Abstract
We assess the cross-reactivity of both cellular as well as recombinant E- and N-cadherins using functionalized bead arrays assembled on atomic-force-microscope cantilevers. This new approach builds upon and enhances the utility of a recently developed force probe that integrates a custom-built, horizontal atomic force microscope with micropipette manipulation. It enables us to test multiple biomolecular interactions of the same cell in a swift sequential or cyclic manner and thus to resolve subtle differences between individual interactions that otherwise would be obscured by cell-cell baseline variability. For each cell, we contrast heterophilic E:N-cadherin binding with the respective homophilic bonds and with a suitable control. Clarifying previous literature reports, we establish that specific bonds between E- and N-cadherins form readily, albeit less frequently than homophilic bonds of either cadherin. We support this assessment with a rough estimate of the ratio of on-rate constants of E/N-cadherin binding.
Cadherins are calcium-dependent adhesion proteins that mediate vital physiological functions like cell-cell interactions, coordinated cell migration, and tissue formation. They also are key players in pathological processes like cancer-cell propagation (1). In particular, the increase of cancer-cell motility during the epithelial-mesenchymal transition at the onset of metastasis has been attributed in part to a switch from E- to N-cadherin expression (2). Although heterotypic cell interactions are common in this process, it remains unclear whether E- and N-cadherins are able to cross-react and support such interactions (3–8). The single-cell assay introduced here allows us to answer this question more directly than previously possible.
Single-live-cell studies are uniquely suited to examine the mechanistic underpinnings of processes like cadherin-mediated cell-cell adhesion. However, as long as each selected cell can only be used in one type of measurement or control, natural baseline variability between cells will obscure subtle features of the behavior of individual cells. To overcome this limitation, we have developed a powerful approach to directly compare multiple biomolecular interactions on a per-cell basis (Fig. 1). We first give a brief overview of this approach and then analyze E- and N-cadherin adhesion frequencies in well-controlled contacts between functionalized beads as well as between beads and cadherin-expressing L cells.
Figure 1.
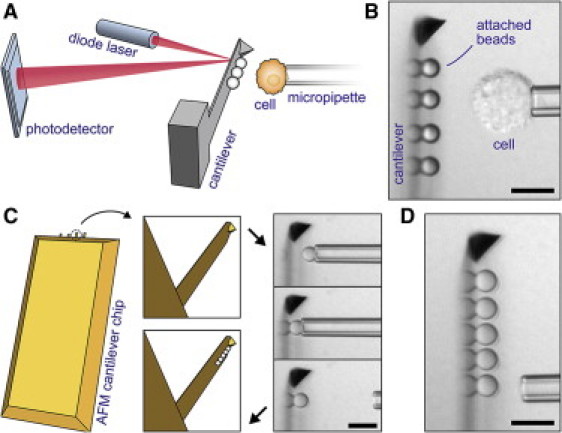
Integration of cantilever-based bead arrays into our custom-built force probe (11). (A) The conventional AFM core is turned on its side and combined with micropipetting. Cells or particles can be probed against an array of beads affixed to the cantilever. (B) Videomicrograph of a typical experiment showing a side view of the cantilever. (Reflections of the attached polystyrene beads form at the flat of the cantilever.) (C) Illustration of our procedure to assemble bead arrays on commercial AFM cantilevers. One-by-one, beads are aligned and attached to the rectangular cantilever using a micropipette. (D) Completed five-bead array; here: assembled from silica beads. (All scale bars denote 10 μm.)
We have previously reported the development of a force-probe instrument that combines the precision and robustness of the atomic force microscope (AFM (9)) with the versatility of micropipetting (10) and the convenience of a side view of ongoing experiments (11–13). Adhesion measurements with this instrument—or any AFM—generally require that the AFM cantilevers be functionalized with molecules of interest. Instead, our force probe allows us to assemble arrays of prefunctionalized microspheres (or other particles) on the flat of the cantilever (Fig. 1), thus greatly expanding the spectrum of adhesive interactions that can be tested with a single cantilever.
Probe beads are routinely affixed to the rectangular cantilever immediately before adhesion experiments, as illustrated in Fig. 1, C and D, and in Movie S1 in the Supporting Material. The beads usually are densely coated with proteins and physisorb strongly (in protein-free buffer) to a clean cantilever. (If necessary, the buffer may later be exchanged for a more suitable medium to maintain healthy cells. Alternatively, specific chemistry can be used to attach the beads.) Each selected bead is maneuvered to the desired position near the cantilever using a micropipette. Aligning both the cantilever tip as well as the bead within the microscope focal plane ensures that all beads are placed along the centerline of the cantilever. We then push the bead firmly against the cantilever for a few seconds before releasing it from the pipette. Importantly, our calibration of this instrument takes into account that both the local spring constant of the cantilever as well as the optical-lever sensitivity of the cantilever-deflection measurement are functions of the probe-bead position (11).
Adhesion tests are performed by picking up a cell with the pipette and moving it repeatedly to/from feedback-controlled contact with one of the beads on the cantilever (11). Because probe beads may be chosen to present different ligands to the cell, multiple adhesive interactions of the same cell can be tested in a swift sequential or cyclic manner. Moreover, any number of cells (or other test objects; see (11)) can be probed against the same bead array.
Fig. 2 summarizes how this technique is used to compare interactions between different combinations of E- and N-cadherins. Three types of functionalized beads (4.8 μm diameter) and two cell types were used in our adhesion tests. Details of their preparation are described in the Supporting Material. In short, recombinant cadherin-Fc chimeras (consisting of the extracellular E- or N-cadherin domain fused with the Fc region of human IgG) were linked to protein-A-coated beads (Fig. 2 A). Substitution of the chimeras with plain Fc fragments provided negative control beads. The cells were L cells (murine fibroblasts that do not normally express cadherin) transfected with DsRed- or GFP-tagged E- or N-cadherin, respectively. The fluorescent tag (attached to the cytoplasmic domain of the cadherins) allowed us to verify the type and level of cadherin expression in each tested cell (e.g., Fig. 2 C).
Figure 2.
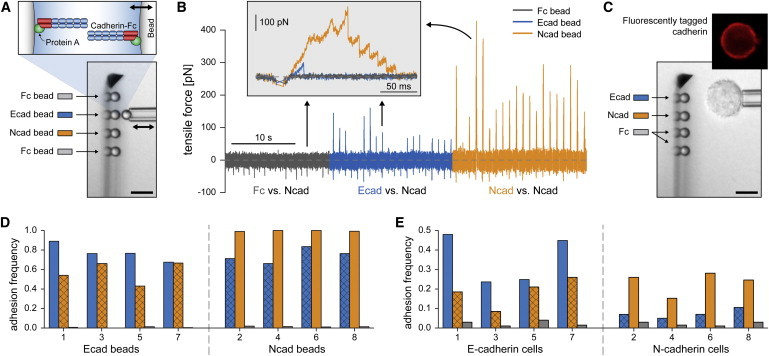
Summary of bead-bead (A, B, and D) and cell-bead (C and E) experiments. (A) A cadherin-coated, pipette-held test bead is probed against an array of four functionalized microspheres. Recombinant cadherin-Fc chimeras were attached to beads via surface-bound protein A (inset at top). The pipette-held bead is repeatedly moved to/from contact with one of the stationary cantilever beads (double arrow). (B) Examples of force-time curves recorded during bead-bead tests. Here, 20 cycles are shown for each tested interaction between a pipette-held Ncad bead and one of the three probe surfaces (Fc, Ecad, and Ncad). Adhesion events produce positive forces during retraction of the test bead. Homophilic Ncad:Ncad bonds form most frequently. (Inset) Enlarged view of superimposed individual test cycles (one of each interaction). High interaction forces are due to multiple bonds, as revealed by consecutive bond-rupture events in the Ncad-Ncad adhesion test (orange). (C) Bright-field videomicrograph of a cell-bead experiment. Also included is a fluorescent image of a pipette-held test cell, confirming expression of cadherin. (D and E) Adhesion frequencies from contacts between different combinations of probe beads and either test beads (D) or test cells (E). (Note that the y scales are different.) Colors encode the specific probe surfaces of the cantilever beads (see legends in A and C). Overlaid crosshatch patterns highlight heterophilic E:N-cadherin binding. Numbers on the x axes indicate the chronological order in which the data sets were gathered. (Bars in panels A and C denote 10 μm.)
Fresh bead arrays of four probe beads—an E-cadherin (Ecad) bead, an N-cadherin (Ncad) bead, and two control beads (Fc)—were assembled on the cantilever for each experiment (Fig. 2, A and C). We performed two types of experiments testing either pipette-held functionalized beads (bead-bead, Fig. 2, A, B, and D) or cells (cell-bead, Fig. 2, C and E) against the bead arrays. Together, these experiments allowed us to compare the adhesive behaviors of recombinant extracellular cadherin domains and full cadherin proteins. The pipette-held test objects alternated between Ecad and Ncad beads in bead-bead experiments and between E-cadherin and N-cadherin cells in cell-bead experiments. Each object was tested against the bead array in a sequential manner (200–300 contacts per probe bead; see Movie S2). The feedback-controlled touch force (negative in Fig. 2 B) was set to −30 pN in all tests; the nominal force-loading rate was 20,000 pN/s in bead-bead experiments and 50,000 pN/s in cell-bead experiments.
We defined the adhesion frequency as the number of attachments surviving at least 30 pN of tensile force (positive in Fig. 2 B) per total number of contacts. Note that many adhesion events consisted of multiple cadherin bonds (see inset of Fig. 2 B), especially when the measured adhesion frequency was close to 1. Each triplet of columns in Fig. 2, D and E, depicts the adhesion frequencies from tests of a single, pipette-held bead or cell against the three probe surfaces (Ecad, Ncad, and Fc).
Columns in gray (negative control) indicate that our bead preparation successfully suppressed nonspecific interactions. For both types of test beads (i.e., Ecad and Ncad), nonspecific adhesion to probe Fc beads occurred in <2% of touches. As expected, nonspecific adhesion of cells occurred more often but still only in <4% of touches.
Specific adhesion is evident in all combinations of E- and N-cadherin interactions. In particular, each of the 16 used test objects—be it a cadherin-coated bead or a cadherin-expressing cell—formed a significant number of heterophilic E:N-cadherin attachments (data highlighted by a crosshatch pattern in Fig. 2, D and E). These results leave little doubt that E- and N-cadherin cross-react under the tested conditions.
How then does this heterophilic adhesion compare to homophilic cadherin interactions? It is important to keep in mind that the frequency of specific adhesion events depends on the cadherin surface density of the bead or cell. Especially when working with individual cells, this density is hard to control and may vary considerably from cell to cell, as also seen in Fig. 2 E (14). However, if both cadherin types are tested against the same probe-bead array, and if an opposite trend in the relative adhesion frequencies is consistently observed, this trend will reflect the inherent behavior of the pertinent molecular interactions. Thus, the results in Fig. 2, D and E, establish that during both bead-bead as well as cell-bead contacts, homophilic E:E- and N:N-cadherin bonds form more frequently than the heterophilic E:N attachments (also clearly seen in Fig. 2 B). As explained in the Supporting Material, our bead-bead tests allowed us to roughly estimate the ratio of the respective on-rate constants as
In conclusion, our new approach to examine multiple biomolecular interactions of the same cell (or other test object) has allowed us to directly compare homophilic and heterophilic binding between recombinant and cellular E- and N-cadherins. Our results demonstrate that functionalized bead arrays affixed to AFM cantilevers are a powerful tool in studies of cellular and biomolecular interactions, with potential applications that go far beyond the adhesion-frequency measurements presented here.
Acknowledgments
We thank T. N. Nguyen for generating the cadherin-expressing cell lines used in this study.
This work was supported in part by National Institutes of Health grant No. R01 A1072391 (V.H.) and Beckman Young Investigator Award (S.Y.). C.O. was supported by University of California Systemwide Biotechnology Research & Education Program GREAT training grant No. 2008-13.
Supporting Material
References and Footnotes
- 1.Thiery J.P. Epithelial-mesenchymal transitions in tumor progression. Nat. Rev. Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 2.Wheelock M.J., Shintani Y., Johnson K.R. Cadherin switching. J. Cell Sci. 2008;121:727–735. doi: 10.1242/jcs.000455. [DOI] [PubMed] [Google Scholar]
- 3.Prakasam A.K., Maruthamuthu V., Leckband D.E. Similarities between heterophilic and homophilic cadherin adhesion. Proc. Natl. Acad. Sci. USA. 2006;103:15434–15439. doi: 10.1073/pnas.0606701103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panorchan P., Thompson M.S., Wirtz D. Single-molecule analysis of cadherin-mediated cell-cell adhesion. J. Cell Sci. 2006;119:66–74. doi: 10.1242/jcs.02719. [DOI] [PubMed] [Google Scholar]
- 5.Leckband D., Prakasam A. Mechanism and dynamics of cadherin adhesion. Annu. Rev. Biomed. Eng. 2006;8:259–287. doi: 10.1146/annurev.bioeng.8.061505.095753. [DOI] [PubMed] [Google Scholar]
- 6.Shi Q., Chien Y.H., Leckband D. Biophysical properties of cadherin bonds do not predict cell sorting. J. Biol. Chem. 2008;283:28454–28463. doi: 10.1074/jbc.M802563200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niessen C.M., Gumbiner B.M. Cadherin-mediated cell sorting not determined by binding or adhesion specificity. J. Cell Biol. 2002;156:389–399. doi: 10.1083/jcb.200108040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duguay D., Foty R.A., Steinberg M.S. Cadherin-mediated cell adhesion and tissue segregation: qualitative and quantitative determinants. Dev. Biol. 2003;253:309–323. doi: 10.1016/s0012-1606(02)00016-7. [DOI] [PubMed] [Google Scholar]
- 9.Binnig G., Quate C.F., Gerber C. Atomic force microscope. Phys. Rev. Lett. 1986;56:930–933. doi: 10.1103/PhysRevLett.56.930. [DOI] [PubMed] [Google Scholar]
- 10.Heinrich V., Rawicz W. Automated, high-resolution micropipet aspiration reveals new insight into the physical properties of fluid membranes. Langmuir. 2005;21:1962–1971. doi: 10.1021/la047801q. [DOI] [PubMed] [Google Scholar]
- 11.Ounkomol C., Xie H., Heinrich V. Versatile horizontal force probe for mechanical tests on pipette-held cells, particles, and membrane capsules. Biophys. J. 2009;96:1218–1231. doi: 10.1016/j.bpj.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heinrich V., Ounkomol C. Force versus axial deflection of pipette-aspirated closed membranes. Biophys. J. 2007;93:363–372. doi: 10.1529/biophysj.107.104091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinrich V., Ounkomol C. Biophysics in reverse: using blood cells to accurately calibrate force-microscopy cantilevers. Appl. Phys. Lett. 2008;92:153902. [Google Scholar]
- 14.Although our instrument allows us to verify cadherin expression in each cell fluorescently (Fig. 2 C), quantifying correlations between adhesion frequency and cadherin surface density will require a more sensitive camera than has been available for this study.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


