Abstract
The host immune system functions as a guardian against tumor development. It has been demonstrated that cytotoxic T lymphocyte (CTL)-mediated cytotoxic pathways function to inhibit or delay human colorectal cancer development. However, the host anti-tumor immune responses also 'edit' the tumor and select for more aggressive variants, resulting in immune evasion and tumor escape. Fas is a death receptor that mediates one of the major cytotoxic effector mechanisms of the CTLs. Fas is highly expressed in normal human colon epithelial cells but is frequently silenced in colorectal carcinoma, especially in metastatic colorectal carcinoma, suggesting that loss of Fas expression and function may be an immune evasion and tumor escape mechanism. In addition, recent studies indicated that Fas also mediates cellular proliferation signaling pathways to promote tumor development. Therefore, the death receptor Fas may not only transduce death signals to suppress tumor development but also activate cellular proliferation and the migration process to promote tumor growth and progression. Thus, understanding the mechanisms by which the Fas receptor and its associated protein complex transduces the death and survival signals may identify molecular targets for the development of therapeutic strategy to enhance the Fas-mediated death signals to increase the efficacy of cancer immunotherapy.
Keywords: Apoptosis, Fas, Metastasis, Immune evasion
INTRODUCTION
Over the past two decades, the notion that the host immune system can recognize and suppress tumor development in the absence of external therapeutic intervention has experienced a new resurgence. Thanks to the advances in our understanding of the molecular interactions between tumor cells and immune cells, immunotherapy including monoclonal antibodies, immune adjuvant, vaccines and cytotoxic T lymphocyte (CTL) adoptive transfer, have now become an effective treatment approach for a variety of cancers[1-6]. Accumulating evidence from studies in mouse models and human cancer patients suggests that tumorigenesis and progression is not only governed by genetic alterations intrinsic to the tumor cells but also by epigenetic and tumor microenvironmental factors. It has become clear that the host immune system is a microenvironmental factor that modulates tumor development[7-15]. The role of the host immune system in suppression of colorectal cancer (CRC) development has been well documented. Studies with a large cohort of human CRC patient specimens showed a significant role of the host immune effector mechanisms in suppression of colorectal carcinoma progression and recurrence[16-20]. However, current evidence suggests the host immune system might be a two-edged sword. On the one hand, the immune effector mechanism can suppress tumor development. On the other hand, the immune system can “edit” the tumors and select for variants that exhibit more aggressive and often apoptosis-resistant phenotype, resulting in immune evasion[20-23].
ANTI-TUMOR CYTOTOXICITY PATHWAYS
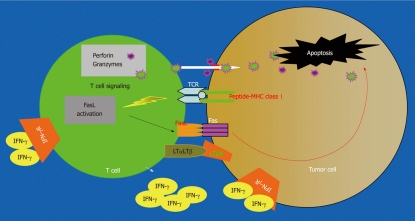
CTLs are the primary immune cells that directly attack tumor cells[24,25]. The perforin exocytosis pathway and the Fas-mediated apoptosis pathway were the first identified cytotoxic effector mechanisms of CTLs[26] (Figure 1). The perforin pathway depends on the polarized secretion of perforin and granzymes and utilizes perforin to traffic granzymes to the target cells where they cleave functional proteins to cause DNA fragmentation and subsequent apoptosis. The Fas/FasL system was originally thought to be essential for activation-induced cell death during an immune response and for normal homeostasis of the immune system. However, it has recently become clear that the Fas/FasL effector mechanism also plays an important role in suppressing tumor growth and progression[27-29]. Despite the fact that the perforin pathway is the dominant anti-tumor cytotoxic mechanism[9,30-32], recent studies have begun to shed light on the importance of other cytotoxic mechanisms of tumor-specific CTLs in suppression of tumor growth[33-37]. For example, it was reported that the perforin pathway of tumor-specific CTLs mediates potent anti-tumor effects in a minimal disease setting but were overwhelmed and became significantly less effective under conditions of an extensive tumor burden and that the FasL-dependent CTL cytotoxic mechanism was essential for optimal tumor regression under extensive tumor burden[11]. It was also observed that, although perforin-mediated killing is of paramount importance for CTL-mediated lysis in vitro, some in vivo cytotoxic mechanisms clearly are independent of perforin, as illustrated in a Renca pulmonary metastases model[38]. Furthermore, it is known that therapeutic effects of CTL-based immunotherapy were dependent, in part, on cytotoxic cell-derived LTα in a B16 lung metastasis model[39]. It has also been demonstrated that TNF and Lymphotoxin are involved in anticancer cytotoxicity exerted by NK cells and monocytes[40-42] and both D122 Lewis lung carcinoma and melanoma were rejected by tumor-specific CTLs through a cytolytic mechanism that is independent of both perforin and Fas pathways in vivo[43,44]. These observations suggest that perforin- and Fas-independent cytotoxic pathways, including IFN-γ and LTα/LTβ, also play significant roles in inhibition of tumor growth (Figure 1). Nevertheless, perforin and Fas/FasL pathways are the two primary anti-tumor effector mechanisms of CTLs.
Figure 1.
Anti-tumor cytotoxicity pathways. Tumor-specific cytotoxic T lymphocytes (CTLs) recognize tumor cells through TCR and Ag-bound MHC class I. Interactions between the CTL and the tumor cell involve direct cell-cell physical contact and release of modulator molecules (i.e. IFN-γ and other molecules). CTLs primarily use the perforin exocytosis pathway and the Fas-mediated apoptosis pathways to destruct the tumor cells. However, other effector mechanisms, such as the LTβR-mediated apoptosis pathway, also play a role in tumor cell destruction.
ANTI-TUMOR IMMUNITY AND COLORECTAL CANCER
Several prominent studies in large cohorts of human CRC patients have pointed to a significant role of the anti-tumor effector mechanisms of the host immune system in suppression of CRC growth, progression and recurrence[16-20,45,46]. Analysis of tumor-infiltrating immune cells and immune effector molecules in more than 400 colorectal carcinoma patients revealed an inverse correlation between the expression of a cluster of genes related to the Th1 adaptive immunity and tumor recurrence[16]. Furthermore, it seems that the immune cell (CD3, CD8) densities in the tumor microenvironment is inversely correlated with recurrence and positively correlated with overall disease-free survival[16]. The host immune cells, particularly the T cells, also play an effective role in suppressing or delaying CRC metastasis. A study of 959 CRC specimens indicated that increased level of infiltrating cytotoxic T cells is associated with the absence of signs of early metastasis, a less advanced pathological stage and increased survival[17]. Consistent with these observations, adoptive transfer immunotherapy of tumor-reactive T cells significantly increased CRC patient survival[19]. In a study with 16 CRC patients, T cells were isolated from sentinel nodes, expanded in vitro and administered to the patients. In 4 out of the 9 stage IV patients, complete tumor regression occurred and medium survival time in the stage IV patients increased from 0.8 to 2.6 years[19].
LOSS OF FAS EXPRESSION AND FUNCTION CONTRIBUTES TO COLORECTAL CANCER PROGRESSION
As discussed above, the Fas-mediated apoptosis pathway is one of the major cytotoxic effector mechanisms of CTLs and was originally identified to function in depletion of self-reactive lymphocytes and thus is essential for shutdown of chronic immune responses and prevention of autoimmunity[47-51]. However, the Fas-mediated apoptosis pathway is also directly involved in elimination of unwanted or diseased cells and thus functions as a guardian to suppress tumor development[52-55]. The tumor-specific CTLs use FasL on their surface to engage the Fas receptor on the tumor cell surface to initiate the Fas-mediated apoptosis and thereby playing a key role in immune cell-mediated suppression of tumor development[11,20,38,56]. An overwhelming amount of data from animal models, together with compelling data from human patients, indicate that Fas function as a tumor suppressor. It has been shown that deficiency of the Fas-mediated apoptosis pathway directly leads to increased spontaneous tumor metastasis in FasL knock out mice[53]. In human CRC patients, loss of Fas expression and acquisition of resistance to Fas-mediated apoptosis is a common phenomenon[20,57,58]. Fas is constitutively expressed at high level in normal human colon tissues. In human primary colorectal carcinoma, however, Fas expression is often diminished and complete loss of Fas expression is frequently observed in human metastatic colorectal carcinoma[57,58]. In our earlier studies, we have also observed an inverse correlation between Fas expression/function and tumor progression[59].
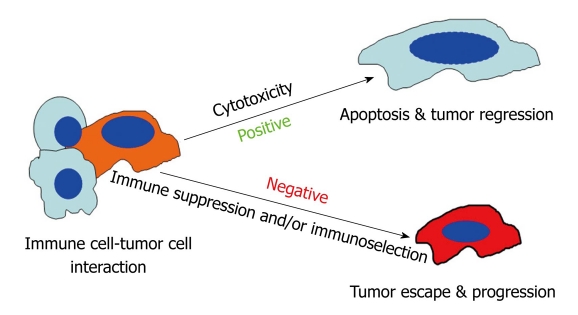
The above observations raised the possibility that metastatic colorectal carcinoma cells use loss of Fas expression and/or function to gain a metastatic phenotype. To determine the role of Fas-FasL interactions in CRC progression, we conducted several studies in the past decade to elucidate the molecular mechanisms underlying loss of Fas expression/function and the metastatic potential in colon carcinoma. A matched pair of human primary and metastatic colon carcinoma cell lines, termed SW480 and SW620, was used as a model system. The SW480 and SW620 tumor cell lines have been previously characterized as primary and metastatic colon adenocarcinoma cell lines respectively, established from the same patient. The SW620 cell line was derived as a lymph node-metastasis identified 6 mo later during disease recurrence[60]. Furthermore, both cell lines were isolated from the patient without any prior chemotherapy[60]. SW620 cells are essentially resistant to FasL-induced apoptosis whereas SW480 cells are sensitive to FasL-induced apoptosis[61-63]. To determine whether the decreased Fas expression and function in the metastatic colon carcinoma cells contribute to the metastatic capability, SW620 and SW480 cells were injected s.c. to nude mice. An RT-PCR assay of human keratin 18 for detection of human tumor cells of epithelial origin in mouse lymphoid tissue was developed for analysis of spontaneous distal metastasis. The detection sensitivity of such an assay is approximately 102-103 cells per mouse spleen. We detected tumor cells in spleens from 7 of the 8 mice that received SW620 cells. In contrast, no tumor cells were detected in spleens from mice that received SW480 cells. Using this sensitive assay and the SW480 and SW620 cell system, we next tested the hypothesis that the metastatic cells might pre-exist in the primary tumor population and anti-Fas selection leads to enrichment and emergence of the Fas-resistant subsets of metastatic tumor cells. One approach taken was to select for Fas-resistant sublines from the primary SW480 cells using an agonistic anti-Fas stimulus to deplete the Fas-sensitive subpopulations in vitro. Thus, any functional difference observed with these SW480-derived sublines could be compared with the naturally occurring metastatic SW620 cells. Overall, our data revealed that SW480-derived and Fas-resistant sublines morphologically, biologically and molecularly resembled the naturally occurring metastatic SW620 cells[23]. Our data thus supported the notion that subsets of metastatic colon carcinoma cells pre-exist in the primary colon carcinoma population and interactions between Fas (tumor cells) and FasL (immune cells) leads to selection and emergence of Fas-resistant subsets of tumor cells with metastatic potential[21,22,64,65] (Figure 2).
Figure 2.
Model of immune cell-tumor cell interaction. Interactions between immune cells and tumor cells involve direct cell-cell physical contact and release of modulator molecules, primarily cytokines and chemokines. In this survival and death battle, on the one hand, the immune cells may prevail and eradicate the tumor cells. On the other hand, tumor cells can counterattack the immune cells by producing inhibitory molecules, activating the immune suppressive cells or acquiring apoptosis-resistant mechanisms to avoid destruction by the immune system. Thus, the anti-tumor immune response can be a two-edged sword and can result in both positive and negative consequences.
The anti-tumor immune response, once viewed as always favorable for suppressing cancer development, is potentially a two-edged sword. While immune cells can eliminate cancer cells, they can also edit them and select for resistant variants. These variants are very aggressive; that is, they can resist apoptosis and possess metastatic potential. This notion is in line with observations in human colorectal patients that Fas-mediated apoptosis exerted by the tumor infiltrating CTLs contributes to CRC recurrence and regression[20]. In addition, therapeutic approaches to sensitize colon carcinoma cells to Fas-mediated apoptosis has enhanced efficacy of colon cancer therapy[66,67]. Therefore, CTL-induced and Fas-mediated anti-tumor cytotoxicity can have both positive and negative consequences. The positive outcome is Fas-mediated tumor cell apoptosis and tumor suppression and the negative consequence is immune selection of Fas-resistant tumor variants that possess metastatic potential (Figure 2)[22,64,65].
It should be noted that although loss of Fas expression and function is associated with enhanced metastatic potential of colon carcinoma cells, it is apparent that loss of Fas expression and/or function alone is insufficient to confer non-metastatic tumor cell with full metastatic capability. Rather, the ability of Fas-resistant tumor cells to achieve metastatic competence requires co-possession of additional metastatic characteristics[68]. In mutant mice a point mutation in the cytoplasmic domain of Fas abolishes the Fas-mediated signal transduction[69-71]. As expected, mice homozygous for this mutated allele develop lymphadenopathy and a lupus-like autoimmune disease[69-71]. However, spontaneous carcinoma formation is rare in these Fas mutant mice[69-71]. Therefore, it is likely that loss of Fas expression and function acts as an “enhancer” not an “initiator” of tumor initiation and/or progression[68].
FAS COUNTERATTACK AND TUMOR IMMUNE EVASION THEORY
Although FasL is primarily expressed in activated immune cells (i.e. CTLs), it has been reported that FasL is also expressed in human cancers of diverse origin, including CRC[72-76]. Because activated CTLs express Fas and are sensitive to Fas-mediated apoptosis[49], the expression of FasL in tumor cells raise the possibility that FasL might mediate immune privilege by inducing apoptosis of anti-tumor immune cells in the tumor microenvironment. Indeed, it has been shown that tumor-expressed FasL induces apoptosis of tumor-infiltrating lymphocytes[73,77,78]. In addition, inhibition of FasL expression in colon carcinoma cells led to increased lymphocyte infiltration and decreased tumor development[72,79]. However, on the other hand, the Fas counterattack theory has been a controversial one ever since it was proposed[80]. In contrast to the observations that tumor-expressing FasL induces tumor-infiltrating T lymphocytes apoptosis, FasL expression by tumor cells led to neutrophil infiltration, proinflammatory response and tumor rejection[28,74]. Of notice is a recent finding that only the membrane-bound FasL is essential for cytotoxic activity and constitutes the guardian against disease, lymphadenopathy, autoimmunity and cancer, whereas excess sFasL might promote autoimmunity and tumorigenesis through non-apoptotic activities[55]. In most of the Fas counterattack reports, it is not clear whether the membrane FasL or the sFasL is involved. In summary, the majority of the in vivo data suggest FasL expressed in tumor cells is unfavorable for tumors. Therefore, it is not entirely clear whether Fas counterattack really is a relevant immune evasion and tumor escape mechanism[28,80].
FAS IS ALSO A TUMOR PROMOTER
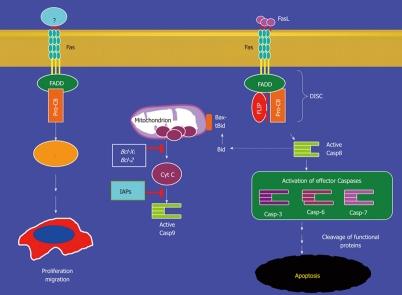
Fas is undoubtedly a death receptor and, when it is engaged by its ligand, mediates apoptosis in a wide variety of cells, including CRC cells. Some new evidence suggest, however, that Fas mediates not only apoptosis but also non-apoptotic signaling pathways including cellular activation, proliferation, differentiation and migration, all of which promote tumor development[50,81-84]. Under conditions of low receptor stimulation or under conditions where apoptosis is blocked, Fas signaling switches to proliferative signaling, leading to activation of ERK1/2 and consequently NF-κB activation in gastric mucosal cells[82,85]. Furthermore, it was observed that co-expression of FasL with apoptosis inhibitor FLIP increased Fas-mediated proliferation and enabled colon carcinoma cells to metastasize to the liver[83]. Therefore, loss of Fas expression and acquisition of apoptosis resistance might alter the Fas-exerted signal from pro-apoptosis to pro-proliferation, resulting in promotion of spontaneous colon carcinoma liver metastasis[83]. Although NF-κB and MAP kinase signaling pathways have be implicated and it is known that the Fas DISC components such as caspase 8, FADD and FLIP are involved in the Fas-mediated non-apoptotic signaling transduction, the molecular links that connect Fas receptor DISC components (Caspase 8, FADD and FLIP) to the ERK and NF-κB signaling pathways is largely undefined. Nevertheless, it has firmly established that Fas not only mediates apoptosis but also exerts non-apoptotic signals to activate the cellular survival signaling pathway, an opposing cellular process of apoptosis, in a broad range of cells including colon cancer cells (Figure 3).
Figure 3.
Fas-mediated signaling pathways. Engagement of the Fas receptor induces DISC formation and subsequently caspase 8 activation. The active caspase 8 can either directly activate caspase 3 to initiate apoptosis, or active caspase 8 may cleave and activate Bid (tBid). tBid interacts with Bax to activate the caspase 9 cascade to induce apoptosis. On the other hand, Fas signaling may also lead to activation of cellular proliferation. Therefore, Fas engagement may leads to two conflicting cellular processes: apoptosis and survival. The molecular mediators controlling the switcher between these two opposing pathways remain to be determined.
CONCLUSION
The Fas-mediated apoptosis pathway is an important effector mechanism that the cytotoxic T lymphocytes use to suppress tumor development. It has been firmly established that engagement of Fas receptor by its ligand FasL induces tumor cell apoptosis and tumor growth inhibition. Fas-FasL interaction, however, also leads to immune selection and generation of apoptosis-resistant tumor escape variant which might explain the phenomenon of the frequent loss of Fas expression and function in metastatic colorectal carcinoma. In addition, recent studies revealed that Fas also mediates non-apoptotic signals that activate cellular survival process, resulting in promotion of tumor development. Thus, Fas functions as a multi-faced “death” receptor that mediates both pro- and anti-apoptotic signaling pathways. Next, we need to identify the molecular switchers inside the Fas death receptor DISC complex that dictate the signaling transduction pathways because they may provide new molecular targets for targeting apoptosis resistance to enhance the efficacy of cancer therapy/immunotherapy.
Footnotes
Peer reviewers: Runjan Chetty, Professor, Department of Pathology and Gene Regulation, University of Glasgow, Western Infirmary (Pathology), Dumbarton Road, Glasgow, G11 6NT, Scotland, United Kingdom; Xiao-Chun Xu, Associate Professor, Department of Clinical Cancer Prevention, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Unit 1360, Houston, TX 77030, United States
S- Editor Wang JL L- Editor Roemmele A E- Editor Yang C
References
- 1.Lutterbuese R, Raum T, Kischel R, Hoffmann P, Mangold S, Rattel B, Friedrich M, Thomas O, Lorenczewski G, Rau D, et al. T cell-engaging BiTE antibodies specific for EGFR potently eliminate KRAS- and BRAF-mutated colorectal cancer cells. Proc Natl Acad Sci USA. 2010;107:12605–12610. doi: 10.1073/pnas.1000976107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- 6.Shapira S, Lisiansky V, Arber N, Kraus S. Targeted immunotherapy for colorectal cancer: monoclonal antibodies and immunotoxins. Expert Opin Investig Drugs. 2010;19 Suppl 1:S67–S77. doi: 10.1517/13543781003737668. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, Schreiber RD. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci USA. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Street SE, Cretney E, Smyth MJ. Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood. 2001;97:192–197. doi: 10.1182/blood.v97.1.192. [DOI] [PubMed] [Google Scholar]
- 9.Smyth MJ, Thia KY, Street SE, MacGregor D, Godfrey DI, Trapani JA. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J Exp Med. 2000;192:755–760. doi: 10.1084/jem.192.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 11.Caldwell SA, Ryan MH, McDuffie E, Abrams SI. The Fas/Fas ligand pathway is important for optimal tumor regression in a mouse model of CTL adoptive immunotherapy of experimental CMS4 lung metastases. J Immunol. 2003;171:2402–2412. doi: 10.4049/jimmunol.171.5.2402. [DOI] [PubMed] [Google Scholar]
- 12.Abrams SI. A multi-functional role of interferon regulatory factor-8 in solid tumor and myeloid cell biology. Immunol Res. 2010;46:59–71. doi: 10.1007/s12026-009-8125-6. [DOI] [PubMed] [Google Scholar]
- 13.Boshoff C, Weiss R. AIDS-related malignancies. Nat Rev Cancer. 2002;2:373–382. doi: 10.1038/nrc797. [DOI] [PubMed] [Google Scholar]
- 14.Birkeland SA, Storm HH, Lamm LU, Barlow L, Blohmé I, Forsberg B, Eklund B, Fjeldborg O, Friedberg M, Frödin L. Cancer risk after renal transplantation in the Nordic countries, 1964-1986. Int J Cancer. 1995;60:183–189. doi: 10.1002/ijc.2910600209. [DOI] [PubMed] [Google Scholar]
- 15.Sheil AG. Cancer after transplantation. World J Surg. 1986;10:389–396. doi: 10.1007/BF01655298. [DOI] [PubMed] [Google Scholar]
- 16.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 17.Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 18.Camus M, Tosolini M, Mlecnik B, Pagès F, Kirilovsky A, Berger A, Costes A, Bindea G, Charoentong P, Bruneval P, et al. Coordination of intratumoral immune reaction and human colorectal cancer recurrence. Cancer Res. 2009;69:2685–2693. doi: 10.1158/0008-5472.CAN-08-2654. [DOI] [PubMed] [Google Scholar]
- 19.Karlsson M, Marits P, Dahl K, Dagöö T, Enerbäck S, Thörn M, Winqvist O. Pilot study of sentinel-node-based adoptive immunotherapy in advanced colorectal cancer. Ann Surg Oncol. 2010;17:1747–1757. doi: 10.1245/s10434-010-0920-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sträter J, Hinz U, Hasel C, Bhanot U, Mechtersheimer G, Lehnert T, Möller P. Impaired CD95 expression predisposes for recurrence in curatively resected colon carcinoma: clinical evidence for immunoselection and CD95L mediated control of minimal residual disease. Gut. 2005;54:661–665. doi: 10.1136/gut.2004.052696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 22.Liu K, Caldwell SA, Greeneltch KM, Yang D, Abrams SI. CTL adoptive immunotherapy concurrently mediates tumor regression and tumor escape. J Immunol. 2006;176:3374–3382. doi: 10.4049/jimmunol.176.6.3374. [DOI] [PubMed] [Google Scholar]
- 23.Liu K, McDuffie E, Abrams SI. Exposure of human primary colon carcinoma cells to anti-Fas interactions influences the emergence of pre-existing Fas-resistant metastatic subpopulations. J Immunol. 2003;171:4164–4174. doi: 10.4049/jimmunol.171.8.4164. [DOI] [PubMed] [Google Scholar]
- 24.Leen AM, Rooney CM, Foster AE. Improving T cell therapy for cancer. Annu Rev Immunol. 2007;25:243–265. doi: 10.1146/annurev.immunol.25.022106.141527. [DOI] [PubMed] [Google Scholar]
- 25.Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 26.Russell JH, Ley TJ. Lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 2002;20:323–370. doi: 10.1146/annurev.immunol.20.100201.131730. [DOI] [PubMed] [Google Scholar]
- 27.Carter LL, Dutton RW. Relative perforin- and Fas-mediated lysis in T1 and T2 CD8 effector populations. J Immunol. 1995;155:1028–1031. [PubMed] [Google Scholar]
- 28.Fingleton B, Carter KJ, Matrisian LM. Loss of functional Fas ligand enhances intestinal tumorigenesis in the Min mouse model. Cancer Res. 2007;67:4800–4806. doi: 10.1158/0008-5472.CAN-06-4473. [DOI] [PubMed] [Google Scholar]
- 29.Yang D, Wang S, Brooks C, Dong Z, Schoenlein PV, Kumar V, Ouyang X, Xiong H, Lahat G, Hayes-Jordan A, et al. IFN regulatory factor 8 sensitizes soft tissue sarcoma cells to death receptor-initiated apoptosis via repression of FLICE-like protein expression. Cancer Res. 2009;69:1080–1088. doi: 10.1158/0008-5472.CAN-08-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kägi D, Ledermann B, Bürki K, Seiler P, Odermatt B, Olsen KJ, Podack ER, Zinkernagel RM, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 31.van den Broek ME, Kägi D, Ossendorp F, Toes R, Vamvakas S, Lutz WK, Melief CJ, Zinkernagel RM, Hengartner H. Decreased tumor surveillance in perforin-deficient mice. J Exp Med. 1996;184:1781–1790. doi: 10.1084/jem.184.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voskoboinik I, Smyth MJ, Trapani JA. Perforin-mediated target-cell death and immune homeostasis. Nat Rev Immunol. 2006;6:940–952. doi: 10.1038/nri1983. [DOI] [PubMed] [Google Scholar]
- 33.Poehlein CH, Hu HM, Yamada J, Assmann I, Alvord WG, Urba WJ, Fox BA. TNF plays an essential role in tumor regression after adoptive transfer of perforin/IFN-gamma double knockout effector T cells. J Immunol. 2003;170:2004–2013. doi: 10.4049/jimmunol.170.4.2004. [DOI] [PubMed] [Google Scholar]
- 34.Saha A, Chatterjee SK, Foon KA, Bhattacharya-Chatterjee M. Anti-idiotype antibody induced cellular immunity in mice transgenic for human carcinoembryonic antigen. Immunology. 2006;118:483–496. doi: 10.1111/j.1365-2567.2006.02391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prévost-Blondel A, Roth E, Rosenthal FM, Pircher H. Crucial role of TNF-alpha in CD8 T cell-mediated elimination of 3LL-A9 Lewis lung carcinoma cells in vivo. J Immunol. 2000;164:3645–3651. doi: 10.4049/jimmunol.164.7.3645. [DOI] [PubMed] [Google Scholar]
- 36.Winter H, van den Engel NK, Poehlein CH, Hatz RA, Fox BA, Hu HM. Tumor-specific T cells signal tumor destruction via the lymphotoxin beta receptor. J Transl Med. 2007;5:14. doi: 10.1186/1479-5876-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang D, Ud Din N, Browning DD, Abrams SI, Liu K. Targeting lymphotoxin beta receptor with tumor-specific T lymphocytes for tumor regression. Clin Cancer Res. 2007;13:5202–5210. doi: 10.1158/1078-0432.CCR-07-1161. [DOI] [PubMed] [Google Scholar]
- 38.Seki N, Brooks AD, Carter CR, Back TC, Parsoneault EM, Smyth MJ, Wiltrout RH, Sayers TJ. Tumor-specific CTL kill murine renal cancer cells using both perforin and Fas ligand-mediated lysis in vitro, but cause tumor regression in vivo in the absence of perforin. J Immunol. 2002;168:3484–3492. doi: 10.4049/jimmunol.168.7.3484. [DOI] [PubMed] [Google Scholar]
- 39.Dobrzanski MJ, Reome JB, Hollenbaugh JA, Hylind JC, Dutton RW. Effector cell-derived lymphotoxin alpha and Fas ligand, but not perforin, promote Tc1 and Tc2 effector cell-mediated tumor therapy in established pulmonary metastases. Cancer Res. 2004;64:406–414. doi: 10.1158/0008-5472.can-03-2580. [DOI] [PubMed] [Google Scholar]
- 40.Kashii Y, Giorda R, Herberman RB, Whiteside TL, Vujanovic NL. Constitutive expression and role of the TNF family ligands in apoptotic killing of tumor cells by human NK cells. J Immunol. 1999;163:5358–5366. [PubMed] [Google Scholar]
- 41.Janjic BM, Lu G, Pimenov A, Whiteside TL, Storkus WJ, Vujanovic NL. Innate direct anticancer effector function of human immature dendritic cells. I. Involvement of an apoptosis-inducing pathway. J Immunol. 2002;168:1823–1830. doi: 10.4049/jimmunol.168.4.1823. [DOI] [PubMed] [Google Scholar]
- 42.Lu G, Janjic BM, Janjic J, Whiteside TL, Storkus WJ, Vujanovic NL. Innate direct anticancer effector function of human immature dendritic cells. II. Role of TNF, lymphotoxin-alpha(1)beta(2), Fas ligand, and TNF-related apoptosis-inducing ligand. J Immunol. 2002;168:1831–1839. doi: 10.4049/jimmunol.168.4.1831. [DOI] [PubMed] [Google Scholar]
- 43.Winter H, Hu HM, Urba WJ, Fox BA. Tumor regression after adoptive transfer of effector T cells is independent of perforin or Fas ligand (APO-1L/CD95L) J Immunol. 1999;163:4462–4472. [PubMed] [Google Scholar]
- 44.Lee SH, Bar-Haim E, Machlenkin A, Goldberger O, Volovitz I, Vadai E, Tzehoval E, Eisenbach L. In vivo rejection of tumor cells dependent on CD8 cells that kill independently of perforin and FasL. Cancer Gene Ther. 2004;11:237–248. doi: 10.1038/sj.cgt.7700678. [DOI] [PubMed] [Google Scholar]
- 45.Simpson JA, Al-Attar A, Watson NF, Scholefield JH, Ilyas M, Durrant LG. Intratumoral T cell infiltration, MHC class I and STAT1 as biomarkers of good prognosis in colorectal cancer. Gut. 2010;59:926–933. doi: 10.1136/gut.2009.194472. [DOI] [PubMed] [Google Scholar]
- 46.McGough JM, Yang D, Huang S, Georgi D, Hewitt SM, Röcken C, Tänzer M, Ebert MP, Liu K. DNA methylation represses IFN-gamma-induced and signal transducer and activator of transcription 1-mediated IFN regulatory factor 8 activation in colon carcinoma cells. Mol Cancer Res. 2008;6:1841–1851. doi: 10.1158/1541-7786.MCR-08-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hughes PD, Belz GT, Fortner KA, Budd RC, Strasser A, Bouillet P. Apoptosis regulators Fas and Bim cooperate in shutdown of chronic immune responses and prevention of autoimmunity. Immunity. 2008;28:197–205. doi: 10.1016/j.immuni.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hutcheson J, Scatizzi JC, Siddiqui AM, Haines GK 3rd, Wu T, Li QZ, Davis LS, Mohan C, Perlman H. Combined deficiency of proapoptotic regulators Bim and Fas results in the early onset of systemic autoimmunity. Immunity. 2008;28:206–217. doi: 10.1016/j.immuni.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 49.Krammer PH. CD95's deadly mission in the immune system. Nature. 2000;407:789–795. doi: 10.1038/35037728. [DOI] [PubMed] [Google Scholar]
- 50.Peter ME, Budd RC, Desbarats J, Hedrick SM, Hueber AO, Newell MK, Owen LB, Pope RM, Tschopp J, Wajant H, et al. The CD95 receptor: apoptosis revisited. Cell. 2007;129:447–450. doi: 10.1016/j.cell.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 51.Weant AE, Michalek RD, Khan IU, Holbrook BC, Willingham MC, Grayson JM. Apoptosis regulators Bim and Fas function concurrently to control autoimmunity and CD8+ T cell contraction. Immunity. 2008;28:218–230. doi: 10.1016/j.immuni.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 52.Owen-Schaub L, Chan H, Cusack JC, Roth J, Hill LL. Fas and Fas ligand interactions in malignant disease. Int J Oncol. 2000;17:5–12. [PubMed] [Google Scholar]
- 53.Owen-Schaub LB, van Golen KL, Hill LL, Price JE. Fas and Fas ligand interactions suppress melanoma lung metastasis. J Exp Med. 1998;188:1717–1723. doi: 10.1084/jem.188.9.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jost PJ, Grabow S, Gray D, McKenzie MD, Nachbur U, Huang DC, Bouillet P, Thomas HE, Borner C, Silke J, et al. XIAP discriminates between type I and type II FAS-induced apoptosis. Nature. 2009;460:1035–1039. doi: 10.1038/nature08229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O' Reilly LA, Tai L, Lee L, Kruse EA, Grabow S, Fairlie WD, Haynes NM, Tarlinton DM, Zhang JG, Belz GT, et al. Membrane-bound Fas ligand only is essential for Fas-induced apoptosis. Nature. 2009;461:659–663. doi: 10.1038/nature08402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kägi D, Vignaux F, Ledermann B, Bürki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994;265:528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- 57.Möller P, Koretz K, Leithäuser F, Brüderlein S, Henne C, Quentmeier A, Krammer PH. Expression of APO-1 (CD95), a member of the NGF/TNF receptor superfamily, in normal and neoplastic colon epithelium. Int J Cancer. 1994;57:371–377. doi: 10.1002/ijc.2910570314. [DOI] [PubMed] [Google Scholar]
- 58.Krammer PH. CD95(APO-1/Fas)-mediated apoptosis: live and let die. Adv Immunol. 1999;71:163–210. doi: 10.1016/s0065-2776(08)60402-2. [DOI] [PubMed] [Google Scholar]
- 59.Yang D, Stewart TJ, Smith KK, Georgi D, Abrams SI, Liu K. Downregulation of IFN-gammaR in association with loss of Fas function is linked to tumor progression. Int J Cancer. 2008;122:350–362. doi: 10.1002/ijc.23090. [DOI] [PubMed] [Google Scholar]
- 60.Hewitt RE, McMarlin A, Kleiner D, Wersto R, Martin P, Tsokos M, Stamp GW, Stetler-Stevenson WG. Validation of a model of colon cancer progression. J Pathol. 2000;192:446–454. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH775>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 61.Bergmann-Leitner ES, Abrams SI. Differential role of Fas/Fas ligand interactions in cytolysis of primary and metastatic colon carcinoma cell lines by human antigen-specific CD8+ CTL. J Immunol. 2000;164:4941–4954. doi: 10.4049/jimmunol.164.9.4941. [DOI] [PubMed] [Google Scholar]
- 62.Liu K, Abrams SI. Coordinate regulation of IFN consensus sequence-binding protein and caspase-1 in the sensitization of human colon carcinoma cells to Fas-mediated apoptosis by IFN-gamma. J Immunol. 2003;170:6329–6337. doi: 10.4049/jimmunol.170.12.6329. [DOI] [PubMed] [Google Scholar]
- 63.Huerta S, Heinzerling JH, Anguiano-Hernandez YM, Huerta-Yepez S, Lin J, Chen D, Bonavida B, Livingston EH. Modification of gene products involved in resistance to apoptosis in metastatic colon cancer cells: roles of Fas, Apaf-1, NFkappaB, IAPs, Smac/DIABLO, and AIF. J Surg Res. 2007;142:184–194. doi: 10.1016/j.jss.2006.12.551. [DOI] [PubMed] [Google Scholar]
- 64.Liu K, Caldwell SA, Abrams SI. Cooperative disengagement of Fas and intercellular adhesion molecule-1 function in neoplastic cells confers enhanced colonization efficiency. Cancer Res. 2005;65:1045–1054. [PubMed] [Google Scholar]
- 65.Liu K, Caldwell SA, Abrams SI. Immune selection and emergence of aggressive tumor variants as negative consequences of Fas-mediated cytotoxicity and altered IFN-gamma-regulated gene expression. Cancer Res. 2005;65:4376–4388. doi: 10.1158/0008-5472.CAN-04-4269. [DOI] [PubMed] [Google Scholar]
- 66.Chakraborty M, Abrams SI, Camphausen K, Liu K, Scott T, Coleman CN, Hodge JW. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol. 2003;170:6338–6347. doi: 10.4049/jimmunol.170.12.6338. [DOI] [PubMed] [Google Scholar]
- 67.Xiao ZY, Wu W, Eagleton N, Chen HQ, Shao J, Teng H, Liu TH, Jiang ZM, Yao HR. Silencing Fas-associated phosphatase 1 expression enhances efficiency of chemotherapy for colon carcinoma with oxaliplatin. World J Gastroenterol. 2010;16:112–118. doi: 10.3748/wjg.v16.i1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu K, Abrams SI. Alterations in Fas expression are characteristic of, but not solely responsible for, enhanced metastatic competence. J Immunol. 2003;170:5973–5980. doi: 10.4049/jimmunol.170.12.5973. [DOI] [PubMed] [Google Scholar]
- 69.Takahashi T, Tanaka M, Brannan CI, Jenkins NA, Copeland NG, Suda T, Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76:969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 70.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 71.Chu K, Niu X, Williams LT. A Fas-associated protein factor, FAF1, potentiates Fas-mediated apoptosis. Proc Natl Acad Sci USA. 1995;92:11894–11898. doi: 10.1073/pnas.92.25.11894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Houston AM, Michael-Robinson JM, Walsh MD, Cummings MC, Ryan AE, Lincoln D, Pandeya N, Jass JR, Radford-Smith GL, O'Connell J. The "Fas counterattack" is not an active mode of tumor immune evasion in colorectal cancer with high-level microsatellite instability. Hum Pathol. 2008;39:243–250. doi: 10.1016/j.humpath.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 73.Ryan AE, Shanahan F, O'Connell J, Houston AM. Fas ligand promotes tumor immune evasion of colon cancer in vivo. Cell Cycle. 2006;5:246–249. doi: 10.4161/cc.5.3.2413. [DOI] [PubMed] [Google Scholar]
- 74.Behrens CK, Igney FH, Arnold B, Möller P, Krammer PH. CD95 ligand-expressing tumors are rejected in anti-tumor TCR transgenic perforin knockout mice. J Immunol. 2001;166:3240–3247. doi: 10.4049/jimmunol.166.5.3240. [DOI] [PubMed] [Google Scholar]
- 75.O'Connell J, O'Sullivan GC, Collins JK, Shanahan F. The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med. 1996;184:1075–1082. doi: 10.1084/jem.184.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Song E, Chen J, Ouyang N, Su F, Wang M, Heemann U. Soluble Fas ligand released by colon adenocarcinoma cells induces host lymphocyte apoptosis: an active mode of immune evasion in colon cancer. Br J Cancer. 2001;85:1047–1054. doi: 10.1054/bjoc.2001.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bennett MW, O'Connell J, O'Sullivan GC, Brady C, Roche D, Collins JK, Shanahan F. The Fas counterattack in vivo: apoptotic depletion of tumor-infiltrating lymphocytes associated with Fas ligand expression by human esophageal carcinoma. J Immunol. 1998;160:5669–5675. [PubMed] [Google Scholar]
- 78.Niehans GA, Brunner T, Frizelle SP, Liston JC, Salerno CT, Knapp DJ, Green DR, Kratzke RA. Human lung carcinomas express Fas ligand. Cancer Res. 1997;57:1007–1012. [PubMed] [Google Scholar]
- 79.Ryan AE, Shanahan F, O'Connell J, Houston AM. Addressing the "Fas counterattack" controversy: blocking fas ligand expression suppresses tumor immune evasion of colon cancer in vivo. Cancer Res. 2005;65:9817–9823. doi: 10.1158/0008-5472.CAN-05-1462. [DOI] [PubMed] [Google Scholar]
- 80.Igney FH, Krammer PH. Tumor counterattack: fact or fiction? Cancer Immunol Immunother. 2005;54:1127–1136. doi: 10.1007/s00262-005-0680-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen L, Park SM, Tumanov AV, Hau A, Sawada K, Feig C, Turner JR, Fu YX, Romero IL, Lengyel E, et al. CD95 promotes tumour growth. Nature. 2010;465:492–496. doi: 10.1038/nature09075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li H, Cai X, Fan X, Moquin B, Stoicov C, Houghton J. Fas Ag-FasL coupling leads to ERK1/2-mediated proliferation of gastric mucosal cells. Am J Physiol Gastrointest Liver Physiol. 2008;294:G263–G275. doi: 10.1152/ajpgi.00267.2007. [DOI] [PubMed] [Google Scholar]
- 83.Li H, Fan X, Stoicov C, Liu JH, Zubair S, Tsai E, Ste Marie R, Wang TC, Lyle S, Kurt-Jones E, et al. Human and mouse colon cancer utilizes CD95 signaling for local growth and metastatic spread to liver. Gastroenterology. 2009;137:934–944, 944.e1-4. doi: 10.1053/j.gastro.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Strasser A, Jost PJ, Nagata S. The many roles of FAS receptor signaling in the immune system. Immunity. 2009;30:180–192. doi: 10.1016/j.immuni.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barnhart BC, Legembre P, Pietras E, Bubici C, Franzoso G, Peter ME. CD95 ligand induces motility and invasiveness of apoptosis-resistant tumor cells. EMBO J. 2004;23:3175–3185. doi: 10.1038/sj.emboj.7600325. [DOI] [PMC free article] [PubMed] [Google Scholar]