Abstract
Exposure to hypoxia-induced replication arrest initiates a DNA damage response which includes both ATR and ATM-mediated signalling. DNA fiber analysis was used to show that these conditions lead to a replication arrest during both the initiation and elongation phases and that this correlated with decreased levels of nucleotides. The DNA damage response induced by hypoxia is distinct from the classical pathways induced by damaging agents primarily due to the lack of detectable DNA damage but also due to the coincident repression of DNA repair in hypoxic conditions. The principle aims of the hypoxia-induced DNA damage response appear to be the induction of p53-dependent apoptosis or the preservation of replication fork integrity. The latter is of particular importance should reoxygenation occur. Tumor reoxygenation occurs as a result of spontaneous changes in blood flow and also therapy. Cells experiencing hypoxia/reoxygenation are therefore sensitive to loss or inhibition of components of the DNA damage response including; Chk1, ATM, ATR and PARP. In addition, restoration of hypoxia-induced p53-mediated signalling may well be effective in the targeting of hypoxic cells. The DNA damage response is also induced in endothelial cells at moderate levels of hypoxia which do not induce replication arrest. In this situation phosphorylation of H2AX has been shown to be required for proliferation and angiogenesis and is therefore an attractive potential therapeutic target.
Background
Most solid tumors develop in an environment of below optimal oxygen concentration (hypoxia). This occurs as a result of inefficient tumor vasculature and the high metabolic demand for oxygen, essentially an issue of low supply, high demand. Many elegant studies have demonstrated that this is therapeutically significant as hypoxic cells are more resistant to both chemo and radio-therapy (1, 2) (3). Hypoxia has also been demonstrated to increase both invasion and metastasis therefore contributing to more aggressive disease (4-6). For these reasons the ability to image hypoxic areas and target these cells has become an area of intense scrutiny. The ability of cancer cells to survive and thrive in these conditions results from their ability to hijack pathways necessary for embryonic development in hypoxic conditions. The principle mediators of the hypoxic response are the HIF transcription factors, which are composed of an oxygen-labile α subunit (HIF-1α, HIF-2α, HIF-3α and HIF-4α) and a shared constitutively expressed protein (HIF-1β/ARNT)(7). In in vivo settings hypoxia occurs as a gradient of oxygen tensions ranging between normal levels (6%), mild hypoxia (0.5-3%) and anoxia (0%) (8). The HIF proteins are responsive to a wide range of oxygen tensions. HIF-1α and HIF-2α posses structurally similar domains and their stability is regulated through two oxygen-dependent degradation domains (NODDD and CODDD) that allow their proteolytic degradation (9). However, expression of HIF-1α and HIF-2α has been shown to differ between hypoxic tissues indicating they may have different roles (10). For example, HIF-1α has been shown to be involved in causing cell cycle arrest following moderate hypoxia by inhibition of c-Myc, whilst HIF-2α may enhance cell cycle progression by promoting the activation of c-Myc and some of its target genes (11).
In contrast, severe levels of hypoxia (<0.1% O2) have been demonstrated to induce a specific hypoxic-response not observed at milder hypoxia levels. This includes the unfolded protein response (UPR), cell death and the DNA damage response (DDR) which are induced at severe levels of hypoxia (12-15). The DDR involves a complex collaboration between signalling pathways activated as a result of different types of DNA damaging stresses. In brief, signals such as a double strand break are detected by a group of proteins known collectively as sensors, including the MRN complex (Mre11-Rad50-NBS1). This initial detection of DNA damage leads to activation of the PI3-kinase, ATM and subsequently ATR. This response is amplified by a group of mediator proteins including MDC1 and 53BP1 (16). Ultimately, these pathways are involved in mediating DNA repair cell cycle checkpoint activation and/or apoptosis in order to maintain genomic stability following such insults (17).
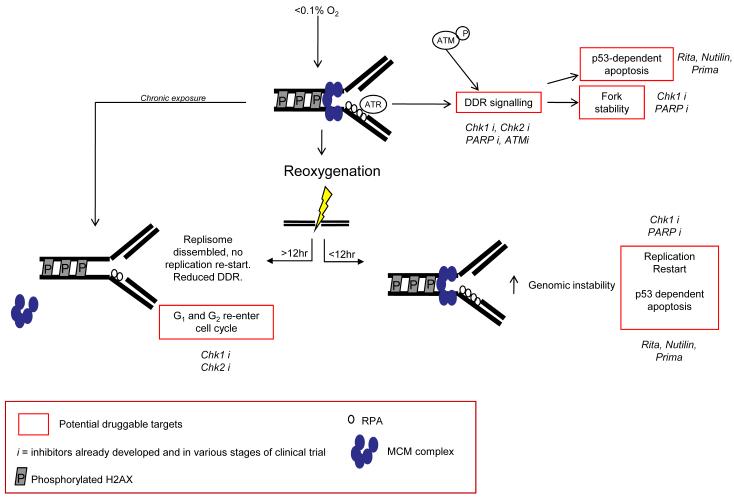
The DDR activated at severe levels of hypoxia (<0.1% O2) involves an induction of rapid replication arrest. The enzyme responsible for nucleotide production is ribonucleotide reductase, which is dependent on cellular oxygen for its function and is therefore likely to be severely compromised in hypoxic conditions (18). In support of this, we recently measured nucleotide levels in hypoxic cells in vitro and found a rapid and significant decrease in levels in response to hypoxia (19). Regions of single stranded DNA (ssDNA) accumulate at stalled replication forks in hypoxic conditions and in turn become coated with RPA (20). This is believed to be the signal for the hypoxic-induction of the DDR which includes the ATR-dependent phosphorylation of, for example, p53, H2AX and Chk1, figure 1 (21, 22). Interestingly, this occurs in the apparent absence of DNA damage unless factors essential to replication fork stability are also inhibited/depleted. Despite this finding the ATM kinase is also active in hypoxia as shown by increased autophosphorylation and an ability to phosphorylate Chk2 (23-25). ATM has previously been demonstrated to be active in the absence of DNA damage although, hypoxia is one of the few physiologically relevant stresses to do this (26, 27). ATM dependent-Chk2 phosphorylation under hypoxic conditions has been shown to lead to phosphorylation of p53 at serine 20 and BRCA1 at serine 988 (28). The trigger that initiates ATM-mediated signalling is currently unclear. However, it seems likely that replication-stress induced ATR in hypoxic conditions contributes (29). Hypoxia-induced replication arrest is reversible if oxygen levels are restored within an acute time frame (up to approximately 8-12 hours). After longer more chronic exposures a disassembly of the replisome is observed as well as a failure to restart DNA synthesis even in the presence of available nucleotides. Specifically, in response to chronic hypoxia exposure the MCM complex is transcriptionally repressed and becomes detached from the chromatin, figure 1 (19).
Figure 1. Hypoxia-induced replication arrest triggers a DNA damage response.
Chronic exposure to levels of oxygen below 0.1% lead to replisome disassembly therefore preventing any possible replication restart during reoxygenation. In contrast, hypoxic cells arrested for only short periods of time do undergo replication restart but do so in the presence of reoxygenation-induced DNA damage and hypoxia-repressed DNA repair. Highlighted in red are the potential therapeutic targets for targeting cells cycling through hypoxia/reoxygenation or angiogenesis discussed in the text. Specific targets which have inhibitors in clinical trials are indicated in italics.
Whilst hypoxia does not lead to an accumulation of DNA damage as detected by either comet or 53BP1 foci formation assay, reoxygenation induces significant levels of DNA damage through the action of reactive oxygen species (ROS) (30). This in turn leads to an ATM-Chk2 mediated G2 arrest to allow repair. Tumor cells lacking Chk2 show reduced reoxygenation-induced arrest and increased apoptosis (23, 25, 31).
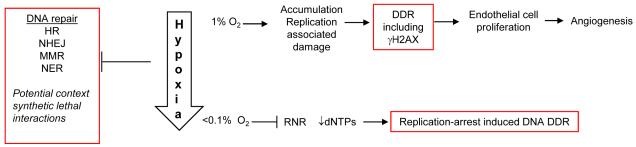
More recently, a hypoxia-mediated induction of a DDR has been observed in conditions which do not cause replication arrest, figure 2 (32). This work demonstrated that in response to hypoxia, (1% O2), γH2AX was induced in proliferating endothelial cells and that even more surprisingly this was required to maintain proliferation and hypoxia-induced neovascularisation in these conditions (32) (reviewed in (33, 34)). Intriguingly, there was no apparent role for γH2AX in developmental angiogenesis as loss of γH2AX only reduced hypoxia induced neovascularisation in pathologic settings, for example hind leg ischemia, retinopathy and tumor angiogenesis. The induction of a DDR in these conditions was attributed to the accumulation of the low level of DNA damage, which occurs during normal replication. This DNA damage may be potentially more prevalent in hypoxic conditions as many essential components of the DNA repair pathways have been shown to be repressed in hypoxic conditions, for a recent review see (35). Homologous recombination, mismatch repair and non-homologous end joining have all been shown to be less effective in hypoxic conditions suggesting that a general response to hypoxia is repression of DNA repair. The mechanisms of repression are varied and include roles for HIF and micro RNAs (miRs) (36, 37). For example, components of the mismatch repair pathway MLH1 and MLH2 have been shown to be repressed under hypoxic conditions. MLH1 repression seems to correlate with increased levels of di- and tri-methylations on H3K9 due to an increase in histone methyltransferase G9a (38). Key members of the homologous recombination pathway, RAD51 and BRCA1 have also been shown to be down regulated in hypoxia. A proposed mechanism for RAD51 and BRACA1 down regulation is the formation of a repressive E2F4/p130 complex at the E2F site on the promoter of these genes (39).
Figure 2. Schematic representation of the DDR to hypoxia.
The DDR to hypoxia is both oxygen and cell type specific, for example endothelial cells have been shown to be reliant on γH2AX for proliferation and angiogenesis. At severe levels of hypoxia nucleotide levels fall leading to stalled replication and a DDR. Highlighted in red are the potential therapeutic targets for targeting cells cycling through hypoxia/reoxygenation or angiogenesis discussed in the text.
Why a cell actively represses these pathways is unclear, although perhaps it is simply an energy saving measure. Importantly, the hypoxia-mediated repression of DNA repair seems to occur at a variety of oxygen tensions i.e. this does not just occur in regions of severe hypoxia (<0.1% O2) which occur at the border of necrotic areas. This is highlighted by the involvement of HIF which, as previously mentioned is stabilised in relatively moderate hypoxic conditions. Our own in vitro data demonstrates that although the kinetics of repression of BRCA1 or Rad51 may differ between exposure to 0.02% and 0.2% oxygen for example, expression levels do decrease in both cases. The implications of this are that larger proportions of tumors will have repressed DNA repair. Repression of genes involved in DNA repair have been proposed to have a substantial role in increasing genomic instability in tumor cells which may contribute to the aggressiveness of hypoxic tumors (35). Interestingly, the hypoxia-induced DDR also appears to be repressed after chronic hypoxia exposure, for example Chk1 is rapidly and robustly phosphorylated during the acute time frame but then decreases (19). The reason behind this observation is not clear although it was also noted that the number of RPA foci in hypoxia-arrested cells also decreases with increasing exposure to hypoxia. This would suggest that the hypoxia-induced signal leading to ATR activation decreases with exposure time. It is possible that this is due to residual polymerase activity although this remains to be shown conclusively.
Clinical-Translational Advances
Targeting the DDR has become a popular strategy for the development of novel therapeutics with many now reaching clinical trials and showing promise (40). Both ATM and Chk1 inhibitors have been developed. Unfortunately, toxicity was observed with some of the early versions of these compounds (41). Second generation Chk1 inhibitors such as AZD7762, however, are proving to have some encouraging effects (42). For example, it was recently demonstrated in vitro that AZD7762 in combination with the nucleoside analog gemcitabine showed enhanced lethality and that AZD7762 acts a radiation sensitizer both in vitro and in in vivo xenograft experiments (43, 44). There is increasing evidence to suggest that DDR inhibitors may be able to effectively target hypoxic cells since loss or inhibition of several key players in the DDR such as ATR and ATM have been shown to sensitize cells to hypoxia/reoxygenation. Cells experiencing hypoxic conditions severe enough to induce a replication arrest are reliant on factors such as ATR and Chk1 to preserve replication fork integrity and prevent DNA breaks (20). Reoxygenation of cells in this state induces DNA damage and a checkpoint response. Indeed, in in vitro studies cells exposed to hypoxia/reoxygenation are sensitive to loss or inhibition of Chk1 or Chk2 therefore suggesting that the inhibitors of these kinases currently in clinical trials may show increased toxicity to hypoxic cells (20, 31, 45). Sensitization of tumor cells to hypoxia/reoxygenation by inhibition of members of the damage response pathway may be of particular therapeutic importance, since it is those cells that are cycling through hypoxia/reoxygenation that are responsible for the worst prognosis (45).
Unfortunately, when considering the targeting of hypoxic cells in vivo a problem arises, the one of drug delivery. Hypoxic regions occur in tumors due to a limited blood supply resulting from an inefficient and chaotic vasculature. This leads to the limited delivery of chemotherapeutic agents to hypoxic regions. For this reason the value of Chk inhibitors to target hypoxic regions will probably be in combination with agents known to induce either reoxygenation or vessel normalisation (46). For example, it has been proposed that the addition of anti-angiogenic therapies such as VEGFR antagonists to conventional chemotherapy may lead to a transient increase in vessel normalisation, resulting in a more efficient delivery of drugs and an increase in tumor oxygen levels (47). Furthermore, reoxygenation as well as an increase in blood flow and tumor shrinkage occur following fractionated radiotherapy, which can again improve the efficiency of subsequent radiotherapy and chemotherapy (48). Some studies have also suggested that chemo- and radiotherapy may target tumor and circulating endothelial cells as well as endothelial progenitor cells and hence have a direct anti-angiogenic effect (49). A further complexity arises from the need to quantitatively measure hypoxia in vivo in order to evaluate novel therapy combinations. As mentioned imaging and measuring tumor hypoxia has been an area of intense scrutiny. Options include the further development/validation of biomarkers amenable to measurement in bodily fluids, the imaging of hypoxic regions in tumors using, for example nitroimidazole derivatives or measurement of tumor oxygenation directly using an Eppendorf electrode (50-52).
The repression of DNA repair pathways in hypoxia also renders cells sensitive to the loss (or inhibition) of alternative pathways, resulting in context synthetic lethality. This term has been adopted to describe the synthetic lethal interaction between the loss of pathway A through therapeutic intervention and the loss of pathway B through its repression by the cellular context. Inhibitors of PARP are now in phase II clinical trials and showing some promise for the treatment of breast cancers with BRCA1 mutations. Given the repression of BRCA1 and other factors essential to homologous recombination in hypoxia, we and others have proposed that hypoxic cells may be sensitive to PARP inhibitors. The PARP inhibitor ABT-888 has already been shown to radiosensitize tumor cell lines in hypoxic conditions (53). The clinical implications of this are that a wider range of tumor types might be sensitive to PARP inhibitors i.e. solid tumors with hypoxic fractions rather than just those showing BRCA loss or BRCAness (Bristow/Hammond, unpublished observations) (54).
The combination of Chk1 inhibitors with other therapies capable of inducing damage such as radiotherapy, inhibitors of DNA replication or topoisomerase inhibitors has also been studied. As previously mentioned, the use of the second generation Chk1 inhibitor AZD7762 and the nucleoside analogue gemcitabine has been shown to have some synergistic effects, attributed to activation of origin firing, destabilization of stalled replication forks and entry of cells with unrepaired DNA damage into mitosis (55). These effects may be further potentiated in hypoxic cells that, as mentioned above, show an increased sensitivity to Chk1 inhibition and harbor defects in DNA repair. Importantly, checkpoint and homologous recombination defects have also been proposed to have a major contribution to the radiosensitization observed by the combination of AZD7762 with radiation (56).
The pharmacological reactivation of p53 may be an effective way of targeting hypoxic tumors since loss of p53 has been shown to select for a loss of the apoptotic response in hypoxia (57). PRIMA, Nutilin and RITA are amongst some of the compounds which are currently under investigation (58). RITA (reactivation of p53 and induction of tumor cell apoptosis) is a small molecule activator of p53. RITA has been shown to inhibit growth and induce p53 dependent apoptosis in vivo (59). Furthermore, RITA has been found to induce a DDR which could lead to increased p53 and H2AX phosphorylation. A block in HIF1α and a down regulation of HIF1α target proteins such as VEGF may also be mediated by RITA. These results suggest that reactivation of p53 in the hypoxic tumor could prove to be an important strategy for targeting the death of cells by reactivating p53-dependent apoptosis and potentially decreasing aberrant angiogenesis (59). Many of the chemotherapy drugs in current use are also reliant on p53 dependent apoptosis for their effects, so RITA and other small molecule reactivators of p53 may also have an important role to play in combination with conventional cancer treatments (60).
Concluding remarks
The hypoxic fraction of a tumor represents the most therapy resistant, likely to metastasise and aggressive tumor cells. It has been suggested that this fraction also potentially contains the highest numbers of cancer stem cells (61). For these reasons any advance in the eradication of hypoxic cells during therapy is likely to have a positive effect on disease progression and patient survival. Whilst DDR inhibitors as single agents are unlikely to be effective against hypoxic cells they may well have significant effects used in combination. The design of clinical trials will be critical in determining these potential benefits i.e. the scheduling of DDR inhibitors with, for example irradiation or anti-angiogenic therapies. The development of accurate biomarkers, able to provide reliable predictive and prognostic information will also be of great aid when choosing those patients that will benefit the most from therapies targeting the DDR.
Acknowledgements
This work was supported by Cancer Research UK grant reference C6515/A9321 awarded to EMH
References
- 1.Hockel M, Schlenger K, Mitze M, Schaffer U, Vaupel P. Hypoxia and Radiation Response in Human Tumors. Semin Radiat Oncol. 1996;6:3–9. doi: 10.1053/SRAO0060003. [DOI] [PubMed] [Google Scholar]
- 2.Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 1998;58:1408–16. [PubMed] [Google Scholar]
- 3.Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56:4509–15. [PubMed] [Google Scholar]
- 4.Brizel DM, Scully SP, Harrelson JM, et al. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 1996;56:941–3. [PubMed] [Google Scholar]
- 5.Erler JT, Bennewith KL, Nicolau M, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–6. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 6.Le QT, Denko NC, Giaccia AJ. Hypoxic gene expression and metastasis. Cancer Metastasis Rev. 2004;23:293–310. doi: 10.1023/B:CANC.0000031768.89246.d7. [DOI] [PubMed] [Google Scholar]
- 7.Brahimi-Horn MC, Pouyssegur J. HIF at a glance. J Cell Sci. 2009;122:1055–7. doi: 10.1242/jcs.035022. [DOI] [PubMed] [Google Scholar]
- 8.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–65. [PubMed] [Google Scholar]
- 9.Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:343–54. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 10.Maxwell PH. The HIF pathway in cancer. Semin Cell Dev Biol. 2005;16:523–30. doi: 10.1016/j.semcdb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC. HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell. 2007;11:335–47. doi: 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koumenis C, Wouters BG. “Translating” tumor hypoxia: unfolded protein response (UPR)-dependent and UPR-independent pathways. Mol Cancer Res. 2006;4:423–36. doi: 10.1158/1541-7786.MCR-06-0150. [DOI] [PubMed] [Google Scholar]
- 13.Papandreou I, Krishna C, Kaper F, Cai D, Giaccia AJ, Denko NC. Anoxia is necessary for tumor cell toxicity caused by a low-oxygen environment. Cancer Res. 2005;65:3171–8. doi: 10.1158/0008-5472.CAN-04-3395. [DOI] [PubMed] [Google Scholar]
- 14.Hammond EM, Kaufmann MR, Giaccia AJ. Oxygen sensing and the DNA-damage response. Current Opinion in Cell Biology. 2007;19:680–4. doi: 10.1016/j.ceb.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Rzymski T, Harris AL. The unfolded protein response and integrated stress response to anoxia. Clin Cancer Res. 2007;13:2537–40. doi: 10.1158/1078-0432.CCR-06-2126. [DOI] [PubMed] [Google Scholar]
- 16.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–8. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou BB, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408:433–9. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]
- 18.Nordlund P, Reichard P. Ribonucleotide reductases. Annu Rev Biochem. 2006;75:681–706. doi: 10.1146/annurev.biochem.75.103004.142443. [DOI] [PubMed] [Google Scholar]
- 19.Pires IM, Bencokova Z, Milani M, et al. Effects of Acute versus Chronic Hypoxia on DNA Damage Responses and Genomic Instability. Cancer Res. 2010;70:925–35. doi: 10.1158/0008-5472.CAN-09-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammond EM, Dorie MJ, Giaccia AJ. Inhibition of ATR Leads to Increased Sensitivity to Hypoxia/Reoxygenation. Cancer Res. 2004;64:6556–62. doi: 10.1158/0008-5472.CAN-04-1520. [DOI] [PubMed] [Google Scholar]
- 21.Zou L, Elledge SJ. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science. 2003;300:1542–8. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]
- 22.Hammond EM, Denko NC, Dorie MJ, Abraham RT, Giaccia AJ. Hypoxia links ATR and p53 through replication arrest. Mol Cell Biol. 2002;22:1834–43. doi: 10.1128/MCB.22.6.1834-1843.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freiberg RA, Hammond EM, Dorie MJ, Welford SM, Giaccia AJ. DNA damage during reoxygenation elicits a Chk2-dependent checkpoint response. Mol Cell Biol. 2006;26:1598–609. doi: 10.1128/MCB.26.5.1598-1609.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bencokova Z, Kaufmann MR, Pires IM, Lecane PS, Giaccia AJ, Hammond EM. ATM activation and signaling under hypoxic conditions. Mol Cell Biol. 2009;29:526–37. doi: 10.1128/MCB.01301-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibson SL, Bindra RS, Glazer PM. Hypoxia-induced phosphorylation of Chk2 in an ataxia telangiectasia mutated-dependent manner. Cancer Res. 2005;65:10734–41. doi: 10.1158/0008-5472.CAN-05-1160. [DOI] [PubMed] [Google Scholar]
- 26.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 27.Hunt CR, Pandita RK, Laszlo A, et al. Hyperthermia activates a subset of ataxia-telangiectasia mutated effectors independent of DNA strand breaks and heat shock protein 70 status. Cancer Res. 2007;67:3010–7. doi: 10.1158/0008-5472.CAN-06-4328. [DOI] [PubMed] [Google Scholar]
- 28.Gibson SL, Bindra RS, Glazer PM. CHK2-dependent phosphorylation of BRCA1 in hypoxia. Radiat Res. 2006;166:646–51. doi: 10.1667/RR0660.1. [DOI] [PubMed] [Google Scholar]
- 29.Stiff T, Walker SA, Cerosaletti K, et al. ATR-dependent phosphorylation and activation of ATM in response to UV treatment or replication fork stalling. Embo J. 2006;25:5775–82. doi: 10.1038/sj.emboj.7601446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hammond EM, Dorie MJ, Giaccia AJ. ATR/ATM targets are phosphorylated by ATR in response to hypoxia and ATM in response to reoxygenation. J Biol Chem. 2003;278:12207–13. doi: 10.1074/jbc.M212360200. [DOI] [PubMed] [Google Scholar]
- 31.Freiberg RA, Krieg AJ, Giaccia AJ, Hammond EM. Checking in on hypoxia/reoxygenation. Cell Cycle. 2006;5:1304–7. doi: 10.4161/cc.5.12.2811. [DOI] [PubMed] [Google Scholar]
- 32.Economopoulou M, Langer HF, Celeste A, et al. Histone H2AX is integral to hypoxia-driven neovascularization. Nat Med. 2009;15:553–8. doi: 10.1038/nm.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coleman ML, Ratcliffe PJ. Angiogenesis: escape from hypoxia. Nat Med. 2009;15:491–3. doi: 10.1038/nm0509-491. [DOI] [PubMed] [Google Scholar]
- 34.Rankin EB, Giaccia AJ, Hammond EM. Bringing H2AX into the angiogenesis family. Cancer Cell. 2009;15:459–61. doi: 10.1016/j.ccr.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer. 2008;8:180–92. doi: 10.1038/nrc2344. [DOI] [PubMed] [Google Scholar]
- 36.Koshiji M, To KK, Hammer S, et al. HIF-1alpha induces genetic instability by transcriptionally downregulating MutSalpha expression. Mol Cell. 2005;17:793–803. doi: 10.1016/j.molcel.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 37.Crosby ME, Kulshreshtha R, Ivan M, Glazer PM. MicroRNA regulation of DNA repair gene expression in hypoxic stress. Cancer Res. 2009;69:1221–9. doi: 10.1158/0008-5472.CAN-08-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen H, Yan Y, Davidson TL, Shinkai Y, Costa M. Hypoxic stress induces dimethylated histone H3 lysine 9 through histone methyltransferase G9a in mammalian cells. Cancer Res. 2006;66:9009–16. doi: 10.1158/0008-5472.CAN-06-0101. [DOI] [PubMed] [Google Scholar]
- 39.Bindra RS, Glazer PM. Repression of RAD51 gene expression by E2F4/p130 complexes in hypoxia. Oncogene. 2007;26:2048–57. doi: 10.1038/sj.onc.1210001. [DOI] [PubMed] [Google Scholar]
- 40.Helleday T, Petermann E, Lundin C, Hodgson B, Sharma RA. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer. 2008;8:193–204. doi: 10.1038/nrc2342. [DOI] [PubMed] [Google Scholar]
- 41.Tse AN, Carvajal R, Schwartz GK. Targeting checkpoint kinase 1 in cancer therapeutics. Clin Cancer Res. 2007;13:1955–60. doi: 10.1158/1078-0432.CCR-06-2793. [DOI] [PubMed] [Google Scholar]
- 42.Dai Y, Grant S. New insights into checkpoint kinase 1 in the DNA damage response signaling network. Clin Cancer Res. 2010;16:376–83. doi: 10.1158/1078-0432.CCR-09-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitchell JB, Choudhuri R, Fabre K, et al. In vitro and in vivo radiation sensitization of human tumor cells by a novel checkpoint kinase inhibitor, AZD7762. Clin Cancer Res. 2010;16:2076–84. doi: 10.1158/1078-0432.CCR-09-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McNeely S, Conti C, Sheikh T, et al. Chk1 inhibition after replicative stress activates a double strand break response mediated by ATM and DNA-dependent protein kinase. Cell Cycle. 9:995–1004. doi: 10.4161/cc.9.5.10935. [DOI] [PubMed] [Google Scholar]
- 45.Hammond EM, Freiberg RA, Giaccia AJ. The roles of Chk 1 and Chk2 in hypoxia and reoxygenation. Cancer Lett. 2005 doi: 10.1016/j.canlet.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 46.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 47.Cairns R, Papandreou I, Denko N. Overcoming physiologic barriers to cancer treatment by molecularly targeting the tumor microenvironment. Mol Cancer Res. 2006;4:61–70. doi: 10.1158/1541-7786.MCR-06-0002. [DOI] [PubMed] [Google Scholar]
- 48.Bussink J, Kaanders JH, Rijken PF, Raleigh JA, Van der Kogel AJ. Changes in blood perfusion and hypoxia after irradiation of a human squamous cell carcinoma xenograft tumor line. Radiat Res. 2000;153:398–404. doi: 10.1667/0033-7587(2000)153[0398:cibpah]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 49.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–27. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 50.Duda DG, Ancukiewicz M, Jain RK. Biomarkers of antiangiogenic therapy: how do we move from candidate biomarkers to valid biomarkers? J Clin Oncol. 2009;28:183–5. doi: 10.1200/JCO.2009.24.8021. [DOI] [PubMed] [Google Scholar]
- 51.Hanrahan EO, Lin HY, Kim ES, et al. Distinct patterns of cytokine and angiogenic factor modulation and markers of benefit for vandetanib and/or chemotherapy in patients with non-small-cell lung cancer. J Clin Oncol. 2010;28:193–201. doi: 10.1200/JCO.2009.22.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tatum JL, Kelloff GJ, Gillies RJ, et al. Hypoxia: importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol. 2006;82:699–757. doi: 10.1080/09553000601002324. [DOI] [PubMed] [Google Scholar]
- 53.Liu SK, Coackley C, Krause M, Jalali F, Chan N, Bristow RG. A novel poly(ADP-ribose) polymerase inhibitor, ABT-888, radiosensitizes malignant human cell lines under hypoxia. Radiother Oncol. 2008;88:258–68. doi: 10.1016/j.radonc.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 54.McCabe N, Turner NC, Lord CJ, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006;66:8109–15. doi: 10.1158/0008-5472.CAN-06-0140. [DOI] [PubMed] [Google Scholar]
- 55.McNeely S, Conti C, Sheikh T, et al. Chk1 inhibition after replicative stress activates a double strand break response mediated by ATM and DNA-dependent protein kinase. Cell Cycle. 2010;9:995–1004. doi: 10.4161/cc.9.5.10935. [DOI] [PubMed] [Google Scholar]
- 56.Morgan MA, Parsels LA, Zhao L, et al. Mechanism of Radiosensitization by the Chk1/2 Inhibitor AZD7762 Involves Abrogation of the G2 Checkpoint and Inhibition of Homologous Recombinational DNA Repair. Cancer Res. 2010 doi: 10.1158/0008-5472.CAN-09-3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Graeber TG, Osmanian C, Jacks T, et al. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumors. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- 58.Di Cintio A, Di Gennaro E, Budillon A. Restoring p53 function in cancer: novel therapeutic approaches for applying the brakes to tumorigenesis. Recent Pat Anticancer Drug Discov. 2010;5:1–13. doi: 10.2174/157489210789702172. [DOI] [PubMed] [Google Scholar]
- 59.Yang J, Ahmed A, Poon E, et al. Small-molecule activation of p53 blocks hypoxia-inducible factor 1alpha and vascular endothelial growth factor expression in vivo and leads to tumor cell apoptosis in normoxia and hypoxia. Mol Cell Biol. 2009;29:2243–53. doi: 10.1128/MCB.00959-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.El-Deiry WS. The role of p53 in chemosensitivity and radiosensitivity. Oncogene. 2003;22:7486–95. doi: 10.1038/sj.onc.1206949. [DOI] [PubMed] [Google Scholar]
- 61.Heddleston JM, Li Z, McLendon RE, Hjelmeland AB, Rich JN. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle. 2009;8:3274–84. doi: 10.4161/cc.8.20.9701. [DOI] [PMC free article] [PubMed] [Google Scholar]