Abstract
Microdeletion of 17q21.31 causes a recurrent recognisable dysmorphic syndrome. Four further patients with 17q21.31 microdeletions are reported here where previously the diagnosis of cardio-facio-cutaneous (CFC) syndrome was suggested. These patients have significant similarities of facial gestalt to previously reported 17q21.31 microdeletion patients, but a striking feature that has not been emphasised previously is the large number of naevi and other pigmentary skin abnormalities that may be present. These features, together with a coarse facial appearance, relative macrocephaly and significant learning disabilities, had led to the previous diagnostic suggestion of CFC syndrome in each of these four cases.
Introduction
17q21.31 microdeletion syndrome is a recently delineated recognisable dysmorphic syndrome which is due to misalignment of low copy number repeat sequences and the unusual genomic architecture at this locus: an inversion polymorphism is present in 20% of the Caucasian population [1,2]. Currently underdiagnosed due to its recent recognition, it is thought that 1 in 16000 individuals may be affected [3]. Haploinsufficiency for the MAPT gene within the deleted region appears the most likely cause for the learning disabilities and other anomalies seen in this condition [1,2].
CFC syndrome was first described in 1986 [4], and shows considerable overlap with other neuro-cardio-facio-cutaneous syndromes, particularly Noonan and Costello syndromes. The reason for these observed overlaps is that the protein products of all genes known to be mutated in these conditions interact within the RAS-MAPK pathway [5]. A previously published series of 17q21.31 microdeletion patients included several with features reminiscent of Noonan syndrome, including one in whom this diagnosis was thought sufficiently likely for Noonan syndrome genes to be tested [6]. Several individuals in that series also had features of ectodermal dysplasia, whilst 3/11 had pulmonary stenosis, and other cardiac lesions were also described [6]. Normal birthweight and relative macrocephaly are features which appear to be present in the majority of patients both with 17q21.31 deletion [6] and RAS pathway disorders [5]. Variable degrees of developmental delay have been noted in patients with 17q21.31 deletion, and this spectrum is still liable to expansion. Similarly, patients with CFC syndrome may have variable degrees of intellectual disability [5].
Patients
Patient 1
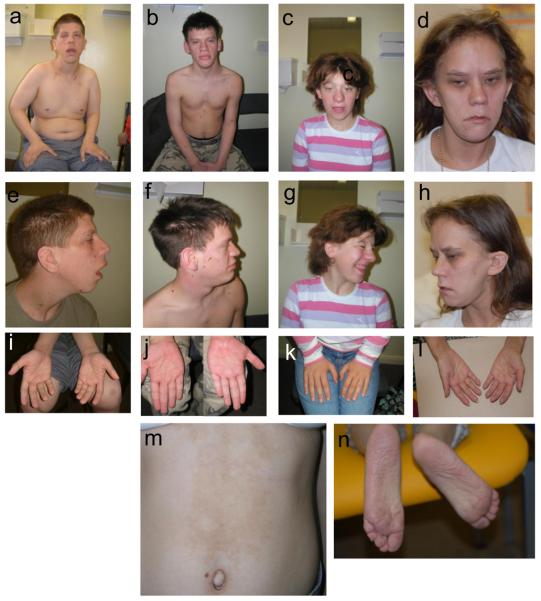
Now 21 years of age, this male patient was born at term, weighing 3.4kg. He required tube feeding in the first three weeks of life, and was hypotonic. Growth has continued between the 25th and 50th centile curves, his current height being 176cm. He has a predominantly truncal fat distribution. His head circumference has followed the 97th centile, and is now 57.6cm. He has had unusual skin since birth, with redundant skin folds, coarse hair with a chaotic growth pattern and keratosis pilaris. He has always been more highly pigmented than other family members. A very large number of naevi developed all over his body through childhood and he also has several café au lait patches. He has slight coarsening of his facial features, including a broad nose, thick lips and posteriorly rotated ears with large lobes, as shown in Figure 1(a,e). He has severe learning difficulties and longstanding epilepsy, which is well-controlled on carbemazepine, but has also had challenging behaviour for which he has been prescribed thioridazine in addition. He also has strabismus, difficulty chewing (from weakness of masseters) and subluxed patellae. His hands were fleshy with deep skin creases (Figure 1i). Endocrinological assessment was undertaken because of his hyperpigmentation and showed normal short synacthen response and oral glucose tolerance. Chromosome analysis demonstrated a 46, XY karyotype, with normal FISH for Prader-Willi and Smith-Magenis syndromes. Cranial CT and muscle biopsy were normal. Transferrin isoelectric focusing and steroid sulphatase activity were normal, as was a urinary mucopolysaccharide screen.
Figure 1 a-h.
facial appearances of the three patients, demonstrating coarse features, multiple naevi and the characteristic nose of 17q21.31 deletion. Figure 1i-l: hands of the four patients, showing thickened skin, particularly marked in patient 1. m: area of hyperpigmentation on the trunk of patient 3. n: soles of patient 4, demonstrating deep creases and dry skin.
Patient 2
Now 22 years of age, this male patient weighed 2.8kg at birth by Caesarian delivery at 41 weeks' gestation for breech presentation. He was admitted to the special care unit on the second day of life with poor feeding and stridor due to laryngomalacia. Feeding difficulties continued until weaning. A small secundum ASD and mild pulmonary stenosis were demonstrated on echocardiography. He had undescended testes and hypospadias which were surgically corrected, and several urinary tract infections. A small umbilical hernia resolved spontaneously. He has joint laxity. Mild delay of motor milestones was present, but speech delay was marked: at age 5, he was still only using single words. Aged 8, he could put three words together. His height (180cm) and weight (75kg) lie between the 50th and 75th centiles, whilst his head circumference is on the 91st (59.7cm). Pubertal development was normal. Aged 16, he sat several national school examinations, attaining average grades in some. He currently lives with his parents, works part time in a supermarket and is attending adult education life skills courses. On review at age 20, his coarse facial features, as shown in Figure 1(b,f) were more marked than in childhood, in particular his broad nose and thick lips. Naevi are remarkably numerous over his entire body. His karyotype was normal, 46, XY.
Patient 3
Now 16 years old, this female patient has short stature and mild to moderate learning difficulties. She had a birthweight of 2.3kg at 41 weeks' gestation, and placental insufficiency was suspected. She would not breast feed, but had established bottle feeds by day 10 of life. Joint laxity and muscular hypotonia were prominent in infancy and early childhood. She first walked at 20 months. She had recurrent chest infections in the first 5 years of life, but has been in good health since, with normal pubertal development. She has coarse textured, dry hair, relatively coarse facial features, as shown in Figure 1(c,g), and an unusual thickened hyperpigmented area of skin on her upper abdomen, as shown in Figure 1m. She is developing naevi, particularly over her face and trunk, and has a mild pectus deformity. Her karyotype is 46, XX. Her height remains around the third centile, and her head circumference of 55.4cm is on the 50th centile.
Patient 4
Now 28 years of age, this female patient was born at term, weighing 2.78kg. She had global developmental delay, first sitting unsupported aged 2.5 years and walking aged 3. She spoke no words until age 14. She was initially referred to a genetics clinic aged 12 years. She was noted to have generalised hyperpigmentation for which no cause could be identified. Porphyria, haemochomatosis and ACTH excess were excluded. Optic atrophy, left pelvi-ureteric junction obstruction and delayed bone age were also present. Her OFC measured 51cm (3rd centile) and height 109cm (<< 0.4th centile). She has sensorineural hearing loss and had chronic serous otitis media. She developed pneumonia and a pericardial effusion aged 11, the latter being persistent to the age of 15, but stable. Swollen proximal interphalangeal joints were noted from age 14, as were several café au lait patches. A diagnosis within the Noonan/CFC/Watson spectrum was therefore considered at this stage. Puberty was delayed, with pelvic ultrasound aged 15 demonstrating a small uterus and delayed ovarian development. Menarche was at 17 years. MRI brain scanning showed left medial temporal sclerosis and a small hippocampus. Aged 19, a thoracic scoliosis and limitation of elbow extension were noted. Aged 28, her height is 131.2cm (<<0.4th centile), OFC is 51.5cm (0.4th centile) and weight is 35.3kg (<0.4th centile). She has a prematurely aged appearance to her skin. She has a broad nasal tip, upslanting palpebral fissures, and a long face with a thin upper lip (Figure 1d,h). Her palmar and plantar creases are deep (see Figure 1l,n), and she has recently been diagnosed with cataracts. Additionally, a small larynx with a small anterior laryngeal web has been noted. Her karyotype is 46, XX. Chromosome breakage studies, creatine kinase, urine amino acids and mucopolysaccharide screen, white cell enzymes, VLCFA, copper levels, sweat test, and thyroid function were all also normal, as was FISH testing for deletion at the NF1 locus.
Methods and results
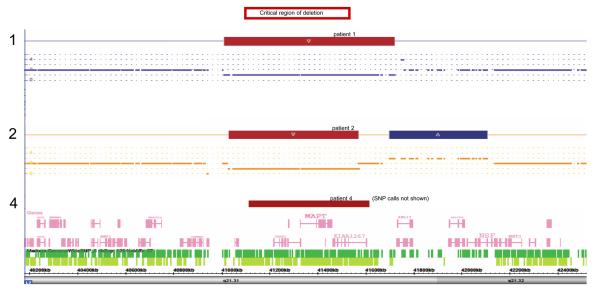
Mutation analysis by sequencing of all exons of BRAF, KRAS, HRAS, MEK1 and MEK2 in which CFC-associated mutations have been reported revealed no such mutation in patient 1. Array comparative genomic hybridisation (aCGH), using the Genechip SNP6.0 array (Affymetrix, Santa Clara, CA, USA), was undertaken on patients 1 and 2, and demonstrated deletions at 17q21.31, of 639kb in patient 1, and 519kb in patient 2, as shown in Figure 2. These deletions include the critical region identified for the condition as indicated, overlapping as they do with those previously reported in 17q21.31 microdeletion syndrome [3]. Additionally, patient 1 was found to have a 2.4Mb duplication of 22q11.21 (data not shown). His 17q21.31 deletion and 22q11.21 duplication were both confirmed by multiplex ligation probe analysis using the MLPA P245 kit (MRC Holland, Amsterdam, Netherlands). Patient 2 was noted to have an additional copy number variant, a duplication of 17q21, just distal to his deletion, as shown in Figure 2.
Figure 2.
Diagram of the 17q21.31 locus, with the deletions in patients 1, 2 and 4 shown. Red indicates deleted material, whilst blue is adjacent duplicated material in patient 2, in this region rich in copy number variants. The critical region for the deletion phenotype is shown at the top of the figure.
In patients 3 and 4, the del17q21.31 phenotype was recognised clinically at a review appointment, and fluorescent in-situ hybridisation (FISH) was then used to demonstrate deletion of MAPT. In patient 4, deletion breakpoints of 17: 41107486..41610161 were indicated by the heterozygous loss of 26 oligonucleotide probes on aCGH, giving an approximate deletion size of 502kb, as represented in Figure 2. The de novo nature of the deletions in patients 2 and 3 was confirmed by normal FISH results in both parents, whilst parental samples were not available for patients 1 and 4.
Discussion
The phenotypic features of 17q21.31 deletion and CFC syndrome are compared in Table 1. A large number of naevi, in conjunction with other cutaneous features, coarse facial characteristics, relative macrocephaly, poor feeding in infancy and significant learning disabilities, were what led to the clinical suggestion of CFC syndrome in the four patients described here. Additionally, the striking skin appearance due to increased pigmentation, sufficient to warrant endocrine investigation, in patients 1 and 4 in this series is another distinctive feature that is reminiscent of RAS-MAPK pathway disorders. It bears particular resemblance to the bronzed skin reported in many patients with Costello syndrome, including those in Costello's original report [7].
Table 1. Comparison of key features of 17q21.31 microdeletion syndrome, CFC syndrome and patients in this series.
(numerators are number of patients with the feature, denominators are the number of patients on whom the data was available)
| 17q21.31 microdeletion (from Koolen et al, 2008) |
CFC syndrome (from Armour & Allanson, 2008 [10]) |
Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|---|---|
| Growth | ||||||
| Normal or high birthweight |
16/22 | 37/38 | yes | yes | no | yes |
| Feeding difficulties |
common | 19/30 | yes | yes | mild | not known |
| Failure to thrive | not common | frequently severe | no | no | mild | not known |
| Normal OFC | 21/22 | yes | yes | yes | yes | |
| Relative macrocephaly (OFC more than +1SD compared to height) |
not characterised | 17/33 | yes | yes | yes | yes |
| Absolute macrocephaly (more than +3SD) |
- | 4/33 | no | no | no | no |
| Short stature (below 3rd centile) |
4/22 | 27/38 | no | no | mild | severe |
| Neurological development | ||||||
| Developmental delay |
22/22, variable degree |
present; variable | severe | mild to moderate |
mild to moderate |
severe |
| Hypotonia | 21/22 | 34/36 | yes | yes | yes | not known |
| Seizures | 11/22 | 18/37 | no | no | no | no |
| Engaging or amiable personality |
16/18 | 25/32 | - | yes | yes | - |
| Heart | ||||||
| Pulmonary stenosis |
- | 14/33 | no | yes | no | no |
| ASD or VSD | 6/27 | 9/32 | no | yes | no | no |
| Other cardiac defect |
common | no | no | no | no | |
| Skin and hair | ||||||
| Naevi | Present in some photos | 28/37 | yes | yes | yes | yes |
| Other skin pigmentary abnormality |
very common, including café au lait patches and generalised hyperpigmentation |
yes, generalised hyperpigmentation |
- | yes, discrete area on abdomen (see figure) |
yes, generalised hyperpigmentation |
|
| Keratosis pilaris/ hyperkeratosis/ ichthyosis dry skin |
A few patients | 20/28 | yes | yes | yes | yes |
| Unusual hair (colour, texture, thickness) |
13/22 | 34/38 | yes | yes | yes | yes |
| Eyes | ||||||
| Strabismus | 10/22 | 28/35 | yes | no | no | no |
| Optic nerve hypoplasia |
- | 11/26 | no | no | no | yes |
22q11.2 duplications, as identified in patient 1, have been associated with a diverse range of features, but overall phenotypes are most commonly mild, and duplications are also frequently inherited from phenotypically normal parents [8]. Whilst it is not possible to exclude increased copy number at 22q11.2 as a contributor to patient 1's clinical presentation, his features did not correlate to those commonly reported in association with this duplication. This is in contrast to his marked similarities to other individuals with 17q21.31 microdeletion syndrome.
The phenotypic similarity of each of the four individuals described here to patients with molecularly proven CFC syndrome raises the question as to whether the 17q21.31 locus could harbour a gene or genes implicated in this condition. Such genes causing a phenotype by deletion would suggest haploinsufficiency as a mechanism, with loss of an allele encoding a protein which inhibits RAS-MAPK pathway activity perhaps being sufficient to cause pathway overactivation, analogous to the mechanism by which inactivating mutations in NF1 cause neurofibromatosis type I [9]. The commonly deleted area on 17q21.31 spans 6 known genes. At present none of these are known to interact with the RAS-MAPK pathway, but two, CRHR1 and MAPT, are each highly expressed in brain, and therefore represent prime candidates for causing the neurological phenotype seen in 17q21.31 microdeletion [3].
CFC syndrome demonstrates considerable variability in the severity of learning disability, physical and behavioural problems demonstrated by affected individuals. A broad spectrum of capabilities is also observed in patients with 17q21.31 deletion, as shown by the four patients described here. The variable severity and manifestations of both CFC syndrome and 17q21.31 microdeletion means that there may be a significant number of individuals for whom both conditions should be considered within the differential diagnosis.
Conclusion
The diagnosis of 17q21.31 deletion may be achievable clinically in some patients, but it may not be easy to recognise in all. Large numbers of naevi and/or pigmentary skin changes, reminiscent of those seen in patients with CFC syndrome, and like those discussed here, should prompt consideration of testing for 17q21.31 deletion by FISH, MLPA or array CGH, particularly if a large, pear-shaped or tubular nose is also present. Patients in whom a clinical diagnosis of CFC syndrome has previously been suggested, or those in whom no mutation has been found in any of the known genes for CFC syndrome, should also be reassessed in the light of this possibility.
These findings demonstrate the particular value of long-term clinical review of patients without a molecular diagnosis, and suggest that aCGH should be considered in all patients with a clinical diagnosis of CFC syndrome who do not have a mutation in known CFC genes. The phenotypic similarities between several patients with 17q21.31 deletions and those with molecularly confirmed CFC syndrome suggests the possibility that one or more genes at 17q21.31 might influence RAS-MAPK pathway activity in some way.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no other competing interests to declare.
References
- 1.Koolen DA, Vissers LE, Pfundt R, de Leeuw N, Knight SJ, Regan R, Kooy RF, Reyniers E, Romano C, Fichera M, Schinzel A, Baumer A, Anderlid BM, Schoumans J, Knoers NV, van Kessel AG, Sistermans EA, Veltman JA, Brunner HG, de Vries BB. A new chromosome 17q21.31 microdeletion syndrome associated with a common inversion polymorphism. Nat Genet. 2006;38:999–1001. doi: 10.1038/ng1853. [DOI] [PubMed] [Google Scholar]
- 2.Shaw-Smith C, Pittman AM, Willatt L, Martin H, Rickman L, Gribble S, Curley R, Cumming S, Dunn C, Kalaitzopoulos D, Porter K, Prigmore E, Krepischi-Santos AC, Varela MC, Koiffmann CP, Lees AJ, Rosenberg C, Firth HV, de Silva R, Carter NP. Microdeletion encompassing MAPT at chromosome 17q21.3 is associated with developmental delay and learning disability. Nat Genet. 2006;38:1032–7. doi: 10.1038/ng1858. [DOI] [PubMed] [Google Scholar]
- 3.Koolen DA, Sharp AJ, Hurst JA, Firth HV, Knight SJ, Goldenberg A, Saugier-Veber P, Pfundt R, Vissers LE, Destrée A, Grisart B, Rooms L, Van der Aa N, Field M, Hackett A, Bell K, Nowaczyk MJ, Mancini GM, Poddighe PJ, Schwartz CE, Rossi E, De Gregori M, Antonacci-Fulton LL, McLellan MD, 2nd, Garrett JM, Wiechert MA, Miner TL, Crosby S, Ciccone R, Willatt L, Rauch A, Zenker M, Aradhya S, Manning MA, Strom TM, Wagenstaller J, Krepischi-Santos AC, Vianna-Morgante AM, Rosenberg C, Price SM, Stewart H, Shaw-Smith C, Brunner HG, Wilkie AO, Veltman JA, Zuffardi O, Eichler EE, de Vries BB. Clinical and molecular delineation of the 17q21.31 microdeletion syndrome. J Med Genet. 2008;45:710–20. doi: 10.1136/jmg.2008.058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reynolds JF, Neri G, Herrmann JP, Blumberg B, Coldwell JG, Miles PV, Opitz JM. New multiple congenital anomalies/mental retardation syndrome with cardio-facio-cutaneous involvement - the CFC syndrome. Am J Med Genet. 1986;28:413–427. doi: 10.1002/ajmg.1320250303. [DOI] [PubMed] [Google Scholar]
- 5.Burkitt Wright EMM, Kerr B. RAS pathway disorders: Important causes of congenital heart disease, feeding difficulties, developmental delay and short stature. Arch Dis Child. 2010 doi: 10.1136/adc.2009.160069. online first 6th April 2010, doi:10.1136/adc.2009.160069. [DOI] [PubMed] [Google Scholar]
- 6.Tan TY, Aftimos S, Worgan L, Susman R, Wilson M, Ghedia S, Kirk EP, Love D, Ronan A, Darmanian A, Slavotinek A, Hogue J, Moeschler JB, Ozmore J, Widmer R, Bruno D, Savarirayan R, Peters G. Phenotypic expansion and further characterisation of the 17q21.31 microdeletion syndrome. J Med Genet. 2009;46:480–9. doi: 10.1136/jmg.2008.065391. [DOI] [PubMed] [Google Scholar]
- 7.Costello JM. A new syndrome: mental subnormality and nasal papillomata. Aust Paediatr J. 1977;13:114–118. doi: 10.1111/j.1440-1754.1977.tb01135.x. [DOI] [PubMed] [Google Scholar]
- 8.Firth HV. 22q11.2 duplication. In: Pagon RA, Bird TC, Dolan CR, Stephens K, editors. GeneReviews [Internet] University of Washington, Seattle; Seattle (WA): 2009. [Google Scholar]
- 9.Huson S. Neurofibromatosis: emerging phenotypes, mechanisms and management. Clin Med. 2008;8:611–7. doi: 10.7861/clinmedicine.8-6-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armour CM, Allanson JE. Further delineation of cardio-facio-cutaneous syndrome: clinical features of 38 individuals with proven mutations. J Med Genet. 2008;45:249–54. doi: 10.1136/jmg.2007.054460. [DOI] [PubMed] [Google Scholar]