Abstract
The small leucine-rich proteoglycans (SLRPs) are involved in many aspects of mammalian biology, both in health and disease. They are now being recognized as key signaling molecules with an expanding repertoire of molecular interactions affecting not only growth factors, but also various receptors involved in controlling cell growth, morphogenesis and immunity. The complexity of SLRP signaling and the multitude of affected signaling pathways can be reconciled with a hierarchical affinity-based interaction of various SLRPs in a cell- and tissue-specific context. Below, we review this interacting network, describe new relationships of the SLRPs with tyrosine kinase and Toll-like receptors and critically assess their roles in cancer and innate immunity.
Keywords: Decorin, biglycan, lumican, signal transduction, EGFR, Met, IGF-IR, Toll-like receptor, cancer, inflammation
Introduction
The small leucine-rich proteoglycans, abbreviated “SLRP”, were originally grouped on the basis of their relatively small protein core (36–42 kDa), as compared to the larger aggregating proteoglycans such as aggrecan and versican, and on their unique structural organization composed of tandem leucine-rich repeats (LRRs) [1,2]. It became also evident that at least three SLRP classes could be distinguished based upon additional unique features such as the organization of disulfide bonds at their N- and C-termini with the cysteine residues following a class-specific topology, and on the basis of their genomic organization with each individual class harboring a nearly identical number and size of exons and often positioned in a similar sequential pattern within chromosomes [3,4]. More recently, five distinct classes of SLRPs have been proposed based on shared biological activity and functions, albeit some of SLRPs are not classical proteoglycans [5]. SLRP biology and function is further complicated by their posttranslational modifications including substitution with sugars and glycosaminoglycan side chains of various types. For instance, the canonical class I members decorin and biglycan contain chondroitin or dermatan sulfate side chains with the exception of asporin. In contrast, all class II members harbor polylactosamine or keratan sulfate chains in their LRRs and sulfated tyrosine residues in their N-termini. Class III members contain chondroitin/dermatan sulfate (epiphycan), keratan sulfate (osteoglycin) or no glycosaminoglycan (opticin) chain. Finally, non canonical class IV and V members lack any glycosaminoglycan chain with the exception of chondroadherin which is substituted with keratan sulfate [6]. Thus, the presence of finite sugar chains, together with further posttranslational refinements, including modification in their degree of sulfation or epimerization, endows this class of proteoglycans with an extra layer of structural complexity.
Initially thought to act exclusively as structural components, SLRPs are now recognized as key players in cell signaling capable of influencing a host of cellular functions such as proliferation, differentiation, survival, adhesion, migration and inflammatory response. All of these functions are mediated by the intrinsic SLRP ability to interact with both cytokines and ligands as well as with surface receptors. This minireview will critically assess recent advances on the modulation of various signaling pathways that are affected by SLRPs including signaling through receptor tyrosine kinase such as the EGFR, Met and IGF-IR, as well as receptors involved in innate immunity and inflammation such as Toll-like receptors and purinergic P2X receptors. We will focus specifically on decorin, biglycan and lumican, the best studied SLRP members so far. More extensive and specialized reviews on the subject have been published covering other aspects of SLRP biology [6–13].
Anti-proliferative effects on cancer cells via EGFR and Met suppression
The first demonstration for an anti-proliferative effect of decorin, at that time called PG40 to reflect its apparent size, was achieved over two decades ago when Ruoslahti and coworkers discovered that stable transfection of decorin causes growth arrest in Chinese hamster ovary cells [14]. They subsequently discovered that this growth inhibition was actually due to decorin’s ability to bind and block TGFβ [15], a property also shared by other SLRPs [16]. This original observation has led to a large number of studies focusing on decorin’s ability to inhibit fibrosis, whose main pathogenetic mechanism involves overactivation of the TGFβ signaling pathway. However, other studies using a variety of transformed cells showed that de novo decorin expression causes severe growth retardation in vitro [17] and suppression of tumorigenicity in animal models of human tumor xenografts [18]. Because most of these transformed cells are not dependent on TGFβ for their growth, it was hypothesized that another receptor system had to be involved insofar as decorin is a soluble proteoglycan. One of the key observations that emerged from these studies was that the decorin-expressing tumor cells become arrested in the G1 phase of the cell cycle and overproduce the cyclin-dependent kinase inhibitor p21WAF1 [19] supporting earlier observations that decorin gene expression is markedly induced during quiescence [20,21]. Indeed, both the mouse and decorin structural organization of their gene and promoter are quite complex [22–24] and subject to an intricate transcriptional regulation [1,25,26]. It was soon discovered that decorin directly interacts with the epidermal growth factor receptor (EGFR) with a KD~87 nM [27]. This interaction evokes a transient activation [28,29] followed by a profound downregulation of the receptor and inhibition of its downstream signaling activity [30,31]. Subsequent studies using the yeast two-hybrid system revealed that decorin binds to a narrow region within the ligand-binding domain L2 of the EGFR overlapping with the EGF binding domain [32]. The structural constraints of the EGFR binding region support a stochiometry of 1:1 for decorin protein core and EGFR, suggesting that decorin is biologically active as a monomer [33]. This interaction prevents receptor dimerization and targets the EGFR to a sustained internalization via caveolin-mediated endocytosis [34], eventually leading to its degradation (Fig. 1). Notably heparanase induces EGFR phosphorylation [35], using similar Tyr residues that are activated by decorin. However, the results are quite different since heparanase leads to EGFR activation [35] whereas decorin leads to EGFR down-regulation [36]. Another effect of decorin is its activation of caspase-3, one of the key enzymes involved in programmed cell death, thereby increasing decorin’s anti-oncogenic activity [37]. Similar effects are also observed in normal mesangial cells where overexpression of decorin activates caspase-3, induces apoptosis and arrests the cells in the G0/G1 phase of the cell cycle via EGFR downregulation [38]. Also caspase-8 activation has been detected in a wide variety of transformed cells when decorin is overexpressed using adenoviral vectors [39].
Fig. 1.
Schematic representation of decorin effects as an antiproliferative (left panel) and proliferative (right panel) molecule. In most cancer cells so far investigated, decorin causes a downregulation of EGFR and Met with consequent activation of p21 and caspase-3, which leads to apoptosis. Decorin also interferes with the non-canonical β-catenin pathway via the Met receptor. In normal cells such renal tubular epithelial cells, decorin evokes a pro-survival and proliferative response via the IGF-IR and downstream signaling. Please, refer to the text for additional information.
The consequences of decorin signaling through RTKs are exemplified by several observations using the decorin-null animals. First, crossing the decorin-null mice, which exhibit a skin fragility phenotype [40], with the p53-null mice causes an early lethality of the double mutant animals with massive organ infiltration by a T cell lymphoma [41]. This is in contrast to the p53-null mice which show a wide variety of tumor types, including carcinomas and sarcomas, and a prolonged survival as compared to the double mutant mice. The second key observation is that about one-third of decorin-deficient mice develop intestinal adenomas that eventually develop into adenocarcinomas, and this process is accelerated and amplified by subjecting the decorin-null mice to a western diet enriched in lipids and low in calcium and vitamin D [42]. Notably, tumorigenesis in the decorin-deficient mice is associated with a down-regulation of both CDK-inhibitors p21WAF1and p27Kip1 and a concurrent upregulation of β-catenin. Together, these in vivo observations suggest that decorin deficiency is permissive for tumorigenesis.
Adenovirus-mediated gene delivery or systemic administration of decorin gene in various tumor xenograft models has revealed an effective inhibition of tumor growth, downregulation of both EGFR and ErbB2, and an inhibitory effect on metastatic spreading [39, 43–48]. Some of these in vivo effects might be mediated by decorin’s ability to inhibit the endogenous tumor cell production of VEGFA [49].
In an animal model of prostate carcinoma generated by a targeted deletion of the tumor suppressor PTEN in the prostate, systemic delivery of decorin causes a marked downregulation of the EGFR in the treated tumors with an associated reduction in tumor growth [50]. Notably, decorin also interferes with a crosstalk between the EGFR and the androgen receptor in prostate carcinoma cells [50]. The interplay between decorin and the EGFR is further underscored by osteosarcoma cells which escape the decorin-suppressing activity via a protracted expression and activation of their endogenous EGFR [51,52].
The complex binding repertoire of decorin would predict that this SLRP could modulate the bioactivity of other RTKs. Indeed, decorin binds directly and with high affinity (KD ~ 1.5 nM) to Met, the receptor for hepatocyte growth factor [53]. Notably, binding of decorin to Met can be efficiently displaced by hepatocyte growth factor, and less efficiently by internalin B, a known bacterial ligand of Met with structural homology to decorin leucine-rich repeats. The interaction between decorin and Met induces transient activation of the receptor, recruitment of the E3 ubiquitin ligase c-Cbl, followed by rapid intracellular degradation of Met. Tumor growth is further suppressed through caspase-3-mediated apoptosis. Notably, signaling through Met leads to phosphorylation of β-catenin, a known downstream Met effector, directing it to proteosomal degradation thereby decreasing cellular motility, tissue invasion and metastasis (Fig. 1). These findings indicate that decorin exerts its anti-proliferative activity by antagonistically targeting multiple tyrosine kinase receptors, thereby contributing to reduction in primary tumor growth and metastastic spreading. The decorin role as a marker for prognosis as well as an anticancer therapeutic is reviewed in this issue by Theocharis et al [54].
Proliferative effects on normal cells via the IGF-IR
By contrast, in normal cells decorin signaling through insulin-like growth factor receptor type 1 (IGF-IR) exerts anti-apoptotic and proliferative effects, favoring cellular growth. Decorin binds IGF-IR with affinity in the low nanomolar range (KD ~1–2 nM) in endothelial cells [55], renal fibroblasts [56], and human tubular epithelial cells [57]. In addition, decorin binds to and sequesters the IGF-I (KD ~18 nM), the natural ligand of this RTK [55]. By binding to the IGF-IR, decorin triggers phosphorylation and downstream activation of phosphoinositide-3 kinase (PI3K), Akt/protein kinase B (Akt/PKB) and p21WAF1 inducing an anti-apoptotic effect [55,57,58] (Fig. 1). The relevance of decorin in the IGF-IR pathway is reinforced in two experimental animal models of inflammatory angiogenesis and unilateral ureteral obstruction. In both cases, decorin-deficiency causes a significant increase in IGF-IR levels as compared to controls [55,56]. More over, lack of decorin promotes renal tubular epithelial cell apoptosis in experimental diabetic nephropathy [57,58] and in a renal obstruction model with interstitial inflammation and fibrosis [55,57]. In renal fibroblasts, decorin activates the mTOR (mammalian target of rapamycin) and p70S6 kinase (p70S6K) downstream of IGF-IR/PI3K/Akt signaling [58]. This ultimately results in increased translation and synthesis of fibrillin-1, thereby indirectly promoting cell proliferation [59]. These pathways might represent the intricate regulatory mechanisms whereby decorin modulates IGF-IR signaling in a cell type-specific manner, thereby giving rise to different biological outcomes. In contrast to the well-characterized interactions of decorin with the EGFR family, the biological necessity for decorin-triggered activation of the canonical IGF signaling cascade is not well characterized. Decorin appears to mimic the effects of IGF-I and stimulates the IGF-IR without inhibiting signaling as it has been shown for its interaction with receptors of the ErbB family. However, the significance of decorin/IGF-IR interaction is not clear. In endothelial cells, decorin promotes transient receptor phosphorylation and activation and subsequent degradation, but it also promotes adhesion and migration on fibrillar collagen [55,60]. In extravillus trophoblasts, instead, decorin inhibits migration by affecting the IGF-IR pathway [61]. All of these studies were performed with “normal” cells. Thus, there are no published data on the role of decorin in modulating cancer growth via the IGF-IR in transformed cells or in tumor models. Further studies are needed to elucidate the role of decorin in the regulation of IGF-IR and to clarify whether decorin/IGF-IR signaling might be operative in carcinoma cells as well.
The complexity of decorin signaling is further expanded by additional degradative pathways involved in decorin catabolism. The endocytosis and lysosomal degradation of decorin comprises multiple pathways including those mediated by the EGFR [34] and low density lipoprotein receptor-related protein (LRP) [62]. Interestingly, lipid-raft-dependent EGFR signaling also modulates decorin uptake, a process that could constitute a regulatory mechanism for desensitization of decorin-evoked signaling [63]. Thus, there are numerous opportunities for feedback control of decorin activity and its efficiency for signaling. The ability of decorin to bind to more than one RTK suggests that decorin is directly involved in the intricate crosstalk between receptors and their downstream signaling cascades.
Biglycan, a danger signal that induces cooperativity of innate immunity receptors
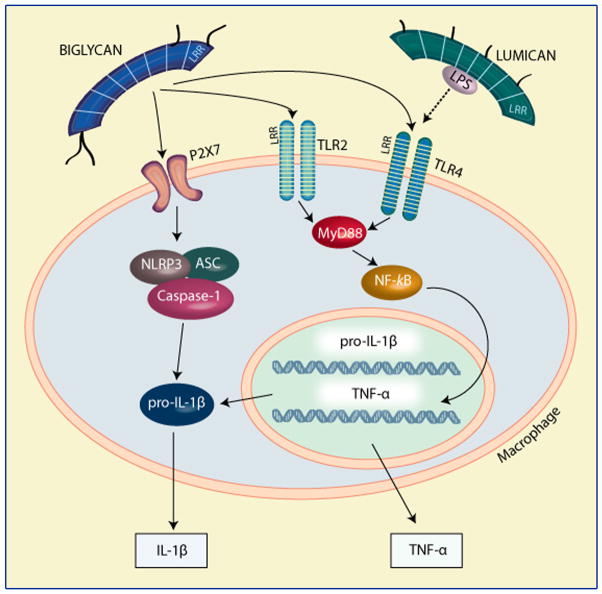
Biglycan, a class I SLRP structurally related to decorin, serves as an agonist of different cell surface receptors thereby giving rise to diverse biological outcomes [64]. The initial observation was made during studies of a renal obstruction model caused by pressure injury. In these studies biglycan was markedly over-expressed in resident renal tubular epithelial cells prior to the infiltration of macrophages, suggesting that biglycan might be involved in the initiation of the inflammatory response [58]. More recently, a number of reports have firmly established that biglycan, in analogy to decorin, acts as a signaling molecule especially important in the innate immune system [65,66]. Under physiological conditions, biglycan is sequestered in the extracellular milieu, acting as a structural component with no apparent immunological function. Upon tissue stress or injury, biglycan is released from the extracellular matrix by a proteolytic processing which is not yet characterized. In contrast to the sequestered proteoglycan, soluble biglycan turns into an endogenous ligand of innate immunity receptors and interacts with Toll-like receptors (TLR)-2 and -4 on macrophages, thereby triggering a robust inflammatory response. It is intriguing that both TLRs and biglycan contain LRR-motifs with the potential to interact with each other. Downstream of TLRs, biglycan signaling involves MyD88, p38, Erk and NFκB and results in the synthesis and secretion of TNFα and MIP-2. Consequently, additional neutrophils and macrophages are recruited to the site of tissue injury. This initial step does not require de novo synthesis of the pro-inflammatory agents and therefore generates a fast response to tissue damage. Moreover, macrophages stimulated by proinflammatory cytokines can synthesize biglycan de novo [65], thereby boosting the inflammatory response in an autocrine and paracrine manner (Fig. 2). Thus, soluble biglycan appears to represent a “danger” motif (DAMP, danger-associated molecular pattern) in analogy to pathogen-associated molecular patterns (PAMPs) in pathogen-driven inflammation. Besides its interaction with TLRs [65], biglycan also acts as a ligand for selectin L/CD44 and is thus directly involved in the recruitment of CD16(-) natural killer cells [67].
Fig. 2.
Schematic representation of biglycan and lumican effects on the innate immune system. Please, refer to the text for detailed information.
Soluble biglycan, as a pivotal DAMP, is not only secured by its interaction with TLR2/4 but is also involved in signaling through the cytoplasmic NOD-like receptors (NLRs) (Fig. 2). This is due to an interaction with and clustering of membrane-bound Toll-like- and purinergic P2X receptors, whereby biglycan induces receptor cooperativity within these newly-formed multireceptor complexes. By signaling through TLR2/4, biglycan stimulates the expression of NLRP3, a member of NLRs, and pro-IL-1β mRNA. Importantly, biglycan is simultaneously capable of interacting with P2X4/P2X7 receptors which will activate the NLRP3/ASC inflammasome in a reactive oxygen species- and heat shock protein 90-dependent manner. These combined signaling events culminate in the activation of caspase-1 and in the processing of pro-IL-1β into its mature form, without the need for additional co-stimulatory factors [66]. Collectively, these findings provide solid evidence for the multi-functional involvement of biglycan within the innate immune system. In particular biglycan appears to specifically interact with two classes of receptors thereby providing cross-talk between their downstream signaling, a function that might be facilitated by the presence of tandem LRRs and glycosaminoglycan side chains. Notably, a recent report has shown that biglycan gene expression is specifically upregulated in human aortic valve stenosis and that the enhanced accumulation of biglycan within the stenotic valves contributes to the production of phospholipid transfer protein, a key factor in atherosclerotic aortic valve development, via TLR2 [68]. Thus, biglycan is well suited to serve as a cross-linker for different cell surface receptors.
In a model of non-infectious inflammation in the kidney, the so called unilateral ureteral obstruction model, biglycan-deficient mice display lower levels of active caspase-1 and mature IL-1β, resulting in reduced infiltration of mononuclear cells and less kidney damage. In a prototypical innate immune process such as lipopolysaccharide-induced sepsis, lack of biglycan results in a clear survival benefit associated with lower levels of circulating TNF-α and IL-1β, reduced activation of the NLRP3 inflammasome and less infiltration in the lung, a major target organ of sepsis in mice [65,66]. These findings have led to a new understanding of the regulation of pathogen-independent (“sterile”) inflammation. Sterile inflammation appears to be driven by soluble biglycan as an endogenous agonist for two crucial TLRs acting as an autonomous trigger of the innate immunity system. In contrast, in PAMP-mediated conditions, biglycan would serve as an amplifier of the inflammatory response by signaling through the second TLR which is not involved in pathogen sensing. This concept describes a fundamental paradigm of how tissue injury is monitored by innate immune receptors detecting the release of minute amounts of components from the extracellular matrix and turning such a signal into a robust inflammatory response. This clearly implicates biglycan as a novel target of anti-inflammatory strategies.
Besides being a strong trigger of pro-inflammatory signaling within the innate immune system, biglycan can also affect bone morphogenetic protein (BMP) signaling, thereby influencing the differentiation of tendon stem/progenitor cells and subsequent tendon formation [69]. Biglycan forms complexes with BMP-4 and modulates osteoblast differentiation [70] as well as enhances its binding to chordin [71]. The latter in turn leads to BMP-4 inactivation by the chordin-Tsg (Twisted gastrulation) complex [71].
Lumican signaling in cell growth and inflammation
The role of lumican in the regulation of cell signaling has not been studied in great detail. In analogy to decorin, lumican inhibits tumor cell growth in soft agar by increasing the expression of the CDK inhibitor p21WAF1 [72]. Again, similar to decorin, these growth inhibitory effects of lumican occur in a variety of cell types including fibrosarcoma, carcinoma and normal embryonic cells [72]. Notably, expression of membrane type metalloprotease 1 (MT1-MMP) reduces lumican secretion and abrogates lumican-mediated p21WAF1 induction [72]. Also decorin is cleaved by MT1-MMP [72] suggesting that protease processing is important in SLRP biology. The role of shedding of cell surface syndecans is reviewed in this series by Manon-Jensen et al [73].
Lumican reduces colony formation in soft agar and tumorigenicity in nude mice of cells transformed by v-src and K-ras oncogenes [74]. In mouse embryonic fibroblasts lumican-evoked upregulation of p21WAF1 occurs through a p53-mediated mechanism with a subsequent decline in the cyclins A, D1 and E [75]. Lumican deficiency is associated with proliferation of stromal keratinocytes and embryonic fibroblasts [76]. Its inhibitory effects on cell growth have also been observed in tumor cells, with some of these cells secreting lumican in an autocrine manner [77]. In melanoma cells, lumican regulates vertical growth, suppresses anchorage-independent proliferation, and inhibits cyclin D1 expression [78, 79]. A recent study has further shown that lumican not only inhibits melanoma invasion and metastasis, but also induces tumor cell apoptosis and inhibits angiogenesis [80]. Thus, lumican might contribute as a therapeutic agent to combat melanoma metastasis.
Lumican can interact with β1-containing integrin receptors and this signaling leads to inhibition of melanoma cell migration by enhancing cell adhesion [81]. Indeed, several components of the focal adhesion complex are modulated by lumican-evoked signaling, including vinculin and focal adhesion kinase [82]. Lumican alters the relationship between actin filaments and β1 integrin, which in turn would affect focal adhesion formation, thereby explaining the anti-invasive effects of this SLRP [82]. A commonality of signaling between lumican and decorin is also supported by recent studies showing involvement of decorin in modulating various integrins in controlling proliferation, adhesion and migration [60,83]. Notably, lumican manufactured by endothelial cells binds to the cell surface of extravasated neutrophilic leukocytes via β2-containing integrin receptors and promotes migration during the inflammatory response [84]. Thus, there is a possible endothelial-dependent lumican expression that might mediate in a paracrine fashion neutrophil recruitment and migration. Lumican also is involved in Fas-FasL-induced apoptosis by upregulating Fas (CD95) in mouse embryonic fibroblasts [75].
In terms of TLR signaling, lumican presents PAMPs to the receptor complex. The protein core of lumican is capable of binding and presenting LPS to CD14, thereby activating TLR4 signaling [85] (Fig. 2). Lumican also binds to and signals through the FasL, it increases the synthesis and secretion of proinflammatory cytokines and accelerates the recruitment of macrophages and neutrophils [76,86]. Via its protein core, lumican interacts with the CXC-chemokine KC (CXCL1), thereby creating a chemokine gradient in the tissue along which neutrophil will infiltrate the site of injury [87].
Conclusions and perspectives
Undoubtedly SLRPs are structural components especially important during development and the maturation of various tissues enriched in mesenchyme. Utilization of animal models including the mouse [7,40, 88–101] and zebrafish [102], or cellular systems with finite SLRP deficiencies [83, 103–105], has revealed fundamental roles for SLRPs in embryonic life and disease progression. The past decade has further witnessed many members of the SLRP gene family emerging as signaling molecules. The discovery that soluble SLRPs engage various cell surface receptors resulting in a triggering of downstream signaling events has shed a new light on how SLRPs might regulate cell behavior. This is possible because of several characteristics of these proteoglycans. First, their makeup is conducive to protein/protein interactions. Second, many surface receptors are made up of protein modules that are often shared by extracellular matrix proteins, including leucine-rich repeats, fibronectin and immunoglobulin repeats, among others. Thus, there is the likely possibility that during evolution some of these modules have been utilized by both matrix (structural) and ligand (signaling) molecules. Third, SLRPs are abundant and ubiquitous, and thus might signal in a different way than traditional ligands whose kinetics are often very rapid, that is, both triggering of signals and transferring of this information to the nucleus takes just a few minutes. In contrast SLRPs can induce protracted signaling leading to growth inhibition in most of the cases studied. An additional layer of complexity is provided by SLRP’s ability to bind and sequester various cytokines, growth factors, and morphogens involved in multiple signaling pathways affecting differentiation, survival, adhesion, migration, cancer and inflammatory responses.
In spite of their conserved and highly similar structural composition, various SLRPs such as decorin, biglycan and lumican have distinct interacting receptors. How could SLRPs bind to multiple receptors and still be specific in their action? One way to answer this important question is to consider a “hierarchical” possibility of receptor binding and activation. For example, decorin binds to EGFR, Met and IGF-IR with diverse affinity constants, with KD ranging from 87 nM for the EGFR to 1–2 nM for the Met and IGF-IR. Thus, when decorin encounters a cancer composed of a mixed population of cells, it might differentially affect the tumor cells depending upon the expression and cellular density of a given RTK. This cell-specific context might also apply to other members of the SLRP gene family. Finally, another key concept emerging from the studies summarized above is that some SLRPs, such as biglycan, might work through clustering and activating multireceptor complexes. This concept provides a novel mechanism of how tissue injury could be sensed by innate immune receptors: detecting the release of minute amounts of matrix constituents and turning such a signal into a robust inflammatory response.
Acknowledgments
We thank Angela McQuillan for her excellent work with the graphic designs. We also like to thank our numerous collaborators who have contributed to our work on SLRPs throughout the past two decades. This work was supported in part by National Institutes of Health grants RO1 CA39481, RO1 CA47282, and RO1 CA120975 (R.V.I.) and by the Deutsche Forschungsgemeinschaft (SFB 815, project A5, SCHA 1082/2-1, Excellence Cluster ECCPS), and Else Kröner-Fresenius-Stiftung (to L.S.).
Abbreviations
- SLRP
small leucine-rich proteoglycan
- LRR
leucine-rich repeat
- RTK
receptor tyrosine kinase
- EGFR
epidermal growth factor receptor
- IGF-IR
insulin-like growth factor receptor type 1
- Met
hepatocyte growth factor receptor
- TLR
Toll-like receptor
- NLR
nucleotide binding oligomerization domain-like receptor
- CDK
cyclin-dependent kinase
- DAMP
danger-associated molecular pattern
- BMP
bone morphogenetic protein
- LPS
lipopolysaccharide
- PI3K
phosphoinositide-3 kinase,
- Akt/PKB
Akt/protein kinase B
- mTOR
mammalian target of rapamycin
- p70S6K
p70S6 kinase
- MyD88
myeloid differentiation primary response gene 88
- Erk
extracellular signal-regulated kinase
- NFκB
nuclear factor κB
- TNFα
tumor necrosis factor α
- MIP-2
macrophage inflammatory protein 2
- P2X
purinoreceptor
References
- 1.Iozzo RV, Murdoch AD. Proteoglycans of the extracellular environment: clues from the gene and protein side offer novel perspectives in molecular diversity and function. FASEB J. 1996;10:598–614. [PubMed] [Google Scholar]
- 2.Iozzo RV. The family of the small leucine-rich proteoglycans: key regulators of matrix assembly and cellular growth. Crit Rev Biochem Mol Biol. 1997;32:141–174. doi: 10.3109/10409239709108551. [DOI] [PubMed] [Google Scholar]
- 3.Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem. 1998;67:609–652. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- 4.Iozzo RV. The biology of the small leucine-rich proteoglycans. Functional network of interactive proteins. J Biol Chem. 1999;274:18843–18846. doi: 10.1074/jbc.274.27.18843. [DOI] [PubMed] [Google Scholar]
- 5.Schaefer L, Iozzo RV. Biological functions of the small leucine-rich proteoglycans: from genetics to signal transduction. J Biol Chem. 2008;283:2135–2139. doi: 10.1074/jbc.R800020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaefer L, Schaefer RM. Proteoglycans: From structural compounds to signaling molecules. Cell Tissue Res. 2010;339:237–246. doi: 10.1007/s00441-009-0821-y. [DOI] [PubMed] [Google Scholar]
- 7.Ameye L, Young MF. Mice deficient in small leucine-rich proteoglycans: novel in vivo models for osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology. 2002;12:107R–116R. doi: 10.1093/glycob/cwf065. [DOI] [PubMed] [Google Scholar]
- 8.Reed CC, Iozzo RV. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj J. 2002;19:249–255. doi: 10.1023/A:1025383913444. [DOI] [PubMed] [Google Scholar]
- 9.Brandan E, Cabello-Verrugio C, Vial C. Novel regulatory mechanisms for the proteoglycans decorin and biglycan during muscle formation and muscular dystrophy. Matrix Biol. 2008;27:700–708. doi: 10.1016/j.matbio.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Chakravarti S. Functions of lumican and fibromodulin: Lessons from knockout mice. Glycoconj J. 2003;19:287–293. doi: 10.1023/A:1025348417078. [DOI] [PubMed] [Google Scholar]
- 11.Kalamajski S, Oldberd Å. The role of small leucine-rich proteoglycans in collagen fibrillogenesis. Matrix Biol. 2010;29:248–253. doi: 10.1016/j.matbio.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Heinegård D. Proteoglycans and more - from molecules to biology. Int J Exp Path. 2009;90:575–586. doi: 10.1111/j.1365-2613.2009.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iozzo RV, Goldoni S, Berendsen A, Young MF. In: Extracellular Matrix: An overview. Mecham RP, editor. Springer; 2010. In press. [Google Scholar]
- 14.Yamaguchi Y, Ruoslahti E. Expression of human proteoglycan in Chinese hamster ovary cells inhibits cell proliferation. Nature. 1988;336:244–246. doi: 10.1038/336244a0. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-β by the proteoglycan decorin. Nature. 1990;346:281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- 16.Hildebrand A, Romaris M, Rasmussen LM, Heinegård D, Twardzik DR, Border WA, Ruoslahti E. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor β. Biochem J. 1994;302:527–534. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santra M, Skorski T, Calabretta B, Lattime EC, Iozzo RV. De novo decorin gene expression suppresses the malignant phenotype in human colon cancer cells. Proc Natl Acad Sci USA. 1995;92:7016–7020. doi: 10.1073/pnas.92.15.7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santra M, Mann DM, Mercer EW, Skorski T, Calabretta B, Iozzo RV. Ectopic expression of decorin protein core causes a generalized growth suppression in neoplastic cells of various histogenetic origin and requires endogenous p21, an inhibitor of cyclin-dependent kinases. J Clin Invest. 1997;100:149–157. doi: 10.1172/JCI119507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Luca A, Santra M, Baldi A, Giordano A, Iozzo RV. Decorin-induced growth suppression is associated with upregulation of p21, an inhibitor of cyclin-dependent kinases. J Biol Chem. 1996;271:18961–18965. doi: 10.1074/jbc.271.31.18961. [DOI] [PubMed] [Google Scholar]
- 20.Coppock DL, Kopman C, Scandalis S, Gilleran S. Preferential gene expression in quiescent human lung fibroblasts. Cell Growth & Differ. 1993;4:483–493. [PubMed] [Google Scholar]
- 21.Mauviel A, Santra M, Chen YQ, Uitto J, Iozzo RV. Transcriptional regulation of decorin gene expression. Induction by quiescence and repression by tumor necrosis factor-α. J Biol Chem. 1995;270:11692–11700. doi: 10.1074/jbc.270.19.11692. [DOI] [PubMed] [Google Scholar]
- 22.Scholzen T, Solursh M, Suzuki S, Reiter R, Morgan JL, Buchberg AM, Siracusa LD, Iozzo RV. The murine decorin. Complete cDNA cloning, genomic organization, chromosomal assignment and expression during organogenesis and tissue differentiation. J Biol Chem. 1994;269:28270–28281. [PubMed] [Google Scholar]
- 23.Danielson KG, Fazzio A, Cohen I, Cannizzaro LA, Eichstetter I, Iozzo RV. The human decorin gene: intron-exon organization, discovery of two alternatively spliced exons in the 5′ untranslated region, and mapping of the gene to chromosome 12q23. Genomics. 1993;15:146–160. doi: 10.1006/geno.1993.1022. [DOI] [PubMed] [Google Scholar]
- 24.Santra M, Danielson KG, Iozzo RV. Structural and functional characterization of the human decorin gene promoter. J Biol Chem. 1994;269:579–587. [PubMed] [Google Scholar]
- 25.Mauviel A, Korang K, Santra M, Tewari D, Uitto J, Iozzo RV. Identification of a bimodal regulatory element encompassing a canonical AP-1 binding site in the proximal promoter region of the human decorin gene. J Biol Chem. 1996;271:24824–24829. doi: 10.1074/jbc.271.40.24824. [DOI] [PubMed] [Google Scholar]
- 26.Iozzo RV, Danielson KG. Transcriptional and post-transcriptional control of proteoglycan gene expression. Progr Nucl Acids Res Mol Biol. 1999;62:19–53. doi: 10.1016/s0079-6603(08)60504-8. [DOI] [PubMed] [Google Scholar]
- 27.Iozzo RV, Moscatello D, McQuillan DJ, Eichstetter I. Decorin is a biological ligand for the epidermal growth factor receptor. J Biol Chem. 1999;274:4489–4492. doi: 10.1074/jbc.274.8.4489. [DOI] [PubMed] [Google Scholar]
- 28.Moscatello DK, Santra M, Mann DM, McQuillan DJ, Wong AJ, Iozzo RV. Decorin suppresses tumor cell growth by activating the epidermal growth factor receptor. J Clin Investig. 1998;101:406–412. doi: 10.1172/JCI846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel S, Santra M, McQuillan DJ, Iozzo RV, Thomas AP. Decorin activates the epidermal growth factor receptor and elevates cytosolic Ca2+ in A431 cells. J Biol Chem. 1998;273:3121–3124. doi: 10.1074/jbc.273.6.3121. [DOI] [PubMed] [Google Scholar]
- 30.Santra M, Eichstetter I, Iozzo RV. An anti-oncogenic role for decorin: downregulation of ErbB2 leads to growth suppression and cytodifferentiation of mammary carcinoma cells. J Biol Chem. 2000;275:35153–35161. doi: 10.1074/jbc.M006821200. [DOI] [PubMed] [Google Scholar]
- 31.Csordás G, Santra M, Reed CC, Eichstetter I, McQuillan DJ, Gross D, Nugent MA, Hajnóczky G, Iozzo RV. Sustained down-regulation of the epidermal growth factor receptor by decorin. A mechanism for controlling tumor growth in vivo. J Biol Chem. 2000;275:32879–32887. doi: 10.1074/jbc.M005609200. [DOI] [PubMed] [Google Scholar]
- 32.Santra M, Reed CC, Iozzo RV. Decorin binds to a narrow region of the epidermal growth factor (EGF) receptor, partially overlapping with but distinct from the EGF-binding epitope. J Biol Chem. 2002;277:35671–35681. doi: 10.1074/jbc.M205317200. [DOI] [PubMed] [Google Scholar]
- 33.Goldoni S, Owens RT, McQuillan DJ, Shriver Z, Sasisekharan R, Birk DE, Campbell S, Iozzo RV. Biologically active decorin is a monomer in solution. J Biol Chem. 2004;279:6606–6612. doi: 10.1074/jbc.M310342200. [DOI] [PubMed] [Google Scholar]
- 34.Zhu J-X, Goldoni S, Bix G, Owens RA, McQuillan D, Reed CC, Iozzo RV. Decorin evokes protracted internalization and degradation of the EGF receptor via caveolar endocytosis. J Biol Chem. 2005;280:32468–32479. doi: 10.1074/jbc.M503833200. [DOI] [PubMed] [Google Scholar]
- 35.Barash U, Cohen-Kaplan V, Dowek I, Ilan N, Vlodawsky I. Proteoglycan roles in health and disease: Heparanase function in tumor progression and metastasis, new concepts arising. FEBS J. 2010 doi: 10.1111/j.1742-4658.2010.07799.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldoni S, Iozzo RV. Tumor microenvironment: Modulation by decorin and related molecules harboring leucine-rich tandem motifs. Int J Cancer. 2008;123:2473–2479. doi: 10.1002/ijc.23930. [DOI] [PubMed] [Google Scholar]
- 37.Seidler DG, Goldoni S, Agnew C, Cardi C, Thakur ML, Owens RA, McQuillan DJ, Iozzo RV. Decorin protein core inhibits in vivo cancer growth and metabolism by hindering epidermal growth factor receptor function and triggering apoptosis via caspase-3 activation. J Biol Chem. 2006;281:26408–26418. doi: 10.1074/jbc.M602853200. [DOI] [PubMed] [Google Scholar]
- 38.Wu H, Wang S, Xue A, Liu Y, Liu Y, Liu Y, Wang H, Chen Q, Guo M, Zhang Z. Overexpression of decorin induces apoptosis and cell growth arrest in cultured rat mesangial cells in vitro. Nephrology. 2008;13:607–615. doi: 10.1111/j.1440-1797.2008.00961.x. [DOI] [PubMed] [Google Scholar]
- 39.Tralhão JG, Schaefer L, Micegova M, Evaristo C, Schönherr E, Kayal S, Veiga-Fernandes H, Danel C, Iozzo RV, Kresse H, et al. In vivo selective and distant killing of cancer cells using adenovirus-mediated decorin gene transfer. FASEB J. 2003;17:464–466. doi: 10.1096/fj.02-0534fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iozzo RV, Chakrani F, Perrotti D, McQuillan DJ, Skorski T, Calabretta B, Eichstetter I. Cooperative action of germline mutations in decorin and p53 accelerates lymphoma tumorigenesis. Proc Natl Acad Sci USA. 1999;96:3092–3097. doi: 10.1073/pnas.96.6.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bi X, Tong C, Dokendorff A, Banroft L, Gallagher L, Guzman-Hartman G, Iozzo RV, Augenlicht LH, Yang W. Genetic deficiency of decorin causes intestinal tumor formation through disruption of intestinal cell maturation. Carcinogenesis. 2008;29:1435–1440. doi: 10.1093/carcin/bgn141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reed CC, Gauldie J, Iozzo RV. Suppression of tumorigenicity by adenovirus-mediated gene transfer of decorin. Oncogene. 2002;21:3688–3695. doi: 10.1038/sj.onc.1205470. [DOI] [PubMed] [Google Scholar]
- 44.Reed CC, Waterhouse A, Kirby S, Kay P, Owens RA, McQuillan DJ, Iozzo RV. Decorin prevents metastatic spreading of breast cancer. Oncogene. 2005;24:1104–1110. doi: 10.1038/sj.onc.1208329. [DOI] [PubMed] [Google Scholar]
- 45.Biglari A, Bataille D, Naumann U, Weller M, Zirger J, Castro MG, Lowenstein PR. Effects of ectopic decorin in modulating intracranial glioma progression in vivo, in a rat syngeneic model. Cancer Gene Therapy. 2004;11:721–732. doi: 10.1038/sj.cgt.7700783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X, Pennisi A, Yaccoby S. Role of decorin in the antimyeloma effects of osteoblasts. Blood. 2008;112:159–168. doi: 10.1182/blood-2007-11-124164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldoni S, Seidler DG, Heath J, Fassan M, Baffa R, Thakur ML, Owens RA, McQuillan DJ, Iozzo RV. An anti-metastatic role for decorin in breast cancer. Am J Pathol. 2008;173:844–855. doi: 10.2353/ajpath.2008.080275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shintani K, Matsumine A, Kusuzaki K, Morikawa J, Matsubara T, Wakabayashi T, Araki K, Satonaka H, Wakabayashi H, Lino T, et al. Decorin suppresses lung metastases of murine osteosarcoma. Oncology Reports. 2008;19:1533–1539. [PubMed] [Google Scholar]
- 49.Grant DS, Yenisey C, Rose RW, Tootell M, Santra M, Iozzo RV. Decorin suppresses tumor cell-mediated angiogenesis. Oncogene. 2002;21:4765–4777. doi: 10.1038/sj.onc.1205595. [DOI] [PubMed] [Google Scholar]
- 50.Hu Y, Sun H, Owens RT, Wu J, Chen YQ, Berquin IM, Perry D, O’Flaherty JT, Edwards IJ. Decorin suppresses prostate tumor growth through inhibition of epidermal growth factor and androgen receptor pathways. Neoplasia. 2009;11:1042–1053. doi: 10.1593/neo.09760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zafiropoulos A, Nikitovic D, Katonis P, Tsatsakis A, Karamanos NK, Tzanakakis GN. Decorin-induced growth inhibition is overcome through protracted expression and activation of epidermal growth factor receptors in osteosarcoma cells. Mol Cancer Res. 2008;6:785–794. doi: 10.1158/1541-7786.MCR-07-0165. [DOI] [PubMed] [Google Scholar]
- 52.Zafiropoulos A, Tzanakakis GN. Decorin-mediated effects in cancer cell biology. Connective Tissue Res. 2008;49:244–248. doi: 10.1080/03008200802147746. [DOI] [PubMed] [Google Scholar]
- 53.Goldoni S, Hunphries A, Nyström A, Sattar S, Owens RT, McQuillan DJ, Ireton K, Iozzo RV. Decorin is a novel antagonistic ligand of the Met receptor. J Cell Biol. 2009;185:743–754. doi: 10.1083/jcb.200901129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Theocharis AD, Tzanakakis G, Karamanos NK. Proteoglycan roles in health and disease: Novel proteoglycan roles in malignancy and their pharmacological targeting. FEBS J. 2010 doi: 10.1111/j.1742-4658.2010.07800.x. In press. [DOI] [PubMed] [Google Scholar]
- 55.Schönherr E, Sunderkötter C, Iozzo RV, Schaefer L. Decorin, a novel player in the insulin-like growth factor system. J Biol Chem. 2005;280:15767–15772. doi: 10.1074/jbc.M500451200. [DOI] [PubMed] [Google Scholar]
- 56.Schaefer L, Tsalastra W, Babelova A, Baliova M, Minnerup J, Sorokin L, Gröne H-J, Reinhardt DP, Pfeilschifter J, Iozzo RV, et al. Decorin-mediated regulation of fibrillin-1 in the kidney involves the insulin-like growth factor-1 receptor and mammalian target of rapamycin. Am J Pathol. 2007;170:301–315. doi: 10.2353/ajpath.2007.060497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Merline R, Lazaroski S, Babelova A, Tsalastra-Greul W, Pfeilschifter J, Schluter KD, Gunther A, Iozzo RV, Schaefer RM, Schaefer L. Decorin deficiency in diabetic mice: aggravation of nephropathy due to overexpression of profibrotic factors, enhanced apoptosis and mononuclear cell infiltration. J Physiol Pharmacol. 2009;60 (suppl 4):5–13. [PMC free article] [PubMed] [Google Scholar]
- 58.Schaefer L, Macakova K, Raslik I, Micegova M, Gröne H-J, Schönherr E, Robenek H, Echtermeyer FG, Grässel S, Bruckner P, et al. Absence of decorin adversely influences tubulointerstitial fibrosis of the obstructed kidney by enhanced apoptosis and increased inflammatory reaction. Am J Pathol. 2002;160:1181–1191. doi: 10.1016/S0002-9440(10)64937-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Porst M, Plank C, Bieritz B, Konik E, Dötsch J, Hilgers KF, Reinhardt DP, Hartner A. Fibrillin-1 regulates mesangial cell attachment, spreading, migration and proliferation. Kidney Int. 2016;69:450–456. doi: 10.1038/sj.ki.5000030. [DOI] [PubMed] [Google Scholar]
- 60.Fiedler LR, Schönherr E, Waddington R, Niland S, Seidler DG, Aeschlimann D, Eble JA. Decorin regulates endothelial cell motility on collagen I through activation of Insulin-like grwoth factor I receptor and modulation of α2β1 integrin activity. J Biol Chem. 2008;283:17406–17415. doi: 10.1074/jbc.M710025200. [DOI] [PubMed] [Google Scholar]
- 61.Iacob D, Cai J, Tsonis M, Babwah A, Chakraborty RN, Lala PK. Decorin-mediated inhibition of proliferation and migration of the human trophoblast via different tyrosine kinase receptors. Endocrinology. 2008;149:6187–6197. doi: 10.1210/en.2008-0780. [DOI] [PubMed] [Google Scholar]
- 62.Brandan E, Retamal C, Cabello-Verrugio C, Marzolo M-P. The low density lipoprotein receptor-related protein functions as an endocytic receptor for decorin. J Biol Chem. 2006;281:31562–31571. doi: 10.1074/jbc.M602919200. [DOI] [PubMed] [Google Scholar]
- 63.Feugaing DDS, Tammi R, Echtermeyer FG, Stenmark H, Kresse H, Smollich M, Schönherr E, Kiesel L, Götte M. Endocytosis of the dermatan sulfate proteoglycan decorin utilizes multiple pathways and is modulated by epidermal growth factor receptor signaling. Biochimie. 2007;89:637–657. doi: 10.1016/j.biochi.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 64.Schaefer L. Extracellular matrix molecules: endogenous danger signals as new drug targets in kidney diseases. Curr Opin Pharmacol. 2010;10:185–190. doi: 10.1016/j.coph.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 65.Schaefer L, Babelova A, Kiss E, Hausser H-J, Baliova M, Krzyzankova M, Marsche G, Young MF, Mihalik D, Gotte M, et al. The matrix component biglycan is proinflammatory and signals through toll-like receptors 4 and 2 in macrophages. J Clin Invest. 2005;115:2223–2233. doi: 10.1172/JCI23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Babelova A, Moreth K, Tsalastra-Greul W, Zeng-Brouwers J, Eickelberg O, Young MF, Bruckner P, Pfeilschifter J, Schaefer RM, Gröne H-J, et al. Biglycan, a danger signal that activates the NLRP3 inflammasome via Toll-like and P2X receptors. J Biol Chem. 2009;284:24035–24048. doi: 10.1074/jbc.M109.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yan W, Wank P, Zhao CX, Tang J, Xiao X, Wang DW. Decorin gene delivery inhibits cardiac fibrosis in spontaneously hypertensive rats by modulation of transforming growth factor-β/Smad and p38 mitogen-activated protein kinase signaling pathways. Hum Gene Ther. 2009;20:1190–1199. doi: 10.1089/hum.2008.204. [DOI] [PubMed] [Google Scholar]
- 68.Derbali H, Bossé Y, Côté N, Pibarot P, Audet A, Pépin A, Arsenault B, Couture C, Després J-P, Mathieu P. Increased biglycan in aortic valve stenosis leads to the overexpression of phospholipid transfer protein via Toll-like receptor 2. Am J Pathol. 2010;176:2638–2645. doi: 10.2353/ajpath.2010.090541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, Li L, Leet AI, Seo B-M, Zhang L, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 70.Chen X-D, Fisher LW, Robey PG, Young MF. The small leucine-rich proteoglycan biglycan modulates BMP-4-induced osteoblast differentiation. FASEB J. 2004;18:948–958. doi: 10.1096/fj.03-0899com. [DOI] [PubMed] [Google Scholar]
- 71.Moreno M, Muñoz R, Aroca F, Labarca M, Brandan E, Larraín J. Biglycan is a new extracellular component of the chordin-BMP4 signaling pathway. EMBO J. 2005;24:1397–1405. doi: 10.1038/sj.emboj.7600615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li Y, Aoki T, Mori Y, Ahmad M, Miyamori H, Takino T, Sato H. Cleavage of lumican by membrane-type matrix metalloprotease-1 abrogates this proteoglycan-mediated suppression of tumor cell colony formation in soft agar. Cancer Res. 2004;64:7058–7064. doi: 10.1158/0008-5472.CAN-04-1038. [DOI] [PubMed] [Google Scholar]
- 73.Manon-Jensen T, Itoh Y, Couchman JR. Proteoglycan roles in health and disease: The multiple roles of syndecan shedding. FEBS J. 2010 doi: 10.1111/j.1742-4658.2010.07798.x. In press. [DOI] [PubMed] [Google Scholar]
- 74.Yoshioka N, Inoue H, Nakanishi K, Oka K, Yutsudo M, Yamashita A, Hakura A, Nojima H. Isolation of transformation suppressor genes by cDNA substraction: Lumican suppresses transformation induced by v-src and v-K-ras. J Virol. 2000;74:1008–1013. doi: 10.1128/jvi.74.2.1008-1013.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vij N, Roberts L, Joyce S, Chakravarti S. Lumican suppresses cell proliferation and aids Fas-Fas ligand mediated apoptosis: implications in the cornea. Exp Eye Res. 2004;78:957–971. doi: 10.1016/j.exer.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 76.Vij N, Roberts L, Joyce S, Chakravarti S. Lumican regulates corneal inflammatory responses by modulating Fas-Fas ligand signaling. Invest Ophthalmol Vis Sci. 2005;46:88–95. doi: 10.1167/iovs.04-0833. [DOI] [PubMed] [Google Scholar]
- 77.Sifaki M, Assouti M, Nikitovic D, Krasagakis K, Karamanos NK, Tzanakakis GN. Lumican, a small leucine-rich proteoglycan substituted with keratan sulfate chains is expressed and secreted by human melanoma cells and not normal melanocytes. IUBMB Life. 2008;58:606–610. doi: 10.1080/15216540600951605. [DOI] [PubMed] [Google Scholar]
- 78.Brézillon S, Venteo L, Ramont L, D’Onofrio M-F, Perreau C, Pluot M, Maquart F-X, Wegrowski Y. Expression of lumican, a small leucine-rich proteoglycan with antitumour activity, in human malignant melanoma. Clin Exp Derm. 2007;32:405–416. doi: 10.1111/j.1365-2230.2007.02437.x. [DOI] [PubMed] [Google Scholar]
- 79.Vuillermoz B, Khoruzhenko A, D’Onofrio MF, Ramont L, Venteo L, Perreau C, Antonicelli F, Maquart FX, Wegrowski Y. The small leucine-rich proteoglycan lumican inhibits melanoma progression. Exp Cell Res. 2004;296:294–306. doi: 10.1016/j.yexcr.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 80.Brézillon S, Zeltz C, Schneider L, Terryn C, Vuillermoz B, Ramont L, Perreau C, Pluot M, Diebold MD, Radwanska A, et al. Lumican Inhibits B16F1 melanoma cell lung metastasis. J Physiol Pharmacol. 2009;60 (suppl 4):15–22. [PubMed] [Google Scholar]
- 81.D’Onofrio M-F, Brézillon S, Baranek T, Perreau C, Roughley P, Maquart F-X, Wegrowski Y. Identification of β1 integrin as mediator of melanoma cell adhesion to lumican. Biochem Biophys Res Commun. 2008;365:266–272. doi: 10.1016/j.bbrc.2007.10.155. [DOI] [PubMed] [Google Scholar]
- 82.Brézillon S, Radwanska A, Zeltz C, Malkowski A, Ploton D, Bobichon H, Perreau C, Malicka-Blaszkiewicz M, Maquart F-X, Wegrowski Y. Lumican core protein inhibits melanoma cell migration via alterations of focal adhesion compleses. Cancer Lett. 2009;283:92–100. doi: 10.1016/j.canlet.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 83.Ferdous Z, Peterson SB, Tseng H, Anderson DK, Iozzo RV, Grande-Allen KJ. A role for decorin in controlling proliferation, adhesion, and migration of murine embryonic fibroblasts. J Biomed Mater Res Part A. 2010;93:419–428. doi: 10.1002/jbm.a.32545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee S, Bowrin K, Hamad AR, Chakravarti S. Extracellular matrix lumican deposited on the surface of neutrophils promotes migration by binding to β2 integrin. J Biol Chem. 2009;284:23662–23669. doi: 10.1074/jbc.M109.026229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu F, Vij N, Roberts L, Lopez-Briones S, Joyce S, Chakravarti S. A novel role of the lumican core protein in bacterial lipopolysaccharide-induced innate immune response. J Biol Chem. 2007;282:26409–26417. doi: 10.1074/jbc.M702402200. [DOI] [PubMed] [Google Scholar]
- 86.Funderburgh JL, Mitschler RR, Funderburgh ML, Roth MR, Chapes SK, Conrad GW. Macrophage receptors for lumican, a corneal keratan sulfate proteoglycan. Invest Ophthalmol Vis Sci. 1997;38:1159–1167. [PubMed] [Google Scholar]
- 87.Carlson EC, Lin M, Liu C-Y, Kao WWY, Perez VL, Pearlman E. Keratocan and lumican regulate neutrophil infiltration and corneal clarity in lipopolysaccharide-induced keratitis by direct interaction with CXCL1. J Biol Chem. 2007;282:33502–33509. doi: 10.1074/jbc.M705823200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Häkkinen L, Strassburger S, Kahari VM, Scott PG, Eichstetter I, Iozzo RV, Larjava H. A role for decorin in the structural organization of periodontal ligament. Lab Invest. 2000;80:1869–1880. doi: 10.1038/labinvest.3780197. [DOI] [PubMed] [Google Scholar]
- 89.Weis SM, Zimmerman SD, Shah M, Covell JW, Omens JH, Ross J, Jr, Dalton N, Jones Y, Reed CC, Iozzo RV, et al. A role for decorin in the remodeling of myocardial infarction. Matrix Biol. 2005;24:313–324. doi: 10.1016/j.matbio.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 90.Järveläinen H, Puolakkainen P, Pakkanen S, Brown EL, Höök M, Iozzo RV, Sage H, Wight TN. A role for decorin in cutaneous wound healing and angiogenesis. Wound Rep Reg. 2006;14:443–452. doi: 10.1111/j.1743-6109.2006.00150.x. [DOI] [PubMed] [Google Scholar]
- 91.Bi Y, Stueltens CH, Kilts T, Wadhwa S, Iozzo RV, Robey PG, Chen X-D, Young MF. Extracellular matrix proteoglycans control the fate of bone marrow stromal cells. J Biol Chem. 2005;280:30481–30489. doi: 10.1074/jbc.M500573200. [DOI] [PubMed] [Google Scholar]
- 92.Wadhwa S, Bi Y, Ortiz AT, Embree MC, Kilts T, Iozzo R, Opperman LA, Young MF. Impaired posterior frontal sutural fusion in the biglycan/decorin double deficient mice. Bone. 2007;40:861–866. doi: 10.1016/j.bone.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Williams KJ, Qiu G, Usui HK, Dunn SR, McCue P, Bottinger E, Iozzo RV, Sharma K. Decorin deficiency enhances progressive nephropathy in diabetic mice. Am J Pathol. 2007;171:1441–1450. doi: 10.2353/ajpath.2007.070079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Robinson PS, Lin TW, Jawad AF, Iozzo RV, Soslowsky LJ. Investigating tendon fascicle structure-function relationship in a transgenic age mouse model using multiple regression models. Ann Biomed Eng. 2004;32:924–931. doi: 10.1023/b:abme.0000032455.78459.56. [DOI] [PubMed] [Google Scholar]
- 95.Zhang G, Ezura Y, Chervoneva I, Robinson PS, Beason DP, Carine ET, Soslowsky LJ, Iozzo RV, Birk DE. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem. 2006;98:1436–1449. doi: 10.1002/jcb.20776. [DOI] [PubMed] [Google Scholar]
- 96.Salemo FG, Pinelli V, Pini L, Tuma B, Iozzo RV, Ludwig MS. Effect of PEEP on induced constriction is enhanced in decorin-deficient mice. Am J Physiol. 2007;293:L1111–1117. doi: 10.1152/ajplung.00095.2007. [DOI] [PubMed] [Google Scholar]
- 97.Fust A, LeBellego F, Iozzo RV, Roughley PJ, Ludwig MS. Alterations in lung mechanics in decorin deficient mice. Am J Physiol Lung Cell Mol Physiol. 2005;288:L159–L166. doi: 10.1152/ajplung.00089.2004. [DOI] [PubMed] [Google Scholar]
- 98.Zhang G, Chen S, Goldoni S, Calder BW, Simpson HC, Owens RT, McQuillan DJ, Young MF, Iozzo RV, Birk DE. Genetic evidence for the coordinated regulation of collagen fibrillogenesis in the cornea by decorin and biglycan. J Biol Chem. 2009;284:8888–8897. doi: 10.1074/jbc.M806590200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Haruyama N, Sreenath TL, Suzuki S, Yao X, Wang Z, Wang Y, Honeycutt C, Iozzo RV, Young MF, Kulkarni AB. Genetic evidence for key roles of decorin and biglycan in dentin mineralization. Matrix Biol. 2009;28:129–136. doi: 10.1016/j.matbio.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sanches JCT, Jones CJP, Aplin JD, Iozzo RV, Zorn TMT, Oliveira SF. Collagen fibril organization in the pregnant endometrium of decorin-deficient mice. J Anat. 2010;216:144–155. doi: 10.1111/j.1469-7580.2009.01170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Embree MC, Kilts TM, Ono M, Inkson CA, Seyed-Picard F, Karsdal MA, Oldberd Å, Bi Y, Young MF. Biglycan and fibromodulin have essential roles in regulating chondrogenesis and extracellular matrix turnover in temporomandibular joint osteoarthritis. Am J Pathol. 2010;176:812–826. doi: 10.2353/ajpath.2010.090450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zoeller JJ, Pimtong W, Corby H, Goldoni S, Iozzo AE, Owens RT, Ho S-Y, Iozzo RV. A central role for decorin during vertebrate convergent extension. J Biol Chem. 2009;284:11728–11737. doi: 10.1074/jbc.M808991200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ferdous Z, Wei VM, Iozzo RV, Höök M, Grande-Allen KJ. Decorin-transforming growth factor-β interaction regulates matrix organization and mechanical characteristics of three-dimensional collagen matrices. J Biol Chem. 2007;282:35887–35898. doi: 10.1074/jbc.M705180200. [DOI] [PubMed] [Google Scholar]
- 104.Ferdous Z, Lazaro LD, Iozzo RV, Höök M, Grande-Allen KJ. Influence of cyclic strain and decorin deficiency on 3D cellularized collagen matrices. Biomaterials. 2008;29:2740–2748. doi: 10.1016/j.biomaterials.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rühland C, Schönherr E, Robenek H, Hansen U, Iozzo RV, Bruckner P, Seidler DG. The glycosaminoglycan chain of decorin plays an important role in collagen fibril formation at the early stages of fibrillogenesis. FEBS J. 2007;274:4246–4255. doi: 10.1111/j.1742-4658.2007.05951.x. [DOI] [PubMed] [Google Scholar]