Abstract
Cyclic-AMP response element binding (CREB) protein regulates the expression of many genes involved in the pathophysiology of depression. Increased CREB levels were found in the brain of antidepressants-treated rats and decreased protein and mRNA expression of CREB was reported in the postmortem brain of depressed suicide victims. We determined CREB protein expression, using Western blot technique, and CRE-DNA binding, using gel shift assay, in neutrophils obtained from 22 drug-free patients with major depressive disorder (MDD) and 23 normal control subjects. Diagnosis of patients was based on DSM-IV criteria; severity of illness was rated by Hamilton Depression Rating Scale (HDRS). We found that the CRE-DNA binding activity and CREB protein expression was significantly decreased in the neutrophils of drug-free MDD patients compared with normal control subjects. Our findings suggest that CREB may play an important role in the pathophysiology of depression and that it may be an important target for the therapeutic action of antidepressant drugs. Neutrophil CREB levels may also serve as a useful biomarker for patients with MDD.
Keywords: CREB, CRE-DNA binding, Depression, Mood disorders, Neutrophils
1. Introduction
Abnormalities in the signal transduction system have been implicated in the pathophysiology of major depressive disorders (MDD). For example, serotonin and thrombin-mediated abnormalities in the phosphoinositide (PI) signaling in the platelets of depressed patients have been reported by us (Pandey et al., 2001) and other investigators (Mikuni et al., 1991). It has shown that both serotonin and thrombin-mediated IP formation is decreased in the platelets of depressed patients (Mikuni et al., 1991; Pandey et al., 2001). Beta- and α-adrenergic receptor-mediated adenylyl cyclase (AC) system has also been reported by us and other investigators to be abnormal in depressed patients (Wang et al., 1974; Pandey et al., 1979; Siever et al., 1984; Mann et al., 1985; Kanof et al., 1986). For example, we have shown that isoproterenol- and norepinephrine-stimulated cyclic AMP (cAMP) formation was decreased in the leukocytes of depressed patients (Pandey et al., 1979). These observations led to the possibility that one or more of the components of this signaling cascade may be abnormal in depression, and there may be altered functional consequences of this signaling system in depression. cAMP response element binding (CREB) protein is a transcription factor that is activated by enzymes such as protein kinase A (PKA) and protein kinase C (PKC), which are components of the PI and AC signaling system (Hardingham et al., 2001; Lonze and Ginty, 2002; Carlezon et al., 2005).
CREB is a member of the basic leucine zipper family of transcription factors (Borrelli et al., 1992). Phosphorylation of CREB at serine-133 leads to its dimerization and activation by binding to cAMP response element (CRE) at the consensus motif 5’-TGACGTCA, which is found in many neuronally expressed genes (Montminy et al., 1990; Lee and Masson, 1993). In its active form, the phosphorylated form of CREB regulates the transcription of many genes that are involved in several aspects of neuronal function (Imaki et al., 1994; Moore et al., 1996; Walton and Dragunow, 2000). CREB plays a crucial role in regulating gene expression and in the development of the nervous system, learning, memory, and cell survival (Shaywitz and Greenberg, 1999; Viola et al., 2000; Hardingham et al., 2001; Lonze and Ginty, 2002; Carlezon et al., 2005).
CREB’s potential involvement in the pathophysiology of depression is based on the observation that treatment with antidepressant drugs and electroconvulsive shock (ECS) causes an increase in the levels of CREB in the rat brain (Tiraboschi et al., 2004; Laifenfeld et al., 2005; Sairanen et al., 2007). The protein and mRNA expression of CREB has also been reported to be altered in the postmortem brain of depressed patients (Dowlatshahi et al., 1999; Odagaki et al., 2001; Yamada et al., 2003; Laifenfeld et al., 2005) and in human fibroblasts of patients with major depression (Manier et al., 2002). There are several studies that show alterations of CREB mRNA in the peripheral cells, such as lymphocytes of depressed patients (Koch et al., 2003; Iga et al., 2007). It has also been shown that CREB mRNA is altered in the lymphocytes and leukocytes of depressed patients (Mantamadiotis et al., 2002). Antidepressant treatment has also been reported to cause changes in the phosphorylation of CREB in the brain of rats (Tiraboschi et al., 2004; Laifenfeld et al., 2005; Sairanen et al., 2007).
Recently, we reported that there is a reduction in mRNA and protein levels of CREB, CRE DNA binding activity in the prefrontal cortex (PFC), Brodmann area-9 (BA-9) and hippocampus of suicide victims compared with normal control subjects, irrespective of diagnosis (Dwivedi et al., 2003). We have also observed that the CRE-DNA binding, the protein expression of CREB, and mRNA expression of CREB were significantly decreased in the PFC of teenage suicide victims compared with normal control subjects (Pandey et al., 2007). CREB is also present in peripheral tissue, such as neutrophils. In order to examine the role of CREB in the pathophysiology of depression, in the present investigation we determined the protein expression of CREB and CRE-DNA binding activity in the neutrophils of depressed patients.
2. Methods and Materials
2.1 Subjects and clinical assessments
Subjects for this study were 22 patients with MDD who were admitted to the psychiatric research unit at the University of Illinois at Chicago and 23 normal control subjects. The main instrument used for determining the diagnosis of the patients was the Structured Clinical Interview for DSM-IV (SCID). SCID was administered to all patients in order to derive the DSM-IV diagnosis. In order to assess the severity of illness, the 24-item Hamilton Depression Rating Scale (HDRS) was administered to patients by two trained raters. Depressed patients who had a score of at least 21 on HDRS were included in the study. Depressed patients on prior antidepressants with long half-life, such as fluoxetine, were excluded. The exclusion criteria for the patients were any significant medical disease, such as renal, cardiovascular or neurological disorders, recent drug or alcohol abuse. Written informed consent was obtained after the procedures were fully explained. The normal control subjects recruited for this study were free of any history of psychiatric or major medical disorders. All patients were drug-free from any psychotropic medication for up to 2 weeks prior to their assessments. This study was approved by the Institutional Review Board (IRB) of the University of Illinois at Chicago.
2.2 Isolation of neutrophils
Thirty to 40 ml of venous blood was collected in a tube containing 3.8% (w/v) sodium citrate (1 vol: 9 vol blood). The blood was centrifuged immediately at 210 g for 15 min. The platelet-rich plasma (PRP) was removed for platelet isolation. To the red blood cell (RBC) layer, 15 ml of saline was added, mixed gently, and then transferred on Ficoll (2:1 respectively). The sample was then centrifuged at 400 g for 40 min. The upper layer above the interface layer was removed and discarded. The interface layer, which contains the lymphocytes, was taken out and processed for the isolation of lymphocytes.
An equal volume of saline was added to the remaining RBCs from the Ficoll tubes and mixed gently. Ten ml of dextran was added and allowed to stand for 45-60 min. The top layer of the supernatant of dextran and saline solution was removed and spun at 150 g for 15 min. The pellet obtained was the neutrophil pellet. In order to get rid of the contaminating red cells, the pellet was suspended in 2 ml of 150 mM NaCl and 6 ml of ice-cold double-distilled water. This mixture was immediately vortexed for 30 sec and then 2 ml of 3.5% NaCl was added to the tube to get rid of the contaminating RBCs, and then it was centrifuged at 150 g for 10 min. The resulting pellets were the neutrophils, which were washed 2 times with an isotonic sodium chloride solution. The neutrophil pellet, thus obtained, was stored at -80°C until used.
2.3 Preparation of nuclear fractions
The preparation of nuclear fraction followed the protocol from Pierce Biotechnology Inc. (Rockford, IL). Briefly, tissue was homogenized in ice-cold cytoplasmic extraction reagent 1 (CER 1) containing 0.5 mg/ml benzamidine, 2 μg/ml aprotinin, 2 μg/ml leupeptin, and 0.75 mM phenylmethylsulfonyl fluoride (PMSF). The homogenate was added to cytoplasmic extraction reagent-II (CER-II) and then centrifuged at 16,000 g for 5 min. The resulting pellet was suspended in ice-cold nuclear extraction reagent (NER) containing 0.5 mg/ml benzamide, 2 μg/ml aprotinin, 2 μg/ml leupeptin, and 2 mM PMSF and incubated for 40 min on ice with frequent agitation. The nuclear extracts were separated by centrifugation at 16,000 g for 10 min. The protein content of the nuclear fraction was determined by the method of Lowry et al. (1951). This nuclear fraction was used to determine the protein expression of CREB and CRE-DNA binding activity.
2.4 Immunolabeling of CREB
The procedure for Western blotting has been described in detail (Dwivedi et al., 2003). Protein samples (30 μg protein) were loaded onto 10% (w/v) sodium dodecyl sulphate (SDS)-polyacrylamide gel. The gels were run and transferred electrophoretically to an enhanced chemiluminescence (ECL) nitrocellulose membrane (Amersham, Arlington Heights, IL). The membranes were washed with TBST buffer (10 mM Tris-base, 0.15 M NaCl, and 0.05% [w/v] Tween 20) for 10 min. The blots were blocked by incubating with 5% (w/v) powdered non-fat milk in TBST, 0.02% nonidet P-40, and 0.02% (w/v) SDS (pH 8.0). Then the bolts were incubated overnight at 4° C with primary polyclonal anti-CREB antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA) with a dilution of 1:3000. The membranes were washed with TBST and incubated with horseradish-peroxidase-linked secondary antibody (anti-rabbit immunoglobulin G [IgG]; 1:3000) for 5 hrs at room temperature. The membranes were extensively washed with TBST and exposed to ECL autoradiography film. The same nitrocellulose membrane was stripped and re-probed with β-actin antibody (Sigma Chemical Co., St. Louis, MO). The bands on the autoradiogram were quantified using the Loats Image Analysis system (Westminster, MD), and the optical density of each sample was corrected by the optical density of the corresponding β-actin band. The values are represented as a percent of control.
2.5 Determination of CRE-DNA binding activity by gel mobility shift assay
Preparation of DNA probe
Commercially available (Stratagene, La Jolla, CA) oligonucleotides incorporating regulatory elements of the CREB sequence (5’-GATTGGCTG ACGTCAGAGAGCT) were used. The probes were end-labeled with (γ-32P) ATP using T4 polynucleotide kinase according to the manufacturer’s methods.
Gel mobility DNA binding assay
Binding reactions were carried out by incubating 10 μg of nuclear extract with 1 μg of poly (DI-DC) and BSA (6 μg) in a reaction mixture (20 mM Hepes (pH 7.9); 1mM DTT; 0.3 mM EDTA; 0.2 mM EGTA; 80 mM NaCl; 10% glycerol; and 0.2 mM PMSF) for 15 min at room temperature. Approximately 5000 CPM of 32P-labeled CREB oligonucleotide were added and incubated for another 30 min. DNA-protein complexes were resolved on a 4.0% non-denaturing polyacrylamide gel in a buffer containing 25 mM Tris-borat (pH 8.2) and 0.5 mM EDTA. The gel was dried and autoradiographed with intensifying screens on film (Kodak, Rochester, NY) at -8°C. The bands of the DNA-protein complex were estimated quantitatively on the autoradiogram using the Loats Image Analysis system.
2.6 Statistical analysis
Statistical analysis of the data was performed using SPSS software for Windows version 15 (SPSS). Results are expressed as the mean ± standard deviation. The comparison of the data between normal control and depressed subjects was performed using an independent sample t-test. Equal variance was assumed and p < 0.05 was considered significant. Pearson correlation matrix was used to determine the effect of age, gender and behavioral rating scores on CREB variables. The effect of gender was determined by comparing CREB variables between male and female subjects in the patient and control groups.
3. Results
We determined protein expression of CREB and CRE-DNA binding activity in 22 MDD patients and 23 normal control subjects. The demographic clinical characteristics of the study subjects are presented in Table I. The symptom scores of HDRS are also provided in Table I. There were no significant differences in age between normal controls and depressed patients and there was no significant correlation between CREB protein levels or CRE-DNA binding and age. There were 12 males and 11 female subjects in normal control group and 11 male and 11 female subjects in depressed group. There were no significant differences in CREB protein or CRE-DNA binding between male and female subjects either in the control or the patient group.
Table I.
Demographic Characteristics of Adult Depressed Patients and Normal Control Subjects
| Group | Age (Years) | Gender (M/F) | Race | HDRS |
|---|---|---|---|---|
| Normal Controls (n = 23) | 36.14 ± 10.52 | 12 M / 11 F | 3 Asian 5 Black 1 Hispanic 14 White |
-- |
| Depressed Patients (n = 22) | 31.95 ± 12.64 | 11 M / 11 F | 5 Asian 2 Black 2 Hispanic 13 White |
27.90 ± 9.10 |
Values are the mean ± SD.
Abbreviations: HDRS, Hamilton Depression Rating Scale; F, female; M, male;
3.1 CRE-DNA binding activity in neutrophils of depressed patients and normal control subjects
We determined the functional status of CREB by determining the CRE-DNA binding activity using a gel mobility shift assay in nuclear fraction of neutrophils from MDD patients and normal control subjects. A representative autoradiogram showing CRE-DNA binding activity in the neutrophils of MDD patients and normal control subjects is shown in Figure 1A. The mean CRE-DNA binding activity was significantly decreased (p < 0.001) in the neutrophils of depressed patients compared with normal control subjects, as shown in Figure 1B. The mean CRE-DNA biding activity was 100 ± 26 for the controls vs. 59 ± 25 for the depressed patients (t = 6.617, df = 43, p = 0.084)
Figure 1.
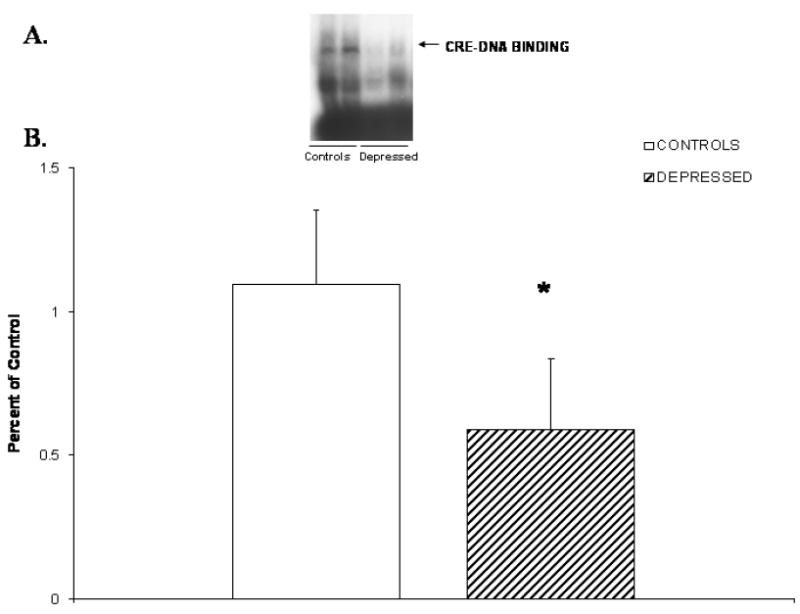
(A) Representative autoradiogram showing CRE-DNA binding activity in nuclear fraction of neutrophils from two controls and two medication-free MDD patients.
(B) CRE-DNA binding activity in nuclear fraction of neutrophils from control subjects (n = 23) and medication-free MDD patients (n = 22). Values are the mean ± S.D.
* p < 0.001
3.2 Immunolabeling of CREB in the neutrophils of depressed patients and normal control subjects
Because we observed a decrease in CRE-DNA binding activity in nuclear fraction of depressed patients compared with normal control subjects, we examined if this decrease in CRE-DNA binding activity was related to an altered protein expression of CREB. We, therefore, determined the immunolabeling of CREB in the nuclear fraction of neutrophils obtained from depressed patients and normal control subjects. Representative Western blots showing immunolabeling of CREB protein in the neutrophils from two depressed patients and two normal control subjects are presented in Figure 2A. As can be seen, the protein expression levels of CREB in these two depressed patients tended to be lower than those in the control subjects. When we compared the mean protein expression levels of CREB in the neutrophils obtained from 22 depressed patients with 23 control subjects, we found that the protein levels were significantly decreased in the neutrophils of depressed patients as shown in Figure 2B. The mean CREB levels were 100 ± 20 for the controls vs. 63 ± 25 for the depressed patients (t = 5.397, df = 43, p < 0. 001).
Figure 2.
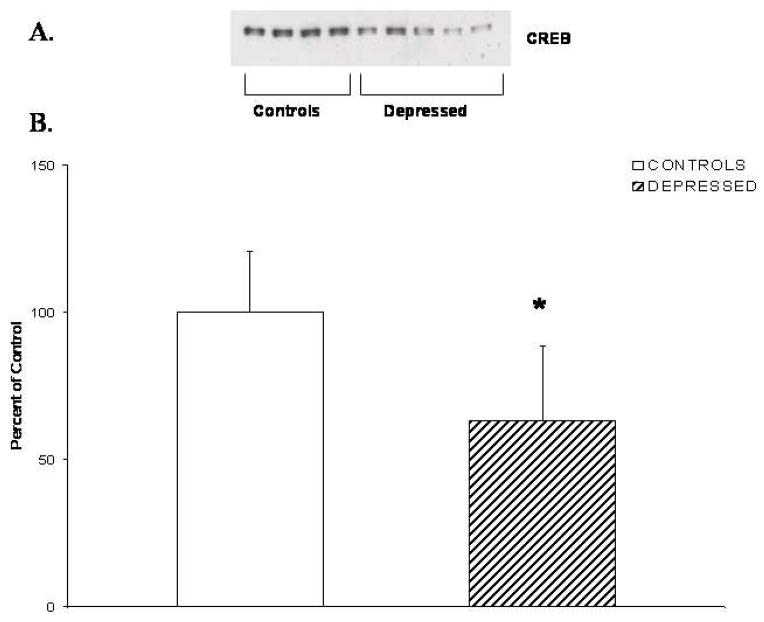
(A) Representative Western blot of total CREB in nuclear fraction of neutrophils obtained from four control subjects and four medication-free MDD patients.
(B) CREB immunolabeling in nuclear fraction of neutrophils from control subjects (n = 23) and medication-free MDD patients (n = 22). Values are the mean ± S.D.
* p < 0.001
3.3 Relationship between HDRS Scores and CREB Protein or CRE-DNA Binding Activity
In order to examine if CREB protein levels or CRE-DNA binding activity are related to the severity of illness we have correlated these with HDRS scores. In depressed patients, we found a significant correlation (r = 0.436; p = 0.043) between the CREB protein levels in neutrophils, but we did not find any significant correlation (r = − 0.132; p = 0.559) between CRE-DNA binding activity in lymphocytes and HDRS scores in depressed patients.
These results suggest that CREB protein levels, but not CRE-DNA binding activity, are indicative of the severity of depression.
4. Discussion
In order to examine the role of CREB in the pathophysiology of depression, in this study we determined the protein expression of CREB and CRE-DNA binding activity in the nuclear fraction of neutrophils obtained from depressed patients during a medication-free period and matched normal control subjects. We found a significant decrease in the protein expression of CREB and CRE-DNA binding activity in the nuclear fraction of neutrophils from depressed patients compared with normal control subjects. We also found a significant correlation between CREB protein levels and HDRS scores, suggesting that CREB protein levels may be a good marker for the severity of depressive illness. These studies, thus, for the first time demonstrated alterations of CREB protein levels and CRE-DNA binding activity in the neutrophils of depressed patients.
Since we earlier showed an abnormality of both PI (Pandey et al., 2001) and AC signaling (Pandey et al., 1979), we attempted to examine if the abnormalities in the signaling system could cause or are related to changes in their target, such as the transcription factor CREB. The activation of CREB is regulated by protein kinases such as PKA and PKC (Riabowol et al., 1988; Nichols et al., 1992; Xie and Rothstein, 1995). PKA, which is a component of the AC signaling system, is activated by cAMP that is formed after stimulation of AC linked receptors such as β-adrenergic receptors. After activation by cAMP, PKA catalytic subunits dissociate and translocate to the nucleus where they phosphorylate and activate CREB. Similarly, PKC is a member of the PI signal transduction system and is activated by diacylglycerol, which is formed as a result of stimulation by serotonin2A (5HT2A) receptors or other receptors linked to the PI system (Berridge and Irvine, 1989; Tanaka and Nishizuka, 1994). Stimulation of these receptors causes the activation of phospholipase C (PLC) and the formation of DAG. PKC activated by DAG phosphorylates and activates CREB (Riabowol et al., 1988; Xie and Rothstein, 1995). Both the signaling systems converge at the level of CREB, and thus abnormalities in PI or AC signaling or both may cause abnormalities in CREB.
On the other hand, abnormalities in one signaling system may be balanced by changes in the other signaling system in such a way that the activation of CREB remains unchanged. The decreased expression of CREB protein and CRE-DNA binding in the neutrophils of depressed patients suggest that it may result due to abnormalities in the PI or the AC signaling system. The precise mechanism behind the decreased CRE-DNA binding activity and expression of CREB in the neutrophils of depressed patients is unclear; however, the most likely possibility for decreased CRE-DNA binding activity appears to be related to a low availability of CREB protein. Another factor that may be responsible for decreased CRE-DNA binding activity could be decreased levels of CREB binding proteins (CBP), as CBP has been shown to regulate CRE-DNA binding activity by interacting with phosphorylated CREB (Chrivia et al., 1993). Then again, there are several mechanisms through which CREB expression is regulated. As mentioned above, PKA not only phosphorylates and activates CREB, but it also phosphorylates CREB/ATF-like proteins, some of which are involved in regulation of the transcription of CREB. It is quite possible that observed abnormalities in PKA may be involved in the decreased phosphorylation of CREB/ATF-like proteins — thereby causing the decreased expression of CREB. Further studies are required to delineate the precise mechanisms for CREB regulation in the neutrophils of depressed patients.
The role of CREB has been studied both in the peripheral cells, such as lymphocytes and leukocytes, as well as the postmortem brain obtained from depressed patients. Although the findings are inconsistent due to various methodological and other confounding variables, these studies in general do show an alteration of CREB in depressive disorder. For example, Yamada et al. (2003) observed that CREB is decreased in the orbitofrontal cortex of antidepressant drug-free depressed patients, whereas Odagaki et al. (2001) reported an increase in levels of CREB in the prefrontal cortex (PFC) of antidepressant drug-free depressed subjects. Laifenfeld et al. (2004) found that there is an increase in levels of pCREB in the PFC of depressed subjects; whereas Young et al. (2004) reported that there are no differences in number of pCREB stained cells between control subjects and diagnostic groups with bipolar disorder, MDD, or schizophrenia, but increased number of pCREB stained cells in several amygdala nuclei in subjects who had died by suicide.
We have found that protein and mRNA expression of CREB is significantly decreased in the PFC and hippocampus of depressed suicide victims and in the PFC of teenage suicide victims compared with controls. The studies of CREB in leukocytes of depressed patients also provided inconsistent results. Whereas Lai et al (2003) did not find only significant difference in CREB mRNA levels between depressed and normal control subjects, Iga et al. (2007) found an increase in CREB mRNA levels in depressed patients compared with controls. We, on the other hand, found a decrease in CREB protein expression and CRE-DNA binding activity in depressed patients compared with controls. The main difference between our methods is that we determined protein rather than mRNA expression of CREB. Whether this difference could explain the discrepancy in the results is not clear. The other reason may be the duration of drug-free period in depressed patients. Since antidepressant treatment has been shown to increase CREB levels in rats (Tiraboschi et al., 2004; Laifenfeld et al., 2005; Sairanen et al., 2007), previous exposure to antidepressant treatment may account for the difference in the observed results.
One of the major lines of evidence suggesting the involvement of CREB in the pathophysiology of depression is derived from the observation that antidepressants, such as desipramine and fluoxetine, cause changes in the expression of CREB or the phosphorylation of CREB, both in vivo (Tiraboschi et al., 2004; Laifenfeld et al., 2005; Sairanen et al., 2007) and in vitro (Manier et al., 2002; Koch et al., 2003) conditions, thus suggesting that alterations in CREB may be involved in the pathophysiology of depression.
In conclusion, our studies of CREB in the neutrophils of depressed patients have several important implications. They indicate (1) that depression may be associated with a decrease in protein expression of CREB in the nuclear fraction of neutrophils; (2) this decrease may also cause changes in the functions of CREB since CRE-DNA binding activity is also found to be decreased in the nuclear fractions of neutrophils of these patients; (3) since we observed a significant correlation between CREB protein levels and HDRS scores, this study also suggest that CREB protein may be a good marker for the severity of depressive illness. Thus, CREB may play an important role in the pathophysiology of depression and one of the mechanisms for the therapeutic actions of antidepressant drugs may be related to their ability to increase CREB levels. It also suggests that neutrophil CREB levels may be a useful biomarker for MDD.
Acknowledgments
This work was supported by a grant RO1-MH-56528 (Dr. Pandey) from the National Institute of Mental Health, Rockville, MD. We thank Barbara Brown and Miljana Petkovic for their help on this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Borrelli E, Montmayeur JP, Foulkes NS, Sassone-Corsi P. Signal transduction and gene control: the cAMP pathway. Critical Reviews in Oncology/Hematology. 1992;3:321–338. [PubMed] [Google Scholar]
- Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends in Neurosciences. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Chrivia JC, Kwok RP, Lamb N, Hagiwara M, Montminy MR, Goodman RH. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- Dowlatshahi D, MacQueen GM, Wang JF, Reiach JS, Young LT. G Protein-coupled cyclic AMP signaling in postmortem brain of subjects with mood disorders: effects of diagnosis, suicide, and treatment at the time of death. Journal of Neurochemistry. 1999;73:1121–1126. doi: 10.1046/j.1471-4159.1999.0731121.x. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rao JS, Rizavi HS, Kotowski J, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Abnormal expression and functional characteristics of cyclic adenosine monophosphate response element binding protein in postmortem brain of suicide subjects. Archives of General Psychiatry. 2003;60:273–282. doi: 10.1001/archpsyc.60.3.273. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Arnold FJ, Bading H. Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nature Neuroscience. 2001;4:261–267. doi: 10.1038/85109. [DOI] [PubMed] [Google Scholar]
- Iga J, Ueno S, Yamauchi K, Numata S, Kinouchi S, Tayoshi-Shibuya S, Song H, Ohmori T. Altered HDAC5 and CREB mRNA expressions in the peripheral leukocytes of major depression. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2007;31:628–632. doi: 10.1016/j.pnpbp.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Imaki J, Yoshida K, Yamashita K. A developmental study of cyclic AMP-response element binding protein (CREB) by in situ hybridization histochemistry and immunocytochemistry in the rat neocortex. Brain Research. 1994;651:269–274. doi: 10.1016/0006-8993(94)90706-4. [DOI] [PubMed] [Google Scholar]
- Kanof PD, Johns C, Davidson M, Siever LJ, Coccaro EF, Davis KL. Prostaglandin receptor sensitivity in psychiatric disorders. Archives of General Psychiatry. 1986;43:987–993. doi: 10.1001/archpsyc.1986.01800100081011. [DOI] [PubMed] [Google Scholar]
- Koch JM, Kell S, Aldenhoff JB. Differential effects of fluoxetine and imipramine on the phosphorylation of the transcription factor CREB and cell-viability. Journal of Psychiatric Research. 2003;37:53–59. doi: 10.1016/s0022-3956(02)00061-4. [DOI] [PubMed] [Google Scholar]
- Lai IC, Hong CJ, Tsai SJ. Expression of cAMP response element-binding protein in major depression before and after antidepressant treatment. Neuropsychobiology. 2003;48:182–185. doi: 10.1159/000074635. [DOI] [PubMed] [Google Scholar]
- Laifenfeld D, Karry R, Klein E, Ben-Shachar D. Alterations in cell adhesion molecule L1 and functionally related genes in major depression: a postmortem study. Biological Psychiatry. 2005;57:716–725. doi: 10.1016/j.biopsych.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Lee KA, Masson N. Transcriptional regulation by CREB and its relatives. Biochim Biophys Acta. 1993;1174:221–233. doi: 10.1016/0167-4781(93)90191-f. [DOI] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- Manier DH, Shelton RC, Sulser F. Noradrenergic antidepressants: does chronic treatment increase or decrease nuclear CREB-P? Journal of Neural Transmission. 2002;109:91–99. doi: 10.1007/s702-002-8239-6. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Brown RP, Halper JP, Sweeney JA, Kocsis JH, Stokes PE, Bilezikian JP. Reduced sensitivity of lymphocyte beta-adrenergic receptors in patients with endogenous depression and psychomotor agitation. The New England Journal of Medicine. 1985;313:715–720. doi: 10.1056/NEJM198509193131202. [DOI] [PubMed] [Google Scholar]
- Mikuni M, Kusumi I, Kagaya A, Kuroda Y, Mori H, Takahashi K. Increased 5-HT-2 receptor function as measured by serotonin-stimulated phosphoinositide hydrolysis in platelets of depressed patients. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 1991;15:49–61. doi: 10.1016/0278-5846(91)90040-8. [DOI] [PubMed] [Google Scholar]
- Montminy MR, Gonzalez GA, Yamamoto KK. Regulation of cAMP-inducible genes by CREB. Trends Neurosci. 1990;13:184–188. doi: 10.1016/0166-2236(90)90045-c. [DOI] [PubMed] [Google Scholar]
- Moore AN, Waxham MN, Dash PK. Neuronal activity increases the phosphorylation of the transcription factor cAMP response element-binding protein (CREB) in rat hippocampus and cortex. The Journal of Biological Chemistry. 1996;271:14214–14220. doi: 10.1074/jbc.271.24.14214. [DOI] [PubMed] [Google Scholar]
- Nichols M, Weih F, Schmid W, DeVack C, Kowenz-Leutz E, Luckow B, Boshart M, Schutz G. Phosphorylation of CREB affects its binding to high and low affinity sites: implications for cAMP induced gene transcription. The EMBO Journal. 1992;11:3337–3346. doi: 10.1002/j.1460-2075.1992.tb05412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odagaki Y, Garcia-Sevilla JA, Huguelet P, La Harpe R, Koyama T, Guimon J. Cyclic AMP-mediated signaling components are upregulated in the prefrontal cortex of depressed suicide victims. Brain Research. 2001;898:224–231. doi: 10.1016/s0006-8993(01)02188-6. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Ren X, Rizavi HS, Roberts RC, Conley RR. Cyclic AMP response element-binding protein in post-mortem brain of teenage suicide victims: specific decrease in the prefrontal cortex but not the hippocampus. The International Journal of Neuropsychopharmacology. 2007;10:621–629. doi: 10.1017/S1461145706007231. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dysken MW, Garver DL, Davis JM. Beta-adrenergic receptor function in affective illness. Am J Psychiatry. 1979;136:675–678. doi: 10.1176/ajp.136.5.675. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Ren X, Pandey SC, Dwivedi Y, Sharma R, Janicak PG. Hyperactive phosphoinositide signaling pathway in platelets of depressed patients: effect of desipramine treatment. Psychiatry Research. 2001;105:23–32. doi: 10.1016/s0165-1781(01)00337-7. [DOI] [PubMed] [Google Scholar]
- Riabowol KT, Fink JS, Gilman MZ, Walsh DA, Goodman RH, Feramisco JR. The catalytic subunit of cAMP-dependent protein kinase induces expression of genes containing cAMP-responsive enhancer elements. Nature. 1988;336:83–86. doi: 10.1038/336083a0. [DOI] [PubMed] [Google Scholar]
- Sairanen M, O’Leary OF, Knuuttila JE, Castren E. Chronic antidepressant treatment selectively increases expression of plasticity-related proteins in the hippocampus and medial prefrontal cortex of the rat. Neuroscience. 2007;144:368–374. doi: 10.1016/j.neuroscience.2006.08.069. [DOI] [PubMed] [Google Scholar]
- Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annual Review of Biochemistry. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- Siever LJ, Kafka MS, Targum S, Lake CR. Platelet alpha-adrenergic binding and biochemical responsiveness in depressed patients and controls. Psychiatry Research. 1984;11:287–302. doi: 10.1016/0165-1781(84)90003-9. [DOI] [PubMed] [Google Scholar]
- Tiraboschi E, Tardito D, Kasahara J, Moraschi S, Pruneri P, Gennarelli M, Racagni G, Popoli M. Selective phosphorylation of nuclear CREB by fluoxetine is linked to activation of CaM kinase IV and MAP kinase cascades. Neuropsychopharmacology. 2004;29:1831–1840. doi: 10.1038/sj.npp.1300488. [DOI] [PubMed] [Google Scholar]
- Viola H, Furman M, Izquierdo LA, Alonso M, Barros DM, de Souza MM, Izquierdo I, Medina JH. Phosphorylated cAMP response element-binding protein as a molecular marker of memory processing in rat hippocampus: effect of novelty. The Journal of Neuroscience. 2000;20:RC112. doi: 10.1523/JNEUROSCI.20-23-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton MR, Dragunow I. Is CREB a key to neuronal survival? Trends in Neurosciences. 2000;23:48–53. doi: 10.1016/s0166-2236(99)01500-3. [DOI] [PubMed] [Google Scholar]
- Wang YC, Pandey GN, Mendels J, Frazer A. Platelet adenylate cyclase responses in depression: implications for a receptor defect. Psychopharmacologia. 1974;36:291–300. doi: 10.1007/BF00422561. [DOI] [PubMed] [Google Scholar]
- Xie H, Rothstein TL. Protein kinase C mediates activation of nuclear cAMP response element-binding protein (CREB) in B lymphocytes stimulated through surface Ig. Journal of Immunology. 1995;154:1717–1723. [PubMed] [Google Scholar]
- Yamada S, Yamamoto M, Ozawa H, Riederer P, Saito T. Reduced phosphorylation of cyclic AMP-responsive element binding protein in the postmortem orbitofrontal cortex of patients with major depressive disorder. Journal of Neural Transmission. 2003;110:671–680. doi: 10.1007/s00702-002-0810-8. [DOI] [PubMed] [Google Scholar]
- Young LT, Bezchlibnyk YB, Chen B, Wang JF, MacQueen GM. Amygdala cyclic adenosine monophosphate response element binding protein phosphorylation in patients with mood disorders: effects of diagnosis, suicide, and drug treatment. Biological Psychiatry. 2004;55:570–577. doi: 10.1016/j.biopsych.2003.10.023. [DOI] [PubMed] [Google Scholar]


