatal complications, reviewed by Engle et al14 and Makari and Groothuis,16 a high incidence of respiratory distress has been reported as one of the most common adverse outcomes among late-preterm infants.7,17–21 There has been little information on the long-term consequences of preterm birth and essentially none for late-preterm birth beyond the first year of life. Results of a recent review on the respiratory consequences of preterm birth suggested that many adverse respiratory consequences of preterm birth are likely the result of persistent pulmonary structural abnormalities.22
Differences in respiratory development between term and late-preterm infants may be illustrated by the susceptibility of preterm infants (33–36 weeks' GA) to infection by such pathogens as respiratory syncytial virus (RSV), for which infection rates among preterm infants (33–36 weeks' GA) more resembled those of younger preterm infants (<33 weeks' GA) than those of term infants.23
The original purpose of this review was to summarize the recent literature regarding the increased respiratory morbidity in preterm infants aged 34 to 36 weeks' GA, a population that largely presents with a low incidence of surfactant-deficiency–related neonatal respiratory problems. We then provide a physiologic explanation as to why these apparently healthy infants have a high incidence of respiratory problems compared with term infants. The physiologic and developmental characteristics underpinning the immediate vulnerability of this population are described along with emerging evidence of long-term sequelae associated with birth during the saccular stage of lung development. Additional background is presented to provide context for the data summary and possible explanation of the results. This review is intended to inform providers who make decisions regarding delivery of late-preterm infants and who are involved in their care in early life of the higher risks faced by these children.
The American Academy of Pediatrics and the American College of Obstetricians and Gynecologists have defined late-preterm infants as those born between and weeks' GA.24 As stated above, our original intent was to focus on this narrow subpopulation; however, for the purposes of this review, we have included studies for which outcomes in infants born between 32 and 36 weeks' GA were reported, because many of the publications of interest often included patient populations a few weeks below the 34- to 36-weeks' GA window.
Online databases (PubMed, Medline, Ovid Biosis, Embase) from 2000 to 2009 were searched by using medical subject headings “morbidity in late preterm infants” and “preterm infants and lung development” to identify studies that included infants of 34 to 36 weeks' GA and reported ≥1 objective quantified outcome (including respiratory morbidity) without regard for sample size. Additional Embase searches included Emtree Thesaurus and free terms “prematurity and morbidity” and “prematurity and lung development.” The articles selected were limited to those published during or after the year 2000 to capture studies that examined lung morbidity such as bronchopulmonary dysplasia (BPD) using the more recent definition from a workshop sponsored by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Heart, Lung, and Blood Institute, and the Office of Rare Diseases.25 The primary objectives, study design and methods, comparison populations, pertinent results (including morbidity and mortality rates of appropriate studies), and study limitations were summarized. Twenty-four studies were identified for inclusion in this review. A summary of the salient features of these studies is provided in Table 1. To describe the underlying respiratory physiology, additional publications are cited that were not limited to the years 2000–2009.
TABLE 1.
Characteristics of Studies That Evaluated the Epidemiology and Outcomes of Preterm (32–36 Weeks' GA) Birth
| Study | Primary Objective | Design and Methods | Comparison Populations | Results | Study Quality or Limitations |
|---|---|---|---|---|---|
| General morbidity | |||||
| Mclntire and Leveno7 (2008) | To analyze neonatal mortality and morbidity rates in 34- to 36-wk-GA and term (39-wk-GA) infants | Retrospective cohort study of all live-born singleton infants of 34–40 wk GA born over a period of 18 y in a center in Dallas, TX | 34-wk-GA infants (n = 3498); 35-wk-GA infants (n = 6571); 36-wk-GA infants (n = 11 702); 37-wk-GA infants (n = 26 504); 39-wk-GA infants (n = 84 747; referent) | Neonatal mortality rate per 1000 live births of 1.1, 1.5, and 0.5 at 34, 35, and 36 wk GA, respectively, vs 0.2 at 39 wk GA (P < .001); increased neonatal morbidity rate in 34- to 36-wk-GA infants | Did not examine long-term effects of prematurity; results from this population might not be generalizable to other populations |
| Melamed et al21 (2009) | To estimate the effect of GA on neonatal morbidity and identify predictors of adverse neonatal outcome | Retrospective study of all spontaneous, low-risk deliveries of 34–36 wk GA from 1997 to 2006 in a center in Tel Aviv, Israel | 34- to 36-wk-GA infants (n = 2478); term infants (n = 7434) | Increased risk of neonatal morbidity in 34- to 36-wk-GA infants, including respiratory distress syndrome, sepsis, intraventricular hemorrhage, hypoglycemia, and jaundice requiring phototherapy (all P < .001) | Retrospective design precluded blinding of infant age during analysis; did not examine long-term effects of prematurity |
| Pulver et al8 (2009) | To compare neonatal and infant mortality rates of late-preterm, early-term, and term newborns;to determine the RR of neonatal and infant death for categories of weight for GA; and to examine causes of neonatal and infant death | Retrospective cohort analysis of birth- and death-certificate data for all infants born in Utah between 1999 and 2005 at ≥34 wk GA | 343 322 infants total, categorized as 34–36 wk GA, 37–38 wk GA, 39–42 wk GA, small for GA, appropriate for GA, and large for GA;9 strata total; appropriate for GA at 39–42 wk GA was the referent | 34- to 36-wk-GA small-for-GA infants ~44 times more likely than 39-to 42-wk-GA appropriate-for-GA newborns to die in their first month and ~21 times more likely to die in their first year | Relied on data from birth and death certificates, which could not be corrected if questionable; results from this population might not be generalizable to other populations |
| Santos et al5 (2008) | To investigate the consequences of term and 34- to 36-wk-GA birth on infant health through 3 mo of age | Population-based birth-cohort study of infants born in Pelotas, Brazil, during 2004 | 34- to 36-wk-GA infants (n = 447); 37- to 41-wk-GA infants (n = 3262; referent) | The risk of neonatal morbidity was ~3 times higher in 34- to 36-wk-GA infants than 37- to 41-wk-GA infants (25.6% vs 8.1%; P < .001); hospital admission rates in first 3 mo of life were 30% higher in 34- to 36-wk-GA infants; neonatal and infant mortality rates were ~5 times higher in 34- to 36-wk-GA infants | Information on maternal morbidity during pregnancy and on delivery was obtained through maternal recall; the quality of care provided to newborns could not be evaluated |
| Shapiro-Mendoza et al26 (2008) | To compare morbidity risk in 34- to 36-wk-GA and 37- to 41-wk-GA infants | Population-based cohort of singleton, 34- to 36-wk-GA and 37- to 41-wk-GA infants who were born in Massachusetts hospitals to Massachusetts residents from January 1, 1998, to November 30, 2003 | 34- to 36-wk-GA infants (n = 26 170); 37- to 41-wk-GA infants (n = 377 638; referent) | 34- to 36-wk-GA infants were 7 times more likely to have newborn morbidity than term infants (22% vs 3%); infants born at 34 wk GA had 20 times higher risk of morbidity compared with infants born at 40 wk GA; doubling of morbidity rate for each week born before 38 wk | Other confounding variables may not have been identified because of use of secondary sources; misclassification of medical conditions would not be identified in secondary sources; results from this population might not be generalizable to other populations |
| Tomashek et al12 (2006) | To understand how 34- to 36-wk-GA infants are affected by discharge policies created for 37- to 41-wk-GA infants | Retrospective population-based cohort of singleton, 34- to 36-wk-GA and 37- to 41-wk-GA newborns born to Massachusetts residents in Massachusetts hospitals from January 1, 1998, to November 30, 2002, at a hospital and discharged home early after vaginal delivery | 34- to 36-wk-GA infants (n = 1004); 37- to 41-wk-GA infants (n = 24 320; referent) | 4.3% 34- to 36-wk-GA vs 2.7% 37- to 41-wk-GA infants were either readmitted or had an observational stay | Hospital readmissions and observational stays could not all be linked to the birth records; unable to account for all neonatal morbidity with data sources used; GA and adequacy of prenatal care were estimated from birth certificates; results from this population might not be generalizable to other populations |
| Tomashek et al6 (2007) | To assess differences in mortality rate between 34- to 36-wk-GA and 37- to 41-wk-GA infants | Retrospective analysis of data from the US period-linked birth/infant death data for 1995–2002 for singleton 34- to 36-wk-GA and 37- to 41-wk-GA infants born to residents of the 50 states and the District of Columbia | 34- to 36-wk-GA infants (n = 2 221 545); 37- to 41-wk-GA infants (n = 24 973 117; referent) | Despite a significant decline in infant mortality rates between 1995 and 2002, infant mortality rates were 3 times higher in 34- to 36-wk-GA infants than 37- to 41-wk-GA infants; early, late, and postneonatal mortality rates were 6, 3, and 2 times higher, respectively, in 34- to 36-wk-GA than in 37- to 41-wk-GA infants | Relied on data from birth and death certificates, which could not be corrected if questionable; cause of death limited to information on death certificate; underlying cause of death may have been misclassified; GA was estimated from birth certificate |
| Young et al10 (2007) | To determine RR for mortality and causes and ages of death in 34- to 42-wk-GA infants | Retrospective cohort analysis of birth- and death-certificate data for all infants born in Utah between 1999 and 2004 at ≥34 to ≤42 wk GA | 34-wk-GA infants (n = 3194); 35-wk-GA infants (n = 5722); 36-wk-GA infants (n = 12 190); 39-wk-GA infants (n = 26 175); 40-wk-GA infants (n = 61 764; referent); 41-wk-GA infants (n = 14 361); 42-wk-GA infants (n = 1081) | Most common cause of death in both groups was birth defects; mortality rates were still higher in 34- to 36-wk-GA than in 40-wk-GA infants after excluding those who died of birth defects | GA and causes and ages of death were based on birth- and death-certificate data; results from this population might not be generalizable to other populations; cause for preterm delivery unknown |
| General respiratory morbidity | |||||
| Clark17 (2005) | To examine causes of a need for ventilation in near-term infants and to determine rates of mortality, CLD, and neurologic morbidity | Prospective cohort analysis of infants of ≥34 wk GA who were intubated within 72 h and required ventilation for <6 h | Mean GA (SD): 37 wk (2wk) (n = 1011) | 51 (5%) patients died; 109 (11%) patients had CLD; 86 (9%) patients had neurologic complications | Small population; patients were selected for the need for a mechanical ventilator |
| Dani et al20 (2009) | To compare the rate of clinical complications (hyperbilirubinemia, hypoglycemia, hypothermia, poor feeding, and respiratory distress) in 34- to 36-wk-GA and ≥37-wk-GA infants | Observational population-based study of infants born in 2008 in a center in Florence, Italy, with birth weight of >2000 g and ≥34 wk GA | 34- to 36-wk-GA infants (n = 137); ≥37-wk-GA infants (n = 2708; referent) | 34- to 36-wk-GA infants had higher rates of clinical complications than ≥37-wk-GA infants | Results from this population might not be generalizable to other populations; only healthy infants were examined |
| De Luca et al27 (2009) | To compare mortality rates and incidence of clinically significant morbidities in <37-wk-GA and ≥37-wk-GA infants according to delivery mode | Prospective analysis of obstetric and neonatal outcomes of all deliveries performed between January 1982 and September 2004 in a center in Geneva, Switzerland | N = 56 549; neonates were stratified according to GA in strata of 1 wk, from 34–42 completed weeks, and grouped into <37-wk-GA and ≥37-wk-GA neonates | Depression at birth, admission to special care, and respiratory morbidity showed a strong age-related trend independent of delivery mode; there was an up to >10-fold decrease in respiratory morbidity from 34 wk GA to term depending on delivery mode | Results from this population might not be generalizable to other populations; restricted indications for elective cesarean delivery may have selected for higher-risk pregnancies |
| Escobar et al9 (2006) | Quantification of short-term hospital outcomes in 35- to 36-wk-GA infants | Review of published data and reanalysis of databases or retrospective cohort analysis | n = 47 495; 35- to 36-wk-GA infants; ≥37-wk-GA infants (referent) | Considerable mortality and morbidity in 35- to 36-wk-GA infants; mortality rate = 0.8% in those who survived for 6 h; ~8% required oxygen support for at least 1 h; more likely to be rehospitalized within 14 or 15–182 d | Conclusions were drawn from limited available data |
| Khashu et al19 (2009) | To compare the mortality and morbidity rates in 33- to 36-wk-GA and 37- to 40-wk-GA infants | Population-based study that compared mortality and morbidity data from the British Columbia Perinatal Database Registry of all singleton newborns born at 33–40 wk GA between April 1, 1999, and March 31, 2002 | 33- to 36-wk-GA infants (n = 6381); 37- to 40-wk-GA infants (n = 88 867; referent) | Stillbirth, perinatal, neonatal, and infant mortality rates were higher in the 33- to 36-wk-GA infants; higher incidence of respiratory morbidity and longer hospital stays in 33- to 36-wk-GA infants | Lack of stratification of certain variables based on underlying diseases; inability to analyze according to week of gestation |
| Kitsommart et al28 (2009) | To compare the morbidity and mortality rates of 34- to 36-wk-GA and ≥37-wk-GA infants | Retrospective chart review of 34- to 36-wk-GA infants who were admitted to the NICU, intermediate care nursery (level 2), or postpartum wards at a center in Hamilton, ON, Canada, from April 1, 2004, to March 31, 2008 | 34- to 36-wk-GA infants (n = 1481); ≥37-wk-GA infants (n = 9332; referent) | Worse respiratory outcomes in 34- to 36-wk-GA infants than in ≥37-wk-GA infants; respiratory outcomes included rates of positive pressure, ventilation requirement (either continuous positive airway pressure or mechanical ventilation), and prevalence of pneumothorax (all P < .001) | Results from this population might not be generalizable to other populations; retrospective design may call into question validity of data |
| Wang et al13 (2004) | To test whether 35- to 36-wk-GA vs 37- to 40-wk-GA infants have more postnatal medical problems and longer hospital stays | Electronic medical record database sorting and analysis of neonatal records of infants who were born between October 1997 and October 2000 (inclusive) in a center in Boston, MA | 35- to 36-wk-GA infants (n = 90); 37- to 40-wk-GA infants (n = 95; referent) | Similar median length of stay in 35- to 36-wk-GA infants born vaginally or by cesarean delivery and 37- to 40-wk-GA infants; significant differences in almost all clinical outcomes (clinical jaundice, hypoglycemia, intravenous infusion, respiratory distress, and temperature instability) | Results from this population might not be generalizable to other populations; did not address longer-term outcomes |
| Infectious respiratory morbidity | |||||
| Boyce et al23 (2000) | To determine rates of RSV hospitalization in children with and without specific medical conditions | Retrospective cohort study of all children <3 y old enrolled in the Tennessee Medicaid program from July 1, 1989, to June 30, 1993 | Age strata: 0 to <6 mo, 6 to <12 mo, 12 to <24 mo, and 24 to <36 mo; within each stratum, low-risk (referent): BPD, CHD, ≤28 wk GA, 29–33 wk GA, 33–36 wk GA, and other | RSV hospitalization rates were highest in 0-to <6-mo-olds and decreased with age; 33- to 36-wk-GA infants had 159.6 hospitalizations per 1000 child-years compared with 163.6 for 29-to <33-wk-GA infants and 88.2 for infants born at term | Database only contains information on children enrolled in Medicaid; hospitalization rates among such children might not be generalizable to the entire population; RSV infection was identified by coding, which may have underestimated RSV impact |
| Carbonell-Estrany et al29 (2000) | To collect data on RSV hospitalization rates and to determine risk factors for rehospitalization among premature infants with and without CLD in Spain | Observational, prospective, longitudinal study in 14 Spanish hospitals (annual birth cohort, 57 000 infants) between October 1, 1998, and March 31, 1999 | ≤28-wk-GA infants (n = 101); 29-to 30-wk-GA infants (n = 179); 31- to 32-wk-GA infants (n = 304); infants with CLD (n = 38) | Lower risk for RSV hospitalization with increasing GA (OR: 0.85 [95% CI: 0.72–0.99]; P < .047); higher risk for hospital admission among infants with CLD (OR: 3.1 [95% CI: 1.22–7.91]; P < .016) | 6% of sample lost to follow-up, may have caused underestimation of hospitalization rate; timing of RSV season may have caused underestimation of hospitalization rate; mean sensitivity of RSV test may have underestimated true number of RSV cases |
| Figueras-Aloy et al31 (2004) | To identify risk factors most likely to lead to RSV-related hospitalization in 33- to 35-wk-GA infants | Hospital-based case-control study conducted in 50 centers in Spain during the RSV season (October 2002–April 2003); controls were selected and included in May–June 2003 | 33- to 35-wk-GA infants hospitalized for RSV (n = 186); 33- to 35-wk-GA age-matched controls (n = 371; referent) | Risk factors for RSV-related hospitalization were absolute chronological age at start of RSV season; breastfeeding for ≤2 mo; ≥1 school-aged siblings; ≥4 residents in the home; family history of wheezing | Results from this population might not be generalizable to other populations; case-control design may have introduced bias in selection of controls |
| Holman et al36 (2003) | To examine the epidemiology of and identify risk factors for bronchiolitis-associated deaths among infants in the United States | Analysis of national multiple cause-of-death and linked birth/infant death data for the years 1995–1998 | n = 229 bronchiolitis-associated infant deaths; <32 wk GA; 32–35 wk GA; ≥36 wk GA (referent) | Bronchiolitis-associated mortality rates were 19.7 per 100 000 live births in <32-wk-GA infants, 5.8 in 32- to 35-wk-GA infants, and 1.3 in ≥36-wk-GA infants | Bronchiolitis was identified by coding, which may have underestimated bronchiolitis impact |
| Horn and Smout33 (2003) | To determine if GA is independently associated with hospital-resource use and outcomes among infants hospitalized for RSV | Analysis of retrospective data for infants hospitalized with RSV bronchiolitis or pneumonia admitted to 10 children's medical centers from April 1, 1995, to September 30, 1996 | ≤32-wk-GA infants (n = 28); 33- to 35-wk-GA infants (n = 31); 36-wk-GA infants (n = 30); ≥37-wk-GA infants (n = 215) | 33- to 35-wk-GA infants had higher resource use than ≤32-wk-GA or ≥37-wk-GA infants; these differences remained significant in multiple regression analyses | Data regarding additional risk factors were not collected |
| Law et al30 (2004) | To identify independent risk factors for hospitalization of infants with RSV infection | Prospective, multicenter, observational cohort study of infants born at 33–35 wk GA between November 1 and ~April 30 of 2000–2001 or 2001–2002 in Canada | 1832 infants 33–35 wk GA | 57% (56 of 98) of the infants had an emergency department visit for RSV; 69% (66 of 96) of the infants were hospitalized for RSV | Results from this population might not be generalizable to other populations; relied on culture-confirmed illness; not all patients were tested |
| Leader and Kohlhase32 (2003) | To provide current estimates of the incidence, associated risk factors, and costs of severe RSV infections among infants in the United States, defined as emergency department visits, hospitalization, and death | Retrospective analysis of National Hospital Ambulatory Medical Care Survey data (1997–2000), National Hospital Discharge Survey data (1997–2000), and Perinatal Mortality Linked Files (1998–1999) | Children with comorbidities (n = 372); children without comorbidities (n = 288) | Children ≤35 wk GA vs ≥37 wk GA had significantly higher RSV mortality rates (P < .01) | Retrospective design may call into question validity of data |
| Sampalis35 (2003) | To evaluate the impact of RSV infections or resource utilization in 32- to 35-wk-GA infants in Canada | Analysis of data from infants born at 32–35 wk GA between 1997 and 2000, hospitalized for proven or probable RSV, and followed to December 31, 2001 | 32- to 35-wk-GA infants hospitalized for proven or probable RSV (n = 2415); 32- to 35-wk-GA control infants (n = 20 254) | Hospitalization rates, total hospital stay, outpatient visits, and overall deaths highest in 32- to 35-wk-GA infants | Use of database may call into question validity of RSV diagnosis; data regarding other causes that led to use of health care services were not available; direct causality between RSV hospitalization and the antecedent outcomes evaluated was not absolutely certain |
| Willson et al34 (2003) | To characterize complications among infants of ≤35 wk GA who were hospitalized for bronchiolitis or RSV pneumonia | Analysis of retrospective data for infants hospitalized with RSV bronchiolitis or pneumonia admitted to 10 children's medical centers from April 1, 1995, to September 30, 1996 | ≤32-wk-GA infants (n = 44); 33- to 35-wk-GA infants (n = 43); 36-wk-GA infants (n = 46); ≥37-wk-GA infants (n = 338) | Respiratory complications most common (60%) and most serious; highest complication rates, longer stay, and higher costs in 33- to 35-wk-GA infants | Use of database may call into question validity of diagnoses |
CLD indicates chronic lung disease; OR, odds ratio; CHD, congenital heart disease.
GENERAL MORBIDITY AND MORTALITY
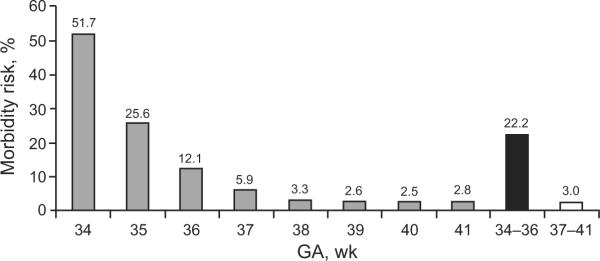
A recent study26 that involved 377 638 term and 26 170 late-preterm (34- to 36-week-GA) infants revealed that 22.2% of the late-preterm infants versus 3.0% of the term infants had newborn morbidity during their birth hospitalization. Preterm infants born at 35 and 36 weeks' GA had 10-fold (relative risk [RR]: 10.2 [95% confidence interval [CI]: 9.7–10.8) and fivefold (RR: 4.8 [95% CI: 4.6–5.1]) higher risk of morbidity, respectively, compared with infants born at 40 weeks' GA. Infants born at 34 weeks' GA had a 20-fold (RR: 20.6 [95% CI: 19.7–21.6]) higher risk of morbidity than infants born at 40 weeks' GA. Relative morbidity increased approximately twofold with every week of decreasing GA earlier than 38 weeks' GA (Fig 1).26 In a retrospective study, McIntire and Leveno7 showed neonatal mortality rates of 1.1, 1.5, and 0.5 per 1000 live births at 34, 35, and 36 weeks' GA, respectively, versus 0.2 per 1000 live births at 39 weeks' GA (P < .001). Pulver et al8 and Santos et al5 showed that 34- to 36-week-GA infants were ~7 and 3 times more likely to die in their first month compared with 39- to 42-week-GA and 37- to 41-week-GA infants, respectively. Two retrospective analyses of records of infants born to US residents revealed higher infant mortality rates in 34- to 36-week-GA infants compared with term infants (37–41 and 40 weeks' GA, respectively).6,10
FIGURE 1.
Proportion of infants with neonatal morbidity as a function of GA. Newborn morbidity was assessed by using a combination of indicators on infants' hospital discharge record and mortality data available from death certificates. Infants born at 34 to 35 weeks' GA were 7 times more likely to have neonatal morbidity than term infants. Infants born at 34 weeks' GA had 20 times the risk of morbidity compared with infants born at 40 weeks' GA. Term (37- to 41-week-GA) infants: n = 377 638; preterm (34- to 36-week-GA) infants: n = 26 170. (Adapted with permission from Shapiro-Mendoza CK, Tomashek KM, Kotelchuck M, et al. Pediatrics. 2008;121[2]:e227. Available at: www.pediatrics.org/cgi/content/full/121/2/e223.)
GENERAL RESPIRATORY MORBIDITY
All forms of respiratory morbidity, including transient tachypnea of the newborn, respiratory distress syndrome, pneumonia, and pulmonary hypertension, affect late-preterm infants at a higher rate than infants of more advanced GA.7,13,17,19,20 A study by De Luca et al27 revealed a strong age-related trend independent of delivery mode in respiratory morbidity and a >10-fold increase in respiratory morbidity in infants of 34 weeks' GA compared with term infants. A retrospective study in Canada by Kitsommart et al28 revealed significantly worse respiratory outcomes (including prevalence of pneumothorax, rates of positive pressure therapy, and mechanical ventilation assistance; all P < .001) in 1481 infants of 34 to 36 weeks' GA compared with 9332 infants of ≥37 weeks' GA.
INFECTIOUS RESPIRATORY MORBIDITY
Very preterm infants (<32 weeks' GA), infants with BPD or congenital heart disease, and preterm infants born at 32 to 36 weeks' GA are particularly vulnerable to developing severe lower respiratory tract infections that require frequent hospitalizations.13,19,29–32 A study by Boyce et al23 revealed that infants born at 33 to 36 weeks' GA had similar rates of admission to the hospital for RSV infection as infants born at <33 weeks' GA, and there were substantially higher rates of admission in these 2 populations compared with those in term infants. Infants born at 33 to 35 weeks' GA had hospital outcomes that were similar to or worse than those of infants born at ≤32 weeks' GA regardless of whether RSV infection was confirmed33 or the infants were hospitalized for nonspecific bronchiolitis.34 After hospitalization for RSV, infants born at 32 to 36 weeks' GA experienced rehospitalization rates twice as high, hospital stays 3 times as long, and outpatient visits twice as frequent as infants of similar GA who were not hospitalized for RSV.35 Prematurity was also associated with an increased risk of bronchiolitis-associated death. The odds ratio for death related to RSV in 32- to 35-week-GA infants compared with term infants was ~5.36
BASIC MECHANISMS OF LUNG DEVELOPMENT
To understand the mechanisms that possibly explain the morbidity in infants born at 34 to 36 weeks' GA, it is necessary to understand lung physiology at this stage of their development.
Stages of Lung Development
Normal lung development is characterized by a carefully choreographed series of prenatal and postnatal events that can be compromised by a variety of factors.37 Early growth and development of the human lung is a continuous process that is highly variable between individuals but, nonetheless, has traditionally been divided into 5 stages. The first is the embryonic phase (26 days to 6 weeks' GA), followed by the pseudoglandular (6–16 weeks' GA), canalicular (16–28 weeks' GA), saccular (28–36 weeks' GA), and alveolar (36 weeks' GA to term) phases. This final phase continues into childhood.37,38 Interference with this stepwise process of lung development during any of these phases may render the lung less effective as a gas exchanger and more susceptible to disease.39
The formation of conducting airways and terminal bronchioles occurs during the canalicular period and establishes the platform for gas exchange.37 Bronchial branching to roughly the 16th generation of the bronchial tree is complete by 16 weeks' GA.40,41 The 28- to 36-week-GA period, termed the saccular period, is a transitional phase before full maturation of alveoli occurs characterized by an increased number of saccules, primitive alveoli that become gradually more effective as gas exchangers and may be sufficient in number and quality to sustain life in the preterm infant. The alveolar walls in the saccular stage are more compact and thick than the final thin walls of alveoli. They also include a double capillary structure that is reduced to a single one in the mature alveolus. However, the blood vessels are well oriented to the epithelium and protrude into air spaces, forming many thin air-blood interfaces that are capable of carrying out the function of gas exchange that fully matures in the alveolar phase.
Although alveoli may form during the saccular phase, mature alveoli are not uniformly present until 36 weeks' GA.38 During the alveolar phase, the epithelium and interstitium decrease in thickness, air-space walls proliferate, and the capillary network matures to its final single-capillary network.38,42 Blood-vessel development, which begins at the earliest stages, continues throughout lung development.43 It is important to note that these structural changes not only affect gas exchange but have profound effects on the mechanical properties of the lung and, hence, the respiratory system as a whole. As noted, premature birth during this critical period may result in significant alteration in lung function and physiology.38
Stable Functional Residual Capacity and Effectiveness of Gas Exchange
Maintenance of a stable and adequate functional residual capacity (FRC) is important for securing stable gas exchange. FRC is determined by the balance between the opposing forces of the chest wall and lung and, thus, is a direct function of their respective mechanical properties. In early life, a compliant chest wall offers little outward recoil to the respiratory system; thus, the elastic characteristics of the respiratory system approximate those of the lung. The lung is also more compliant (ie, has less elastance) in premature and newborn infants. The lung becomes less compliant (ie, increases in elastance) as it undergoes alveolarization, and the interstitium becomes more intricately woven.44 Compliance of the chest wall declines over the first 2 years of life.45–48 In early life, the lung–chest-wall equilibrium results in a mechanically determined FRC that is low relative to older children and adults and is an important determinant of age-related vulnerability to hypoxia. To circumvent this limitation, infants actively elevate their FRC by using laryngeal braking during tidal expiration and by initiating inspiration at an end-expiratory volume above that determined by the mechanical properties of the chest wall and lung.49,50 An additional mechanism is persistence of inspiratory muscle activity into the expiratory phase, thereby modulating the expiratory flow.51
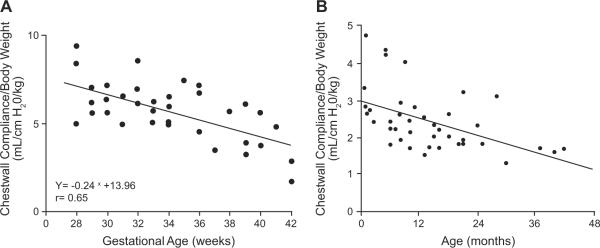
The transition from an actively maintained FRC to one that, as in adults and older children, is mechanically determined occurs in term infants late in the first year and into the second year of life.52 The timing of this transition coincides with the declining compliance (stiffening) of the chest wall and its gradually increasing contribution to the overall stabilization of the respiratory system (Fig 2).46,47 Chest-wall compliance is particularly elevated in premature infants, with the slope of change toward reduced compliance being the steepest between 28 and 40 weeks' GA compared with that at any stage in postnatal life (Fig 2A).46 Thus, it is reasonable to assume that breathing with an overly compliant chest wall, high lung compliance, and reduced number of air-containing units is a challenge for infants delivered before term. The challenge of maintaining an FRC that permits stable gas exchange is likely compounded in the premature infant by apneic events, which have been shown to drive the system to critically low lung volumes and result in rapid desaturation.50
FIGURE 2.
A, Correlation between GA and chest-wall compliance. (Reproduced with permission by Blackwell Publishing, Ltd, from Gerhardt T, Bancalari E. Acta Paediatr Scand. 1980;69[3]:361.) B, Correlation between age and chest-wall compliance. (Reproduced with permission from Papastamelos C, Panitch HB, England SE, Allen JL. J Appl Physiol. 1995;78[1]:182, copyright © 1995 American Physiologic Society.)
Airway Tethering
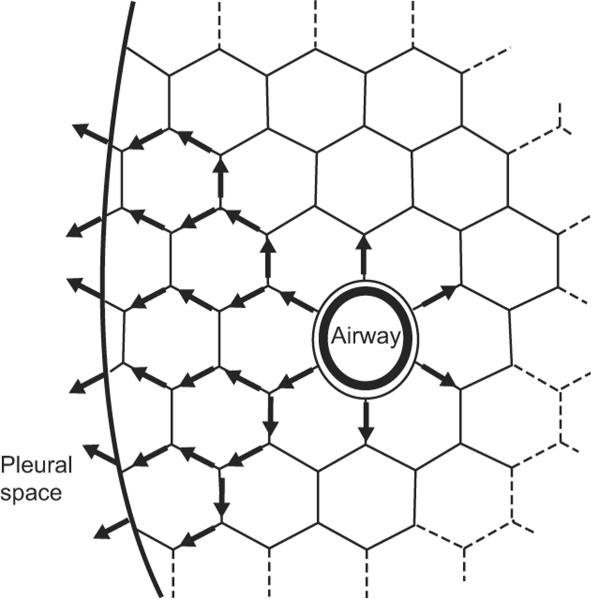
An additional crucial mechanism that secures airway patency and, thus, adequate maintenance of FRC is airway-tethering.53–56 Tethering is mediated through the elastic components in alveolar walls that surround bronchi. These elastic fibers are anchored to each other, creating an extended mesh that exerts a circumferential pull on the intraparenchymal airways (Fig 3). This complex elastic network transmits tension from the pleural surface to the individual bronchi. The tension on the system increases during inspiration, resulting in increased airway caliber. The cross-sectional area of the airway decreases with decline in lung volume. Thus, tethering couples lung-volume changes to airway caliber. Tethering of airways is less effective in infants born prematurely, because alveolarization and the associated development of the parenchymal elastic network are still in the saccular stage of development at 32 to 36 weeks' GA. The effect of reduced tethering is decreased airway stability, increased tendency to closure, increased airway resistance, and, ultimately, a tendency to collapse alveolar units in the lung periphery.53–56
FIGURE 3.
Airway-tethering. Tension is transmitted to the airway through the surrounding alveolar septal walls. Airway diameter, and therefore expiratory flows, are increased at higher lung volumes. (Reprinted with permission from Leff A, Schumacker PT. Respiratory Physiology: Basics and Applications. Philadelphia, PA: W.B. Saunders Company; 1993:43.)
Changes in Lung Volume During the Last Trimester of Gestation
Total lung volume undergoes rapid changes during the last trimester of gestation. Calculations by Langston et al38 revealed that at 30 weeks' GA, the lung volume is only 34% of the ultimate lung volume at mature birth, and at 34 weeks only reaches 47% of the final volume at maturity. In contrast, the air-space walls decrease in thickness such that at 30 and 34 weeks, they are 164% (28 μm) and 135% (23 μm), respectively, relative to the ultimate wall thickness at mature birth (17 μm). In parallel, dramatic increases in air-space surface area occur. Surface area increases from 1.0 to 2.0 m2 at 30 to 32 weeks' GA and to 3.0 to 4.0 m2 at term. These volume changes likely have direct mechanical implications in reducing the vulnerability caused by a low and unstable FRC. Maturation of the alveolar network improves parenchymal elastance and, therefore, airway-tethering.
ASSOCIATION OF PRETERM (30- TO 36-WEEK-GA) BIRTH WITHOUT CLINICAL LUNG DISEASE WITH ALTERED LUNG DEVELOPMENT AND FUNCTION
Lung development occurs mostly in utero. At term, the lung is in its final stage of development: the alveolar stage.57 Normal in utero lung development occurs according to a highly programmed sequence in a stable milieu, one that is significantly more hypoxic relative to the atmosphere (at a fraction of inspired oxygen of 2%–3% at 10–12 weeks' GA, which rises to 8%–10% thereafter). This hypoxic environment represents the norm for lung organogenesis, including vascular development.58–60 Early events of trophoblast differentiation are oxygen regulated.61 It is now recognized that postnatal hyperoxia plays a key role in the development of BPD.62 Premature birth interrupts normal in utero lung development and results in an early transition from the hypoxic intrauterine environment to a comparatively hyperoxic atmospheric environment. An inhaled oxygen concentration of 21% represents significant hyperoxia for the preterm infant. Although this relative hyperoxia has not been directly demonstrated to be an independent cause of altered lung maturation, it may play a role in the subsequent development of chronic lung dysfunction.
There is increasing evidence to support the hypothesis that preterm delivery, even in the absence of any neonatal respiratory disease, may have adverse effects on subsequent lung growth and development and that these alterations may persist and worsen during the first 5 years of life.63–68 Results of a limited number of studies have shown that premature infants of varying GAs, but born without clinical lung disease, have altered pulmonary function. Reduced airway function in the absence of neonatal respiratory disease was demonstrated at 1 year of age in a population of healthy infants born at 29 to 36 weeks' GA.66 Mansell et al reported lower airway conductances and maximum expiratory flows in a group of 5- to 7-year-old children who had been born prematurely but without respiratory problems, which suggests that airway dysfunction persists into childhood.69 These effects may not be dissimilar to those experienced by very preterm infants (<32 weeks' GA), who have generally been the focus of attention because of the severity of their respiratory problems immediately after birth and frequent development of BPD.70,71 A direct association between premature birth and reduced expiratory flows was demonstrated recently by using the raised-volume rapid-thoracic-compression technique.72 In these studies, healthy preterm infants (30–34 weeks' GA [mean: 33.4 weeks' GA]) studied at a mean corrected age of 8 weeks had reduced airway flows in the presence of normal forced vital capacity (FVC) compared with term infants. In a follow-up analysis, the reduced flows did not normalize in these children by 16 months of age, thus demonstrating a lack of “catch-up” growth in airway function by early in the second year of life (Fig 4).73 This result led the authors to conclude that preterm birth was associated with altered lung development. These longitudinal studies that used the raised-volume method confirmed the results of previous studies that suggested a similar conclusion.22
FIGURE 4.
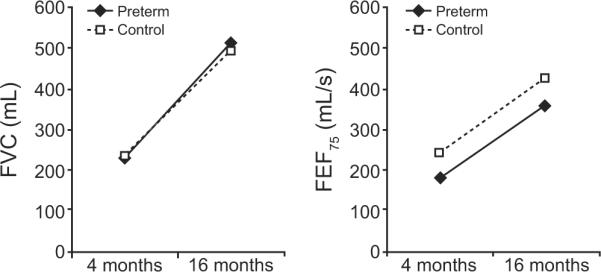
Growth rate of lung function in healthy preterm infants born at <35 weeks' GA (no significant respiratory disease; patients were excluded if they required any mechanical ventilation, supplemental oxygen for >48 hours, or treatment with surfactant). Shown are flow-volume curves in preterm infants (solid line) versus control term group (dotted line), quantified by the FVC and forced expiratory flow at 75% of FVC (forced expiratory flow [FEF75]) (P < .05 between groups). Despite normal lung volume, preterm infants had persistently reduced airflow through the age of 16 months. (Reprinted with permission from Friedrich L, Pitrez PM, Stein RT, Goldani M, Tepper R, Jones MH. Am J Respir Crit Care Med. 2007;176[12]:1272, copyright © 2007 American Thoracic Society.)
The long-term significance of reduced airway function early in life was recently emphasized in a longitudinal study that involved a large group of nonselected (enrolled at birth without any specific criteria) infants who had participated in the Tucson Children's Respiratory Study.74 The study's results showed that infants whose pulmonary function was in the lowest quartile also had pulmonary function in the lowest quartile through the years of follow-up until early adulthood.74 These findings in a normal unselected population suggest that level of pulmonary function in early life tracks and changes little with growth. Data from Weiss and Ware75 have suggested that deficits in lung function during early life, especially if associated with lower respiratory illnesses, increase the risk of chronic obstructive pulmonary disease later in adult life. Of particular importance in this context is the role played by RSV, which is known to affect most children during their first year of life.32
DISCUSSION
Late-preterm infants (– weeks' GA) account for nearly three-quarters of all preterm births in the United States. However, until recently, the emphasis of most research has been on the very preterm infant (<32 weeks' GA) or the very low birth weight infant (≤1500 g). In this review we focused on respiratory disorders, the predominant consequence, but not the only system affected by, preterm birth (34–36 weeks' GA).7,18
A search of the literature from 2000 to 2009 identified 24 studies that reported respiratory morbidity and mortality in populations that included late-preterm infants, 9 of which specifically addressed the impact of RSV bronchiolitis in this population. The evidence presented in the reviewed articles demonstrates that the respiratory vulnerability of preterm infants born at 32 to 36 weeks' GA, usually considered to be at low risk for subsequent respiratory problems, is more similar to that of very preterm infants (<32 weeks' GA) than of term infants. Consequently, these infants are at high risk of developing medical complications that result in higher rates of respiratory morbidity and mortality compared with term infants, as demonstrated by their higher rates of hospital readmission shortly after discharge and during the following 6 months, longer hospital stays, and increased health care use compared with term infants.35,36
Taken together, the results of the published studies suggest that birth at 32 to 36 weeks' GA is a very important risk factor for respiratory morbidity during infancy and early childhood. In this review, we made an attempt to describe physiologic and developmental phenomena that underlie the vulnerability of infants to respiratory diseases. These mechanisms play a role in all infants but are likely to be of particular importance in the preterm population. The conclusions that we draw regarding late-preterm infant lung function are largely extrapolated from physiologic principles and a developmental continuum between very preterm and term infants. Infants born at 30 to 34 weeks' GA without clinical lung disease have altered lung function that persists throughout infancy.72,73 The physiologic deficiencies that result from incomplete lung development described above are likely to account for the early morbidity and vulnerability to infection. With growth in the first to second years of life, the physiologic instability of the chest wall and the inadequate maintenance of lung volume likely correct themselves and should not account for the long-term deficiencies. Therefore, it is apparent that an actual impairment of the potential for full growth is related to early birth. The observation of a direct association between premature birth and reduced forced expiratory flows72 suggests that interruption of lung development is an important determinant of the subsequent increase in respiratory morbidity. Deficits in lung function during early life seem to persist until early adulthood74 and, if associated with lower respiratory illnesses, may increase the risk of chronic obstructive pulmonary disease later in life.75 These findings may have major implications for obstetric and neonatal care.
One of the consequences of altered lung development in preterm infants is their increased vulnerability to RSV infection. Published data have suggested that preterm birth at 33 to 35 weeks' GA significantly increases the risk for severe outcomes among infants who are hospitalized for RSV.33 RSV hospitalization in healthy premature (32- to 35-week-GA) infants is associated with a significant increase in subsequent health care resource use.35 Welliver et al76 proposed that the pathogenesis of viral lower respiratory tract infection is primarily related to the failure to develop an adaptive cytotoxic T-lymphocyte response and that clearance of the virus depends on inefficient innate immune responses mediated by macrophages and neutrophils. The increased vulnerability to RSV in infants born at 32 to 36 weeks' GA suggests that these maturation-dependent deficiencies may be more pronounced in this age group.
The main limitation of the published retrospective analyses is the fact that information on morbidity and mortality was obtained, in most cases, by review of hospital discharge records linked to birth and death certificates, which may have introduced errors in the estimation of GAs and in the accuracy of reporting. Also, the quality of care provided to preterm infants born at 32 to 36 weeks' GA could not be retrospectively evaluated, which made it difficult to compare data between the different studies. Because only published studies were examined, the presence of publication bias cannot be excluded. Despite these limitations, perinatal consequences of late-preterm birth in relation to morbidity and hospitalization ultimately translated into a several-fold greater risk of neonatal mortality among late-preterm infants born at 34 to 36 weeks' GA (1.5–7.9 deaths per 1000 live births) compared with term infants (0.2–2.4 deaths per 1000 live births).4–7,9
Those involved in the care of late-preterm infants should recognize that these infants have, on average, lower airway function than term infants and require diligent evaluation and monitoring, during the neonatal period and throughout early childhood.77,78
The various physiologic studies cited in this review included infants with a spectrum of GAs that spanned prematurity from as early as 30 to 32 weeks' GA, with 34 to 36 weeks' GA representing the upper range of study subjects. Although the demarcation that separated the latter age group is blurred, we think that the conclusions from these studies apply to the older GAs within the study population. Statements regarding long-term and short-term lung function have to be viewed with these limitations in mind.
Substantial research efforts are needed to move toward a more scientific approach in this population. Research attention should be directed to the newborn group without BPD, and within this group, particular emphasis should be placed on late-preterm infants (born at 34–36 weeks' GA). Data from Friedrich et al72,73 reveal lung-function deficits in prematurely born infants without BPD and require confirmation in larger populations. Although it seems likely, it has not yet been demonstrated that the subset of those infants with the lowest lung function is responsible for the majority of the observed later increase in morbidity in this population. The lung function and clinical characteristics of the subset of infants at greatest risk for subsequent morbidity and rehospitalization in the first years of life need to be prospectively characterized. Identifying the characteristics of the group of infants at highest risk for later morbidity will enable the development of trials of therapeutic interventions intended to prevent late respiratory morbidity in these infants. Monoclonal antiviral medications have been shown to be effective in reducing late respiratory morbidity in infants born at ≤32 weeks' GA and infants with BPD. Definition of a target group of 32- to 36-week-GA infants with high rates of rehospitalization resulting from respiratory illnesses would make therapeutic trials of these agents feasible. It will also be important to better characterize the nature and distribution of the lung abnormalities in these at-risk infants by using additional functional measures (plethysmographic fractional lung volumes, lung-clearance index, diffusion capacity) and lung-imaging techniques. The reviewed studies cited herein focused on morbidities and mortality that occur shortly after birth or during the ensuing 6 months. They did not address whether the clinical outcomes they evaluated had long-term implications. Physiologic studies of the at-risk cohort should be extended to childhood and beyond to investigate the long-term physiologic consequences of late-preterm birth.
ACKNOWLEDGMENTS
We acknowledge Physicians World, a division of KnowledgePoint360 Group, LLC, for providing writing and editing support. Additional editorial support was provided by John E. Fincke, PhD, and Gerard P. Johnson, PhD, of Complete Healthcare Communications, Inc. Both were funded by MedImmune.
FINANCIAL DISCLOSURE: Dr Colin has served on advisory boards conducted by MedImmune and serves on Merck's speaker's bureau; Dr McEvoy has served on advisory boards for MedImmune and owns stock in pharmaceutical companies, and MedImmune has provided grant support for her investigator-initiated research; and Dr Castile and Nationwide Children's Hospital receive royalties from nSpire Health, Inc in relation to sales of the Infant Pulmonary Laboratory, and Dr Castile has also served on an advisory board conducted by MedImmune.
ABBREVIATIONS
- GA
gestational age
- RSV
respiratory syncytial virus
- BPD
bronchopulmonary dysplasia
- RR
relative risk
- CI
confidence interval
- FRC
functional residual capacity
- FVC
forced vital capacity
REFERENCES
- 1.Hamilton BE, Martin JA, Ventura SJ. Births: preliminary data for 2007. Natl Vital Stat Rep. 2009;57(12):1–23. [PubMed] [Google Scholar]
- 2.American College of Obstetricians and Gynecologists ACOG educational bulletin. Assessment of fetal lung maturity. Number 230, November 1996. Committee on Educational Bulletins of the American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 1997;56(2):191–198. [PubMed] [Google Scholar]
- 3.American College of Obstetricians and Gynecologists ACOG committee opinion: antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2002;99(5 pt 1):871–873. doi: 10.1016/s0029-7844(02)02023-9. [DOI] [PubMed] [Google Scholar]
- 4.Kramer MS, Demissie K, Yang H, Platt RW, Sauvé R, Liston R. The contribution of mild and moderate preterm birth to infant mortality. Fetal and Infant Health Study Group of the Canadian Perinatal Surveillance System. JAMA. 2000;284(7):843–849. doi: 10.1001/jama.284.7.843. [DOI] [PubMed] [Google Scholar]
- 5.Santos IS, Matijasevich A, Silveira MF, et al. Associated factors and consequences of late preterm births: results from the 2004 Pelotas birth cohort. Paediatr Perinat Epidemiol. 2008;22(4):350–359. doi: 10.1111/j.1365-3016.2008.00934.x. [DOI] [PubMed] [Google Scholar]
- 6.Tomashek KM, Shapiro-Mendoza CK, Davidoff MJ, Petrini JR. Differences in mortality between late-preterm and term singleton infants in the United States, 1995–2002. J Pediatr. 2007;151(5):450–456. e456.e1. doi: 10.1016/j.jpeds.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 7.McIntire DD, Leveno KJ. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol. 2008;111(1):35–41. doi: 10.1097/01.AOG.0000297311.33046.73. [DOI] [PubMed] [Google Scholar]
- 8.Pulver LS, Guest-Warnick G, Stoddard GJ, Byington CL, Young PC. Weight for gestational age affects the mortality of late preterm infants. Pediatrics. 2009;123(6) doi: 10.1542/peds.2008-3288. Available at: www.pediatrics.org/cgi/content/full/123/6/e1072. [DOI] [PubMed] [Google Scholar]
- 9.Escobar GJ, Clark RH, Greene JD. Short-term outcomes of infants born at 35 and 36 weeks gestation: we need to ask more questions. Semin Perinatol. 2006;30(1):28–33. doi: 10.1053/j.semperi.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Young PC, Glasgow TS, Li X, Guest-Warnick G, Stoddard G. Mortality of late-preterm (near-term) newborns in Utah. Pediatrics. 2007;119(3) doi: 10.1542/peds.2006-2486. Available at: www.pediatrics.org/cgi/content/full/119/3/e659. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro-Mendoza CK, Tomashek KM, Kotelchuck M, Barfield W, Weiss J, Evans S. Risk factors for neonatal morbidity and mortality among “healthy,” late preterm newborns. Semin Perinatol. 2006;30(2):54–60. doi: 10.1053/j.semperi.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Tomashek KM, Shapiro-Mendoza CK, Weiss J, et al. Early discharge among late preterm and term newborns and risk of neonatal morbidity. Semin Perinatol. 2006;30(2):61–68. doi: 10.1053/j.semperi.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Wang ML, Dorer DJ, Fleming MP, Catlin EA. Clinical outcomes of near-term infants. Pediatrics. 2004;114(2):372–376. doi: 10.1542/peds.114.2.372. [DOI] [PubMed] [Google Scholar]
- 14.Engle WA, Tomashek KM, Wallman C. “Late-preterm” infants: a population at risk [published correction appears in Pediatrics. 2008;121(2):451] Pediatrics. 2007;120(6):1390–1401. doi: 10.1542/peds.2007-2952. [DOI] [PubMed] [Google Scholar]
- 15.Escobar GJ, Gonzales VM, Armstrong MA, Folck BF, Xiong B, Newman TB. Rehospitalization for neonatal dehydration: a nested case-control study. Arch Pediatr Adolesc Med. 2002;156(2):155–161. doi: 10.1001/archpedi.156.2.155. [DOI] [PubMed] [Google Scholar]
- 16.Makari D, Groothuis J. Health risks of the late-preterm infant. Neonatol Today. 2009;4(1):1–8. [Google Scholar]
- 17.Clark RH. The epidemiology of respiratory failure in neonates born at an estimated gestational age of 34 weeks or more. J Perinatol. 2005;25(4):251–257. doi: 10.1038/sj.jp.7211242. [DOI] [PubMed] [Google Scholar]
- 18.Escobar GJ, Greene JD, Hulac P, et al. Rehospitalisation after birth hospitalisation: patterns among infants of all gestations. Arch Dis Child. 2005;90(2):125–131. doi: 10.1136/adc.2003.039974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khashu M, Narayanan M, Bhargava S, Osiovich H. Perinatal outcomes associated with preterm birth at 33 to 36 weeks' gestation: a population-based cohort study. Pediatrics. 2009;123(1):109–113. doi: 10.1542/peds.2007-3743. [DOI] [PubMed] [Google Scholar]
- 20.Dani C, Corsini I, Piergentili L, Bertini G, Pratesi S, Rubaltelli FF. Neonatal morbidity in late preterm and term infants in the nursery of a tertiary hospital. Acta Paediatr. 2009;98(11):1841–1843. doi: 10.1111/j.1651-2227.2009.01425.x. [DOI] [PubMed] [Google Scholar]
- 21.Melamed N, Klinger G, Tenenbaum-Gavish K, et al. Short-term neonatal outcome in low-risk, spontaneous, singleton, late preterm deliveries. Obstet Gynecol. 2009;114(2 pt 1):253–260. doi: 10.1097/AOG.0b013e3181af6931. [DOI] [PubMed] [Google Scholar]
- 22.Moss TJ. Respiratory consequences of preterm birth. Clin Exp Pharmacol Physiol. 2006;33(3):280–284. doi: 10.1111/j.1440-1681.2006.04359.x. [DOI] [PubMed] [Google Scholar]
- 23.Boyce TG, Mellen BG, Mitchel EF, Jr, Wright PF, Griffin MR. Rates of hospitalization for respiratory syncytial virus infection among children in Medicaid. J Pediatr. 2000;137(6):865–870. doi: 10.1067/mpd.2000.110531. [DOI] [PubMed] [Google Scholar]
- 24.American Academy of Pediatrics. American College of Obstetricians and Gynecologists . Guidelines for Perinatal Care. 5th ed. American Academy of Pediatrics; Elk Grove Village, IL: 2005. [Google Scholar]
- 25.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 26.Shapiro-Mendoza CK, Tomashek KM, Kotelchuck M, et al. Effect of late-preterm birth and maternal medical conditions on newborn morbidity risk. Pediatrics. 2008;121(2) doi: 10.1542/peds.2006-3629. Available at: www.pediatrics.org/cgi/content/full/121/2/e223. [DOI] [PubMed] [Google Scholar]
- 27.De Luca R, Boulvain M, Irion O, Berner M, Pfister RE. Incidence of early neonatal mortality and morbidity after late-preterm and term cesarean delivery. Pediatrics. 2009;123(6) doi: 10.1542/peds.2008-2407. Available at: www.pediatrics.org/cgi/content/full/123/6/e1064. [DOI] [PubMed] [Google Scholar]
- 28.Kitsommart R, Janes M, Mahajan V, et al. Outcomes of late-preterm infants: a retrospective, single-center, Canadian study. Clin Pediatr (Phila) 2009;48(8):844–850. doi: 10.1177/0009922809340432. [DOI] [PubMed] [Google Scholar]
- 29.Carbonell-Estrany X, Quero J, Bustos G, et al. Rehospitalization because of respiratory syncytial virus infection in premature infants younger than 33 weeks of gestation: a prospective study. IRIS Study Group. Pediatr Infect Dis J. 2000;19(7):592–597. doi: 10.1097/00006454-200007000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Law BJ, Langley JM, Allen U, et al. The Pediatric Investigators Collaborative Network on Infections in Canada study of predictors of hospitalization for respiratory syncytial virus infection for infants born at 33 through 35 completed weeks of gestation. Pediatr Infect Dis J. 2004;23(9):806–814. doi: 10.1097/01.inf.0000137568.71589.bd. [DOI] [PubMed] [Google Scholar]
- 31.Figueras-Aloy J, Carbonell-Estrany X, Quero J. Case-control study of the risk factors linked to respiratory syncytial virus infection requiring hospitalization in premature infants born at a gestational age of 33–35 weeks in Spain. Pediatr Infect Dis J. 2004;23(9):815–820. doi: 10.1097/01.inf.0000136869.21397.6b. [DOI] [PubMed] [Google Scholar]
- 32.Leader S, Kohlhase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J Pediatr. 2003;143(5 suppl):S127–S132. doi: 10.1067/s0022-3476(03)00510-9. [DOI] [PubMed] [Google Scholar]
- 33.Horn SD, Smout RJ. Effect of prematurity on respiratory syncytial virus hospital resource use and outcomes. J Pediatr. 2003;143(5 suppl):S133–S141. doi: 10.1067/s0022-3476(03)00509-2. [DOI] [PubMed] [Google Scholar]
- 34.Willson DF, Landrigan CP, Horn SD, Smout RJ. Complications in infants hospitalized for bronchiolitis or respiratory syncytial virus pneumonia. J Pediatr. 2003;143(5 suppl):S142–S149. doi: 10.1067/s0022-3476(03)00514-6. [DOI] [PubMed] [Google Scholar]
- 35.Sampalis JS. Morbidity and mortality after RSV-associated hospitalizations among premature Canadian infants. J Pediatr. 2003;143(5 suppl):S150–S156. doi: 10.1067/s0022-3476(03)00513-4. [DOI] [PubMed] [Google Scholar]
- 36.Holman RC, Shay DK, Curns AT, Lingappa JR, Anderson LJ. Risk factors for bronchiolitis-associated deaths among infants in the United States. Pediatr Infect Dis J. 2003;22(6):483–490. doi: 10.1097/01.inf.0000069765.43405.3b. [DOI] [PubMed] [Google Scholar]
- 37.Joshi S, Kotecha S. Lung growth and development. Early Hum Dev. 2007;83(12):789–794. doi: 10.1016/j.earlhumdev.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 38.Langston C, Kida K, Reed M, Thurlbeck WM. Human lung growth in late gestation and in the neonate. Am Rev Respir Dis. 1984;129(4):607–613. [PubMed] [Google Scholar]
- 39.Maritz GS, Morley CJ, Harding R. Early developmental origins of impaired lung structure and function. Early Hum Dev. 2005;81(9):763–771. doi: 10.1016/j.earlhumdev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 40.Bucher U, Reid L. Development of the intra-segmental bronchial tree: the pattern of branching and development of cartilage at various stages of intra-uterine life. Thorax. 1961;16:207–218. doi: 10.1136/thx.16.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kotecha S. Lung growth for beginners. Paediatr Respir Rev. 2000;1(4):308–313. doi: 10.1053/prrv.2000.0069. [DOI] [PubMed] [Google Scholar]
- 42.Copland I, Post M. Lung development and fetal lung growth. Paediatr Respir Rev. 2004;5(suppl A):S259–S264. doi: 10.1016/s1526-0542(04)90049-8. [DOI] [PubMed] [Google Scholar]
- 43.Stenmark KR, Gebb SA. Lung vascular development: breathing new life into an old problem. Am J Respir Cell Mol Biol. 2003;28(2):133–137. doi: 10.1165/rcmb.F259. [DOI] [PubMed] [Google Scholar]
- 44.Bryan CA, Wohl MEB. Respiratory mechanics in children. In: Macklem PT, Mead J, editors. Handbook of Physiology: The Respiratory System. Vol 3. American Physiological Society; Bethesda, MD: 1986. pp. 183–191. Pt 2. [Google Scholar]
- 45.Davis GM, Coates AL, Papageorgiou A, Bureau MA. Direct measurement of static chest wall compliance in animal and human neonates. J Appl Physiol. 1988;65(3):1093–1098. doi: 10.1152/jappl.1988.65.3.1093. [DOI] [PubMed] [Google Scholar]
- 46.Gerhardt T, Bancalari E. Chestwall compliance in full-term and premature infants. Acta Paediatr Scand. 1980;69(3):359–364. doi: 10.1111/j.1651-2227.1980.tb07093.x. [DOI] [PubMed] [Google Scholar]
- 47.Papastamelos C, Panitch HB, England SE, Allen JL. Developmental changes in chest wall compliance in infancy and early childhood. J Appl Physiol. 1995;78(1):179–184. doi: 10.1152/jappl.1995.78.1.179. [DOI] [PubMed] [Google Scholar]
- 48.Richards CC, Bachman L. Lung and chest wall compliance of apneic paralyzed infants. J Clin Invest. 1961;40:273–278. doi: 10.1172/JCI104253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kosch PC, Hutchinson AA, Wozniak JA, Carlo WA, Stark AR. Posterior cricoarytenoid and diaphragm activities during tidal breathing in neonates. J Appl Physiol. 1988;64(5):1968–1978. doi: 10.1152/jappl.1988.64.5.1968. [DOI] [PubMed] [Google Scholar]
- 50.Kosch PC, Stark AR. Dynamic maintenance of end-expiratory lung volume in full-term infants. J Appl Physiol. 1984;57(4):1126–1133. doi: 10.1152/jappl.1984.57.4.1126. [DOI] [PubMed] [Google Scholar]
- 51.Mortola JP, Milic-Emili J, Noworaj A, Smith B, Fox G, Weeks S. Muscle pressure and flow during expiration in infants. Am Rev Respir Dis. 1984;129(1):49–53. doi: 10.1164/arrd.1984.129.1.49. [DOI] [PubMed] [Google Scholar]
- 52.Colin AA, Wohl ME, Mead J, Ratjen FA, Glass G, Stark AR. Transition from dynamically maintained to relaxed end-expiratory volume in human infants. J Appl Physiol. 1989;67(5):2107–2111. doi: 10.1152/jappl.1989.67.5.2107. [DOI] [PubMed] [Google Scholar]
- 53.Gomes RF, Shardonofsky F, Eidelman DH, Bates JH. Respiratory mechanics and lung development in the rat from early age to adulthood. J Appl Physiol. 2001;90(5):1631–1638. doi: 10.1152/jappl.2001.90.5.1631. [DOI] [PubMed] [Google Scholar]
- 54.Henschen M, Stocks J, Brookes I, Frey U. New aspects of airway mechanics in preterm infants. Eur Respir J. 2006;27(5):913–920. doi: 10.1183/09031936.06.00036305. [DOI] [PubMed] [Google Scholar]
- 55.Moreno RH, Hogg JC, Pare PD. Mechanics of airway narrowing. Am Rev Respir Dis. 1986;133(6):1171–1180. doi: 10.1164/arrd.1986.133.6.1171. [DOI] [PubMed] [Google Scholar]
- 56.Plopper CG, Nishio SJ, Schelegle ES. Tethering tracheobronchial airways within the lungs. Am J Respir Crit Care Med. 2003;167(1):2–3. doi: 10.1164/rccm.2211002. [DOI] [PubMed] [Google Scholar]
- 57.Burri PH. Development and growth of the human lung. In: Macklem PT, Mead J, editors. Handbook of Physiology: The Respiratory System. Vol 3. American Physiological Society; Bethesda, MD: 1997. pp. 1–46. Pt 1. [Google Scholar]
- 58.Burton GJ, Jauniaux E, Watson AL. Maternal arterial connections to the placental intervillous space during the first trimester of human pregnancy: the Boyd collection revisited. Am J Obstet Gynecol. 1999;181(3):718–724. doi: 10.1016/s0002-9378(99)70518-1. [DOI] [PubMed] [Google Scholar]
- 59.Groenman F, Rutter M, Caniggia I, Tibboel D, Post M. Hypoxia-inducible factors in the first trimester human lung. J Histochem Cytochem. 2007;55(4):355–363. doi: 10.1369/jhc.6A7129.2006. [DOI] [PubMed] [Google Scholar]
- 60.Rodesch F, Simon P, Donner C, Jauniaux E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol. 1992;80(2):283–285. [PubMed] [Google Scholar]
- 61.Caniggia I, Mostachfi H, Winter J, et al. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3) J Clin Invest. 2000;105(5):577–587. doi: 10.1172/JCI8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Paepe ME, Mao Q, Powell J, et al. Growth of pulmonary microvasculature in ventilated preterm infants. Am J Respir Crit Care Med. 2006;173(2):204–211. doi: 10.1164/rccm.200506-927OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gappa M, Stocks J, Merkus P. Lung growth and development after preterm birth: further evidence. Am J Respir Crit Care Med. 2003;168(3):399. doi: 10.1164/ajrccm.168.3.955. author reply 400. [DOI] [PubMed] [Google Scholar]
- 64.Greenough A. Late respiratory outcomes after preterm birth. Early Hum Dev. 2007;83(12):785–788. doi: 10.1016/j.earlhumdev.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 65.Hjalmarson O, Sandberg K. Abnormal lung function in healthy preterm infants. Am J Respir Crit Care Med. 2002;165(1):83–87. doi: 10.1164/ajrccm.165.1.2107093. [DOI] [PubMed] [Google Scholar]
- 66.Hoo AF, Dezateux C, Henschen M, Costeloe K, Stocks J. Development of airway function in infancy after preterm delivery. J Pediatr. 2002;141(5):652–658. doi: 10.1067/mpd.2002.128114. [DOI] [PubMed] [Google Scholar]
- 67.Jobe AH. An unknown: lung growth and development after very preterm birth. Am J Respir Crit Care Med. 2002;166(12 pt 1):1529–1530. doi: 10.1164/rccm.2209012. [DOI] [PubMed] [Google Scholar]
- 68.Stocks J, Coates A, Bush A. Lung function in infants and young children with chronic lung disease of infancy: the next steps? Pediatr Pulmonol. 2007;42(1):3–9. doi: 10.1002/ppul.20520. [DOI] [PubMed] [Google Scholar]
- 69.Mansell AL, Driscoll JM, James LS. Pulmonary follow-up of moderately low birth weight infants with and without respiratory distress syndrome. J Pediatr. 1987;110(1):111–115. doi: 10.1016/s0022-3476(87)80301-3. [DOI] [PubMed] [Google Scholar]
- 70.McEvoy C, Bowling S, Williamson K, McGaw P, Durand M. Randomized, double-blinded trial of low-dose dexamethasone: II. Functional residual capacity and pulmonary outcome in very low birth weight infants at risk for bronchopulmonary dysplasia. Pediatr Pulmonol. 2004;38(1):55–63. doi: 10.1002/ppul.20037. [DOI] [PubMed] [Google Scholar]
- 71.Morley CJ, Davis PG, Doyle LW, et al. Nasal CPAP or intubation at birth for very preterm infants. N Engl J Med. 2008;358(7):700–708. doi: 10.1056/NEJMoa072788. [DOI] [PubMed] [Google Scholar]
- 72.Friedrich L, Stein RT, Pitrez PM, Corso AL, Jones MH. Reduced lung function in healthy preterm infants in the first months of life. Am J Respir Crit Care Med. 2006;173(4):442–447. doi: 10.1164/rccm.200503-444OC. [DOI] [PubMed] [Google Scholar]
- 73.Friedrich L, Pitrez PM, Stein RT, Goldani M, Tepper R, Jones MH. Growth rate of lung function in healthy preterm infants. Am J Respir Crit Care Med. 2007;176(12):1269–1273. doi: 10.1164/rccm.200703-476OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stern DA, Morgan WJ, Wright AL, Guerra S, Martinez FD. Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. Lancet. 2007;370(9589):758–764. doi: 10.1016/S0140-6736(07)61379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weiss ST, Ware JH. Overview of issues in the longitudinal analysis of respiratory data. Am J Respir Crit Care Med. 1996;154(6 pt 2):S208–S211. doi: 10.1164/ajrccm/154.6_Pt_2.S208. [DOI] [PubMed] [Google Scholar]
- 76.Welliver TP, Garofalo RP, Hosakote Y, et al. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J Infect Dis. 2007;195(8):1126–1136. doi: 10.1086/512615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Raju TN. Late-preterm births: challenges and opportunities. Pediatrics. 2008;121(2):402–403. doi: 10.1542/peds.2007-2357. [DOI] [PubMed] [Google Scholar]
- 78.Raju TN, Higgins RD, Stark AR, Leveno KJ. Optimizing care and outcome for late-preterm (near-term) infants: a summary of the workshop sponsored by the National Institute of Child Health and Human Development. Pediatrics. 2006;118(3):1207–1214. doi: 10.1542/peds.2006-0018. [DOI] [PubMed] [Google Scholar]