Abstract
Objectives
Differences in white matter structure measured with diffusion tensor imaging (DTI) are associated with late-life depression, but results examining how these differences relate to antidepressant remission are mixed. To better describe these relationships, we examined how one-year change in DTI measures are related to one-year course of depression.
Design
One-year cross-sectional follow-up to a 12-week clinical trial of sertraline.
Setting
Outpatients at an academic medical center.
Participants
29 depressed and 20 never-depressed elderly subjects. Over the one-year period, 16 depressed subjects achieved and maintained remission, while 13 did not.
Measurements
One-year change in fractional anisotropy (FA) and diffusivity in frontal white matter, as measured by DTI.
Results
Contrary to our hypotheses, depressed subjects who did not remit over the study interval exhibited significantly less change in anterior cingulate cortex white matter FA than did never-depressed or depressed-remitted subjects. There were no group differences in other frontal or central white matter regions. Moreover, there was a significant positive relationship between change in MADRS and change in anterior cingulate cortex FA, wherein greater interval decline in FA was associated with greater interval decline in MADRS.
Conclusions
Older depressed individuals who remit exhibit white matter changes comparable to what is observed in never-depressed individuals, while nonremitters exhibit significantly less change in anterior cingulate cortex FA. Such a finding may be related to either antidepressant effects on brain structure or the effects of chronic stress on brain structure. Further work is needed to better understand this relationship.
Keywords: Aging, depression, frontal lobe, anterior cingulate cortex, white matter, diffusion tensor imaging
INTRODUCTION
Depression in older adults can differ substantially from depression in younger adult populations, being characterized by medical comorbidity, age-related brain changes, and cognitive impairment. As depression is associated with suffering, disability, and poorer outcomes of comorbid medical illness, effective antidepressant treatment is crucial. However, despite initial remission rates for late-life depression (LLD) being comparable to rates observed in midlife, relapse rates may be higher. 1 It is possible that age-related brain changes may contribute to this higher relapse rate through deterioration of the neural network elements involved in mood regulation.
Such changes in the neural circuit could occur through several age-related mechanisms. One may be underlying neurodegenerative processes contributing to greater than expected atrophy of frontal and temporal gray matter regions. Alternatively, subclinical cerebrovascular disease may contribute to mood dysregulation by disruption of frontostriatal or frontolimbic connections, a theory supported by studies associating greater progression of cerebral hyperintense lesions with increased risk of poor antidepressant outcomes.2
Recent aging research has utilized diffusion tensor imaging (DTI),3 a magnetic resonance imaging modality that measures the magnitude and direction of water diffusion in tissue. Aging is associated with changes in DTI measures, including lower fractional anisotropy (FA), a measure of the directional magnitude of water diffusion. Specifically, aging is associated with lower white matter FA values in frontal regions than posterior white matter regions 4-6 and there may be differential aging effects on FA within various frontal white matter areas.7 Lower FA is typically associated with degradation of structural barriers to water diffusion, including cell membranes or myelin sheaths, suggesting frontal white matter vulnerability to aging effects.
DTI has also been used to examine white matter structure in LLD. Several cross-sectional studies report that depressed elders exhibit lower frontal and temporal FA.8-12 DTI measures are also correlated with other features observed in LLD, including hyperintense lesions 13 and deficits in executive function and processing speed.14, 15 Despite this fairly consistent set of findings relating lower white matter FA to late-life depression, the relationship between frontal FA and antidepressant response is less clear. In studies examining 12-week course of antidepressants, Alexopoulos and colleagues found that failure to achieve remission was associated with lower FA in frontal and temporal white matter.16, 17 In contrast, our group found the opposite, that failure to obtain remission was associated with higher frontal white matter FA measures.18 The reason for this discrepancy is not clear.
In order to determine how our observed short-term association between FA and acute antidepressant outcome compared with longer-term course of depression and change in FA, we conducted a longitudinal study within our study population. We now report results from a one-year follow-up evaluation of individuals who participated in the previously reported 12-week sertraline trial.18 We sought to determine if there were different rates of change in FA or diffusivity (measured with the apparent diffusion coefficient, or ADC) between depressed and nondepressed subjects. Moreover, we sought to determine if change in frontal FA or ADC over that one-year interval differed based on course of depression or if it was associated with depression severity at follow-up.
METHODS
Sample
Participants were initially recruited via advertisements and outpatient clinical referrals at Duke University Medical Center to participate in a 12-week study examining the relationship between hyperintense lesions, cognition, and 12-week response to sertraline.18, 19 Entry criteria for this parent study included meeting DSM-IV criteria for Major Depressive Disorder without psychosis by the Structured Clinical Interview for DSM-IV Disorders (SCID),20 exhibiting a baseline Montgomery-Asberg Depression Rating Scale (MADRS) 21 score of 18 or greater, and being age 60 years or older. Exclusion criteria included: 1) MRI contraindications; 2) comorbid Axis I diagnosis on SCID; 3) active suicidality; 4) current episode having failed to respond to adequate trials of two antidepressants or a failed trial of sertraline; 5) current psychotherapy; 6)severe or unstable medical conditions; 7) primary neurological disorders, including dementia or clinically evident stroke; 8) Mini Mental Status Examination (MMSE) 22 score < 21. Note that the presence of cerebrovascular ischemia identified on MRI in the absence of neurological symptoms (such as presence of white matter hyperintensities) did not result in subject exclusion.
In addition to the depressed sample, a sample of never-depressed older individuals was also recruited. Eligibility criteria were similar to those used for the depressed cohort, except participants in this cohort could neither meet criteria for MDD nor have any history of mental illness.
For the current study, participants in this previous study were recontacted after one year. All subjects who participated in the previous study were eligible to participate in this follow-up study, unless they met one of the exclusion criteria: 1) new MRI contraindication; 2) new Axis I diagnosis (other than MDD) by clinical examination with a geriatric psychiatrist; 3) active suicidality; 4) severe or unstable medical conditions; 5) new onset of primary neurological disorders, including new diagnosis of dementia or stroke.
All study procedures were explained to each participant, and those who provided written informed consent were enrolled. The study was approved by the Duke University Health System Institutional Review Board.
Clinical Assessment and Treatment
Data acquisition at both baseline and one-year follow-up included assessment of demographic data, measurement of depression severity with the MADRS, assessment of global cognition with the MMSE, and measurement of medical illness burden using the Cumulative Illness Rating Scale (CIRS).23 Concurrent medication use was reviewed at both evaluations.
The initial 12-week open-label trial of sertraline has been previously described.19 Succinctly, subjects received sertraline 25 mg for one day to rule out drug sensitivity, then 50 mg daily. Dose increases were allowed at 2 weeks (to 100 mg daily), 4 weeks (to 150 mg daily), and 6 weeks (to 200 mg daily) based on treatment response and side effects. Dose decreases were allowed for side effects. After completing the 12-week trial, subjects were referred back to community providers. In general, if they had responded to sertraline, the recommendation was to continue on that medication. If they had not, the recommendation was to consider a change to another antidepressant.
MRI Acquisition and Processing
At both the initial and one-year assessments, subjects were imaged with a 1.5 T whole-body MRI (Signa, GE Healthcare) using a standard head (volumetric) radiofrequency coil. The scanner alignment light was used to adjust head tilt and rotation. A rapid sagittal localizer scan was acquired to confirm alignment. The initial MRI was obtained within the first two weeks of study participation, typically at the first follow-up visit after initiating study drug.
The diffusion tensor images were acquired using a single shot 2D diffusion tensor echo planar pulse sequence in the axial plane with a 32cm field-of-view, 3mm slice thickness (no gaps between slices), repetition time (Tr) = 6000, echo time (Te) =100, 128 (phase) x128 (frequency), full imaging bandwidth=180KHz, 1 signal average, 6 diffusion directions, each with a b-value of 1000 sec/mm2 plus an acquisition with b=0 using the Basser scheme.24 To reduce noise, four acquisitions were obtained and combined as described below.
Diffusion tensor images were processed prior to alignment and averaging using custom programs run on MATLAB software (version 7, The MathWorks, Natick, MA) that calculated the diffusion tensor eigenvalues in each voxel which then allowed calculation of the FA and ADC images for each acquisition. The baseline scans were registered to the EPI template from SPM (Wellcome Department of Cognitive Neurology, London) using MIRIT (Multimodality Image Registration from Information Theory)25 from KUL (Katholieke Universiteit, Leuven, Belgium).26 The b0 images for the first acquisition of the baseline scans were registered to the EPI template, and then the b0 images of the other three acquisitions were registered to the realigned b0 images of the first acquisition. The transformations used for these b0 registrations were then applied to the FA and ADC images from each of the four acquisitions. Final FA and ADC images were created by averaging the four aligned FA images to form the final, averaged, aligned FA image with a similar average, final, aligned ADC image from the four aligned ADC images. A similar method was used for the follow-up scans, except the b0 images for the first acquisition were aligned to that subject’s previously processed baseline scan instead of the EPI template.
As previously described,8, 13, 18 regions-of-interest (ROIs) were placed by a single analyst blinded to subject identity and treatment outcome using Analyze software (version 7.0, Mayo Clinic). Oval ROIs of identical size (45.7 mm2) were placed initially on the baseline scan, their placement saved to a file, then applied to the aligned follow-up scan. The same ROI was thus used for FA and ADC measures on both the baseline and follow-up scans for each subject, which assured the region measured in the follow-up scan was the same region measured in the baseline scan. All ROI placement in the follow-up scans was reviewed to assure placement was consistent with placement procedures. If a given ROI in the follow-up scan did not fit with placement procedures (such as extending into ventricular CSF) it was adjusted to be concordant with placement methods. This adjustment was rarely required, and only for the corpus callosum ROIs.
ROI placement procedures have been previously described.8, 13, 18 The superior (SFG) and middle frontal gyri (MFG) ROIs were placed halfway between the precentral sulcus and anterior boundary of the brain, on the most inferior slice where both gyri were visible as separate structures. The anterior cingulate cortex (ACC) ROIs were placed in the white matter lateral to the cingulate gyrus, on the most inferior slice where the anterior horns of the lateral ventricle were still visible. Internal capsule ROIs were placed in the anterior limb on the same slice where the ACC was measured. For the corpus callosum, two ROIs were placed to either side of midline, on the slice ventral to the slice where it was divided by the longitudinal fissure. On review of ROI placement on the follow-up scans, only the ROIs measuring the corpus callosum had to be adjusted due to expansion of the lateral ventricles during the interval between scans.
Reliability was established by repeated measurements on ten baseline DTI scans. Intraclass correlation coefficients (ICCs) of FA measures were: left ACC, 0.949; right ACC, 0.934; left SFG, 0.955; right SFG, 0.951; left MFG, 0.975; right MFG, 0.946; left internal capsule, 0.956; right internal capsule, 0.993; left corpus callosum, 0.975; right corpus callosum, 0.978. ICCs for ADC measures (using the same ROIs as the FA measures) were: left ACC, 0.984; right ACC, 0.994; left SFG, 0.952; right SFG, 0.985; left MFG, 0.989; right MFG, 0.723; left internal capsule, 0.858; right internal capsule, 0.975; left corpus callosum, 0.953; right corpus callosum, 0.762.
Analytic Plan
We have previously associated differences in DTI measures of frontal white matter – specifically, the white matter of the anterior cingulate cortex, superior and frontal gyri – with both depression and response to acute antidepressant administration.8, 18 For the current study, we determined the mean value of those regions (superior frontal gyri, middle frontal gyri, and anterior cingulate cortex) between the two hemispheres, creating an average cross-hemispheric value for each DTI-based measure in each subject. To determine if any findings in the frontal white matter were specific to course of depression, we also examined two central white matter regions which we have not previously associated with depression or the antidepressant response in previous studies: the anterior corpus callosum and the internal capsule. Again, measures were made in each hemisphere, with the mean of the hemispheric measures used in analyses. This was done to reduce the number of statistical comparisons.
All statistical analyses were conducted using SAS (version 9.2, Cary, NC, USA). To test for differences in change in DTI measures over time, we created models comparing three groups: never depressed control subjects, depressed subjects who were remitted (MADRS ≤ 8) at one-year follow-up, and depressed subjects not remitted a one-year follow-up. These models examined change in the DTI measure (FA or ADC, defined as follow-up value – baseline value) as the dependent variable, with group assignment, age, sex, medical comorbidity (by CIRS), time between scans, and baseline DTI measure as independent variables. Within these models, we tested for differences between the three diagnostic cohorts using least square means comparison testing.
Next, we tested for the relationship between change in DTI measures over one year and change in MADRS over one year. These models used MADRS change as the dependent variable, with change in regional DTI measure as an independent variable, along with age, sex, MMSE, CIRS, and baseline DTI measure. In initial models we included all subjects, with a variable designating depressed or nondepressed cohort. To assure that any findings were not being driven by the nondepressed sample, we ran subsequent models including only those individuals depressed at study entry.
RESULTS
As previously reported,18 74 depressed subjects enrolled in the original 12-week trial of sertraline had evaluable DTI data. Thirty-six of those subjects either refused to participate in this follow-up study or were lost to follow-up. Of the 38 individuals who did enroll, DTI scan data was not processable for 9 individuals, primarily due to the MRI being ended early or difficulties with image registration. This resulted in a sample of 29 individuals who were depressed at entry into the parent study who were included in this follow-up study. We found no significant baseline differences between subjects who were and were not included in this follow-up study in age, sex, education, MMSE, or medical comorbidity (by CIRS). We additionally found no significant difference in depression severity (by MADRS) at entry into the parent study or at the final MADRS score upon exiting the 12-week trial (data not shown). At the 1-year assessment of those 29 previously depressed subjects, 16 were remitted (defined as MADRS ≤ 8) and 13 were not remitted, with a MADRS > 8 and continuing to meet DSM-IV criteria for Major Depressive Disorder.
The sample additionally included 20 elderly subjects with no psychiatric history. This population was recruited from an original cohort of 27 never-depressed elders enrolled in the original study. None of this never-depressed population became depressed over the 1-year period, and no subjects were excluded because of new onset of depression. Demographic differences between groups are displayed in Table 1. There was a significant difference between cohorts in age, and a strong trend for a difference in MMSE score, likely driven by the depressed-remitted cohort, which was lower than the other cohorts. However, the largest mean difference between groups in MMSE score was 1.3, which has limited clinical significance.
Table 1.
Cohort Demographic Differences
| Control N = 20 |
Depressed, Remitted N = 16 |
Depressed, Nonremitted N = 13 |
Test value, df |
P value | |
|---|---|---|---|---|---|
| Age (y) | 74.3 (4.4) | 66.4 (5.9) | 68.4 (7.2) | F 2,48 = 9.24 | 0.0004 |
| Sex, % Female (N) |
40.0 (8) | 62.5 (10) | 53.9 (7) | χ2 = 1.86, 1 df |
0.3951 |
| Age of initial depression onset (y) |
- | 50.1 (19.6) | 56.4 (14.9) | t = 0.95, 27 df |
0.3505 |
| Antidepressant use at follow-up, % (N) |
0 | 75.0 (12) | 84.6 (11) | χ2 = 0.40, 1 df |
0.5250 |
| MADRS, entry | 1.9 (2.1) | 25.1 (3.9) | 25.2 (4.4) | F 2,48 = 270.13 |
< 0.0001 |
| MADRS, 1 year | 0.9 (1.2) | 4.0 (2.2) | 14.6 (4.5) | F 2,48 = 101.48 |
< 0.0001 |
| MMSE | 28.9 (1.2) | 27.6 (2.2) | 28.4 (1.0) | F 2,48 = 3.20 | 0.0501 |
| CIRS | 5.7 (2.3) | 5.7 (2.5) | 6.9 (3.7) | F 2,48 = 0.93 | 0.4018 |
| Days between MRIscans |
455.9 (40.7) |
450.6 (116.2) | 450.8 (106.5) | F 2,48 = 0.02 | 0.9809 |
The comparison for antidepressant use only compared depressed cohorts, not the control cohort.
Group differences in baseline DTI measures are displayed in Table 2. After controlling for age, sex, and medical comorbidity severity, only middle frontal gyri FA was significantly associated with one-year group assignment. A comparison of least square means (with 43 df, derived from the model error) showed that both depressed groups had significantly lower FA values than did the nondepressed group (nondepressed vs. remitted, p = 0.0332; nondepressed vs. nonremitted, p = 0.0143), but there was not a significant difference between depressed groups (p = 0.7078).
Table 2.
Baseline DTI measures by group assignment at one year
| ADC | Control N = 20 |
Depressed, Remitted N = 16 |
Depressed, Nonremitted N = 13 |
Test value | P value |
|---|---|---|---|---|---|
| ACC | 81.8 (3.7) | 80.6 (5.0) | 81.6 (4.7) | F 2,48 = 0.33 | 0.7229 |
| SFG | 75.6 (3.9) | 75.0 (5.7) | 76.1 (4.3) | F 2,48 = 0.15 | 0.8647 |
| MFG | 78.5 (4.9) | 76.4 (6.5) | 79.8 (6.0) | F 2,48 = 1.48 | 0.2387 |
| CC | 102.0 (16.8) | 103.0 (11.8) | 104.5 (12.6) | F 2,48 = 1.31 | 0.2799 |
| IC | 75.7 (8.9) | 71.5 (5.5) | 76.0 (8.3) | F 2,48 = 0.75 | 0.4768 |
|
| |||||
| FA | |||||
| ACC | 0.346 (0.049) | 0.371 (0.043) | 0.358 (0.060) | F 2,48 = 0.28 | 0.7582 |
| SFG | 0.411 (0.045) | 0.391 (0.048) | 0.400 (0.051) | F 2,48 = 1.96 | 0.1526 |
| MFG | 0.300 (0.040) | 0.278 (0.048) | 0.269 (0.039) | F 2,48 = 3.73 | 0.0322 |
| CC | 0.468 (0.079) | 0.448 (0.044) | 0.436 (0.053) | F 2,48 = 1.09 | 0.3437 |
| IC | 0.334 (0.041) | 0.335 (0.044) | 0.356 (0.057) | F 2,48 = 1.18 | 0.3163 |
ADC = apparent diffusion coefficient; FA = fractional anisotropy; ACC = anterior cingulate cortex; SFG = superior frontal gyri; MFG = middle frontal gyri; CC = corpus callosum; IC = internal capsule. Models controlled for age, sex, and CIRS.
At follow-up, most of the depressed population were on antidepressants, except for 4 remitted and 2 nonremitted depressed subjects. Of the 23 subjects on antidepressants, 14 were on sertraline (6 nonremitted, 8 remitted). Nine (5 nonremitted, 4 remitted) were on other antidepressants, including venlafaxine (N = 4), bupropion (N = 1), citalopram (N = 1), escitalopram (N = 2), and duloxetine (N = 1). There was no significant difference in frequency of sertraline use at follow-up between remitted (8 of 12 (66.7%) on sertraline) and nonremitted subjects (6 of 11 (54.6%) on sertraline; 1df, χ2 = 0.35, p = 0.552).
Change in DTI Measures: Group Differences
Table 3 shows mean group difference between regional ADC and FA measures, defined as Time 2 – Time 1. In models controlling for age, sex, CIRS, baseline DTI measure, and time between scans, only change in the ACC FA was significantly different between cohort groups. The depressed, nonremitted cohort exhibited less reduction in this region’s FA than did the nondepressed or depressed, remitted cohorts. Differences in the ACC ADC values approached but did not reach a level of statistical significance.
Table 3.
One-Year Change in DTI measures by group
| ADC | Control N = 20 |
Depressed, Remitted N = 16 |
Depressed, Nonremitted N = 13 |
Test value | P value |
|---|---|---|---|---|---|
| ACC | 0.2 (2.4) | 1.5 (3.1) | 0.7 (4.3) | F 2,48 = 3.21 | 0.0509 |
| SFG | 1.1 (0.9) | 0.4 (3.0) | −0.3 (0.9) | F 2,48 = 1.66 | 0.2020 |
| MFG | 1.3 (1.5) | 1.3 (4.2) | −0.1 (1.8) | F 2,48 = 0.53 | 0.5933 |
| CC | 3.6 (4.5) | −0.8 (7.0) | 0.9 (6.5) | F 2,48 = 0.93 | 0.4033 |
| IC | 0.4 (1.6) | 1.3 (4.2) | 0.02 (2.1) | F 2,48 = 0.74 | 0.4847 |
|
| |||||
| FA | |||||
| ACC | −0.031 (0.023) |
−0.031 (0.028) |
−0.008 (0.031) | F 2,48 = 4.33 | 0.0198 |
| SFG | 0.001 (0.016) | 0.002 (0.022) | −0.001 (0.020) | F 2,48 = 0.70 | 0.5039 |
| MFG | −0.001 (0.014) |
0.001 (0.023) | 0.016 (0.031) | F 2,48 = 1.53 | 0.2292 |
| CC | 0.004 (0.034) | 0.003 (0.022) | −0.001 (0.020) | F 2,48 = 0.08 | 0.9215 |
| IC | −0.003 (0.022) |
−0.010 (0.019) |
−0.005 (0.025) | F 2,48 = 0.07 | 0.9370 |
ADC = apparent diffusion coefficient; FA = fractional anisotropy; ACC = anterior cingulate cortex; SFG = superior frontal gyri; MFG = middle frontal gyri; CC = corpus callosum; IC = internal capsule. Values represent change, defined as time 2 – time 1, so negative values represent a decrease in that measure, and positive values represent an increase in that measure. Models controlled for age, sex, CIRS, baseline DTI measure, and time between scans.
To determine if differences in antidepressant use might be affecting this result, we excluded the 6 depressed subjects who were not taking antidepressants at follow-up and re-examined the models. The group differences in change in ACC FA remained significant after excluding these subjects (F 2,42 = 5.74, p = 0.0071), while the differences in ACC ADC no longer approached statistical significance (F 2,42 = 2.15, p = 0.1322).
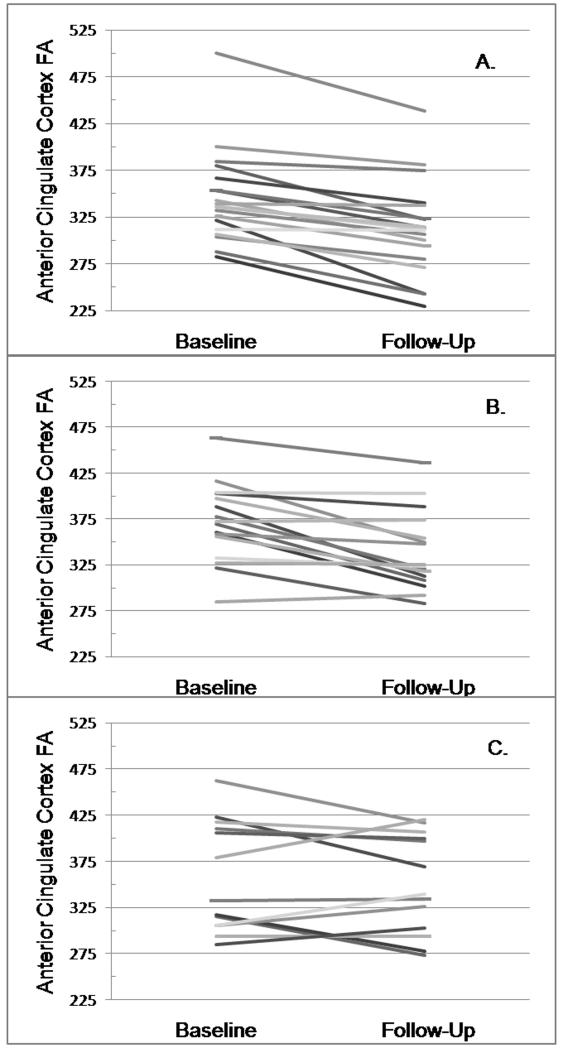
Figure 1 displays change in ACC FA by each subject, for each diagnostic cohort. Although the majority of subjects for the nondepressed and depressed, remitted cohorts (Figure 1 A, B) exhibited a decline in this measure, in the depressed, nonremitted cohort (Figure 1, C), 6 subjects (46%) exhibited an increase in ACC FA while 7 exhibited a decrease. When compared against those subjects who experienced a decrease in ACC FA, the group with increased ACC FA exhibited a significantly greater change in ACC FA (0.020, SD = 0.017; − 0.031, SD = 0.018; t = 5.22, 11 df, p = 0.0003) but a lower baseline ACC FA (0.317, SD = 0.034; 0.393, SD = 0.056; t = −2.91, 11 df, p = 0.0142). At alpha = 0.05, there were no significant differences between these groups in age, age of depression onset, MMSE or CIRS score, MADRS score at baseline or follow-up, sex representation, or days between scans (data not shown). All of the depressed, nonremitting subjects who exhibited a decline in ACC FA were taking antidepressants at followup, while two of the six who exhibited an increase in ACC FA were not taking antidepressants at followup (Fisher’s exact test, p = 0.1923).
Figure 1.
Change in Anterior Cingulate Cortex Fractional Anisotropy
Figures show slope of change in ACC FA for each subject by diagnostic cohort. A) Nondepressed subjects; B) Depressed, remitted subjects; C) Depressed, nonremitted subjects.
Change in DTI Measures: Relationship with Longitudinal Change in MADRS Score
Finally we sought to determine if change in DTI measures over 1 year was associated with change in MADRS over 1 year. In these models, change in MADRS was the dependent variable, while change in DTI measure was the independent variable. Both change variables were calculated as Time 2 – Time 1. We also controlled for age, sex, time between scans, baseline MMSE, and baseline CIRS. We created two sets of models (Table 4): one included all subjects, and had an additional diagnostic variable designating subjects as being depressed or nondepressed at study entry; the second examined only subjects who were depressed at study entry.
Table 4.
Models examining change in MADRS over one year
| All subjects | Depressed only | |||
|---|---|---|---|---|
| Variable | F value | P value | F value | P value |
| Change in ACC FA | 7.66 | 0.0087 | 4.65 | 0.0434 |
| Baseline ACC FA | 0.53 | 0.4716 | 0.04 | 0.8500 |
| BL MADRS | 27.69 | < 0.0001 | 12.49 | 0.0021 |
| MMSE | 0.77 | 0.3869 | 0.45 | 0.5077 |
| CIRS | 1.84 | 0.1835 | 0.92 | 0.3480 |
| Age | 1.48 | 0.2314 | 1.41 | 0.2485 |
| Sex | 0.13 | 0.7216 | 0.00 | 0.9692 |
| Time between assessments |
1.79 | 0.1890 | 1.66 | 0.2124 |
| Depression diagnosis | 5.60 | 0.0232 | ||
FA = fractional anisotropy; ACC = anterior cingulate cortex; MMSE = Mini-Mental State Exam; CIRS = Cumulative illness rating scale. For the “All subjects” model, each variable had 1,48 DF. For the “Depressed only” model, each variable had 1,28 DF.
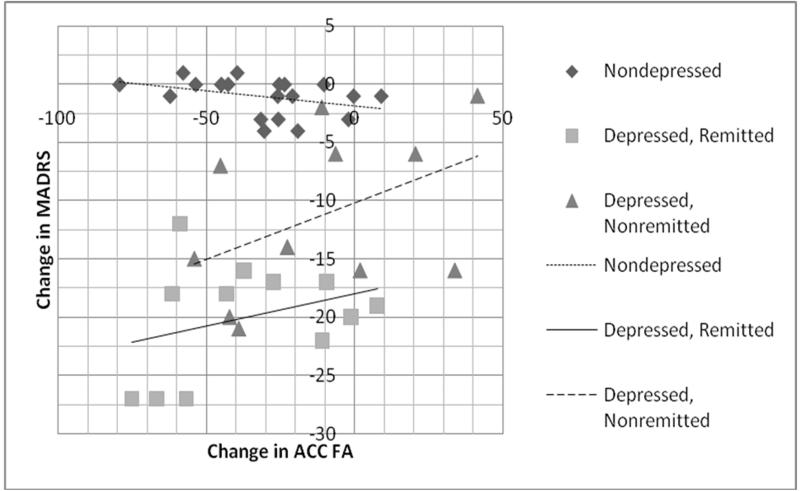
Only change in anterior cingulate cortex FA was significantly associated with change in MADRS (Table 4), exhibiting a positive correlation where a greater decline in FA was associated with greater decline in MADRS (Figure 2). Removal of depressed subjects not taking antidepressants at follow-up increased the strength of the relationship between change in ACC FA and change in MADRS (all subjects: F 1,42 = 15.76, p = 0.0004; depressed only: F 1,22 = 12.93, p = 0.0029). We did not find a similar relationship between change in MADRS and change in other DTI measures (data not shown), regardless of including or excluding previously depressed subjects not taking antidepressants at follow-up.
Figure 2.
Change in MADRS by change in Frontal Anisotropy
Figures display change in MADRS score by change in anterior cingulate cortex FA. Both change values displayed as 1 year – baseline measures.
DISCUSSION
Our primary finding was that the cohort of subjects who were depressed at study entry and did not remit over the following one-year period exhibited less change in ACC white matter FA than did either nondepressed subjects, or depressed subjects who remitted. There was no significant difference in ACC FA change between the nondepressed and remitted subjects. Moreover, we found a statistically significant positive correlation between change in ACC FA and change in MADRS score over one year, wherein a greater decrease in ACC FA was associated with greater decrease in MADRS. These findings were not observed in other frontal or central white matter regions.
To our knowledge, this is the first study to examine longitudinal change in DTI measures and how they may be related to course of depression. Although there are no comparable longitudinal studies, our current findings are not concordant with our previous work examining hyperintense lesions and depression outcomes. We previously associated greater two-year increases in hyperintensity lesion volume with poorer course of depression over that period,2 a finding of particular salience given how we also associated greater hyperintensity volume with widespread decreases in frontal FA measures.13 Pursuing this line of reasoning, we hypothesized that failure to remit at one-year would be associated with a reduction in frontal FA measures, not increased FA measures as we observed. Since this is not what we observed, another mechanism may be at work.
Two potential influences may be contributing to our findings: effects of antidepressant medications or effects of a chronic stress response. Although difficult to quantify in this sample due to heterogeneity in treatment over the study interval, the nonremitted group may have been treated more aggressively over the study interval. There has been recent interest on how antidepressant medications may upregulate sterol regulatory element-binding protein (SREBP) transcription factors in glial cells.27 These SREBPs may play a central role in myelination and may influence neuronal function.28 Thus, we might hypothesize that more aggressive antidepressant treatment would upregulate SREBPs and potentially reverse stress-related myelination deficits while slowing aging effects. Such an antidepressant effect is supported by previous work associating antidepressant exposure with increased orbitofrontal cortex volume.29 Unfortunately we could not demonstrate that the nonremitted cohort did in fact receive more aggressive therapy, and in fact, of those nonremitted subjects who exhibited an increase in ACC FA, only 66% (4 of 6) were taking an antidepressant at followup.
As chronic depression affects the stress response, we may be observing chronic effects of that response. Chronic stress has been demonstrated to contribute to frontostriatal reorganization 30 and reductions in length and branch numbers of apical dendrites in the anterior cingulate cortex.31 In turn, dendrite formation and increased synaptic density is associated with a decrease in FA, so these dendritic changes seen with chronic stress could increase FA,32 or potentially create a confounding effect resulting in less reduction in FA from normal aging processes. Of course, our approach examined ACC white matter, wherein FA may be more related to myelination, and oligodendrocytes appear to decrease in response to chronic stress,33 so this potential explanation may not be completely satisfying.
Given how our findings were contrary to our hypothesis, clearly our findings require further study. Importantly, this study had several notable limitations, such as a relatively small sample which may be particularly problematic given relatively high variability for our measures and the large number of covariates required for our analyses. As our measures have demonstrated reliability, this variability demonstrates the heterogeneity seen in DTI measures over time. Moreover, unmeasured factors may have contributed to our findings, as genetic differences have also been associated with frontal white matter microstructural changes.34 Additionally, other image analysis techniques such as voxel-based morphometry could have been used. Although this method has the advantage of examining the entire brain, it may miss critical regions of interest and does not necessarily replace a region-of-interest approach.35
Importantly, our results may be related to bias in the sample. We initially excluded individuals with obvious cognitive impairment or neurological disease; those individuals may have a different trajectory both in depression course and DTI changes. Additionally, despite finding no differences in baseline characteristics between those who did and did not participate in the follow-up scan, interval changes in mental or physical health may differ between participants and nonparticipants. If all eligible subjects had participated, our results may have differed.
In conclusion, we found failure to achieve remission of depression over a one-year period to be associated with less reduction of FA of the anterior cingulate white matter than was observed in subjects who did remit or were never depressed. Importantly, there was significant heterogeneity of change in ACC FA in this nonremitting group, suggesting different pathways contributing to treatment resistance or different effects of chronic depression on neuronal and glial structure. This finding requires further study with a more standardized antidepressant treatment strategy and assessment of stress response.
Acknowledgements
We would like to acknowledge Cynthia R. Key for preparing and pre-processing of DTI data and Kulpreet Singh for assistance with the figure.
Financial Support: This study was supported by NIMH grants R01 MH078216 and R01 MH074916
Footnotes
Disclosure / Conflicts of Interest: Drs. Taylor and MacFall and Mr. Boyd have no conflicts to report. Dr. Payne has received speaking honoraria from Eli Lilly (Taiwan). Dr. Sheline reports serving on the Eli-Lilly Speakers Bureau. Dr. Krishnan has received consultancy fees from CeNeRx, Corcept, GlaxoSmithKline, Johnson & Johnson, Merck, Roche, and Sonexa. He has ownership interest in Orexigen, CeNeRx and Sonexa. Dr. Doraiswamy has received research grants and honoraria for consulting or speaking from several pharmaceutical companies and antidepressant manufacturers, and has stock in Sonexa Therapeutics.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mitchell AJ, Subramaniam H. Prognosis of depression in old age compared to middle age: a systematic review of comparative studies. Am J Psychiatry. 2005;162:1588–1601. doi: 10.1176/appi.ajp.162.9.1588. [DOI] [PubMed] [Google Scholar]
- 2.Taylor WD, Steffens DC, MacFall JR, et al. White matter hyperintensity progression and late-life depression outcomes. Arch Gen Psychiatry. 2003;60:1090–1096. doi: 10.1001/archpsyc.60.11.1090. [DOI] [PubMed] [Google Scholar]
- 3.Gunning-Dixon FM, Brickman AM, Cheng JC, et al. Aging of cerebral white matter: a review of MRI findings. Int J Geriatr Psychiatry. 2009;24:109–117. doi: 10.1002/gps.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sullivan EV, Adalsteinsson E, Hedehus M, et al. Equivalent disruption of regional white matter microstructure in ageing healthy men and women. Neuroreport. 2001;12:99–104. doi: 10.1097/00001756-200101220-00027. [DOI] [PubMed] [Google Scholar]
- 5.Pfefferbaum A, Adalsteinsson E, Sullivan EV. Frontal circuitry degredation marks healthy adult aging: Evidence from diffusion tensor imaging. Neuroimage. 2005;26:891–899. doi: 10.1016/j.neuroimage.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 6.Davis SW, Dennis NA, Buchler NG, et al. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage. 2009;46:530–541. doi: 10.1016/j.neuroimage.2009.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salat DH, Tuch DS, Hevelone ND, et al. Age-related changes in prefrontal white matter measured by diffusion tensor imaging. Ann N Y Acad Sci. 2005;1064:37–49. doi: 10.1196/annals.1340.009. [DOI] [PubMed] [Google Scholar]
- 8.Bae JN, MacFall JR, Krishnan KR, et al. Dorsolateral prefrontal cortex and anterior cingulate cortex white matter alterations in late-life depression. Biol Psychiatry. 2006;60:1356–1363. doi: 10.1016/j.biopsych.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 9.Nobuhara K, Okugawa G, Sugimoto T, et al. Frontal white matter anisotropy and symptom severity of late-life depression: a magnetic resonance diffusion tensor imaging study. J Neurol Neurosurg Psychiatry. 2006;77:120–122. doi: 10.1136/jnnp.2004.055129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor WD, MacFall JR, Payne ME, et al. Late-life depression and microstructural abnormalities in dorsolateral prefrontal cortex white matter. Am J Psychiatry. 2004;161:1293–1296. doi: 10.1176/appi.ajp.161.7.1293. [DOI] [PubMed] [Google Scholar]
- 11.Yang Q, Huang X, Hong N, et al. White matter microstructural abnormalities in late-life depression. Int Psychogeriatr. 2007;19:757–766. doi: 10.1017/S1041610207004875. [DOI] [PubMed] [Google Scholar]
- 12.Taylor WD, MacFall JR, Gerig G, et al. Structural integrity of the uncinate fasciculus in geriatric depression: Relationship with age of onset. Neuropsychiatr Dis Treat. 2007;3:669–674. [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor WD, Bae JN, MacFall JR, et al. Widespread effects of hyperintense lesions on cerebral white matter structure. Am J Roentgenol. 2007;188:1695–1704. doi: 10.2214/AJR.06.1163. [DOI] [PubMed] [Google Scholar]
- 14.Murphy CF, Gunning-Dixon FM, Hoptman MJ, et al. White-matter integrity predicts Stroop performance in patients with geriatric depression. Biol Psychiatry. 2007;61:1007–1010. doi: 10.1016/j.biopsych.2006.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vernooij MW, Ikram MA, Vrooman HA, et al. White matter microstructural integrity and cognitive function in a general elderly population. Arch Gen Psychiatry. 2009;66:545–553. doi: 10.1001/archgenpsychiatry.2009.5. [DOI] [PubMed] [Google Scholar]
- 16.Alexopoulos GS, Kiosses DN, Choi SJ, et al. Frontal white matter microstructure and treatment response in late-life depression: a preliminary study. Am J Psychiatry. 2002;159:1929–1932. doi: 10.1176/appi.ajp.159.11.1929. [DOI] [PubMed] [Google Scholar]
- 17.Alexopoulos GS, Murphy CF, Gunning-Dixon FM, et al. Microstructural white matter abnormalities and remission of geriatric depression. Am J Psychiatry. 2008;165:238–244. doi: 10.1176/appi.ajp.2007.07050744. [DOI] [PubMed] [Google Scholar]
- 18.Taylor WD, Kuchibhatla M, Payne ME, et al. Frontal white matter anisotropy and antidepressant response in late-life depression. PLoS ONE. 2008;3:e3267. doi: 10.1371/journal.pone.0003267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheline YI, Pieper CF, Barch DM, et al. Support for the vascular depression hypothesis in late life depression: results from a two site, prospective, antidepressant treatment trial. Arch Gen Psychiatry. 2009;67:277–285. doi: 10.1001/archgenpsychiatry.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spitzer RL, Williams JB, Gibbon M, et al. The structured clinical interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 21.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41:237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 24.Basser PJ, Pierpaoli C. A simplified method to measure the diffusion tensor from seven MR images. Magn Reson Med. 1998;39:928–934. doi: 10.1002/mrm.1910390610. [DOI] [PubMed] [Google Scholar]
- 25.Maes F, Collignon A, Vandermeulen D, et al. Multimodality image registration by maximization of mutual information. IEEE Trans Med Imaging. 1997;16:187–198. doi: 10.1109/42.563664. [DOI] [PubMed] [Google Scholar]
- 26.Van Leemput K, Maes F, Vandermeulen D, et al. A unifying framework for partial volume segmentation of brain MR images. IEEE Trans Med Imaging. 2003;22:105–119. doi: 10.1109/TMI.2002.806587. [DOI] [PubMed] [Google Scholar]
- 27.Raeder MB, Ferno J, Glambek M, et al. Antidepressant drugs activate SREBP and up-regulate cholesterol and fatty acid biosynthesis in human glial cells. Neurosci Lett. 2006;395:185–190. doi: 10.1016/j.neulet.2005.10.096. [DOI] [PubMed] [Google Scholar]
- 28.Camargo N, Smit AB, Verheijen MH. SREBPs: SREBP function in glia-neuron interactions. FEBS J. 2009;276:628–636. doi: 10.1111/j.1742-4658.2008.06808.x. [DOI] [PubMed] [Google Scholar]
- 29.Lavretsky H, Roybal DJ, Ballmaier M, et al. Antidepressant exposure may protect against decrement in frontal gray matter volumes in geriatric depression. J Clin Psychiatry. 2005;66:964–967. doi: 10.4088/jcp.v66n0801. [DOI] [PubMed] [Google Scholar]
- 30.Dias-Ferreira E, Sousa JC, Melo I, et al. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325:621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- 31.Radley JJ, Sisti HM, Hao J, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Baloch S, Verma R, Huang H, et al. Quantification of brain maturation and growth patterns in C57BL/6J mice via computational neuroanatomy of diffusion tensor images. Cereb Cortex. 2009;19:675–687. doi: 10.1093/cercor/bhn112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banasr M, Valentine GW, Li XY, et al. Chronic unpredictable stress decreases cell proliferation in the cerebral cortex of the adult rat. Biol Psychiatry. 2007;62:496–504. doi: 10.1016/j.biopsych.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Alexopoulos GS, Murphy CF, Gunning-Dixon FM, et al. Serotonin transporter polymorphisms, microstructural white matter abnormalities and remission of geriatric depression. J Affect Disord. 2009 doi: 10.1016/j.jad.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giuliani NR, Calhoun VD, Pearlson GD, et al. Voxel-based morphometry versus region of interest: a comparison of two methods for analyzing gray matter differences in schizophrenia. Schizophr Res. 2005;74:135–147. doi: 10.1016/j.schres.2004.08.019. [DOI] [PubMed] [Google Scholar]