Abstract
The primary objective of the current prospective study was to examine developmental patterns of voxel-by-voxel gray and white matter volumes (GMV, WMV, respectively) that would predict psychosis in adolescents with 22q11.2 deletion syndrome (22q11.2DS), the most common known genetic risk factor for schizophrenia. We performed a longitudinal voxel-based morphometry analysis using structural T1 MRI scans from 19 individuals with 22q11.2DS and 18 typically developing individuals. In 22q11.2DS, univariate analysis showed that greater reduction in left dorsal prefrontal cortical (dPFC) GMV over time predicted greater psychotic symptoms at Time2. This dPFC region also showed significantly reduced volumes in 22q11.2DS compared to typically developing individuals at Time1 and 2, greater reduction over time in 22q11.2DS COMTMet compared to COMTVal, and greater reduction in those with greater decline in verbal IQ over time. Leave- one-out multivariate pattern analysis (MVPA) on the other hand, showed that patterns of GM and WM morphometric changes over time in regions including but not limited to the dPFC predicted risk for psychotic symptoms (94.7-100% accuracy) significantly better than using univariate analysis (63.1%). Additional predictive brain regions included medial PFC and dorsal cingulum. This longitudinal prospective study shows novel evidence of morphometric spatial patterns predicting the development of psychotic symptoms in 22q11.2DS, and further elucidates the abnormal maturational processes in 22q11.2DS. The use of neuroimaging using MVPA may hold promise to predict outcome in a variety of neuropsychiatric disorders.
1. Introduction
The 22q11.2DS, also known as velocardiofacial syndrome (Shprintzen et al., 1978), is the most common microdeletion syndrome in humans occurring in at least 1 to 5,000 live births (Botto et al., 2003). It has been shown that at least 25% of individuals with 22q11.2DS develop a schizophrenia-like psychosis by young adulthood (Murphy, Jones, & Owen, 1999). Being the most common identifiable genetic risk factor for schizophrenia, 22q11.2DS serves as an important model from which to elucidate the path leading from a well defined genetic defect to variation in brain development and eventually to the evolution of psychotic symptoms.
Research has shown links between the development of psychotic symptoms and VIQ decline or catechol-O-methyltransferase (COMT) hemizygosity(Gothelf et al., 2005), but no studies have demonstrated whether neuroanatomical patterns can predict the development of psychotic symptoms in 22q11.2DS. This may be due to the fact that past longitudinal studies have used univariate analysis of more crude volumetric or lobar volume measures (Gothelf et al., 2005) rather than multivariate analysis of voxel-based measures, which could be a more sensitive and powerful measure in detecting subtle regional changes. Indeed, studies have begun to elucidate neuroanatomical patterns that predict disease transition in at-risk mental states of psychosis (Koutsouleris et al., 2009). Therefore, the main purpose of the current study was to identify neuroanatomical patterns that predicted risk of psychotic symptoms with high accuracy using cross-validation support vector machine (SVM) algorithms and to compare that with univariate methods.
2. Methods
2.1. Subjects
Time1 and Time2 data included 19 children with 22q11.2DS and 18 typically developing (TD) controls. The presence of the 22q11.2 microdeletion was confirmed in all subjects with 22q11.2DS by fluorescence in situ hybridization (FISH). All controls were screened and were not included in the study if they had a history of major psychiatric disorder or neurological or cognitive impairment. The follow-up interval was 4.9 ± 0.7 for the 22q11.2DS group and 4.9 ± 0.9 years for the controls. The demographic and clinical characteristics of the sample are presented in Table 1. None of the subjects had history of substance abuse and the sample was well matched across diagnostic groups in mean age, parents’ years of education, male to female ratio, ethnicity, and handedness (Gothelf, Penniman, Gu, Eliez, & Reiss, 2007). None of the subjects had a psychotic disorder at Time1. In general, the 22q11.2DS group had significantly lower IQ scores compared to the TD group. There were also significant IQ interactions such that the TD group showed general increase while the 22q11.2DS group showed a general decrease in IQ over time.
Table 1.
Demographic Information for the 22q11.2DS and TD Groups
| 22q11.2DS | Controls | ANOVA | T-test, Chi-square | |||||
|---|---|---|---|---|---|---|---|---|
| Time 1 | Time 2 | Time 1 | Time 2 | P (group) | P (time) | P (interaction) | P | |
| N | 19 | 18 | ||||||
| Age | 13.05 | 17.92 | 13.40 | 18.31 | 0.78 | <0.001 | 0.90 | |
| 3.96 | 3.81 | 4.04 | 4.48 | |||||
| Gender (F:M) | 8:11 | 8:10 | 0.89 | |||||
| Handedness (Lt:Mixed:Rt) | 2:1:16 | 0:0:18 | 0.21 | |||||
| COMT (Met:Val) | 11:8 | |||||||
| BPRS | 36.32 | |||||||
| 12.59 | ||||||||
| VIQ | 80.05 | 75.50 | 114.82 | 118.50 | <0.001 | 0.16 | 0.001 | 22q11.2DS T1 > T2: 0.09 Controls T1 < T2: 0.04 |
| 14.31 | 15.4 | 8.52 | 10.44 | |||||
| GMV [ml] | 795.98 | 760.87 | 872.49 | 833.27 | 0.006 | <0.001 | 0.74 | |
| 80.61 | 89.85 | 70.45 | 78.44 | |||||
| WMV [ml] | 389.81 | 410.89 | 438.49 | 450.91 | 0.02 | <0.001 | 0.17 | |
| 55.56 | 57.05 | 54.06 | 51.12 | |||||
Notes:  :Not applicable
:Not applicable
Italicized font: Standard deviation
By the time of the Time2 scan, 10 participants with 22q11.2DS had received atypical antipsychotics (6 subjects) or mood stabilizers (10 subjects) for more than six months. All six subjects receiving antipsychotics had a psychotic disorder. After providing a complete description of the study to the subjects and their parents, written informed consent was obtained at both time points, according to protocols approved by the institutional review board at Stanford University School of Medicine.
2.2. Genotyping
Blood samples were drawn from the 22q11.2DS group to determine genotype. The COMT Val108/158Met polymorphism (rs165688) was genotyped using a standard method (Lachman et al., 1996). Eleven individuals had COMTMet and eight had COMTVal genotypes. The demographic and clinical characteristics of the sample are presented in Table 2.
Table 2.
Demographic Information for the 22q11.2DS COMTMet and COMTVal Groups
| 22q11.2DS Met | 22q11.2DS Val | ANOVA | T-test, Chi-square | |||||
|---|---|---|---|---|---|---|---|---|
| Time 1 | Time 2 | Time 1 | Time 2 | P (group) | P (time) | P (interaction) | P | |
| N | 11 | 8 | ||||||
| Age | 14.40 | 19.10 | 11.19 | 16.30 | 0.10 | <0.001 | 0.20 | |
| 3.78 | 3.59 | 3.61 | 3.71 | |||||
| Gender (F:M) | 5:6 | 3:5 | 0.55 | |||||
| Handedness (Lt:Mixed:Rt) | 2:0:9 | 0:1:7 | 0.24 | |||||
| COMT (Met:Val) | 11:0 | 0:8 | ||||||
| BPRS | 40.45 | 30.63 | 0.09 | |||||
| 12.38 | 11.17 | |||||||
| VIQ | 80.45 | 75.09 | 75.75 | 74.88 | 0.73 | 0.12 | 0.26 | |
| 11.93 | 12.57 | 20.05 | 19.04 | |||||
| GMV [ml] | 771.21 | 738.93 | 830.04 | 791.05 | 0.16 | <0.001 | 0.69 | |
| 77.56 | 89.97 | 76.33 | 86.01 | |||||
| WMV [ml] | 386.87 | 404.45 | 393.84 | 419.76 | 0.68 | <0.001 | 0.29 | |
| 52.88 | 53.25 | 62.55 | 64.53 | |||||
| CSF [ml] | 442.28 | 456.24 | 417.39 | 437.00 | 0.72 | 0.57 | 0.92 | |
| 143.98 | 158.19 | 100.71 | 160.07 | |||||
Notes:  :Not applicable
:Not applicable
Italicized font: Standard deviation
2.3. Cognitive and psychiatric measures
Cognitive and psychiatric assessments were conducted at both time points. For the cognitive assessment, the Wechsler Intelligence Scale for Children, 3rd edition (WISC III) was used for subjects 17 years and younger and the Wechsler Adult Intelligence Scale, 3rd edition (WAIS III) was used for subjects older than 17 years. For screening of psychotic disorders, the Screening Question portion of the Schedule for Affective Disorders and Schizophrenia for School Age Children-Present and Lifetime Version (K-SAD-PL) was used. In addition, subjects above the age of 18 years were also evaluated with the Structured Clinical Interview for DSM-IV Diagnoses (SCID). At Time2, all 22q11.DS individuals were tested by a child and adolescent psychiatrist, who completed the Brief Psychiatric Rating Scale (BPRS) to measure psychotic symptoms.
2.4. Magnetic resonance imaging (MRI) acquisition
All imaging data were acquired at the Richard M. Lucas Research Center (Stanford University, Palo Alto, CA USA) using the same Signa 1.5 T scanner (General Electric, Milwaukee, WI). Data were acquired at two time-points with a slow spoiled gradient echo (SPGR) sequence: flip angle=45°, repetition time (TR)=6 s, echo time (TE)=1 s, matrix size=256×256, field of view (FOV)=240×240 mm, pixel size=0.9375×0.9375 mm, slice number=124, thickness=1.5 mm.
2.5. Image processing: Voxel-based morphometry (VBM) analyses
VBM analyses of T1 MR images were performed using SPM5 (http://www.fil.ion.ucl.ac.uk/spm) and VBM5.1 (http://dbm.neuro.uni-jena.de/vbm). T1 images were bias corrected, segmented to GM, WM and cerebro-spinal fluid (CSF), spatially normalized and modulated, followed by smoothing with an isotropic Gaussian kernel with full-width at half-maximum (FWHM) of 12 mm. Since the results from standard and customized templates were essentially unchanged, the results from the standard template are reported here.
2.6. Statistical Analysis
2.6.1. Analyses of GM and WM volumes
We examined total GMV, WMV and total tissue volume (TTV, GMV+ WMV) obtained from VBM analyses using repeated measures analyses of variance (ANOVA).
2.6.2. VBM analysis
We examined regional GM and WM volume differences between 22q11.2DS and TD controls using whole-brain analysis of covariance (ANCOVA) covarying out age and total GMV / WMV (for regional GM and WM analyses, respectively). As supplementary analyses, VIQ was also included as a nuisance variable. Comparisons between 22q11.2DS and TD groups were performed also examining Time1 and Time2 data separately. Similarly, comparisons between Time1 and Time2 data were performed examining 22q11.2DS and TD groups separately.
2.6.3. Covariation between GM and WM volumes and BPRS, VIQ and COMT genotypes
Whole brain multiple regression analyses were performed with Time2 BPRS score as the outcome variable and change in GMV or WMV as the predictor. The effects of total GMV or WMV at Time1 and age were regressed out of these models. Within the brain regions that showed a significant effect, small-volume correction (SVC) was performed to examine whether there were significant differences between 22q11.2DS and TD groups at both Time1 and Time2.
Mean values from significant brain regions in the whole brain VBM multiple regression analyses described above were extracted for each subject (significant effects were only found for GMV). These values were then adjusted for age and total GMV by performing linear multiple regression analysis with age and total GMV as independent variables, and obtaining the residuals. These adjusted brain volumes were used to examine whether there were differences between 22q11.2DS individuals with COMTMet and COMTVal. In addition, decrease in VIQ as a function of time (i.e., VIQ slope=[Time2 VIQ − Time1 VIQ] / duration [yr]) was evaluated for correlations with adjusted brain volumes.
A statistical threshold with a joint-expected probability of p=0.01 with a correction for non-stationary cluster extent threshold (to correct for non-isotropic smoothness) was used in the VBM analyses. P = 0.05 corrected for family-wise-error (FWE) was used for SVC analysis.
2.6.4. Multivariate Pattern Analysis (MVPA)
We performed leave-one-out linear SVM analysis (regularization parameter C=1) using in-house tools based on Matlab. First, we constructed a class vector constituting either ‘+1’s and ‘-1’s depending on whether the individual with 22q11.2DS had more or less psychotic symptoms (median split, where median value was a BPRS score of 34). As expected, there was a significant difference in Time2 BPRS score (group with more symptoms: n=10, mean=45.6, SD=9.75; group with less symptoms: n=9, mean=26.0, SD=4.72; t(17)=5.47, p=0.00004).
Next, we converted contrast images into a S-by-N matrix where S is the number of subjects (19) and N is the number of features/voxels (4×4×4mm) and normalized the matrix so that mean=1 and SD=1. The number of features was reduced by recursive feature elimination iteratively, removing 30% of worst-discriminating voxels at a time until the performance started deteriorating. This was compared with classification performance based on the results from univariate GLM analyses, i.e., mean average of left dPFC GM volume, which showed significant negative correlation with Time2 BPRS score and change in regional GM volume, as the only feature. All procedures were performed by keeping training data, which were used to construct the classifier, and test data independent using leave-one-out cross-validation. Significance was determined using permutation analysis by randomly reassigning class labels 2000 times (p<0.05). Results were similar when classes were determined based on the existence of a full-blown psychotic disorder (cut-off: BPRS=35, psychosis: n=7).
3. Results
3.1 Between-group differences using univariate analysis
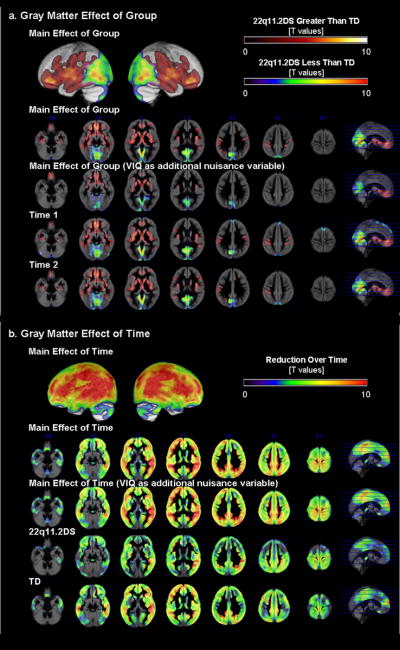
Summaries of baseline (Time1) and follow-up (Time2) brain TTV is presented in Table 1, and results of regional brain volume are presented in Table 3. When repeated measures ANCOVA was performed (total GMV and age as covariates), there was a main effect of diagnostic group, a main effect of time, but no significant interaction (p=0.01 corrected, Fig. 1a,b). Differences in regional GMV between 22q11.2DS and TD groups were very similar at Time1 and Time 2 with the 22q11.2DS group showing significantly reduced GMV in posterior medial parieto-occipital and cerebellar regions (posterior vermis, inferior semi-lunar lobule, uvula and pyramis, and anterior culmen), inferior / middle occipital, lingual, parahippocampal, posterior cingulate gyri, (pre)cuneus, and midbrain. In contrast, the 22q11.2DS group showed significantly greater GMV compared to TD in anterior medial cortical and sub-cortical regions: bilateral rectal, orbital, inferior / middle / superior / medial frontal, subcallosal, inferior / middle / superior / transverse temporal, supramarginal (and inferior parietal lobule), fusiform, parahippocampal gyri, anterior cingulate, insula, claustrum, thalamus, putamen, lateral global pallidus, caudate, uncus, hippocampus and midbrain red nucleus. Most GM regions, except for some midline cerebellar and medial cortical and subcortical structures, showed significant reduction over time in both the 22q11.2DS and TD groups. The results did not change when the statistical effects of VIQ were controlled in the model.
Table 3.
VBM Results (Note: Only peaks are reported here. See text for details.)
| Region | BA | Talairach Coordinates | T | P (corr) | Cluster | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| GRAY MATTER VOLUME | |||||||
| Main Effect of Group: Control > 22q11.2DS | |||||||
| Left Lingual, Posterior Cingulate Gyri, Cuneus | 7, 18, 30 | 0 | -72 | -1 | 8.75 | <0.001 | 62954 |
| -7 | -58 | 6 | 8.37 | ||||
| -10 | -66 | 30 | 6.69 | ||||
| Main Effect of Group: 22q11.2DS > Control | |||||||
| Left Inferior, Medial Frontal Gyri, Right Caudate Head | 47, 11 | -18 | 34 | -13 | 7.96 | <0.001 | 216349 |
| -8 | 46 | -12 | 7.41 | ||||
| 8 | 2 | 0 | 7.13 | ||||
| Main Effect of Time: Time 1 > Time 2 | |||||||
| Right Middle Temporal, Middle Occipital Gyri, Left Superior Temporal Gyrus | 37, 39 | 32 | -58 | 24 | 15.45 | <0.001 | 1173427 |
| 37 | -61 | 9 | 14.72 | ||||
| -32 | -57 | 29 | 13.82 | ||||
| Main Effect of Time: Time 2 > Time 1 | |||||||
| n.s. | |||||||
| Interaction | |||||||
| n.s | |||||||
| 22q11.2DS: Correlation between Decrease in GMV and Higher 2 BPRS Scores | |||||||
| Left Middle, Medial Frontal Gyri | 8, 9 | -25 | 25 | 43 | 4.76 | <0.001 | 28552 |
| -10 | 32 | 32 | 4.68 | ||||
| -9 | 28 | 47 | 4.59 | ||||
| WHITE MATTER VOLUME | |||||||
| Main Effect of Group: Control > 22q11.2DS | |||||||
| Left Cerebellar Region | 15 | -40 | -35 | 7.68 | 0.005 | 12797 | |
| -34 | -63 | -29 | 6.59 | ||||
| -3 | -39 | -16 | 5.96 | ||||
| Left Occipital, Parietel Regions | -4 | -81 | 1 | 7.49 | <0.001 | 23725 | |
| -9 | -74 | -2 | 6.59 | ||||
| -19 | -83 | 18 | 5.55 | ||||
| Main Effect of Group: 22q11.2DS > Control | |||||||
| Bilateral Medial Frontal, Left Parietal Regions | -9 | 23 | -10 | 7.7 | <0.001 | 125706 | |
| 9 | 29 | -4 | 7.48 | ||||
| 46 | -46 | 46 | 6.82 | ||||
| Main Effect of Time: Time 1 > Time 2 | |||||||
| n.s. | |||||||
| Main Effect of Time: Time 2 > Time 1 | |||||||
| Left Brainstem, Right Medial Frontal Region | -10 | -19 | -5 | 12.67 | <0.001 | 659495 | |
| -12 | -10 | -7 | 12.54 | ||||
| 13 | 2 | -2 | 12.14 | ||||
| Interaction: [22q11.2DS > Controls] × [Time 2 > Time 1] | |||||||
| Left Lateral Frontal, Right Medial Frontal Regions | -40 | 16 | 13 | 4.65 | <0.001 | 166654 | |
| 10 | -34 | 7 | 4.6 | ||||
| 12 | 4 | 37 | 4.56 | ||||
| Interaction: [Controls > 22q11.2DS] × [Time 2 > Time 1] | |||||||
| n.s. | |||||||
Fig 1.
Regional GMV results. a. Group differences between 22q11.2DS and TD groups are displayed by examining the main effect of group, and for Time1 and Time2 separately. b. Differences between Time1 and Time2 data are displayed by examining the main effect of time, and for 22q11.2DS and TD groups separately.
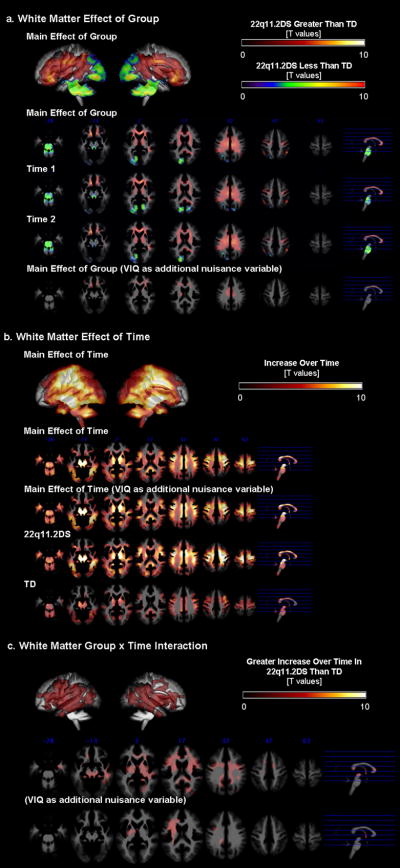
Spatial distribution (anterior-posterior, medial-lateral gradients) of regional WMV results was similar to that of the GMV results. When repeated-measures-ANCOVA was performed (total WMV and age as covariates), there was a main effect of diagnostic group (p=0.01 corrected, Fig. 2a). Both at Time1 and Time2, the 22q11.2DS group showed significantly lower regional WMV in the parieto-occipital and midline cerebellar regions. This was no longer significant when VIQ was included in the model. On the other hand, the 22q11.2DS group had significantly greater regional WMV in frontal and subcortical regions (results unchanged when VIQ was regressed out). There was also a main effect of time (p=0.01 corrected, Fig. 2b) such that WMV increased over time in both groups (results unchanged when VIQ included). The 22q11.2DS group however, had many more brain regions that showed increase in WMV over time. Reflecting this qualitative observation and unlike GMV, there was a significant interaction; regions mainly in the fronto-temporal regions exhibited significantly greater increase in WMV in the 22q11.2DS compared to the TD group (p=0.01 corrected, Fig. 2c). The interaction results did not change when VIQ was included as a covariate of uninterest in the model.
Fig 2.
Regional WMV results. a. Group differences between 22q11.2DS and TD groups are displayed by examining the main effect of group, and for Time1 and Time2 separately. b. Differences between Time1 and Time2 data are displayed by examining the main effect of time, and for 22q11.2DS and TD groups separately. C. Interaction effects between group (22q11.2DS and TD groups) and time (Time1 and Time2).
3.2. Associations between Longitudinal Changes in VBM and Time2 Psychotic Symptoms in the 22q11.2DS Group
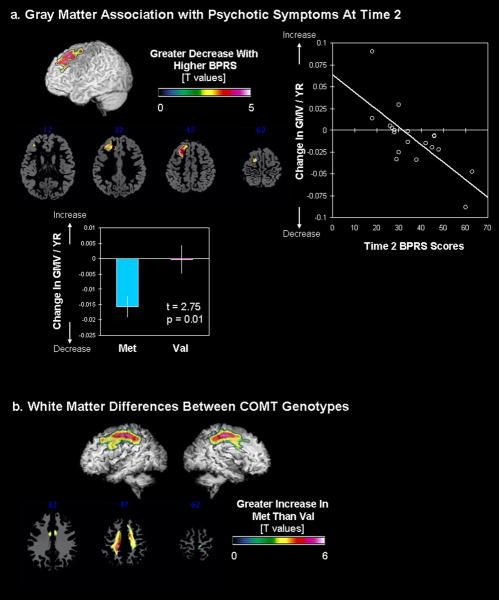
Whole-brain regression analysis revealed significant association between greater decrease in regional GMV of the left dorsal prefrontal cortex (dPFC) over time and greater psychotic symptoms as measured by higher BPRS scores (Fig. 3a). In addition, this prefrontal GM region was significantly reduced in the 22q11.2DS compared to the TD group at both Time1 and Time2 (Time1: t=4.09, p=0.017 SVC, extent threshold (ET) =272; Time2: t=4.02, p=0.021, SVC, ET=229). When individuals with 22q11.2DS were grouped based on COMT status, those with the Met genotype had significantly greater reduction in GMV over time in the same left dPFC region (t(17)=2.75, p=0.014, Fig. 3a). As expected, decrease in VIQ as a function of time (i.e., VIQ slope) was correlated with decrease in left dPFC GMV over time (i.e., those with greater decrease in GMV showed greater decline in VIQ over time were; r=0.46, p=0.05). There were no significant clusters in WM or CSF that predicted the severity of psychotic symptoms at Time2.
Fig. 3.
Covariation between brain regional volumes and Time2 psychotic symptoms and COMT genotypes. a. Brain regions that show significant correlation with GMV and Time2 BPRS scores (left). Extracted and adjusted brain volumes (for age and total GMV) are plotted against Time2 BPRS scores (right) as well as for COMTMet and COMTVal groups (below). b. Brain regions that show significant differences in WMV between COMTMet and COMTVal groups.
3.3. Associations between Longitudinal Changes in VBM and COMT Genotype
Besides the left dPFC region found to be significantly different with genotype status, there were significant differences in regional WMV in a large region along the cingulum to the superior longitudinal fasciculus / arcuate fasciculus (Fig. 3b). The Met group showed significantly greater increase in this WM region compared to the Val group.
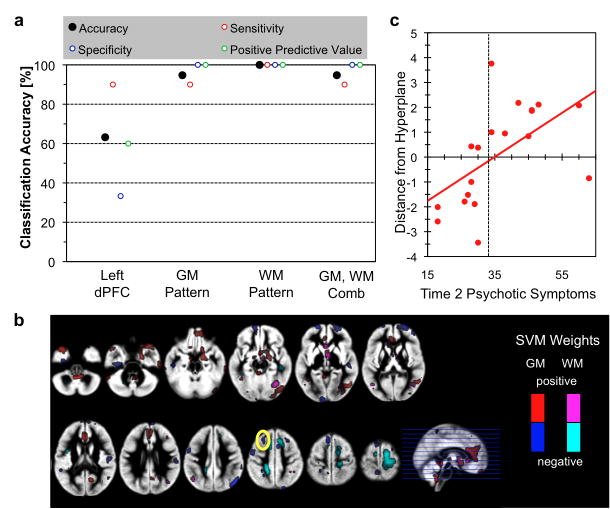
3.4. Multivariate Pattern Analyses Classifying those by Severity of Psychotic Symptoms
Regional GM volumes of left dPFC (cluster shown in Fig. 3a) as the only feature, accuracy of classifying 22q11.2DS individuals with more or less psychotic symptoms was 63.1% and was significantly better than chance (p < 0.05) (Fig. 4a). Results from whole brain pattern classification using voxel-by-voxel GM volumes (accuracy: 94.7%), WM volumes (accuracy: 100%) and a combination of GM and WM volumes (accuracy: 94.7%) were significantly better than the results of univariate analysis (all p’s<0.05), but not significantly different from each other (all p’s>0.1). Distance from the hyperplane of the classifiers for each subject showed significant correlation with Time2 BPRS scores for whole brain pattern classification analyses (GM: r=0.49, p=0.035; WM: r=0.73, p<0.001; GM and WM combined: r=0.70, p<0.001) but only a trend for significant effect for univariate analysis (r=0.42, p=0.072) (Fig. 4b). Patterns of voxels that contributed to the classifier included not only the left dPFC but also other regions such as the medial PFC (mPFC), right amygdala, orbitofrontal and dorsal cingulum (Fig. 4c).
Fig 4.
Multivariate pattern analysis results. a. Classification accuracy using left PFC GM volume as single feature (left PFC), patterns of whole-brain GM volume (GM), patterns of whole-brain WM volume (WM), and combination of GM and WM (GM+WM). b. Association between distance from hyperplane for the whole-brain GM and WM pattern classifier and BPRS scores. r=0.70, p<0.001. c. Morphometric patterns that discriminate between 22q11.2DS individuals with and without psychotic symptoms. Voxels that remained during the recursive feature elimination with positive weights are plotted in red (GM) and violet (WM), and with negative weights in blue (GM) and cyan (WM). Yellow solid circle indicate overlap with univariate analysis showing associations between GMV changes and Time2 psychotic symptoms.
4. Discussion
In this longitudinal study of 22q11.2DS adolescents, we show that later psychotic symptoms can be predicted by developmental changes in morphometric spatial GMV and WMV patterns with very high accuracy using cross-validated MVPA (94.7 and 100%, respectively), and significantly better than using univariate analysis of the PFC GMV (63.1%). We further show that longitudinal VBM analysis replicates and extends previous findings regarding the developmental neuroanatomical characteristics of 22q11.2DS and its association with the emergence of psychotic symptoms and COMT genotype.
In line with previous cross-sectional studies (Bearden et al., 2009; Bearden et al., 2007; Campbell et al., 2006; Eliez, Schmitt, White, & Reiss, 2000; Kates et al., 2001), we found reduced brain volumes in extensive parieto-occipital and cerebellar regions and the midbrain. Conversely, we found significantly increased GMV in anterior medial cortical and subcortical regions. As can be seen in Fig. 1a the antero-posterior between-group gradient of cortical development was observed at both time point measures. An even more dramatic dissociation between the anterior and posterior cortical poles occurs in another intriguing neurogenetic condition - Williams syndrome (Gothelf et al., 2008; Reiss et al., 2004). It is likely that haploinsufficiency of genes in these syndromes is responsible for this antero-posterior neuroanatomical developmental abnormality.
The cortical pattern of volume increase in frontal regions and decrease in posterior regions occurred for both GMV and WMV. While we did not find a group by time interaction for GMV there was a group by time interaction for WMV. There was a more robust increase in WMV in 22q11.2DS than in controls in extensive fronto-temporal brain regions (Fig. 2c). Several previous studies have demonstrated that WMV deficits, especially in posterior cortical regions, are common in 22q11.2DS (Campbell et al., 2006; Eliez et al., 2000; Kates et al., 2001; Simon et al., 2005; van Amelsvoort et al., 2004). In a previous longitudinal analysis of the same sample, using a coarse lobar dissection of the brain, we also observed decreased cranial WMV in adolescents with 22q11.2DS compared to controls (Gothelf et al., 2007). Our VBM results replicate these findings and further show that the accelerated growth in WMV is localized to fronto-temporal cortical regions. It is yet to be determined if the accelerated growth of fronto-temporal WM in adolescents with 22q11.2DS represents aberrant brain maturation or alternatively a functional “catch-up”.
In terms of the association between brain developmental trajectories and the emergence of psychotic symptoms, we found that decrease in the left dPFC over time correlated with the severity of psychotic symptoms at Time2. The same prefrontal cluster was reduced in size in 22q11.2DS individuals at both time points and was also more reduced in size in the 22q11.2DS subgroup carrying the COMTMet allele. Subjects with 22q11.2DS carrying the COMT Met allele putatively have very high levels of prefrontal cortical dopamine and this is likely to interfere with prefrontal cortical maturational processes especially during adolescence- a time of dramatic increase in cortical dopamine levels (Lambe, Krimer, & Goldman-Rakic, 2000). A few studies have shown that the COMT genotype affects prefrontal neuroanatomy (Gothelf et al., 2005; Kates et al., 2006; van Amelsvoort et al., 2008). In line with our results, Van Amelsvoort et al. (van Amelsvoort et al., 2008) found that 22q11.2DS adult COMTMet adult carriers had significantly smaller frontal lobe volumes. Kates et al.(Kates et al., 2006) found that dPFC volumes of COMTMet 22q11.2DS male children were decreased but were increased in COMTMet 22q11.2DS female carriers. In a previous analysis of the same cohort using a simplified (delimiting plane based) cortical parcellation approach, we found that prefrontal volumes declined significantly more in COMTMet compared to COMTVal 22q11.2DS carriers when followed from childhood to late adolescence-young adulthood (Gothelf et al., 2005). However, limitations of this and previous analyses investigating prefrontal neuroanatomy in 22q11.2DS includes the use of a simplified ROI based measure of the PFC (Gothelf et al., 2005; Kates et al., 2006) or cross-sectional samples only (Kates et al., 2006; Vorstman et al., 2008). The simplified PFC measure used in our previous studies may also explain why we were not able to find an association between the severity of psychotic symptoms and prefrontal neuroanatomy in these prior analyses (Gothelf et al., 2005; Gothelf et al., 2007). With the application of a MVPA approach, we were able to identify developmental changes in neuroanatomical patterns of GMV and WMV such as lesser left dPFC and dorsal cingulum, and greater mPFC, right amygdala and orbitofrontal cortex that predicted which 22q11.2DS adolescent would have greater or lesser psychotic symptoms with very high accuracy. This MVPA approach was significantly better than using univariate approaches. Further, how likely one were to be in one group versus the other based on neuroanatomical patterns (i.e., distance from the hyperplane) was associated with later psychotic symptoms, which further supports the validity of this approach. The MVPA is considered as a more sensitive method than the traditional univariate analysis and therefore identified additional regions, besides the dPFC, as contributing to the prediction of psychosis. MVPA is based on the hypothesis that multiple brain regions contribute to a disease progression. The results of our study suggests that dPFC is a very significant region in contributing to the prediction of psychosis as it shows up in univariate as well as multivariate analyses. The MVPA additionally shows the importance of these other regions that on their own may seemingly contribute very little, and hence are nonsignificant in the univariate analysis, but are detected by MVPA.
Several studies with healthy subjects and with subjects with schizophrenia found that the mPFC is strongly and consistently activated while individuals perform mentalizing tasks or perceive emotions (Brunet-Gouet & Decety, 2006). The mPFC was also found to be abnormally activated in patients with schizophrenia while performing tasks involving theory of mind and social cognition (Brunet-Gouet et al., 2006). Recent studies suggest that in addition to psychotic disorders, autism spectrum disorders are also common in 22q11.2DS (Kates et al., 2007). Hence, the abnormal development of the mPFC and yet to be identified other brain regions, possibly contribute to the psychotic and autistic phenotypes of 22q11.2DS. In a recent longitudinal analysis of European cohort of individuals with 22q11.2 DS and measuring cortical thickness, Schaer et al. also showed abnormal development of the prefrontal cortex in individuals with 22q11.2DS (Schaer et al., 2009).
The finding of greater increase in WMV along the dorsal cingulum bundle and superior longitudinal fascisculus / arcuate fasciculus in 22q11.2DS COMTMet vs. COMTVal subgroups is interesting in light of the literature on abnormalities of these fiber tracts in schizophrenia in relation to working memory and other executive functions (Buchsbaum et al., 2007; Green et al., 2009; Takei et al., 2009). Future work elucidating the relationships between the regions observed to predict outcome is warranted.
There are several limitations to the current study. The influence of antipsychotic medications on the neuroanatomical findings is difficult to disentangle in the current study. In addition, although the age of onset of psychosis in 22q11.2DS is earlier than the age of onset of schizophrenia in the general population (Green et al., 2009), some of the participants currently not psychotic might develop psychotic symptoms in the future.
In conclusion, there are probably a large number of interacting factors affecting brain development in subjects with 22q11.2DS. However, it seems that some of the brain maturational processes that are consistently emerging as hallmarks of 22q11.2DS are the antero-posterior dissociation in cortical development and abnormal maturation of WM. Other processes, such as decline in dPFC GMV volume occur in at risk 22q11.2DS subgroups, e.g. COMTMet carriers and 22q11.2DS subjects who later develop psychotic disorders. The interaction between these processes and developmental changes in regions such as the mPFC and dorsal cingulum are likely to be the key risk factors of psychosis. If replicated in a larger independent sample, multivariate machine learning methods may be useful in the future in identifying neuroanatomical patterns that predict clinical outcome in 22q11.2DS.
Acknowledgments
Role of funding sources This study was funded by grants NIMH MH50047-15 (Dr. Reiss), NARSAD Young Investigator Award (Drs. Gothelf and Hoeft), the Child Health Research Program from the Stanford University School of Medicine and NICHD 1K23HD054720-01 (Dr Hoeft). The NIMH, NARSAD and NICHD had no further role in the study design; collection, analysis, and interpretation of data; writing of the report; and decision to submit the paper for publication.
Footnotes
Contributors Doron Gothelf: author, subject recruitment, data collection, data analysis.
Fumiko Hoeft: author, data collection, data analysis.
Takefumi Ueno: author, data analysis
Lisa Sugiura: data management, data analysis
Agatha D. Lee & Paul Thompson: method development, data analysis
Allan Reiss: author, data analysis and oversees the research project.
Conflict of interests I, D. Gothelf, have no conflicts of interest. I have no financial ties to any people or organizations that could have influenced this research study.
Financial Disclosure: None.
References
- Bearden CE, van Erp TG, Dutton RA, Lee AD, Simon TJ, Cannon TD, Emanuel BS, McDonald-McGinn D, Zackai EH, Thompson PM. Alterations in midline cortical thickness and gyrification patterns mapped in children with 22q11.2 deletions. Cereb Cortex. 2009;19:115–26. doi: 10.1093/cercor/bhn064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden CE, van Erp TG, Dutton RA, Tran H, Zimmermann L, Sun D, Geaga JA, Simon TJ, Glahn DC, Cannon TD, Emanuel BS, Toga AW, Thompson PM. Mapping cortical thickness in children with 22q11.2 deletions. Cereb Cortex. 2007;17:1889–98. doi: 10.1093/cercor/bhl097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botto LD, May K, Fernhoff PM, Correa A, Coleman K, Rasmussen SA, Merritt RK, O’Leary LA, Wong LY, Elixson EM, Mahle WT, Campbell RM. A population-based study of the 22q11.2 deletion: phenotype, incidence, and contribution to major birth defects in the population. Pediatrics. 2003;112:101–7. doi: 10.1542/peds.112.1.101. [DOI] [PubMed] [Google Scholar]
- Brunet-Gouet E, Decety J. Social brain dysfunctions in schizophrenia: a review of neuroimaging studies. Psychiatry Res. 2006;148:75–92. doi: 10.1016/j.pscychresns.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Buchsbaum BR, Hazlett EA, Haznedar MM, Newmark R, Tang CY, Hof PR. Relative glucose metabolic rate higher in white matter in patients with schizophrenia. Am J Psychiatry. 2007;164:1072–81. doi: 10.1176/ajp.2007.164.7.1072. [DOI] [PubMed] [Google Scholar]
- Campbell LE, Daly E, Toal F, Stevens A, Azuma R, Catani M, Ng V, van Amelsvoort T, Chitnis X, Cutter W, Murphy DG, Murphy KC. Brain and behaviour in children with 22q11.2 deletion syndrome: a volumetric and voxel-based morphometry MRI study. Brain. 2006;129:1218–28. doi: 10.1093/brain/awl066. [DOI] [PubMed] [Google Scholar]
- Eliez S, Schmitt JE, White CD, Reiss AL. Children and adolescents with velocardiofacial syndrome: a volumetric MRI study. Am J Psychiatry. 2000;157:409–15. doi: 10.1176/appi.ajp.157.3.409. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Eliez S, Thompson T, Hinard C, Penniman L, Feinstein C, Kwon H, Jin S, Jo B, Antonarakis SE, Morris MA, Reiss AL. COMT genotype predicts longitudinal cognitive decline and psychosis in 22q11.2 deletion syndrome. Nat Neurosci. 2005;8:1500–1502. doi: 10.1038/nn1572. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Penniman L, Gu E, Eliez S, Reiss AL. Developmental trajectories of brain structure in adolescents with 22q11.2 deletion syndrome: a longitudinal study. Schizophr Res. 2007;96:72–81. doi: 10.1016/j.schres.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothelf D, Searcy YM, Reilly J, Bellugi U, Lanre-Amos T, Mills D, Korenberg JR, Galaburda A, Reiss AL. Association between Cerebral Shape and Social Use of Language in Williams Syndrome. Am J Med Genet A. 2008 doi: 10.1002/ajmg.a.32507. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green T, Gothelf D, Glaser B, Debbane M, Frisch A, Kotler M, Weizman A, Eliez S. Psychiatric disorders and intellectual functioning throughout development in velocardiofacial (22q11.2 deletion) syndrome. J Am Acad Child Adolesc Psychiatry. 2009;48:1060–8. doi: 10.1097/CHI.0b013e3181b76683. [DOI] [PubMed] [Google Scholar]
- Kates WR, Antshel KM, Abdulsabur N, Colgan D, Funke B, Fremont W, Higgins AM, Kucherlapati R, Shprintzen RJ. A gender-moderated effect of a functional COMT polymorphism on prefrontal brain morphology and function in velo-cardio-facial syndrome (22q11.2 deletion syndrome) Am J Med Genet B Neuropsychiatr Genet. 2006;141:274–80. doi: 10.1002/ajmg.b.30284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates WR, Antshel KM, Fremont WP, Shprintzen RJ, Strunge LA, Burnette CP, Higgins AM. Comparing phenotypes in patients with idiopathic autism to patients with velocardiofacial syndrome (22q11 DS) with and without autism. Am J Med Genet A. 2007;143A:2642–50. doi: 10.1002/ajmg.a.32012. [DOI] [PubMed] [Google Scholar]
- Kates WR, Burnette CP, Jabs EW, Rutberg J, Murphy AM, Grados M, Geraghty M, Kaufmann WE, Pearlson GD. Regional cortical white matter reductions in velocardiofacial syndrome: a volumetric MRI analysis. Biol Psychiatry. 2001;49:677–84. doi: 10.1016/s0006-3223(00)01002-7. [DOI] [PubMed] [Google Scholar]
- Koutsouleris N, Meisenzahl EM, Davatzikos C, Bottlender R, Frodl T, Scheuerecker J, Schmitt G, Zetzsche T, Decker P, Reiser M, Moller HJ, Gaser C. Use of neuroanatomical pattern classification to identify subjects in at-risk mental states of psychosis and predict disease transition. Arch Gen Psychiatry. 2009;66:700–12. doi: 10.1001/archgenpsychiatry.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–50. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Krimer LS, Goldman-Rakic PS. Differential postnatal development of catecholamine and serotonin inputs to identified neurons in prefrontal cortex of rhesus monkey. J Neurosci. 2000;20:8780–7. doi: 10.1523/JNEUROSCI.20-23-08780.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KC, Jones LA, Owen MJ. High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry. 1999;56:940–5. doi: 10.1001/archpsyc.56.10.940. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Eckert MA, Rose FE, Karchemskiy A, Kesler S, Chang M, Reynolds MF, Kwon H, Galaburda A. An experiment of nature: brain anatomy parallels cognition and behavior in Williams syndrome. J Neurosci. 2004;24:5009–15. doi: 10.1523/JNEUROSCI.5272-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaer M, Debbane M, Bach Cuadra M, Ottet MC, Glaser B, Thiran JP, Eliez S. Deviant trajectories of cortical maturation in 22q11.2 deletion syndrome (22q11DS): a cross-sectional and longitudinal study. Schizophr Res. 2009;115:182–90. doi: 10.1016/j.schres.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Shprintzen RJ, Goldberg RB, Lewin ML, Sidoti EJ, Berkman MD, Argamaso RV, Young D. A new syndrome involving cleft palate, cardiac anomalies, typical facies, and learning disabilities: velo-cardio-facial syndrome. Cleft Palate J. 1978;15:56–62. [PubMed] [Google Scholar]
- Simon TJ, Ding L, Bish JP, McDonald-McGinn DM, Zackai EH, Gee J. Volumetric, connective, and morphologic changes in the brains of children with chromosome 22q11.2 deletion syndrome: an integrative study. Neuroimage. 2005;25:169–80. doi: 10.1016/j.neuroimage.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Takei K, Yamasue H, Abe O, Yamada H, Inoue H, Suga M, Muroi M, Sasaki H, Aoki S, Kasai K. Structural disruption of the dorsal cingulum bundle is associated with impaired Stroop performance in patients with schizophrenia. Schizophr Res. 2009;114:119–27. doi: 10.1016/j.schres.2009.05.012. [DOI] [PubMed] [Google Scholar]
- van Amelsvoort T, Daly E, Henry J, Robertson D, Ng V, Owen M, Murphy KC, Murphy DG. Brain anatomy in adults with velocardiofacial syndrome with and without schizophrenia: preliminary results of a structural magnetic resonance imaging study. Arch Gen Psychiatry. 2004;61:1085–96. doi: 10.1001/archpsyc.61.11.1085. [DOI] [PubMed] [Google Scholar]
- van Amelsvoort T, Zinkstok J, Figee M, Daly E, Morris R, Owen MJ, Murphy KC, De Haan L, Linszen DH, Glaser B, Murphy DG. Effects of a functional COMT polymorphism on brain anatomy and cognitive function in adults with velo-cardio-facial syndrome. Psychol Med. 2008;38:89–100. doi: 10.1017/S0033291707000700. [DOI] [PubMed] [Google Scholar]
- Vorstman JA, Chow EW, Ophoff RA, van Engeland H, Beemer FA, Kahn RS, Sinke RJ, Bassett AS. Association of the PIK4CA schizophrenia-susceptibility gene in adults with the 22q11.2 deletion syndrome. Am J Med Genet B Neuropsychiatr Genet. 2008 doi: 10.1002/ajmg.b.30827. [DOI] [PMC free article] [PubMed] [Google Scholar]