Abstract
Recent crystallographic studies revealed that five transporter families without much sequence similarities among them have similar structure folds to LeuT, a bacterial neurotransmitter:sodium symporter homolog. The LeuT fold is characterized by an internal twofold structural pseudosymmetry. The transport cycle of some members of each of these families is dependent on a sodium gradient across the membrane, whereas in some others the role of sodium is mimicked by proton. We report on the identification of common structure-dynamics elements of the transporters with LeuT fold, which are recognizable in the conformational transitions related to function. The findings from comparative computational modeling and simulation studies of LeuT, and ApcT from the amino acid-polyamine-organocation transporter family define the intramolecular mechanisms by which Na+ binding couples to the transport process, and single out the lead/active role of TM1a in the transition to inward-open conformation. These mechanistic insights are derived in the context of collaborative investigations of LeuT dynamics with both single-molecule fluorescence and simulations that have produced excellent agreement of the dynamic details, and are found to be generalizable across the transporter families and to transcend sequence and motif similarities.
The crystal structure of LeuT from the neurotransimitter:sodium symporter (NSS) family was solved with two embedded Na+ in close association with the bound substrate (1). Previous studies have indicated significant effects on LeuT structure and the formation of outward- and inward-facing conformations from binding and dissociation, respectively, of the Na+ in the Na2 site (2,3). Recently, the crystal structure of ApcT, a proton-dependent transporter, from the amino acid-polyamine-organocation (APC) transporter family was solved in an inward-open conformation, with the side chain of Lys158 located in a pocket that can be structurally aligned to the Na2 site in LeuT (4). Thus the protonation and deprotonation of Lys158 of ApcT, which can result in differed coordinating configurations, were proposed to serve similar functions as the binding and dissociation of Na2 in the conformational transition of LeuT (4).
The analysis of structures with LeuT fold shows that the signature difference between outward- and inward-facing conformations is the rearrangements of TM1 and TM6, the unwound regions of which are directly involved in the formation of the Na+ binding site(s) (5). However, it remained unclear how the impact of Na+ binding is propagated to the gating region on either side of the transporter. Recent single-molecule fluorescence (smFRET) studies (6) have identified the nature of conformational transitions triggered by Na+ and substrate. We used molecular dynamics (MD) simulations to interpret the distance changes monitored with smFRET as corresponding to changes that occur in the intracellular interaction network that result in the intracellular portion of TM1, TM1a, moving away from the protein bundle during the transition from outward- to inward-facing conformations (6). Here we report the results of a comparative characterization of the transition to the inward-open conformation from MD simulations extending those described previously for LeuT (2) and carried out here for ApcT. A key role for TM5 in coordinating this conformational transition is revealed, and we discuss the implications of the results for the functional mechanism and modulation of LeuT-like transporters.
In structures with LeuT fold, Na2 sites are lined by residues from TM1 and TM8. In ApcT and in the BetP structure from BCCT family (4,7), TM5 also contributes to the aligned Na2 sites. The protonation of Lys158 of ApcT is believed to mimic Na+ binding (4). Congruently, we found in our homology modeling of eukaryotic serotonin transporter and dopamine transporter from the NSS family that after prolonged equilibration (30 to 60 ns), a conserved Thr from TM5, in association with a conserved Asp from TM8, becomes involved in the Na2 binding site (Fig. S1 of the Supporting Material). The aligned position of this Asp is occupied by a Thr in LeuT and coordinates Na2. Based on the prevailing assumption that the eukaryotic and prokaryotic NSS undergo a similar conformational transition mechanism despite differences in structural details such as the Na2 binding configurations (1,2,4), this involvement of TM5 in Na2 binding suggests that it has a role as well in coordinating the intracellular conformational changes in the NSS family, in addition to its previously demonstrated involvement in the permeation pathway (2,8). Thus the commonality of the Na2 site structure may be broader than that identified between NSS and APC by the sets of directly coordinating residues in the LeuT and ApcT structures. Indeed, other members of the APC family were found to be Na+-dependent and are likely to have an Na2 site (9).
To address this new perspective we used the long MD simulations to investigate the conformational impact of a positive charge in the Na2 site, characterizing the Na+ binding/dissociation in LeuT in comparison to protonation/deprotonation of Lys158 in ApcT. From LeuT trajectories with and without Na+ (600 ns each), we identify a change in Na2 binding with the significant backbone dihedral changes of Val23 and the rotamer changes of Phe203 (Figs. 1, 2, Fig. S3, and Table S1). Similar rearrangements were observed in our steered molecular dynamics/MD studies of LeuT that have induced a more drastic inward-facing conformation, as discussed below. A correspondingly similar backbone rearrangement is seen by comparing ApcT simulations with Lys158 protonated and deprotonated (180 ns each): when Lys158 is protonated, the backbone carbonyl of the Ile22 (aligned with Val23 in LeuT) is flipped from the orientation found in the inward-open ApcT crystal structure, to that observed for Val23 in the inward-closed form of LeuT in the crystal structure (Fig. 1).
Figure 1.
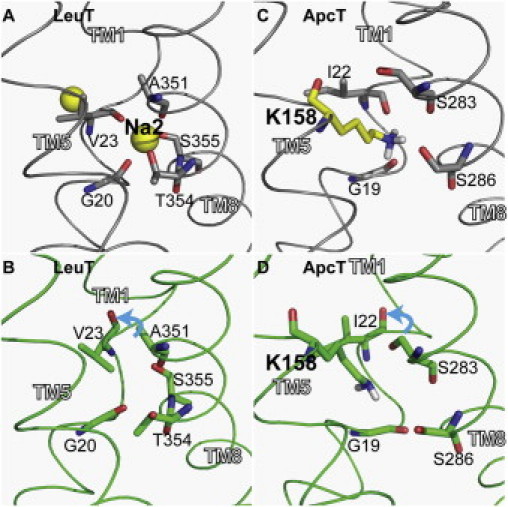
Configurational changes in the Na2 site of LeuT in the presence (A) and absence (B) of Na+, and the corresponding vicinity of Lys158 of ApcT with Lys158 protonated (C) and deprotonated (D).
Figure 2.
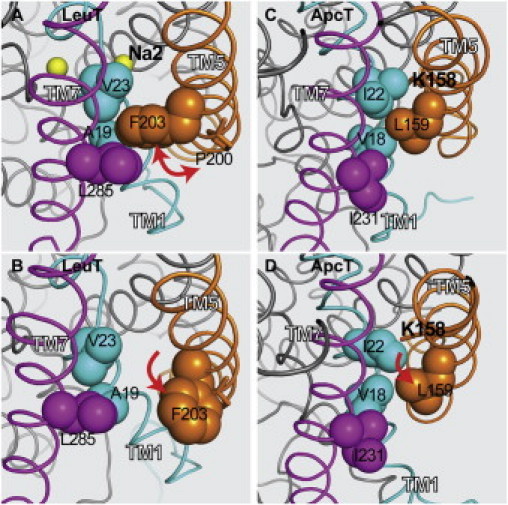
Local conformational changes near the Na2 site in LeuT (A and B), and the vicinity of Lys158 of ApcT (C and D) that allow TM1a to swing outwards during the transition from the inward-closed (A and C, respectively) to the inward-open conformations (B and D, respectively).
These backbone dihedral changes impact strongly the local conformational rearrangement (Table S1). In ApcT, it also has a profound impact on the global rearrangement that is consonant with the conformational rearrangements leading to the LeuT inward-facing conformation, generated by computationally steering the S1 site-bound substrate intracellularly in the presence of S2 and absence of Na2 (2), which was followed by prolonged MD simulations designed to examine the structural equilibration (6). In parallel, the evolution of conformational rearrangements in LeuT expressed in the distance changes of the representative 7/86 residue pair (between N-terminus and intracellular loop 1) was also monitored with smFRET (6). Distance changes deduced from the smFRET measurements agree well with the simulations results (6). Interestingly, the distance change pattern— i.e., increase versus decrease in going from in the inward-closed to the inward-open state, which is monitored by the 7/86 pair in the LeuT simulations and in smFRET— was the same as we observed here for the aligned 6/82 pair in the ApcT simulations of the conformational transition from the Lys158 protonated state to the Lys158 deprotonated one ((6) and Fig. S2). The similarity of this important dynamic element (see details in (6)) in the two transporters is observed here despite significant differences in the nature of local interactions in their functionally important intracellular regions (see Supporting Material for details about these interactions).
The outward movement of TM1a is common to our observations from simulations of LeuT (see (6)) and those described here for ApcT, as well as to the smFRET data for LeuT. This movement is essential to create the space needed for the release of substrate to the cytoplasm. That a similar solution for conformational changes supporting release is observed in the two different transporters suggests a conservation of the intrinsic mode of rearrangement for the LeuT-like structure fold, albeit triggered differently and produced by a different set of local changes. This is likely the case for other members of the different transporter families with LeuT fold but different sequence composition.
Given the difference in the way in which the two transporters incorporate the effect of charge in the Na2 site, it is significant to understand and useful to examine how the changes in that region are connected to the global rearrangements discussed above. In LeuT, the changes are propagated through a network formed by interacting loci in TMs 1, 5, and 7 (Val23-Ala19-Phe203-Leu285), near the Pro200 in TM5, by triggering a change in conformation around this highly conserved proline-kink, which is facilitated by the altered rotamer distribution observed for the conserved Phe203 in the presence and absence of Na+ and substrate (Fig. S3). We propose that the trans χ1 rotamer of the Phe203 allows TM1a to swing outward, which also requires the dissociation of the Trp8-Ile187-Tyr268 interaction network. This combined move is a consequence of both substrate and Na+ binding (2). Remarkably, in the ApcT simulations we observed a similar interaction network (Ile22-Val18-Leu159-Ile231; note that Leu159 is next to Lys158) and changes associated with inward-open to inward-closed conformational rearrangements produced only by the change in Lys158 protonation. This exposes the details of the commonality between mechanisms in LeuT and ApcT which, although different in magnitude, are modulated by Na2 binding or protonation, respectively, and determine the disposition of TM1a (Fig. 2). These consistencies, and the similarities in triggers and conformational changes, offer strong support for the validity of simulation-based findings and conclusions reached for LeuT, and suggest a common molecular mechanism for the transition to inward-facing conformation in transporters with LeuT fold.
The characteristics of the proposed common mechanism are that 1), it is triggered by the elimination of a positive charge from the Na2 site, which induces significant backbone dihedral changes of an Na+ (positive charge)-binding residue from TM1 (Val23 in LeuT and Ile22 in ApcT); 2), it propagates the impact of this local rearrangement through coordinated changes of TM5, which is in close association with Na2 binding, by employing a spatially ordered network of interactions; and 3), it modulates the movement of TM1a downward and away from the bundle in a manner enabled by steps 1 and 2. The specific rearrangement of TM1a is the signature step in the creation of the inward-facing conformation and the opening of the intracellular pathway. In the context of entire transport cycle, the introduction and elimination of a positive charge in the Na2 site alters the energy requirements for the conformational changes, though possibly to different degrees in different transporters (see the difference between LeuT and ApcT regarding intracellular interaction networks described in the Supporting Material). Due to these changes in the energetics the substrate can readily shift the conformational equilibrium in the direction needed for transport (2).
The findings we describe define the intramolecular mechanisms by which Na+ binding couples to the transport process, and single out the lead/active role of TM1a and the significant conformational changes involving the conserved proline-kink in TM5 in the transition to inward-open conformation. The structurally equivalent TM6b segment exhibits a relatively more passive role, and there seems to be no driving force for, or need for, a parallel rearrangement in TM1a and TM6b. This is also consistent with our previous findings in Tyt1 (10). This conclusion is supported by the commonality of mechanisms we observed for LeuT-like transporters in different families, and departs from previously proposed mechanisms suggested entirely by the apparent symmetry of the helices in the crystal structure of the transporter molecule (5,11).
Acknowledgments
Computations were performed on the Ranger at the Texas Advanced Computing Center (TG-MCB090022) and the David A Cofrin computational infrastructure of the Institute for Computational Biomedicine at Weill Cornell Medical College. This work was supported in part by National Institutes of Health Grants DA12408 (to H.A.W.) and DA023694 (to L.S.).
Contributor Information
Lei Shi, Email: les2007@med.cornell.edu.
Harel Weinstein, Email: haw2002@med.cornell.edu.
Supporting Material
References and Footnotes
- 1.Yamashita A., Singh S.K., Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl—dependent neurotransmitter transporters. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- 2.Shi L., Quick M., Javitch J.A. The mechanism of a neurotransmitter:sodium symporter—inward release of Na+ and substrate is triggered by substrate in a second binding site. Mol. Cell. 2008;30:667–677. doi: 10.1016/j.molcel.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noskov S.Y., Roux B. Control of ion selectivity in LeuT: two Na+ binding sites with two different mechanisms. J. Mol. Biol. 2008;377:804–818. doi: 10.1016/j.jmb.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaffer P.L., Goehring A., Gouaux E. Structure and mechanism of a Na+-independent amino acid transporter. Science. 2009;325:1010–1014. doi: 10.1126/science.1176088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krishnamurthy H., Piscitelli C.L., Gouaux E. Unlocking the molecular secrets of sodium-coupled transporters. Nature. 2009;459:347–355. doi: 10.1038/nature08143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao Y., Terry D., Javitch J.A. Single-molecule dynamics of gating in a neurotransmitter transporter homologue. Nature. 2010;465:188–193. doi: 10.1038/nature09057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ressl S., Terwisscha van Scheltinga A.C., Ziegler C. Molecular basis of transport and regulation in the Na(+)/betaine symporter BetP. Nature. 2009;458:47–52. doi: 10.1038/nature07819. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y.W., Rudnick G. The cytoplasmic substrate permeation pathway of serotonin transporter. J. Biol. Chem. 2006;281:36213–36220. doi: 10.1074/jbc.M605468200. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z., Albers T., Grewer C. A conserved Na(+) binding site of the sodium-coupled neutral amino acid transporter 2 (SNAT2) J. Biol. Chem. 2009;284:25314–25323. doi: 10.1074/jbc.M109.038422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quick M., Yano H., Javitch J.A. State-dependent conformations of the translocation pathway in the tyrosine transporter Tyt1, a novel neurotransmitter:sodium symporter from Fusobacterium nucleatum. J. Biol. Chem. 2006;281:26444–26454. doi: 10.1074/jbc.M602438200. [DOI] [PubMed] [Google Scholar]
- 11.Forrest L.R., Rudnick G. The rocking bundle: a mechanism for ion-coupled solute flux by symmetrical transporters. Physiology (Bethesda) 2009;24:377–386. doi: 10.1152/physiol.00030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


