Abstract
To expand the available tools for investigating human sarcomas, we characterized the primary properties of 22 common, uncommon, and newly characterized sarcoma cell lines representing eight different histological subtypes. Throughout the characterization process we noticed that in vitro markers and assays are poor indicators of tumorigenicity and that generated xenografts often bear little resemblance to the original histopathology. In vitro properties examined included morphology, proliferation rate, cell cycle characteristics, invasiveness, and immunohistochemical expression of p53 and phospho-AKT. In vivo properties examined included days to tumor formation in NOD/SCID mice, xenograft morphology in several locations and immunohistochemical expression of Ki67, p53 and phospho-AKT. We believe that such an in depth comparison of a large cohort of sarcoma cell lines will be useful in both designing and interpreting experiments aimed at elucidating both the molecular biology and efficacy of therapeutic agents in sarcomas. However, that data generated also suggests a small set of sarcoma cell lines may be inappropriate for generalizations regarding biological behavior of specific sarcoma subtypes. Integration of functional genomics or other more sophisticated assays of cell lines may help bridge the differences in vitro and in vivo.
Keywords: cell line, properties, sarcoma
INTRODUCTION
As for all cancer subtypes, pre-clinical studies on sarcoma cell lines have been used as a rational prelude for phase I and phase II clinical trials. Recent examples of this include pre-clinical work on the DNA minor groove binder trabectedin (ET-743)1 and on the synergistic combination of gemcitabine and docetaxel.2 Although trabectedin demonstrated significant activity in myxoid/round cell liposarcomas in clinical studies,3 the only liposarcoma cell line (i.e. HS-18) used in the original pre-clinical studies was the second most resistant cell line of all those examined, at odds with the clinical data. Similarly, the synergy of gemcitabine with docetaxel2 was originally observed in an osteosarcoma cell line (i.e. SAOS-2), but the greatest clinical activity seen to date has been in malignant fibrous histiocytomas (i.e. undifferentiated sarcomas) and leiomyosarcomas,4 with even less data supporting any synergy between the two drugs.5
In our opinion, there are two main reasons that might explain the obvious disconnect between pre-clinical and clinical development that is highlighted by the above examples. The first reason is the assumption that sarcoma subtypes are interchangeable. This concept is supported by the historical trend to divide sarcomas into broad categories such as pediatric versus adult, simple versus complex karyotype or soft-tissue versus non-soft tissue sarcomas.6 This rationale is fading as sarcoma subtype-specific treatments are emerging (e.g. imatinib for gastrointestinal stromal tumors7). The second reason that might help explain the disconnect between pre-clinical and clinical development is the relative scarcity of well-characterized sarcoma subtype-specific cell lines available for pre-clinical development.
Even when there are abundant numbers of sarcoma subtype-specific cell lines available for specific studies (e.g. osteosarcoma cell lines, widely available from repositories such as the American Type Culture Collection [ATCC]), it is often difficult to extract appropriate baseline cell line comparative characteristics in order to make meaningful conclusions. Numerous publications (and some more recent ones8–10) clearly demonstrate differential sensitivities to various treatments on osteosarcoma cell lines and these results may indeed be therapeutically meaningful. In one report8 gemcitabine was clearly shown to be 10 times more potent in inhibiting the growth of HOS osteosarcoma cells than MG63 osteosarcoma cells. Is this simply due to the fact that HOS cells proliferate 10 times as fast as MG63 cells? Since it is generally accepted that cytotoxic chemotherapy such as gemcitabine preferentially affects rapidly proliferating cells, the latter conclusion would be much more interesting if the proliferation rates of these two cell types were known. Surprisingly, such comparative data are almost non-existent.
In an attempt to address the above issues, and to provide some standardization for sarcoma cell lines used for both pre-clinical and mechanistic exploratory studies in the sarcoma field, we characterized and compared 22 sarcoma cell lines representing eight different histological subtypes for the following baseline properties: proliferation, cell cycle distribution, invasiveness, p53, phosphor-AKT and in vivo tumorigenesis. Some of these cell lines are standard cell lines that have been used in many studies (e.g. SAOS-2) and are commercially available; some are newer cell lines that have appeared only recently in published reports (e.g. AX, AW11); and some are newly generated cell lines (e.g. CHSA2) provided to us for these purposes by our collaborators. In carrying out these studies we note that in vitro markers and assays are poor indicators of tumorigenicity and that generated xenografts often bear little resemblance to the original histopathology. This latter observation adds yet a third reason for the observed differences between pre-clinical sarcoma research and clinical drug development.
MATERIALS AND METHODS
Cell lines, growth, proliferation, FACS
All cell lines used, original histopathological type, origin and the medium in which they were maintained are listed in Table S1. Cells were fixed, stained and visualized with hematoxylin as previously described.12 Proliferation rate was assessed in triplicate wells via the addition of MTS (Promega, Madison, WI, USA) 24 h after plating. Fluorescence-activated cell sorting (FACS) was carried out as previously described.12
Immunocytochemistry and immunohistochemistry anti p53 (ab-2; mouse monoclonal OP09L; Calbiochem, San Diego, CA, USA); phospho-AKT (3787L; monoclonal, Cell Signaling, Danvers, MA, USA) and Ki67 (rabbit polyclonal; ab15580; Abcam, Cambridge, MA, USA) as previously described.12 Each analysis was repeated at least three times.
Invasion assay
BD BioCoat Growth Factor Reduced MATRIGEL Invasion Chambers were used following the manufacturer’s instructions with the following modifications: The inserts were rehydrated with 500 μL culture medium and maintained for 2 h at 37°C, 5% CO2. The medium was removed without disturbing the Matrigel layer. A sample of 600 μL of culture medium with 5 μg/mL fibronectin was added to the lower chamber. The inserts were transferred to these wells and 2.5 × 104 cells were added to each insert in 500 μL of culture medium plus 0.1% bovine serum albumin (BSA). The chamber was incubated for both 12 and 24 h at 37°C, 5% CO2. After incubation the lower chamber was aspirated, fixed with 500 μL of 10% formalin, washed three times with water, and stained with 500 μL of crystal violet for 5–10 min. The membrane was then removed from the insert with a scalpel and mounted on a microscope slide. Each analysis at each time point was repeated at least three times. Representative data are shown.
Xenografts
Xenografts were generated in NOD/SCID mice (Jackson Laboratories, Bar Harbor, ME, USA) as previously described.12 All pathological reviews of the formed xenografts were done blindly and independently by two pathologists with expertise in sarcoma pathology (Fabrizio Remotti MD and Mireia Castillo-Martin MD PhD). Any discrepancies in histopathological evaluation were kindly further reviewed by Dr Carlos Cordon-Cardo (Columbia University). A concordant evaluation is provided in Table S3. See also supplemental Materials and Methods.
RESULTS
In vitro analysis
In vitro morphological analysis
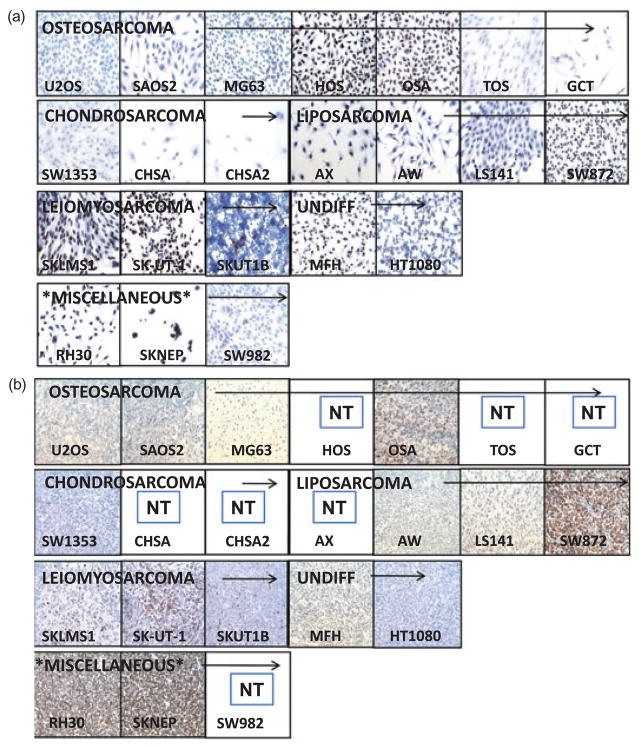
We found that at high densities in vitro the morphological features were obscured by the presence of numerous cells, therefore we reasoned that examination at a low density may make morphological features of individual cells more evident and perhaps reflective of their original histopathology as visualized under light microscopy. Accordingly we stained our 22 sarcoma cell lines with hematoxylin and examined their morphological features at low density (Fig. 1a and Table S2 for complete descriptive details). Of note, that despite numerous culture attempts in our hands SKNEP always grew in clumps and that only the SKNEP cell line’s in vitro morphology of small round blue cells potentially resembled its presumed histological origin. Of note CHSA, CHSA2 (chondrosarcoma cell lines) and OSA (osteosarcoma cell lines) are newly isolated human sarcoma cell lines obtained from primary human sarcomas.
Figure 1.
(a) Morphological characteristics of cell lines in vitro. (b) Morphological characteristics of sarcoma cell lines forming xenografts.
In vitro proliferation (cell number over time)
Assessment of in vitro cell number over time indicated that our 22 sarcoma cell lines displayed one of four patterns (Fig. 2a,b): very fast (doubling time 72–96 h; 3–4 days); fast (doubling time 96–120 h; 4–5 days); moderate (doubling time in the order of 14 days); and slow (doubling time in the order of 21 days). The very fast cell lines were: SKUT1B, HOS, MG63, OSA143BG, HT1080, AX, SKLMS1, SKUT1, and MFH. The fast cell lines were: AW, RH30, LS141, SAOS2, SW872, and SKNEP. The moderate cell lines were SW1353, CHSA, SW982, and GCT. The slow growing cell lines included TOS and CHSA2. No association between histological subtype or morphological appearance and in vitro proliferation were noted.
Figure 2.
(a) Time to doubling for each indicated cell line in days. (b) Time to doubling for the very fast and fast subgroups in hours. (c) Graphical representation of percentage of G1, S, and G2. Each time point was independently analyzed three times. (d) Time to tumor formation in days. Empty bars next to indicated cell lines means no tumors were formed up to 100 days of observations.
In vitro cell cycle analysis
To further assess and describe these cells we carried out a complete cell cycle analysis (Fig. 2c). We observed no obvious relationship between the doubling time of cell lines of similar histology or even with similar doubling times.
Invasiveness
Since invasion assays are highly influenced by doubling times especially for cells with fast in vitro proliferation rates, we carried out invasive assays at the 24 h interval (Fig. 3a). HT1080 displayed the greatest invasive potential (very fast grower), followed by LS141 (a fast grower) and TOS (a slow grower). Although the rest of the cell lines displayed a spectrum of invasive potential, no correlation between invasiveness and in vitro proliferation could be observed at either the 12 (data not shown) or 24 h invasive measurements suggesting that these intervals are appropriate for this analysis. In contrast invasiveness measured over a 36 h period was clearly influenced by cell proliferation (data not shown).
Figure 3.
(a) In vitro invasive assays at 24 h. Each time point was independently analyzed three times. Representative pictures are shown. (b) Cell lines were grown in vitro and stained for Ki67 expression.
In vivo analysis
In vivo morphology
To assess whether or not in vivo analysis may better resemble presumed histological origins, we subcutaneously injected 3 million cells of each cell line into both the right and left flank of two NOD/SCID mice and observed for tumor formation (Fig. 1b [magnification 200×] and Fig. S1 [magnification 400×]). HOS, AX, CHSA, SW982, GCT, TOS, and CHSA2 did not show any signs of tumor formation at our 100-day arbitrarily set end point. Close examination of the matrigel nodule at the site of injection upon killing all non-tumor forming mice at the 100-day endpoint clearly demonstrated no live tumor cells, precluding the possibility that longer observation may have resulted in tumor formation. We have further repeated these experiments to ensure that these cell lines are indeed non-tumorigenic by subcutaneously injecting 6 million cells into both the right and left flank of two additional NOD/SCID mice and, as previously, observed no signs of tumor formation (gross or microscopic) after 100 days of observation. In the majority of the samples the predominant histology was high grade pleomorphic or undifferentiated spindle cells sarcoma (Fig. 1b and Table S3). Only SAOS2 xenografts formed an osteoid producing matrix consistent with an osteosarcoma. In OSA143 xenografts, possible signs of early osteoid production may have been noted in one of four xenografts examined, but are not conclusive enough to conclude a diagnosis of osteosarcoma. In none of the observed tumors assessed for this analysis was there any evidence of necrosis. In all cell lines concluded to be tumorigenic, tumors formed in both the right and left flanks following subcutaneous implantation. Injection at other sites showed no histological variance (see Supplemental Text S1 and Fig. S2).
In vivo tumor growth (growth rate)
Growth rate was measured as the time from inoculation to the formation of a 1-cm tumor (our standard protocol per IUCAC) as grossly measured using calipers (Fig. 2d). No signs of necrosis of any tumors were noted. Additional experiments supported that the frequency of tumor initiating cells is unlikely to account for the differences in our measurements of in vivo tumor growth (see Supplemental Text S1 and Figures).
In vivo Ki-67
We further examined the growth rate of the formed xenografts using Ki67 (a commonly used tumor proliferative index; Fig. 3b 16). We noted a trend to a statistically significant correlation between Ki67 and days to xenograft formation (see Supplement). As stated, we were very careful in this analysis that none of the tumors examined were larger than 1 cm and similarly showed no signs of necrosis, thus predisposing that we were assessing these tumors in their growth phase.
p53 and phospho-AKT Status
p53 mutations have been historically cited as occurring in more than 50% of all sarcomas.17,18 Similarly we have previously published on the importance of disruption of the PTEN pathway and sarcomagenesis.19 Thus to further characterize these cell lines we sought to assess the status of p53 and phospho-AKT.
Since detection of p53 via immunohistochemistry is observed only when p53 is mutated,18 we examined the status of p53 via immunohistochemistry in both cell lines and in formed xenografts (Fig. 4a,b). We noted that HOS, OSA, SW872, SKLMS1, SKUT1, MFH, RH30, and SKNEP showed intense staining for p53 in the majority of cells. U2OS, SW1353, SKU1B, and HT1080 showed focal staining, but the majority of cells in these cell lines were negative for p53. Other cell lines showed no readily identifiable p53 positive cells. No relationship was noted between the proliferation status and p53 status. Fast growing cell lines were noted to be either p53 positive (e.g. HOS) or p53 negative (e.g. MG63).
Figure 4.
(a) Indicated cell lines were grown in vitro and stained for p53 expression. (b) Formed tumors were excised, paraffin embedded, and stained for p53 expression.
Immunohistochemical analysis of phospho-AKT (Fig. 5a,b) indicative of an activated AKT pathway either due to PTEN mutations or other PTEN-pathway aberrancies was noted most obviously only in the liposarcoma cell line SW872 and to a lesser degree in SW1353. However, by far the majority of these cell lines did not show signs of an activated AKT pathway as judged via phospho-AKT immunohistochemistry.
Figure 5.
(a) Indicated cell lines were grown in vitro and stained for phospo-AKT expression. (b) Formed tumors were excised, paraffin embedded, and stained for phospo-AKT expression. Each analysis was repeated independently three times with identical results. Representative pictures are shown.
DISCUSSION
Our initial goal in characterizing this panel of sarcoma cell lines was to establish a significant group of sarcoma cell lines for baseline characteristic properties (akin to the NCI 60 panel), such that when certain other properties of these cell lines are reported (e.g. chemotherapy sensitivity to novel agents) the new properties can be properly interpreted against these standard baseline characteristics. In doing so, we hoped to provide some standardization to an already complex field. Interestingly enough throughout the characterization process, we noticed that many of the fields’ preconceived notions regarding the applicability of sarcoma cell lines to recapitulate primary tumor biology and the ability of certain properties to predict others did not hold true.
Our data indicate that the properties of in vitro proliferation, invasiveness, tumorigenicity, and status of major tumor suppressors are unrelated. The implication is that the genes and pathways that regulate these processes are distinctly different. This is a surprising conclusion since tumor development is believed to go through distinct sequential phases starting with tumor growth, invasiveness, dissemination and metastatic formation.20 Our data would suggest that this is not necessarily a sequential process since the most invasive cell lines were not tumorigenic; while many of the tumorigenic cell lines showed minimal invasive properties, and of course both properties were unrelated to tumor growth measured either in vitro or (when possible) in vivo.
Finally, we were equally surprised that the majority of the xenografts bore little resemblance to the primary diagnosis regardless of site of implantation (e.g. subcutaneously or orthotopically). This could be because the process of cell line establishment and subsequent xenograft formation selects for the higher grade component of the tumor, which may not be the predominant histology observed in the tumor specimen. If the process of cell line formation selects for the high grade component of a primary tumor, significant caution should be taken in assigning histopathological designations to cell lines based on primary tissue histopathology or in relating functional observations in vitro as rationale for clinical trials.
As stated above, this is an initial study designed to promote standardization and a point of reference in the sarcoma field. We recognize that examining 22 cell lines does not have the statistical power to generate significant inferences. By collecting and generating additional sarcoma cell lines we hope to increase the statistical power of this analysis. We also recognize that although no obvious association was observed between the status of p53 and phosho-AKT, the status of other tumor suppressors and oncogenes (both known and unknown) may provide a better correlation. Finally, the most effective future use of these data may be in gene expression or proteomic profiling to look for differences in gene or protein expression between tumorigenic and non-tumorigenic cell lines, invasive and non-invasive cell lines, etc. Performing these studies, followed by in vitro validation and clinical correlation, may lead us to better predict those patients whose tumor may be locally invasive without the propensity to metastasize. Such findings may lead to more sophisticated recommendations for primary resections than has been given to date, or predict for the utility of adjuvant chemotherapy even among a specific sarcoma subtype.
In conclusion, our results support the suspicion of sarcoma researchers that data generated via the use of a small set of sarcoma cell lines is inappropriate for making conclusions regarding sarcoma subtype behavior overall and that either (i) a large panel of sarcoma cell lines is needed that on average is representative of in vivo behavior; or (ii) specific cell lines that have been validated and compared in vitro and in vivo should be used for further sarcoma biological and therapeutic exploration. Performing these studies, followed by in vitro validation and clinical correlation, may lead us to better predict (for example) those patients whose tumor may be locally invasive without the propensity to metastasize. Such findings may lead to more sophisticated recommendations for primary resections than has been given to date, or predict for the utility of adjuvant chemotherapy even among a specific sarcoma subtype.
Supplementary Material
Text S1 Sarcoma cell line properties.
Figure S1 Indicated cell lines were grown in vivo and stained with hematoxylin prior to reaching confluence. Magnification 400×.
Figure S2 (a) Intraperitoneal xenograft formation. (b) RH30 xenograft formation in the anterior tibialis muscle. Each cell line was implanted as described above into three mice and monitored until tumor formation as described in the text. Representative pictures are shown. (b) Serial dilution xenograft experiments of two fast and two slow forming tumor cell lines.
Figure S3 Pairwise plot and univariate regression analysis correlation coefficients. R2 values (coefficients of correlation) are provided below each regression plot.
Table S1 Culture conditions for the indicated cell lines and source of origin.
Table S2 Morphological characteristics of cell lines in vitro. Representative pictures of in vitro morphology are shown. CS, chondrosarcoma; ES, Ewing’s sarcoma; LMS, leiomyosarcoma; LS, liposarcoma; OS, osteosarcoma; SS, synovial sarcoma; US, undifferentiated sarcoma.
Table S3 Morphological characteristics of sarcoma cell lines forming xenografts. Representative pictures of in vivo morphology are shown. CS, chondrosarcoma; ES, Ewing’s sarcoma; LMS, leiomyosarcoma; LS, liposarcoma; OS, osteosarcoma; SS, synovial sarcoma; US, undifferentiated sarcoma. Each cell line was implanted into the right and left flank of three mice and monitored till tumor formation as described in the text.
Footnotes
Additional Supporting Information may be found in the online version of this article:
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Li WW, Takahashi N, Jhanwar S, et al. Sensitivity of soft tissue sarcoma cell lines to chemotherapeutic agents: identification of ecteinascidin-743 as a potent cytotoxic agent. Clin Cancer Res. 2001;7:2908–11. [PubMed] [Google Scholar]
- 2.Leu KM, Ostruszka LJ, Shewach D, et al. Laboratory and clinical evidence of synergistic cytotoxicity of sequential treatment with gemcitabine followed by docetaxel in the treatment of sarcoma. J Clin Oncol. 2004;22:1706–12. doi: 10.1200/JCO.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 3.Grosso F, Jones RL, Demetri GD, et al. Efficacy of trabectedin (ecteinascidin-743) in advanced pretreated myxoid liposarcomas: a retrospective study. Lancet Oncol. 2007;8:595–602. doi: 10.1016/S1470-2045(07)70175-4. [DOI] [PubMed] [Google Scholar]
- 4.Maki RG, Wathen JK, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 corrected. J Clin Oncol. 2007;25:2755–63. doi: 10.1200/JCO.2006.10.4117. [DOI] [PubMed] [Google Scholar]
- 5.Maki RG. Gemcitabine and docetaxel in metastatic sarcoma: past, present, and future. Oncologist. 2007;12:999–1006. doi: 10.1634/theoncologist.12-8-999. [DOI] [PubMed] [Google Scholar]
- 6.Matushansky I, Maki RG. Mechanisms of sarcomagenesis. Hematol Oncol Clin North Am. 2005;19:427–49. v. doi: 10.1016/j.hoc.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364:1127–34. doi: 10.1016/S0140-6736(04)17098-0. [DOI] [PubMed] [Google Scholar]
- 8.Ando T, Ichikawa J, Okamoto A, Tasaka K, Nakao A, Hamada Y. Gemcitabine inhibits viability, growth, and metastasis of osteosarcoma cell lines. J Orthop Res. 2005;23:964–9. doi: 10.1016/j.orthres.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Graat HC, Witlox MA, Schagen FH, et al. Different susceptibility of osteosarcoma cell lines and primary cells to treatment with oncolytic adenovirus and doxorubicin or cisplatin. Br J Cancer. 2006;94:1837–44. doi: 10.1038/sj.bjc.6603189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walters DK, Muff R, Langsam B, Gruber P, Born W, Fuchs B. Taurolidine: a novel anti-neoplastic agent induces apoptosis of osteosarcoma cell lines. Investig New Drugs. 2007;25:305–12. doi: 10.1007/s10637-007-9052-9. [DOI] [PubMed] [Google Scholar]
- 11.Moneo V, Serelde BG, Fominaya J, et al. Extreme sensitivity to Yondelis (Trabectedin, ET-743) in low passaged sarcoma cell lines correlates with mutated p53. J Cell Biochem. 2007;100:339–48. doi: 10.1002/jcb.21073. [DOI] [PubMed] [Google Scholar]
- 12.Matushansky I, Hernando E, Socci ND, et al. Derivation of sarcomas from mesenchymal stem cells via inactivation of the Wnt pathway. J Clin Invest. 2007;117:3248–57. doi: 10.1172/JCI31377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chambers JM. Linear models. In: Chambers SJM, Hastie TJ, editors. Statistical Models. Pacific Grove, California: Wadsworth & Brooks/Cole; 1992. pp. 96–138. [Google Scholar]
- 14.Hastie TJ, Pregibon D. Generalized linear models. In: Chambers SJM, Hastie TJ, editors. Statistical Models. Pacific Grove, California: Wadsworth & Brooks/Cole; 1992. pp. 196–246. [Google Scholar]
- 15.Wilkinson GN, Rogers CE. Symbolic descriptions of factorial models for analysis of variance. Appl Stat. 1973;22:392–9. [Google Scholar]
- 16.Heslin MJ, Cordon-Cardo C, Lewis JJ, Woodruff JM, Brennan MF. Ki-67 detected by MIB-1 predicts distant metastasis and tumor mortality in primary, high grade extremity soft tissue sarcoma. Cancer. 1998;83:490–7. doi: 10.1002/(sici)1097-0142(19980801)83:3<490::aid-cncr18>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 17.Leach FS, Tokino T, Meltzer P, et al. p53 Mutation and MDM2 amplification in human soft tissue sarcomas. Cancer Res. 1993;53:2231–4. [PubMed] [Google Scholar]
- 18.Latres E, Drobnjak M, Pollack D, et al. Chromosome 17 abnormalities and TP53 mutations in adult soft tissue sarcomas. Am J Pathol. 1994;145:345–55. [PMC free article] [PubMed] [Google Scholar]
- 19.Hernando E, Charytonowicz E, Dudas ME, et al. The AKT-mTOR pathway plays a critical role in the development of leiomyosarcomas. Nature Med. 2007;13:748–53. doi: 10.1038/nm1560. [DOI] [PubMed] [Google Scholar]
- 20.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Text S1 Sarcoma cell line properties.
Figure S1 Indicated cell lines were grown in vivo and stained with hematoxylin prior to reaching confluence. Magnification 400×.
Figure S2 (a) Intraperitoneal xenograft formation. (b) RH30 xenograft formation in the anterior tibialis muscle. Each cell line was implanted as described above into three mice and monitored until tumor formation as described in the text. Representative pictures are shown. (b) Serial dilution xenograft experiments of two fast and two slow forming tumor cell lines.
Figure S3 Pairwise plot and univariate regression analysis correlation coefficients. R2 values (coefficients of correlation) are provided below each regression plot.
Table S1 Culture conditions for the indicated cell lines and source of origin.
Table S2 Morphological characteristics of cell lines in vitro. Representative pictures of in vitro morphology are shown. CS, chondrosarcoma; ES, Ewing’s sarcoma; LMS, leiomyosarcoma; LS, liposarcoma; OS, osteosarcoma; SS, synovial sarcoma; US, undifferentiated sarcoma.
Table S3 Morphological characteristics of sarcoma cell lines forming xenografts. Representative pictures of in vivo morphology are shown. CS, chondrosarcoma; ES, Ewing’s sarcoma; LMS, leiomyosarcoma; LS, liposarcoma; OS, osteosarcoma; SS, synovial sarcoma; US, undifferentiated sarcoma. Each cell line was implanted into the right and left flank of three mice and monitored till tumor formation as described in the text.