Abstract
Context
Existing therapies for bipolar depression have a considerable lag of onset of action. Pharmacological strategies that produce rapid antidepressant effects—for instance, within a few hours or days—would have an enormous impact on patient care and public health.
Objective
To determine whether an N-methyl-d-aspartate–receptor antagonist produces rapid antidepressant effects in subjects with bipolar depression.
Design
A randomized, placebo-controlled, double-blind, crossover, add-on study conducted from October 2006 to June 2009.
Setting
Mood Disorders Research Unit at the National Institute of Mental Health, Bethesda, Maryland.
Patients
Eighteen subjects with DSM-IV bipolar depression (treatment-resistant).
Interventions
Subjects maintained at therapeutic levels of lithium or valproate received an intravenous infusion of either ketamine hydrochloride (0.5 mg/kg) or placebo on 2 test days 2 weeks apart. The Montgomery-Asberg Depression Rating Scale was used to rate subjects at baseline and at 40, 80, 110, and 230 minutes and on days 1, 2, 3, 7, 10, and 14 postinfusion.
Main Outcome Measures
Change in Montgomery-Asberg Depression Rating Scale primary efficacy measure scores.
Results
Within 40 minutes, depressive symptoms significantly improved in subjects receiving ketamine compared with placebo (d=0.52, 95% confidence interval [CI], 0.28-0.76); this improvement remained significant through day 3. The drug difference effect size was largest at day 2 (d=0.80, 95% CI, 0.55-1.04). Seventy-one percent of subjects responded to ketamine and 6% responded to placebo at some point during the trial. One subject receiving ketamine and 1 receiving placebo developed manic symptoms. Ketamine was generally well tolerated; the most common adverse effect was dissociative symptoms, only at the 40-minute point.
Conclusion
In patients with treatment-resistant bipolar depression, robust and rapid antidepressant effects resulted from a single intravenous dose of an N-methyl-d-aspartate antagonist.
Trial Registration
clinicaltrials.gov Identifier: NCT00088699
BIPOLAR DISORDER (BPD) IS one of the most severe psychiatric disorders and ranks in the top 10 causes of medical disability worldwide. The lifetime prevalence is approximately 4% in the United States,1 with depressive symptomatology dominating the longitudinal course of the illness.2 Currently approved treatments for bipolar depression include lithium, quetiapine, and the combination olanzapine and fluoxetine. Other treatments used during the depressive phase of the illness include lamotrigine, antidepressants, and atypical antipsychotics. However, many patients with bipolar depression do not respond adequately to these medications despite adequate trials.2-4 Another limitation of existing therapeutics is that they are associated with a considerable lag of onset; only a fraction of patients meet response criteria by the end of the first week of treatment.5-7 This delayed onset of antidepressant effects can result in considerable morbidity, including increased suicide risk.8,9 Therefore, rapid-onset pharmacological strategies with pronounced and sustained effects would have an enormous impact on public health; this is particularly true because BPD is perhaps the psychiatric disorder with the highest mortality rate.10
Reasons for this lack of better therapeutics include our limited understanding of the neurobiological basis of BPD and of the mechanism of action of existing effective medications.11,12 With the exception of lithium, all pharmacological agents currently used to treat BPD were developed to treat other illnesses (ie, antipsychotics, anticonvulsants). However, several lines of evidence have recently converged to suggest that glutamatergic system dysfunction—particularly the N-methyl-d-aspartate (NMDA)–receptor complex—may play an important role in the pathophysiology of BPD. For example, postmortem studies have reported altered NMDA-receptor complexes in the brain tissue of patients with BPD.13-15 At the genetic level, polymorphisms of the GRIN1 and GRIN2B genes coding for the NR1 and NR2B subunits, respectively, have been associated with BPD.16,17 Finally the glutamatergic modulator riluzole was found to have antidepressant properties and to enhance glutamatergic neurotransmission in patients with BPD18,19 as well as enhance the surface expression of the α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptor subunit GluR1 in cultured hippocampal mouse neurons.20
Therefore, testing the efficacy and safety of glutamatergic modulators in patients with BPD could yield a better understanding of the neurobiological processes involved in this complex illness and lead to the development of improved treatments. N-methyl-d-aspartate antagonists are important candidate drugs for this purpose. They are associated with known antidepressant effects in several animal models of depression,21-26 and clinical studies show that they induce a rapid-onset antidepressant response (within 2 hours) in individuals with major depressive disorder.27-29 Based on knowledge gleaned from preclinical and clinical studies, we postulated that directly targeting the NMDA receptor would bring about rapid antidepressant effects in patients with bipolar depression. The objective of the present trial was to determine whether a single intravenous infusion of an NMDA antagonist in patients with treatment-resistant bipolar depression would result in a rapid antidepressant response. Ketamine, a noncompetitive NMDA antagonist, was selected for this study because it has an established safety profile in both research and clinical settings, particularly when used in subanesthetic doses.28,30 We elected to study treatment-resistant patients who had failed to respond to prior treatments, in part because ketamine is associated with dissociative symptoms.
METHODS
PATIENT SELECTION
Subjects were recruited from local inpatient psychiatric units, the Internet, and local and national physician referrals. Eligible participants were men and women aged 18 to 65 years who were inpatients with a diagnosis of bipolar I or II depression without psychotic features, as diagnosed by the Structured Clinical Interview for Axis I DSM-IV Disorders, Patient Version.31 Final diagnoses of bipolar depression and other Axis I disorders (as permitted in the study) were ascertained by general consensus of 3 clinicians using all available information (Structured Clinical Interview for Axis I DSM-IV Disorders, clinical interviews, and [in most cases] interviews with someone who knew the patient well). Subjects were studied at the National Institute of Mental Health (NIMH) Mood Disorders Research Unit in Bethesda, Maryland, between October 2006 and June 2009. Subjects were required to have a score of 20 or more on the Montgomery-Asberg Depression Rating Scale (MADRS) at screening and at the start of each ketamine or placebo infusion. Patients were also required to have a current major depressive episode of at least 4 weeks, to have previously failed at least 1 adequate antidepressant trial (as assessed by the Antidepressant Treatment History Form, modified32), and to have failed a prospective open trial of a mood stabilizer while at the NIMH (either lithium or valproate for a minimum period of 4 weeks at therapeutic levels [serum lithium, 0.6-1.2 mEq/L; or valproic acid, 50-125 μg/mL]).
All subjects were in good physical health as determined by medical history, physical examination, blood laboratory tests, electrocardiography, chest radiography, urinalysis, and toxicology. Subjects were free from comorbid substance abuse or dependence for at least 3 months and judged clinically not to be at serious risk of suicide. Comorbid Axis I anxiety disorder diagnoses were permitted if they were not the primary focus of treatment within 12 months before screening. Patients who were rapid cyclers (≥4 mood episodes within 12 months, based on DSM-IV criteria) were included. Exclusion criteria included any serious unstable medical disorder or condition, previous treatment with ketamine, or concomitant treatment with psychotropic medications other than lithium or valproate in the 2 weeks before randomization (5 weeks for fluoxetine); in addition, female subjects could not be pregnant or nursing. The study was approved by the Combined Neuroscience institutional review board at the National Institutes of Health. All subjects provided written informed consent before entry into the study and were assigned a clinical research advocate from the NIMH Subjects Protection Unit to monitor the consent process and research participation throughout the study.
Conservative estimates of expected change in depressive symptoms in response to ketamine were based on previous studies of individuals with major depressive disorder; thus, a sample size of 19 was initially expected to reach 90% power with a 2-tailed test. The power analysis was based on response rates at day 1.
STUDY DESIGN
This was a single-center, double-blind, randomized, crossover, placebo-controlled study conducted to assess the efficacy and safety of a single intravenous infusion of the NMDA antagonist ketamine combined with lithium or valproate therapy in the treatment of bipolar I or II depression. As noted previously, subjects were first required to have failed to respond to a prospective open trial of therapeutic levels of either lithium or valproate at the NIMH for a minimum of 4 weeks, regardless of whether they were already taking therapeutic levels of lithium or valproate at admission. During the entirety of the study, patients were required to take either lithium or valproate within the specified range and were not allowed to receive any other psychotropic medications (including benzodiazepines) or to receive structured psychotherapy. Lithium and valproate levels were obtained weekly. Vital signs and oximetry were monitored during the infusion and for 1 hour after. Electrocardiograms, complete blood counts, electrolyte panels, and liver function tests were obtained at baseline and at the end of the study.
Following nonresponse to open treatment with lithium or valproate and a 2-week drug-free period (except for treatment with lithium or valproate), subjects received intravenous infusions of saline solution and 0.5-mg/kg ketamine hydrochloride 2 weeks apart using a randomized, double-blind, crossover design. The ketamine dose was based on previous controlled studies of patients with major depressive disorder.30,33,34
Patients were randomly assigned to the order in which they received the 2 infusions via a random-numbers chart. Study solutions were supplied in identical 50-mL syringes containing either 0.9% of saline or ketamine with the additional volume of saline to total 50 mL. Ketamine forms a clear solution when dissolved in 0.9% saline. The infusions were administered over 40 minutes via a Baxter infusion pump (Deerfield, Illinois) by an anesthesiologist in the perianesthesia care unit. All staff, including the anesthesiologist, was blind to whether drug or placebo was being administered.
MAIN OUTCOME MEASURES
Subjects were rated 60 minutes prior to the infusion and at 40, 80, 110, and 230 minutes postinfusion. They were also rated at days 1, 2, 3, 7, 10, and 14 postinfusion. Rating scales included the MADRS, which was the primary outcome measure. Secondary outcome measures were the 17-item Hamilton Scale for Depression,35 the Beck Depression Inventory,36 the visual analog scale,37 the Hamilton Anxiety Rating Scale,38 the Brief Psychiatric Rating Scale,39 the Clinician Administered Dissociative Scale,40 and the Young Mania Rating Scale.41 Ratings for symptoms that could not change over brief periods of time (eg, sleep, appetite) were carried forward from the initial ratings for those time points. The Hamilton Anxiety Rating Scale was performed at all time points except for minutes 40, 80, and 110. Patient ratings were performed by research nurses, a physician, and a psychologist who trained together to establish reliability. High interrater reliability was obtained for the MADRS (interrater correlation coefficient=0.88), Hamilton Scale for Depression (interrater correlation coefficient=0.81), and Young Mania Rating Scale (interrater correlation coefficient = 0.91). Throughout the study, the same rater conducted most ratings for an individual patient.
KETAMINE AND NORKETAMINE PLASMA LEVELS
Ketamine and norketamine (ketamine's active metabolite) plasma levels were obtained at 40, 80, 110, and 230 minutes postinfusion (see the eAppendix for relevant methods, available at http://www.archgenpsychiatry.com).
STATISTICAL ANALYSIS
Linear mixed models with fixed, repeated-measures factors for time and treatment were used with fixed and random intercepts, a random factor for participant, and restricted maximum likelihood estimates. Main effects for time and treatment as well as their interaction were included in the model along with a fixed main effect for treatment order. Schwarz Bayesian criteria were used to determine the best-fitting variance-covariance structure, a first-order autoregressive model. For significant interactions, Bonferroni-corrected simple-effects post hoc tests were used to determine the timing of treatment differences. Cohen d was calculated to illustrate the effect size of specific comparisons. The Shapiro-Wilk test was used to determine the normality of the data. Significance was evaluated at P<.05, 2-tailed.
The primary analysis—intent to treat—included all available data; all randomized patients had baseline and at least 1 postbaseline measure. Carryover effects were examined by comparing the baseline values in each study phase within the various analysis models.
Response (50% improvement from baseline on MADRS) and remission (MADRS scores <10) rates were examined at each time point using the McNemar test and correcting for the number of comparisons using the Hochberg adjusted Bonferroni procedure.
RESULTS
PATIENTS
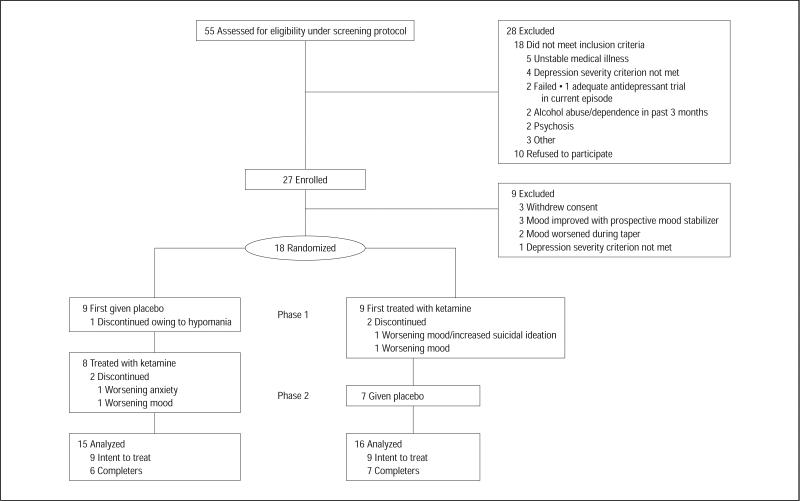
Fifty-five subjects were screened, and 18 who met DSM-IV criteria for bipolar depression were randomized (Figure 1). In total, 13 of 18 (72%) subjects completed both phases. Three patients dropped out in the first randomized phase of the study: 1 patient 1 day after infusion owing to worsening mood/suicidal ideation (this subject had a history of suicide attempts and suicidal ideation on and off prior to study entry), 1 patient 3 days after infusion owing to low mood, and another 10 days after infusion owing to hypomania. In the second phase, 1 patient dropped out after 8 days owing to high anxiety, and another dropped out after 10 days owing to low mood. Four of the 5 dropouts were taking valproate for mood stabilization. The patient who dropped out owing to hypomania was receiving placebo, and the other dropouts occurred during the ketamine phase. Thirteen of 17 (76%) subjects completed the ketamine phase and 15 of 16 (94%) subjects completed the placebo phase. Nine of 10 (90%) patients using lithium as a mood stabilizer completed the study; in contrast, 4 of 8 (50%) patients using valproate completed the study (Fisher exact test, P=.12).
Figure 1.
Enrollment, randomization, withdrawals, and completion of the 2 treatment phases (n=18).
Demographic and clinical characteristics for the subjects are summarized in Table 1. Current and lifetime treatment trials are listed in Table 2.
Table 1.
Demographic and Clinical Characteristics of Patients With Bipolar Disorder (n=18)
| Characteristic | No. (%) |
|---|---|
| Age, mean (SD), y | 47.9 (13.1) |
| Length of illness, mean (SD), y | 27.6 (11.2) |
| Length of current major depressive episode, mean (SD), mo | 15.1 (13.3) |
| Length of hospitalization prior to randomization, mean (SD), d | 64.7 (53.1) |
| No. of psychotropic medications on admission, mean (SD) | 3.0 (1.8) |
| Length of therapeutic mood stabilizer levels prior to randomization, mean (SD), d | 52.1 (28.2) |
| Lifetime failed antidepressant trials, mean (SD)a | 7.2 (4.0) |
| Female sex | 12 (67) |
| Education | |
| College/graduate school | 12 (67) |
| Incomplete college | 3 (17) |
| High school | 3 (17) |
| Disability | 12 (67) |
| Unemployed | 17 (94) |
| Family history | |
| Mood disorder | 17 (94) |
| Alcohol abuse or dependence | 10 (56) |
| Suicide attempt | 7 (39) |
| Life history | |
| Suicide attempt | 9 (50) |
| Sexual abuse | 7 (39) |
| Physical abuse | 4 (22) |
| Lifetime diagnosis | |
| Alcohol abuse or dependence | 8 (44) |
| Substance abuse or dependence | 9 (50) |
| Anxiety disorder | 6 (33) |
| Bipolar disorder subtype | |
| I | 8 (44) |
| II | 10 (56) |
| Mood stabilizer used for the study | |
| Lithium | 10 (56) |
| Valproate | 8 (44) |
Based on modified Antidepressant Treatment History Form criteria.32
Table 2.
Medication Use During Current Major Depressive Episode and Patient's Lifetime (n=18)
| No. (%) by Medication Use |
||
|---|---|---|
| Medication/Somatic Treatment | Current Major Depressive Episode | Lifetime |
| Lithium | 7 (39) | 16 (89) |
| Antidepressants | ||
| Duloxetine | 7 (39) | 13 (72) |
| SSRI | 6 (33) | 18 (100) |
| Bupropion | 5 (28) | 16 (89) |
| Venlafaxine | 2 (11) | 11 (61) |
| Trazodone | 2 (11) | 7 (39) |
| MAOI | 1 (6) | 7 (39) |
| TCA | 0 | 7 (39) |
| Mirtazapine | 0 | 6 (33) |
| Nefazodone | 0 | 3 (17) |
| Antipsychotics | ||
| Quetiapine | 9 (50) | 16 (89) |
| Aripiprazole | 2 (11) | 10 (56) |
| Risperidone | 2 (11) | 8 (44) |
| Ziprasidone | 2 (11) | 7 (39) |
| Olanzapine | 1 (6) | 7 (39) |
| Clozapine | 0 | 2 (11) |
| Conventional antipsychotics | 0 | 5 (28) |
| Anticonvulsants | ||
| Valproate | 6 (33) | 14 (78) |
| Oxcarbazepine | 4 (22) | 5 (28) |
| Lamotrigine | 3 (17) | 13 (72) |
| Carbamazepine | 1 (6) | 9 (50) |
| Topiramate | 1 (6) | 4 (22) |
| Zonisamide | 1 (6) | 1 (6) |
| Gabapentin | 0 | 3 (17) |
| Levetiracetam | 0 | 1 (6) |
| Somatic treatments | ||
| ECT | 2 (11) | 10 (56) |
| VNS | 1 (6) | 1 (6) |
| Other | ||
| Pramipexole | 0 | 2 (11) |
| Thyroid augmentation | 3 (17) | 8 (44) |
| Stimulant | 1 (6) | 8 (44) |
| Modafinil | 0 | 1 (6) |
Abbreviations: ECT, electroconvulsive therapy; MAOI, monoamine oxidase inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant; VNS, vagal nerve stimulation.
Mean lithium levels prior to the first ketamine infusion were 0.77 mEq/L (SD, 0.14 mEq/L) and mean valproate levels were 74.38 μg/mL (SD, 13.02 μg/mL). No patient deviated from therapeutic levels during the study.
EFFICACY
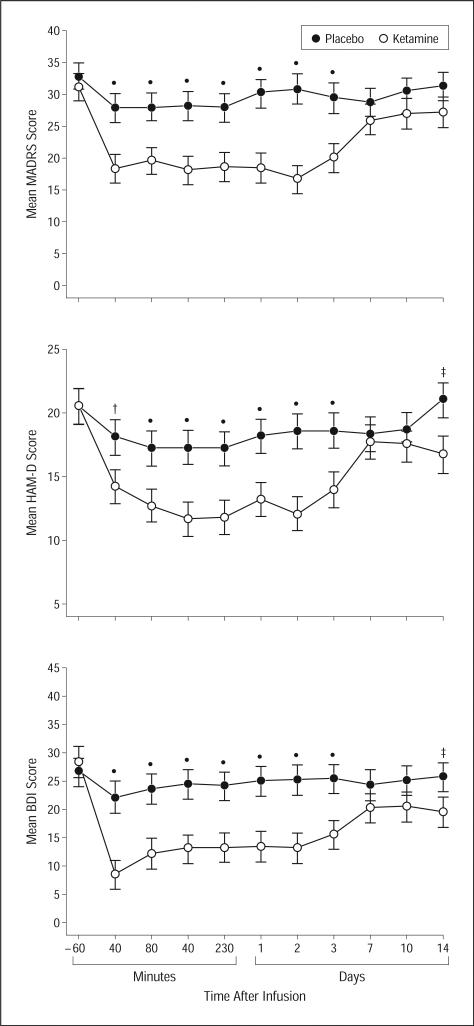
Using all available data in the intent-to-treat sample (see the “Methods” section) and with the MADRS score as the primary outcome measure, the linear mixed model indicated a significant interaction between time and drug (F10,245=3.22, P<.001). Post hoc tests showed significantly fewer depressive symptoms when patients were receiving ketamine compared with placebo from 40 minutes to 3 days postinfusion (P<.001) (Figure 2). Comparisons at baseline and at days 7, 10, and 14 postinfusion were not significant following correction for multiple comparisons (P=.46, P=.21, P=.13, and P=.09, respectively). The effect sizes were 0.52 (95% confidence interval [CI], 0.28-0.76) at 40 minutes, 0.67 (95% CI, 0.42-0.91) at day 1, and 0.22 (95% CI, −0.03 to 0.48) at day 14. The largest effect was seen 2 days after infusion (d=0.80; 95% CI, 0.55-1.04).
Figure 2.
Change in depression scale scores during 2 weeks in patients with bipolar disorder given placebo and ketamine (n=18). Values are expressed as generalized least-square means and standard errors for the intent-to-treat analysis. BDI indicates Beck Depression Inventory; HAM-D, 17-item Hamilton Scale for Depression; MADRS, Montgomery-Asberg Depression Rating Scale; *P<.001; †P<.01; and ‡P<.05.
Examining subjects who completed the study yielded similar results. The interaction between drug and time was significant (F10,191=2.55, P=.007). Post hoc tests indicated significant drug differences from 40 minutes through day 7. The effect sizes were similar in size to the intent-to-treat analysis (eg, d=0.72; 95% CI, 0.44-1.00 at day 1).
To examine potential carryover effects in the intent-to-treat sample, a single linear mixed model was used to compare the baseline MADRS scores for each drug and drug order. The model showed no significant effects (order: F1,16=1.48, P=.24; drug: F1,14=0.01, P=.94; order×drug: F1,14=1.69, P=.22). Patients receiving ketamine first had a nonsignificant decrease in MADRS scores from the first baseline rating (mean MADRS score, 31.2 [SD, 4.4]) to the second-phase baseline rating (mean, 29.4 [SD, 8.1]; F14=0.91, P=.36). Patients receiving placebo first also had a nonsignificant decrease in MADRS scores from the first- (mean, 33.9 [SD, 4.8]) to the second-phase baseline rating (mean, 32.9 [SD, 3.8]; F14=0.78, P=.39).
An additional analysis was conducted to understand the potential for carryover effects. The primary analysis was run using only the first-phase data, so the drug factor was a between-subjects measure. In this case, the drug × time interaction approached significance (F10,148=1.71, P=.08). The drug main effect was not significant (F1,16=1.92, P=.19), but the time effect was significant (F10,148=2.93, P=.002). Given the small sample size, effect sizes were calculated to determine whether they differed between the first phase and the full study. Results indicated that effect sizes were similar to the full study. For instance, the effect at 40 minutes was 0.63 (95% CI, −0.04 to 1.30) and the effect at day 1 was 0.66 (95% CI, −0.01 to 1.33); these values compare with 0.52 and 0.67, respectively, in the full analysis.
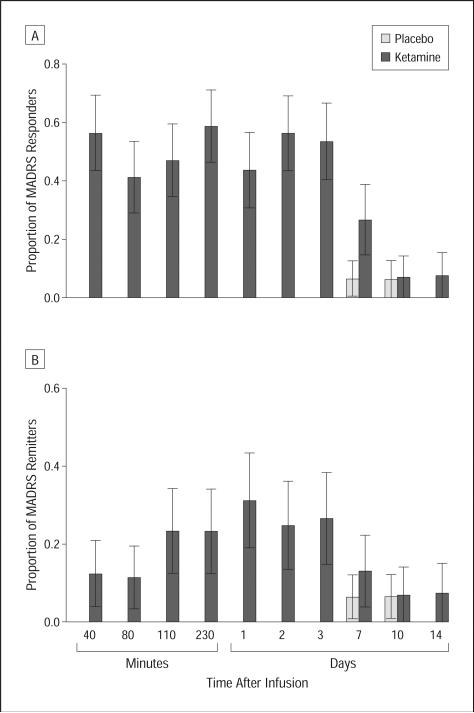
Figure 3 shows the proportion of responders (Figure 3A) and remitters (Figure 3B) at each point in the intent-to-treat sample. No patients responded or remitted while taking placebo through the first 3 days; 1 patient (6%) receiving placebo had response and remission at days 7 and 10. Nine of 16 (56%) patients receiving ketamine responded, and 2 of 16 (13%) remitted at 40 minutes; 7 of 16 (44%) responded and 5 of 16 (31%) patients receiving ketamine remitted after 1 day.
Figure 3.
Proportion of responders and remitters after ketamine or placebo infusion by Montgomery-Asberg Depression Rating Scale (MADRS) score. A, Proportion of responders (50% improvement on MADRS) from 40 minutes to day 14 postinfusion (n=18). B, Proportion of remitters (MADRS score <10) from 40 minutes to day 14 postinfusion (n=18).
When only completers were included in the analysis, the McNemar test showed a significantly higher response rate from 40 minutes through day 3 with ketamine, but only the result at 230 minutes remained significant after applying the Hochberg-adjusted Bonferroni procedure to adjust for multiple comparisons. None of the remission rates were significantly different. Twelve of 17 (71%) patients responded to ketamine and 1 of 16 (6%) responded to placebo at some point during the trial. The median time to initial response was 40 minutes. After responding, losing response was defined as reaching less than 25% improvement from baseline. Under those conditions, the response to ketamine lasted an average of 6.8 days (standard error, 1.4 days); 4 patients responded for 1 week, and 3 additional patients had a response lasting 2 weeks or more.
To confirm the change in depressive symptoms as assessed by the MADRS, similar statistical models were used for the secondary measures. For the Hamilton Scale for Depression, a significant interaction was observed between drug and time (F10,251=2.75, P=.003). When receiving ketamine, patients had fewer depressive symptoms from 40 minutes to 3 days and at 14 days postinfusion. Similar results were obtained for the Beck Depression Inventory (F10,205=3.76, P<.001), in which differences were present for the same times (Figure 2). With the visual analog scale, depressive symptoms were lower with ketamine from 40 minutes to 3 days postinfusion (F10,187=3.92, P<.001).
Anxiety symptoms, as assessed by both the Hamilton Anxiety Rating Scale and the visual analog scale, decreased significantly. A linear mixed model using the Hamilton Anxiety Rating Scale showed significantly less anxiety for subjects receiving ketamine compared with placebo (F7,147=2.10, P=.047) at the first postinfusion observation and at 230 minutes through day 3. Drug differences were significant at day 10 but not at days 7 or 14 (eFigure). Similar findings were obtained with the visual analog scale, which showed significantly fewer symptoms in subjects receiving ketamine (F10,254=3.02, P=.001) from 40 minutes through day 2.
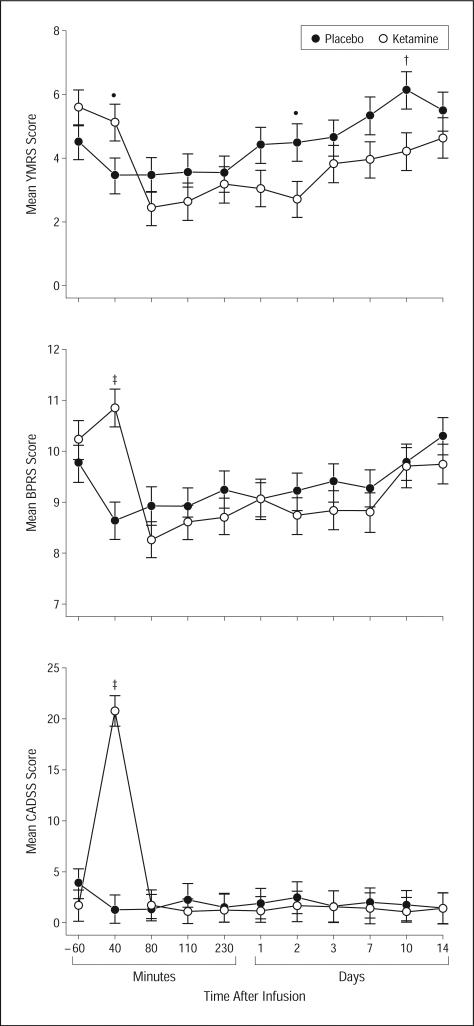
The linear mixed model with manic symptoms, as assessed by the Young Mania Rating Scale, showed a significant interaction between time and drug (F10,220=2.50, P=.007) (Figure 4). Patients receiving ketamine had significantly higher scores at 40 minutes, but significantly lower scores at days 2 and 14. The difference at 40 minutes came from a nonsignificant decrease in Young Mania Rating Scale scores on placebo and a slight, nonsignificant increase when receiving ketamine. Compared with baseline, there was no significant change in manic symptoms in patients receiving placebo, but Young Mania Rating Scale scores significantly declined with ketamine from 80 minutes through day 2 postinfusion. One patient who was concomitantly receiving lithium reached a Young Mania Rating Scale score of 15 at 40 minutes while taking ketamine, but the symptoms were no longer higher than baseline by 80 minutes. Of the patients receiving placebo and valproate, one patient reached a score of 13 on day 7 and a score of 21 on day 10.
Figure 4.
Change in psychiatric scale scores in patients with bipolar disorder given placebo and ketamine (n=18). Values are expressed as generalized least-square means and standard errors for the intent-to-treat analysis.
BPRS indicates Brief Psychiatric Rating Scale; CADSS, Clinician Administered Dissociative Scale; YMRS, Young Mania Rating Scale; *P<.05; †P<.01; and ‡P<.001.
The statistical model for Brief Psychiatric Rating Scale positive symptoms showed a significant drug×time interaction (F10,248=4.45, P<.001) (Figure 4). Ketamine and placebo differed only at 40 minutes postinfusion, and this difference was due to a small, nonsignificant decrease with placebo and an even smaller increase with ketamine. On the Clinician Administered Dissociative Scale, the significant interaction (F10,263=16.52, P<.001) pointed to a ketamine/placebo difference at 40 minutes only where patients had a large increase on ketamine (Figure 4).
Spearman correlations were used to examine the relationship between change in depressive symptoms and in positive and dissociative symptoms. The unusual distribution of changes in positive and dissociative symptoms drove the use of the nonparametric correlation. We found no significant correlations between change in MADRS scores at various points and change in Brief Psychiatric Rating Scale positive or Clinician Administered Dissociative Scale symptoms, either with ketamine or placebo. Forty minutes after ketamine infusion, the correlation between MADRS scores and Brief Psychiatric Rating Scale positive symptoms was r = 0.08 (P = .76); the correlation between MADRS scores and Clinician Administered Dissociative Scale symptoms was r=0.23 (P=.40). At day 1 postinfusion, the correlations were r = 0.09 (P = .75) for Brief Psychiatric Rating Scale positive symptoms and r=−0.08 (P=.76) for Clinician Administered Dissociative Scale symptoms.
Pearson correlations showed that plasma ketamine and norketamine levels after 40 minutes were not significantly related to change in MADRS score at 40 minutes (ketamine: r = −0.18, P = .51; norketamine: r=0.34, P=.20). Correlations did not change after 40 minutes.
An analysis of individual MADRS items showed that 8 of 10 symptoms changed significantly, even after correction for multiple comparisons. Reduced sleep and suicidal thoughts were not significant, and reduced appetite scores were significantly increased. All other symptoms decreased significantly.
The influence of mood stabilizer on response rates was not significant with ketamine (Fisher exact test, P=.59; lithium: 8 of 10 responded [80%], valproate: 4 of 7 responded [57%]) or placebo (Fisher exact test, P=.38; lithium: 0 of 10 responded [0%], valproate: 1 of 6 responded [17%]). Time to first response to ketamine, as assessed by Kaplan-Maier survival analysis, was also not significant (log-rank test, , P=.30; mean: lithium, 4083 minutes [standard error, 2542 minutes]; and valproate, 8679 minutes [standard error, 3758 minutes]). All responders to ketamine had an initial response within the first hours after infusion. An additional linear mixed model was performed after adding the type of mood stabilizer (lithium or valproate) as a factor in the model, but no significant effects were observed (mood stabilizer, F1,15=0.21, P=.65; mood stabilizer×time, F10,207=0.35, P=.97; mood stabilizer×drug, F1,140=0.65, P=.42; mood stabilizer×time×drug, F10,232=0.85, P=.58).
No serious adverse events occurred during the study. Adverse events occurring during the infusion in 10% or more of subjects receiving ketamine or placebo included feeling woozy or loopy, feeling lethargic or drowsy, cognitive impairment, fear or anxiety, nausea, dizziness, odd sensations, blurred vision, and headache. Adverse events associated only with ketamine (≥10% of subjects) included dissociation; feeling strange, weird, or bizarre; dry mouth; tachycardia; and increased blood pressure. The 2 subjects who experienced increased blood pressure and tachycardia returned to normal within minutes after the infusion. No adverse event was significantly different from placebo at 80 minutes or thereafter (eTable). No significant changes occurred in electrocardiography, respiratory, or laboratory values during the study.
COMMENT
This double-blind, placebo-controlled, proof-of-concept study found that a single intravenous infusion of an NMDA antagonist in patients with treatment-resistant bipolar depression resulted in a robust and rapid (within minutes) antidepressant response. To our knowledge, this is the first article detailing the rapid antidepressant effects of a single infusion of an NMDA antagonist in patients with treatment-resistant bipolar depression.
We found that patient depression scores improved significantly more in patients receiving ketamine than in those receiving placebo, and this improvement occurred as early as 40 minutes postinfusion. This difference was statistically significant for 4 different efficacy scales: MADRS, Hamilton Scale for Depression, self-rated Beck Depression Inventory, and visual analog scale depression, as well as the Hamilton Anxiety Rating Scale and visual analog scale anxiety scales. These findings are particularly noteworthy because a substantial proportion of study participants had been prescribed complex polypharmacy regimens in the past with substantial treatment failures. The mean number of past antidepressant trials was 7, and more than 55% of participants failed to respond to electroconvulsive therapy. The toll of this protracted and refractory illness on the subjects was evident, in that two-thirds of participants were on psychiatric disability and nearly all were unemployed.
Compared with our previous study with ketamine in patients with treatment-resistant major depressive disorder,34 we found (1) more rapid onset of antidepressant effects (40 vs 110 minutes), (2) a higher response rate at 40 minutes (56% vs 11%) but lower at day 1 (42% vs 71%), (3) similar remission rates at day 1 (31% for both studies), and (4) shorter duration of antidepressant effects beyond the 2-hour half-life of ketamine42 (3 days in the present study vs 7 in the prior study). Other than the obvious reason of diagnosis, possible explanations for these differences include the concomitant use of lithium or valproate and that the patients in the present study had a more severe treatment-resistant history.
These results lend further credence to earlier controlled studies investigating the glutamatergic system, and NMDA antagonism in particular, as putatively involved in the mechanism of rapid antidepressant response.33,34 Preclinical studies postulated that ketamine's mechanism of action is initially mediated by NMDA antagonism but subsequently involves enhanced AMPA throughput. Ketamine, presumably by disinhibiting GABAergic inputs and thereby enhancing the firing rate of glutamatergic neurons, increases the presynaptic release of glutamate, resulting in increased extracellular levels of glutamate.43 This increased glutamate release preferentially favors AMPA receptors over NMDA receptors, because the latter are blocked by ketamine; the net effect of ketamine's antidepressant effect on a cellular level is thus an increased glutamatergic throughput of AMPA relative to NMDA.44
Future research will need to address whether differences in kinetics associated with intravenous administration—which allows for faster absorption and avoids hepatic metabolism—are important or necessary for rapid antidepressant effects to occur.45 Indeed, several studies of drugs with different mechanisms of action that use this method of administration report rapid onset of antidepressant effects.46-48
This study is associated with several strengths. Notably, subjects were hospitalized for an average of 9 weeks prior to their first infusion, permitting sufficient time to characterize them, conduct a prospective trial of a mood stabilizer, and document the stability of depressive symptoms during their current episode. In addition, the study was randomized and placebo controlled; before randomization, patients were required to have experienced a non-response to a prospective trial of a minimum of 4 weeks of therapeutic levels of lithium or valproate therapy as well as a prior failed antidepressant trial.
Nevertheless, several limitations also exist, and these preliminary results require cautious interpretation. First, the group size was small. In addition, the subjects in this study were a refractory subgroup of patients with treatment-resistant bipolar depression who were relatively late in their course of illness (Table 1); thus, the results may not be generalizable to patients with BPD who have different illness and course characteristics (eg, rapid cycling course and current substance use disorders). Another key issue is the possibility that the response seen in this study resulted from the patients’ use of lithium or valproate rather than ketamine. This seems unlikely because the mean duration of therapeutic levels of mood stabilizers before receiving the first infusion was, on average, 7 weeks. In addition, most patients included in the study had previously failed to respond to trials of either or both of these medicines (Table 2). Next, our study findings could be explained by the notion that subjects cycled out of their major depressive episode. This is also unlikely given that only one subject included in the study met rapid cycling criteria. Furthermore, the use of the placebo phase should protect against findings due to random switching.
Consistent with all published studies using ketamine,30,33,34,49 we found transitory perceptual and dissociative disturbances in patients receiving ketamine that could have compromised the study blinding. Raters and patients were not asked to guess treatment assignment, which would have helped clarify the strength of the blinding. Although most ketamine patients experienced such changes, those changes were not associated with antidepressant response; furthermore, some patients who responded to ketamine had no substantial dissociative symptoms. However, limitations in maintaining the study blinding may have biased patient reporting by diminishing placebo effects, thereby potentially confounding the results. It is interesting to note that although none of the subjects taking placebo met the stringent 50% response criteria in the first week, they had an improvement of 16% at 40 minutes, 14% at 230 minutes, and 10% at day 3. In addition, 31% of patients had at least a 20% improvement at 230 minutes, and 1 had a 40% improvement on day 3. This implies that some clinical improvement occurred with placebo. Furthermore, at least 10% of patients receiving placebo experienced adverse events similar to those occurring with an active drug. While the clinical improvement and adverse events experienced by subjects receiving placebo does not mean that they could not discern placebo from the active drug, it does imply that they experienced changes consistent with having received an active drug. Nevertheless, future studies using ketamine will have to address the limitation of blinding and may include an active comparator with central nervous system effects.
Effective treatments for preventing mood episodes in patients with BPD are urgently needed. However, it is important to note that the purpose of this study was to determine the relevance of the NMDA receptor in initial and rapid onset of antidepressant response, not in preventing relapse. Taken together, the present results support the hypothesis that targeting the NMDA receptor complex brings about a rapid antidepressant effect in patients with bipolar depression. Future studies should examine strategies for long-term maintenance of ketamine's rapid antidepressant response.
Funding/Support
Funding for this work was supported by the Intramural Research Program at the NIMH, National Institutes of Health, Department of Health and Human Services, and by a National Alliance for Research on Schizophrenia and Depression Award (Dr Zarate).
Footnotes
Financial Disclosure: Ioline Henter, MA, provided outstanding editorial assistance. A patent application for the use of ketamine in depression has been submitted listing Drs Zarate and Manji among the inventors; they have assigned their rights on the patent to the US government.
Online-Only Material: The online-only material is available at http://www.archgenpsychiatry.com.
REFERENCES
- 1.Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RM, Petukhova M, Kessler RC. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calabrese JR, Huffman RF, White RL, Edwards S, Thompson TR, Ascher JA, Monaghan ET, Leadbetter RA. Lamotrigine in the acute treatment of bipolar depression: results of five double-blind, placebo-controlled clinical trials. Bipolar Disord. 2008;10(2):323–333. doi: 10.1111/j.1399-5618.2007.00500.x. [DOI] [PubMed] [Google Scholar]
- 3.Sachs GS, Nierenberg AA, Calabrese JR, Marangell LB, Wisniewski SR, Gyulai L, Friedman ES, Bowden CL, Fossey MD, Ostacher MJ, Ketter TA, Patel J, Hauser P, Rapport D, Martinez JM, Allen MH, Miklowitz DJ, Otto MW, Dennehy EB, Thase ME. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711–1722. doi: 10.1056/NEJMoa064135. [DOI] [PubMed] [Google Scholar]
- 4.Nierenberg AA, Ostacher MJ, Calabrese JR, Ketter TA, Marangell LB, Miklowitz DJ, Miyahara S, Bauer MS, Thase ME, Wisniewski SR, Sachs GS. Treatment-resistant bipolar depression: a STEP-BD equipoise randomized effectiveness trial of antidepressant augmentation with lamotrigine, inositol, or risperidone. Am J Psychiatry. 2006;163(2):210–216. doi: 10.1176/appi.ajp.163.2.210. [DOI] [PubMed] [Google Scholar]
- 5.Weisler RH, Calabrese JR, Thase ME, Arvekvist R, Stening G, Paulsson B, Suppes T. Efficacy of quetiapine monotherapy for the treatment of depressive episodes in bipolar I disorder: a post hoc analysis of combined results from 2 double-blind, randomized, placebo-controlled studies. J Clin Psychiatry. 2008;69(5):769–782. doi: 10.4088/jcp.v69n0510. [DOI] [PubMed] [Google Scholar]
- 6.Calabrese JR, Keck PE, Jr, Macfadden W, Minkwitz M, Ketter TA, Weisler RH, Cutler AJ, McCoy R, Wilson E, Mullen J. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. Am J Psychiatry. 2005;162(7):1351–1360. doi: 10.1176/appi.ajp.162.7.1351. [DOI] [PubMed] [Google Scholar]
- 7.Thase ME, Macfadden W, Weisler RH, Chang W, Paulsson B, Khan A, Calabrese JR, BOLDER II Study Group Efficacy of quetiapine monotherapy in bipolar I and II depression: a double-blind, placebo-controlled study (the BOLDER II study). J Clin Psychopharmacol. 2006;26(6):600–609. doi: 10.1097/01.jcp.0000248603.76231.b7. [DOI] [PubMed] [Google Scholar]
- 8.Machado-Vieira R, Salvadore G, Luckenbaugh DA, Manji HK, Zarate CA., Jr Rapid onset of antidepressant action: a new paradigm in the research and treatment of major depressive disorder. J Clin Psychiatry. 2008;69(6):946–958. doi: 10.4088/jcp.v69n0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jick H, Kaye JA, Jick SS. Antidepressants and the risk of suicidal behaviors. JAMA. 2004;292(3):338–343. doi: 10.1001/jama.292.3.338. [DOI] [PubMed] [Google Scholar]
- 10.Nock MK, Hwang I, Sampson N, Kessler RC, Angermeyer M, Beautrais A, Borges G, Bromet E, Bruffaerts R, de Girolamo G, de Graaf R, Florescu S, Gureje O, Haro JM, Hu C, Huang Y, Karam EG, Kawakami N, Kovess V, Levinson D, Posada-Villa J, Sagar R, Tomov T, Viana MC, Williams DR. Cross-national analysis of the associations among mental disorders and suicidal behavior: findings from the WHO World Mental Health Surveys. PLoS Med. 2009;6(8):e1000123. doi: 10.1371/journal.pmed.1000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinowich K, Schloesser RJ, Manji HK. Bipolar disorder: from genes to behavior pathways. J Clin Invest. 2009;119(4):726–736. doi: 10.1172/JCI37703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zarate CA, Jr, Singh J, Manji HK. Cellular plasticity cascades: targets for the development of novel therapeutics for bipolar disorder. Biol Psychiatry. 2006;59(11):1006–1020. doi: 10.1016/j.biopsych.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 13.Law AJ, Deakin JF. Asymmetrical reductions of hippocampal NMDAR1 glutamate receptor mRNA in the psychoses. Neuroreport. 2001;12(13):2971–2974. doi: 10.1097/00001756-200109170-00043. [DOI] [PubMed] [Google Scholar]
- 14.McCullumsmith RE, Kristiansen LV, Beneyto M, Scarr E, Dean B, Meador-Woodruff JH. Decreased NR1, NR2A, and SAP102 transcript expression in the hippocampus in bipolar disorder. Brain Res. 2007;1127(1):108–118. doi: 10.1016/j.brainres.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woo TU, Walsh JP, Benes FM. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2004;61(7):649–657. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- 16.Martucci L, Wong AH, De Luca V, Likhodi O, Wong GW, King N, Kennedy JL. N-methyl-D-aspartate receptor NR2B subunit gene GRIN2B in schizophrenia and bipolar disorder: Polymorphisms and mRNA levels. Schizophr Res. 2006;84(2-3):214–221. doi: 10.1016/j.schres.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Mundo E, Tharmalingham S, Neves-Pereira M, Dalton EJ, Macciardi F, Parikh SV, Bolonna A, Kerwin RW, Arranz MJ, Makoff AJ, Kennedy JL. Evidence that the N-methyl-D-aspartate subunit 1 receptor gene (GRIN1) confers susceptibility to bipolar disorder. Mol Psychiatry. 2003;8(2):241–245. doi: 10.1038/sj.mp.4001218. [DOI] [PubMed] [Google Scholar]
- 18.Brennan BP, Hudson JI, Jensen JE, McCarthy J, Roberts JL, Prescot AP, Cohen BM, Pope HG, Jr, Renshaw PF, Ongür D. Rapid enhancement of glutamatergic neurotransmission in bipolar depression following treatment with riluzole. Neuropsychopharmacology. 2010;35(3):834–846. doi: 10.1038/npp.2009.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zarate CA, Jr, Quiroz JA, Singh JB, Denicoff KD, De Jesus G, Luckenbaugh DA, Charney DS, Manji HK. An open-label trial of the glutamate-modulating agent riluzole in combination with lithium for the treatment of bipolar depression. Biol Psychiatry. 2005;57(4):430–432. doi: 10.1016/j.biopsych.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 20.Du J, Suzuki K, Wei Y, Wang Y, Blumenthal R, Chen Z, Falke C, Zarate CA, Jr, Manji HK. The anticonvulsants lamotrigine, riluzole, and valproate differentially regulate AMPA receptor membrane localization: relationship to clinical effects in mood disorders. Neuropsychopharmacology. 2007;32(4):793–802. doi: 10.1038/sj.npp.1301178. [DOI] [PubMed] [Google Scholar]
- 21.Layer RT, Popik P, Olds T, Skolnick P. Antidepressant-like actions of the polyamine site NMDA antagonist, eliprodil (SL-82.0715). Pharmacol Biochem Behav. 1995;52(3):621–627. doi: 10.1016/0091-3057(95)00155-p. [DOI] [PubMed] [Google Scholar]
- 22.Meloni D, Gambarana C, De Montis MG, Dal Pra P, Taddei I, Tagliamonte A. Dizocilpine antagonizes the effect of chronic imipramine on learned helplessness in rats. Pharmacol Biochem Behav. 1993;46(2):423–426. doi: 10.1016/0091-3057(93)90374-3. [DOI] [PubMed] [Google Scholar]
- 23.Moryl E, Danysz W, Quack G. Potential antidepressive properties of amantadine, memantine and bifemelane. Pharmacol Toxicol. 1993;72(6):394–397. doi: 10.1111/j.1600-0773.1993.tb01351.x. [DOI] [PubMed] [Google Scholar]
- 24.Papp M, Moryl E. Antidepressant activity of non-competitive and competitive NMDA receptor antagonists in a chronic mild stress model of depression. Eur J Pharmacol. 1994;263(1-2):1–7. doi: 10.1016/0014-2999(94)90516-9. [DOI] [PubMed] [Google Scholar]
- 25.Przegaliński E, Tatarczynska E, Deren-Wesolek A, Chojnacka-Wojcik E. Antidepressant-like effects of a partial agonist at strychnine-insensitive glycine receptors and a competitive NMDA receptor antagonist. Neuropharmacology. 1997;36(1):31–37. doi: 10.1016/s0028-3908(96)00157-8. [DOI] [PubMed] [Google Scholar]
- 26.Trullas R, Skolnick P. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur J Pharmacol. 1990;185(1):1–10. doi: 10.1016/0014-2999(90)90204-j. [DOI] [PubMed] [Google Scholar]
- 27.Krystal JH, Sanacora G, Blumberg H, Anand A, Charney DS, Marek G, Epperson CN, Goddard A, Mason GF. Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Mol Psychiatry. 2002;7(suppl 1):S71–S80. doi: 10.1038/sj.mp.4001021. [DOI] [PubMed] [Google Scholar]
- 28.Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7(5):426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zarate CA, Quiroz J, Payne J, Manji HK. Modulators of the glutamatergic system: implications for the development of improved therapeutics in mood disorders. Psychopharmacol Bull. 2002;36(4):35–83. [PubMed] [Google Scholar]
- 30.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans: psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51(3):199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 31.First MB, Spitzer RL, Gibbon M, Williams AR. Structured Clinical Interview for DSM-IV TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York State Psychiatric Institute, Biometrics Research; New York, NY: 2001. [Google Scholar]
- 32.Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(suppl 16):10–17. [PubMed] [Google Scholar]
- 33.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 34.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 35.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beck AT, Beamesderfer A. Assessment of depression: the depression inventory. Mod Probl Pharmacopsychiatry. 1974;7(0):151–169. doi: 10.1159/000395074. [DOI] [PubMed] [Google Scholar]
- 37.Aitken RC. Measurement of feelings using visual analogue scales. Proc R Soc Med. 1969;62(10):989–993. doi: 10.1177/003591576906201005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 39.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 40.Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, Mazure CM. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). J Trauma Stress. 1998;11(1):125–136. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- 41.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 42.White PF, Schuttler J, Shafer A, Stanski DR, Horai Y, Trevor AJ. Comparative pharmacology of the ketamine isomers: studies in volunteers. Br J Anaesth. 1985;57(2):197–203. doi: 10.1093/bja/57.2.197. [DOI] [PubMed] [Google Scholar]
- 43.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17(8):2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63(4):349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 45.Altamura AC, Dell'Osso B, Buoli M, Zanoni S, Mundo E. Intravenous augmentative citalopram versus clomipramine in partial/nonresponder depressed patients: a short-term, low dose, randomized, placebo-controlled study. J Clin Psychopharmacol. 2008;28(4):406–410. doi: 10.1097/JCP.0b013e31817d5931. [DOI] [PubMed] [Google Scholar]
- 46.Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63(10):1121–1129. doi: 10.1001/archpsyc.63.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28(6):631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- 48.Szuba MP, Amsterdam JD, Fernando AT III, Gary KA, Whybrow PC, Winokur A. Rapid antidepressant response after nocturnal TRH administration in patients with bipolar type I and bipolar type II major depression. J Clin Psychopharmacol. 2005;25(4):325–330. doi: 10.1097/01.jcp.0000169037.17884.79. [DOI] [PubMed] [Google Scholar]
- 49.Anand A, Charney DS, Oren DA, Berman RM, Hu XS, Cappiello A, Krystal JH. Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: support for hyperglutamatergic effects of N-methyl-D-aspartate receptor antagonists. Arch Gen Psychiatry. 2000;57(3):270–276. doi: 10.1001/archpsyc.57.3.270. [DOI] [PubMed] [Google Scholar]