Abstract
To carry out realistic in vitro mechanical testing on anatomical tissue, a choice has to be made regarding the buffering environment. Therefore, it is important to understand how the environment may influence the measurement to ensure the highest level of accuracy. The most physiologically relevant loading direction of tendon is along its longitudinal axis. Thus, in this study, we focus on the tensile mechanical properties of two hierarchical levels from human patellar tendon, namely: individual collagen fibrils and fascicles. Investigations on collagen fibrils and fascicles were made at pH 7.4 in solutions of phosphate-buffered saline at three different concentrations as well as two HEPES buffered solutions containing NaCl or NaCl + CaCl2. An atomic force microscope technique was used for tensile testing of individual collagen fibrils. Only a slight increase in relative energy dissipation was observed at the highest phosphate-buffered saline concentration for both the fibrils and fascicles, indicating a stabilizing effect of ionic screening, but changes were much less than reported for radial compression. Due to the small magnitude of the effects, the tensile mechanical properties of collagen fibrils and fascicles from the patellar tendon of mature humans are essentially insensitive to environmental salt concentration and composition at physiological pH.
Introduction
Tendons are a fibrous collagenous connective tissue that is responsible for transmitting forces from muscle to bone to produce joint movement. The importance of tendon in the function of the body is mechanical in nature and investigating the response of tendons under load is essential. When it comes to the examination of tissue mechanical properties, it is often necessary to remove the tissue from its natural environment, thus requiring an adequate substitute. Interstitial fluids contain a complex mixture of a large number of different components including minerals, proteins, sugars, and more.
The most abundant components are Na+ (∼140 mM) (1) and Cl− (∼100 mM) (2) making up ∼80% of the total osmolarity (∼300 mM), and a common substitute is therefore a 150 mM (0.9%) NaCl solution, commonly referred to as physiological saline. Such a simple saline solution does not take into account the physiological pH value of 7.4 (3) and therefore a buffer is often added. A large number of buffers exist with different advantages and disadvantages (4); a common choice is phosphate. However, a number of studies have indicated that such buffered solutions do not correctly mimic the natural environment, but instead yield increased swelling (5,6). Furthermore, it has been reported that buffer solutions have altered the mechanical properties (6–8) compared to freshly harvested tissue tested under ambient conditions. It should be noted that simply testing under ambient conditions is often not realistic, because the tissue will dry quickly, especially if the specimens are small.
Different studies have found that changing the molarity of the buffering solution affects the water content of the tissue (9,10). The hydrating properties of physiological solutions are important, because hydration has been shown to influence mechanical properties (11). Most notably dry tissues are stiffer than hydrated tissues; as hydration increases, so does cyclic relaxation (6), stress relaxation (12,13), and creep (9,10), which are all manifestations of viscous behavior. These studies used sucrose to alter solution molarity; however, this option will be problematic because sucrose solutions are significantly more viscous than water (14).
In a recent study by Grant et al. (15), individual collagen fibrils were investigated by nanoindentation to determine the radial compressive mechanical properties in a number of saline solutions with varying composition, concentration, and pH. It was found that increasing the salt concentration led to a severalfold increase in modulus. However, due to the highly anisotropic structure of collagen fibrils, the mechanical response in the radial direction will be considerably different from that in the axial direction.
A number of recent studies on tensile mechanical properties of individual collagen fibrils report modulus values ranging from 0.2 to 0.86 GPa for hydrated fibrils (16–18). Several studies have looked into the difference between dry and hydrated tissue; however, none have investigated differences in the hydrated environment.
The tendon is known to have a hierarchical structure ranging from the collagen molecule to the whole tendon (Fig. 1). However, the mechanical connection between the levels of the hierarchy is poorly understood. Some studies suggest that fibrils slip significantly during loading (19,20), while the great length of fibrils (21,22) and the inability to obtain unbroken fibrils from mature tissue (23) indicate that fibrils are mechanically and possibly even physically continuous. It is therefore of great interest to investigate how changes at one hierarchical level relate to the other levels.
Figure 1.
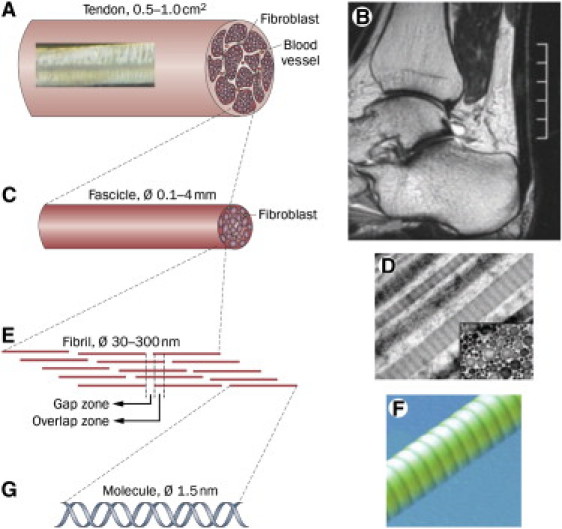
(A) Schematic of the whole tendon. (Inset) Texture of the tendon with crimping. (B) Magnetic resonance image of the human Achilles tendon. (C) Tendon fascicles which are long separable cylindrical structures. (D) Longitudinal transmission electron microscope image of fibrils in a fascicle. (Inset) Transmission electron microscope cross section where the fibrils appear as circles. (E) Schematic of the highly organized structure of molecules in a collagen fibril giving rise to a characteristic ∼68-nm banding pattern of gap and overlap zones. (F) Three-dimensional AFM image of a collagen fibril displaying the banding pattern. (G) The building blocks of the fibrils are the collagen molecules, a peptide triple helix. (Figure reproduced from Magnusson et al. (45).)
In this article, we investigated the axial tensile mechanical properties and swelling of collagen fibrils and fascicles from the human patellar tendon (Fig. 1), to assess the degree to which changes at one hierarchical level would carry over to the other. The environmental factors investigated are osmolarity and presence of calcium. The effect of osmolarity was primarily investigated to determine whether changes to the hydration through osmosis would occur and whether the mechanisms of force transmission would be disrupted or enhanced by salts as indicated by Grant et al. (15). The investigation of calcium was prompted by previous reports showing that energy dissipation in nanoscale pulling and indentation of collagen was significantly increased in CaCl2-containing solutions compared to solutions with only NaCl (24,25).
Materials and Methods
Materials
Human patellar tendon tissue was obtained during anterior cruciate ligament replacement surgery (approved by the Danish Council of Ethics). The tissue had been stored frozen (−20°C) in 150 mM phosphate-buffered saline (PBS). Reports on the effect of frozen storage on mechanical properties are inconsistent (26–30), but in our laboratory we have not observed any measurable differences in mechanical properties between fresh and frozen tissue. Individual fascicles were liberated from the tissue, one used for the fibril level experiments and a further six used for experiments at the fascicular level.
Atomic force microscopy (AFM) was performed on a MultiMode microscope with a IIIa controller (Veeco Instruments, Woodbury, NY). Three different cantilevers were used for AFM experimentation (Olympus, Tokyo, Japan):
-
1.
OMCL-AC160TS (kspring∼42 N/m),
-
2.
OMCL-TR800PSA long cantilever (kspring∼0.15 N/m), and
-
3.
OMCL-TR800PSA short cantilever (kspring∼0.57 N/m).
A PicoForce setup was used for mechanical measurements (Veeco Instruments). The exact spring constant of cantilevers were determined by analysis of their thermal spectrum (thermal tune) (31).
PBS solutions were prepared from a 20× concentrated PBS stock solution (4.00 g/L KCl, 160.20 g/L NaCl, 4.00 g/L NaH2PO4, 23.00 g/L, Na2HPO4) by diluting 3, 20, and 150 times with ultra-pure water (resistivity >18 MΩ·cm) and adjusting to pH 7.4. This yields solutions with concentrations of 1 M, 150 mM, and 20 mM (called PBS1000, PBS150, and PBS20, respectively). Furthermore, two 10-mM HEPES buffers were prepared to investigate the effect of Ca2+, one containing 150 mM NaCl (called HEPES) and another containing 90 mM NaCl, 40 mM CaCl2 (called CaCl2) (pH 7.4). The HEPES solution was used to clear out residual phosphate before the introduction of calcium that would otherwise precipitate as calcium phosphate; it also serves as a control to the effect of HEPES itself.
Experimental overview
Each fascicle and fibril was tested in all five solutions. First, the specimen was tested in each of the three PBS solutions. The order of PBS solutions in this test was varied between experiments in a systematic fashion comprising the six possible permutations of solution order. This allows for the effect of time to be assessed by comparing among the first, second, and third PBS buffers without having the PBS concentration as a confounder (Table 1). Then it was tested in the HEPES solution and finally in the CaCl2 solution. Fascicles and fibrils were tested at two deformation rates of 0.5 and 4 mm/min and 9.81 and 314 μm/s, respectively, to assess viscous effects. To eliminate correlation between rate and time, the order of the testing rates was reversed in every other experiment. The deformation rates for the fascicles translate to ∼0.1%/s and 1%/s strain rate, and for the fibrils they translate to ∼5%/s and 160%/s.
Table 1.
Results grouped by injection order irrespective of PBS concentration
| Time of PBS injection∗ | No. 1 | No. 2 | No. 3 |
|---|---|---|---|
| Fascicles | |||
| Modulus (GPa) | 0.55 ± 14 | 0.54 ± 14 | 0.54 ± 15 |
| Relative energy dissipation (%) | 24.9 ± 3.8 | 24.7 ± 2.6 | 24.5 ± 4.5 |
| Diameter (mm) | 0.28 ± 0.16 | 0.28 ± 0.17 | 0.28 ± 0.16 |
| Fibrils | |||
| Modulus (GPa) | 2.89 ± 0.23 | 2.88 ± 0.23 | 2.93 ± 0.21 |
| Relative energy dissipation (%) | 14.8 ± 3.4 | 14.3 ± 4.5 | 13.9 ± 4.4 |
| Height (nm) | 167 ± 50 | 168 ± 47 | 171 ± 48 |
Values are mean ± SD. None of the parameters differ significantly between time-points.
Time is shown as the injection order (first, second, or third PBS buffer introduced). Each time-point covers two measurements at each PBS concentration.
Fascicle testing
Fascicle mechanical testing was performed in a micro tensile tester (200-N tensile stage, petri dish version; Deben, Suffolk, UK) equipped with a 20 N load cell (shown in Fig. 2 A). The middle of the fascicle was kept moist by gauze soaked in the first solution while the ends dried. The dry ends were glued with cyanoacrylate on to the clamps and an aluminum disk was placed on top, which significantly increased the rate of curing. The first solution was introduced after 15 min.
Figure 2.
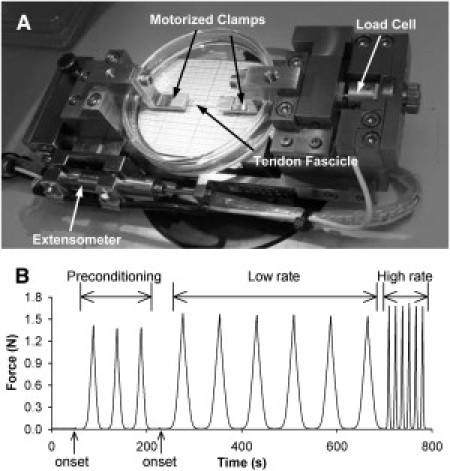
Fascicle mechanical test design. (A) Photograph of the mechanical rig showing the most important components. (B) Example of fascicle mechanical data showing onset determinations, preconditioning, and cyclic tests at two deformation rates. The basic scheme is similar to that used for fibrils.
The fascicle was stretched until reaching the force onset (∼0.01 N) followed by three preconditioning cycles to 4% strain after which the onset was redetermined (see Fig. 2 B). Using the new onset, six cycles to 4% strain were performed at each deformation rate. Data from the sixth cycle was analyzed. At the end of the test the fascicle was returned to a slack position, and the solution was exchanged while keeping the fascicle moist. The fascicle was incubated in the new solution for 1 h before repeating the test protocol.
In each solution, the fascicle diameter was determined from microscope images taken at the first onset. Cross-sectional area (CSA) was calculated assuming a circular profile (32); subsequently, the CSA in the first solution was used to calculate stress for that specimen in all solutions.
Fibril mechanical testing
Fibril mechanical testing was performed using a previously published method (33) with one improvement to the procedure. The glue used for this study was Araldite 2014 (Huntsman Advanced Materials, Everberg, Belgium), which reduces the initial curing period by 24 h. In brief, tissue was spread and dried on a silicon substrate, then two droplets of glue (∼200 μm apart) were placed with an AFM cantilever on a separate fibril. After curing (18 h), the CSA was determined at eight positions along the dry fibril by tapping mode AFM imaging using cantilever 1. Dry CSA was used because it is easier to measure and is not affected by changes in the tapping force. After CSA measurement, one of the glue droplets at the end of the selected fibril was scraped free using cantilever 1. This free region of the collagen fibril was attached with glue to cantilever 3 in such a way that the fibril was sandwiched between the glue pad and the cantilever. After further curing (18 h), the first buffer solution was introduced and given 30-min equilibration time before mechanical testing. The data acquisition technique requires merging of multiple separate curves and therefore only steady-state measurement can be made (33).
To ensure comparability between fibril and fascicle tests, the fibril testing protocol was kept as identical as technically possible to the one used on fascicles. An initial force onset was determined (∼2.5 nN) and preconditioning cycles to 4% strain were performed for 10 min. The final onset was then determined and used throughout the experiment. The test consisted of cycling to 4% strain for 5 min to ensure a steady state before acquiring the force curve in a piecewise manner as previously described (33). When changing deformation rate, the fibril was cycled for 2 min to ensure a steady state at the new rate before acquiring data. The fibril was then relaxed and the buffer solution removed with a syringe, leaving a small amount of solution between the cantilever and the surface to avoid drying out the fibril. The fluid-cell was flushed thoroughly with 10 chamber volumes of the next test solution. The collagen fibril was allowed to equilibrate for 30 min before the testing protocol was repeated.
Fibril height
The effect of solution environment on fibril swelling was investigated by AFM imaging using cantilever 2 for tapping-mode in liquid. AFM samples were prepared as described above. To avoid compression of the fibrils, tapping force was kept low by increasing the amplitude set-point until the tip almost detached from the surface. Even at low tapping force, it is necessary to keep the scan direction parallel to the fibrils to avoid detaching them from the substrate. Due to the low tapping force, tracking was poor along the fibril sides (Fig. 3 B) and therefore only the height was measured. For each fibril, height was measured at the same four points in all solutions.
Figure 3.
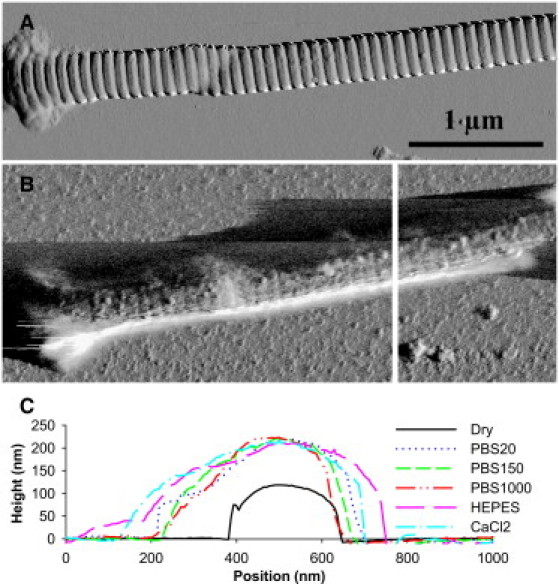
AFM images used for measuring fibril heights to assess swelling. (A) Dry collagen fibril imaged in air. (B) The same fibril imaged in the PBS20 solution. The fibril is casting a shadow to the left due to the low tapping force. (C) Line section profiles used to determine fibril height in each solution. The displayed sections were taken at the position shown with a vertical white line in panel B. As can be seen, the peak height varies little between the solutions.
Data reduction and statistics
Mechanical measurements were made on six fascicles and six fibrils, swelling was assessed on the same six fascicles and 12 other fibrils. Modulus was determined at the lowest common stress in each experiment, by linear regression to the final 20% (stress) of the loading curve. Relative energy dissipation was determined by integrating along the entire hysteresis loop and normalizing to the area under the loading curve. Effect of buffer composition was analyzed by one-way ANOVA with repeated measures. The effect of deformation rate was analyzed by two-way ANOVA with repeated measures. Multiple comparisons were made with Bonferroni corrections. Based on the observed standard deviations, the experiment had a power >98% to detect a 15% change in modulus and relative energy dissipation, at both the fibril and fascicle level. We consider 15% change to be a reasonable requirement in light of the >100% effects of Ca2+ and salt concentration reported by others (15,24,25). For the fibril height and fascicle diameter, the relevant magnitude of change is probably somewhat smaller, and the power of these experiments to detect a 5% change was >90%.
Results
Fibrils
Mechanical testing was performed on single collagen fibrils, producing hysteresis loops as shown in Fig. 4 A. The fibrils had a mean dry diameter of 122 ± 22 nm, which belongs in the larger end of the range seen in human patellar tendon (34). The diameter was very uniform along the length of fibrils with an average standard deviation of 3.5%. The tested fibrils were usually several hundred micrometers in length, but were entangled in other fibrils, allowing only a shorter part to be tested. The mean testing length of fibrils was 192.8 ± 6.0 μm. We tested the collagen fibrils to a mean strain value of 4.25 ± 0.48% corresponding to a stress of 89 ± 15 MPa using dry CSA. The aim was to reach into a linear response region while keeping stress low enough to avoid plastic deformation. Fig. 4 B shows the modulus (slope) of a representative stress-strain curve as a function of the stress and it can be seen that the modulus reaches a plateau. Although the slope does not become completely constant we still consider it to be in a linear region, and because analysis was performed at a common stress value, the slight nonlinearity would not affect the results. We did not observe any plastic deformation during the tensile tests.
Figure 4.
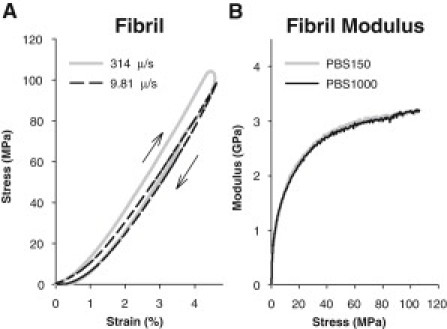
(A) Representative stress-strain curves obtained on a fibril at two deformation rates. Note that the rounded tip in the high-rate curve is caused by a measuring artifact. (Arrows) Loading and unloading direction in the hysteresis loop. (B) Plot of modulus as a function of stress for a single fibril. There is a large initial increase followed by a plateau region.
The effect of osmolarity was investigated in the three PBS solutions at concentrations from 20 mM to 1 M. The modulus of collagen fibrils (see Fig. 6 B, later) showed no significant difference between any of the solutions. Furthermore, the fibril swelling based on height measurements (Fig. 3) was not affected by the PBS concentration either. However, the relative energy dissipation (see Fig. 6 C, later) increased to 17.0 ± 3.8% in PBS1000 from 12.9 ± 3.2% in PBS20 (P < 0.001) and 13.2 ± 3.8% in PBS150 (P < 0.001).
Figure 6.
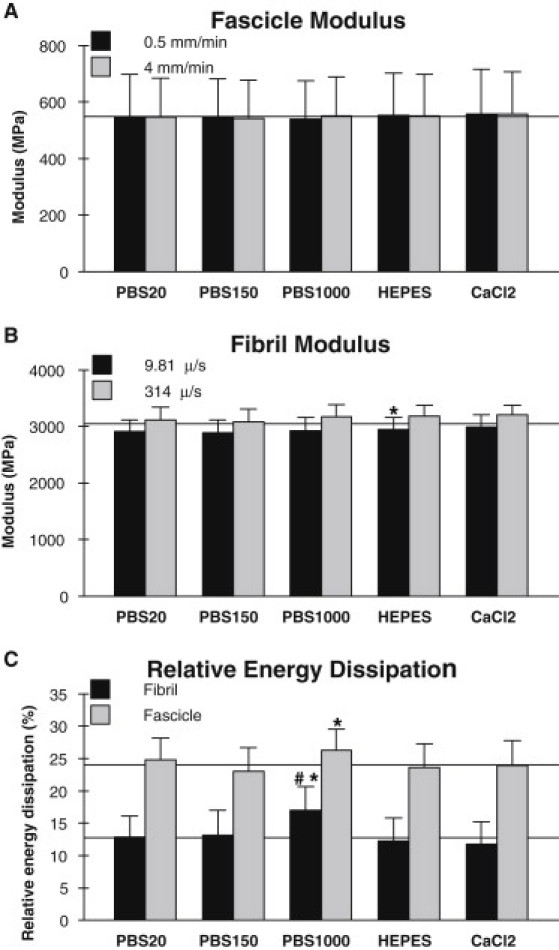
Mechanical results for fascicles and fibrils in each of the five solutions. (A) Modulus of collagen fascicles measured at two deformation rates (0.5 and 4 mm/min). Difference between deformation rates is not significant in any solution. (B) Modulus of collagen fibrils measured at two deformation rates (9.81 and 314 μm/s). Difference between deformation rates is statistically significant in all solutions. (C) Relative energy dissipation of collagen fibrils and fascicles at the low rates (9.81 μm/s and 0.5 mm/min). The pound symbol (#) indicates significantly different from PBS20; the asterisk symbol (∗) indicates significantly different from PBS150 (p < 0.05).) (Horizontal lines) Guide to the eye.
Replacing the phosphate with a HEPES buffer caused a statistically significant increase in fibril modulus of 2.4 ± 1.6% (P < 0.05) compared to the PBS150 solution of equal concentration. Fibril height and relative energy dissipation were not affected by the change from phosphate to HEPES buffer. Investigating the possible effect of calcium on collagen fibril mechanics by including CaCl2 in the HEPES solution did not change any of the measured properties.
The possible viscoelastic effects of the solutions was investigated by increasing the deformation rate from 9.81 μm/s to 314 μm/s. Doing so significantly increased the tensile modulus (see Fig. 6 B, later) in all five solutions (P < 0.01) with a mean increase across all solutions of 7.6 ± 4.3%. Varying the solution environment did not affect the viscoelastic response because there was no interaction between buffer composition and deformation rate in any of the measurements. We did not analyze the effect of deformation rate on relative energy dissipation because the high rate measurements were affected by a measuring artifact seen as a rounded tip in the unloading part of the hysteresis loop in Fig. 4 A.
The initial three PBS solutions were applied in a varied order to eliminate cross talk between composition and time. Analyzing the PBS data by order instead of composition therefore allows us to assess whether changes occurred in the mechanical measurements as the experiment progressed. This was not the case, as there was no significant difference between time points for any of the measured parameters (Table 1).
Fascicles
We performed mechanical tests on fascicles in addition to fibrils to be able to separate intra- and interfibrillar effects (Fig. 5 A). The tested fascicles had a mean diameter of 0.27 ± 0.16 mm and a mean length of 6.93 ± 0.62 mm. The fascicles were tested into the linear region but at sufficiently low stress and strain to avoid plastic deformation. The mean value of the target stress and strain in PBS150 was 12.9 ± 3.9 MPa and 3.87 ± 0.32%, respectively; for comparison, the mean failure stress following the entire experiment was 60 ± 29 MPa and failure strain was 17 ± 7% (see Fig. 5 B).
Figure 5.
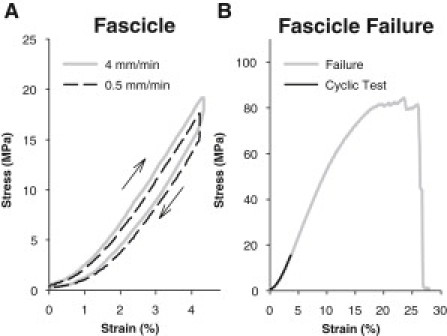
(A) Stress-strain curves obtained on a fascicle at two deformation rates. (B) Comparison between the cyclic test region (∼4% strain) used in this study and the failure properties of a representative fascicle.
Changing the ionic strength of the environment in the range of 20 mM to 1 M PBS did not have any effect on the modulus of the fascicles which remained constant at ∼550 MPa (Fig. 6 A). However, the relative energy dissipation (Fig. 6 C) showed a statistically significant increase from 23.0 ± 3.7% in PBS150 to 26.3 ± 3.3% in PBS1000 (P < 0.01). The swelling of the fascicle as determined by the diameter did not change significantly with solution concentration.
Using a HEPES buffer instead of the phosphate did not significantly affect any of the measured fascicle properties when compared to the PBS150 solution (Fig. 6, A and C). The presence of Ca2+ in the HEPES buffer did not change the mechanical response or swelling either.
Increasing the deformation rate from 0.5 mm/min to 4 mm/min did not significantly affect the modulus (Fig. 6 A) or the relative energy dissipation in any of the solutions. In addition, we found no interaction between buffer composition and deformation rate in any of the measurements.
There was no significant difference between time points for any of the measured parameters (Table 1), showing that none of the measured properties changed over the course of the experiment.
Discussion
The main purpose of this study was to investigate the sensitivity of collagen-based materials to changes in the ionic concentration of their environment. Previously, Ca2+ has been reported to affect mechanical properties of bone and rat tail tendon (24,25,35). The effect of Ca2+ on human tendon at the fibril and fascicle level was therefore also investigated in this study.
Structure
The structural data on fascicle diameter and fibril height did not respond significantly to any of the environmental conditions. At the fascicle level this indicates that the saline solutions utilized in this study had no osmotic effect on the tissue, which is in contrast to previous reports using solutions with sucrose or polyethylene glycol (5,9). At the fibril level, it has previously been shown by x-ray methods that in cornea the molecular spacing, which is assumed to be proportional to fibril diameter, decreases with increasing environmental ionic strength (36), which is in contrast to the present results. However, the reported decrease going from an ionic strength of 0.03 μm to 1 μm was only ∼4%, which is on the order of our measuring uncertainty estimated to be ∼3% from trials using only PBS150. This effect may thus have been too small to detect in this study. For the fibril height, this work agrees well with recent findings by Grant et al. (15), who also reported a lack of changes when adding 1 M KCl at either pH 7 or pH 5.
Modulus
For fascicles, the modulus was unaffected by any of the environmental conditions; however, for fibrils, the modulus increased in HEPES compared to PBS150. Although statistically significant, the physiological importance is questionable, because the relative increase was only 2.3% and the effect was not significant compared to PBS20 or PBS1000. It should further be noted that the HEPES solution was always introduced after the PBS150, making time a confounder; but because no significant time correlation was found in the PBS solutions (Table 1) this is unlikely to be an important factor. We therefore conclude that the effect of HEPES on fibrils is so small that the tensile modulus of both collagen fibrils and fascicles must be considered practically insensitive to all of the investigated environmental conditions.
Increasing the deformation rate of tensile testing led to an increased modulus in the fibrils but not in the fascicles. The effect was not modulated by solution composition, which indicates that the investigated solutions had no impact on the viscous response. However, other measures of viscoelasticity such as creep may be more sensitive.
Comparing our results at the fibril and fascicle level, it is clear that the modulus we report is much greater for the fibrils than the fascicles (Table 1). A thorough discussion of this matter is beyond the scope of this work, but in brief, the majority of the difference can be accounted for by adjusting for the hydrated diameter of the fibrils (36–38), the volume fraction of fibrils (39), and artifacts leading to reduced modulus when testing fascicles of short length in vitro (40).
Energy dissipation
The other mechanical parameter investigated was the relative energy dissipation, which showed a statistically significant increase in PBS1000 compared to PBS20 for fibrils and to PBS150 for both fascicles and fibrils. For fibrils, there was an absolute increase of ∼4% and a trend to increase with osmolarity, although not significant between PBS20 and PBS150. The increase in relative energy dissipation was caused primarily by a reduction in the energy retention during unloading (data not shown). Therefore, if the increase in relative energy dissipation represents a physical effect, a possible mechanism could be stabilization of the deformed state by electrostatic screening, which would reduce the rate of recovery during unloading.
It seems reasonable for the deformed state to be more strongly stabilized by screening than the undeformed state because existing electrostatic interactions would be disrupted by the deformation. For fascicles, the effect was smaller (3.2%), and there was no trend with respect to osmolarity (Fig. 6 C). The less prominent effect in fascicles is expected because fascicles can dissipate energy by interfibrillar mechanisms such as fibril friction and fluid motion through the matrix in addition to that dissipated within the fibrils. This also explains why the magnitude of relative energy dissipation is greater in fascicles than fibrils (Fig. 6 C).
Ca2+ did not significantly affect the relative energy dissipation of fibrils, which indicates that the previously reported increase in energy dissipation during nanoindentation and pulling experiments are related to lateral fibril interactions. The fact that this effect is also present on purified collagen (25) suggests that this interaction is mediated by the collagen itself and not other matrix molecules.
Fibril mechanics
Our finding that the tensile modulus of fibrils is not affected by salt concentration (Fig. 6 B) is in sharp contrast to the radial compressive properties reported by Grant et al. (15). In that work, a twofold increase in modulus was reported when adding 1 M salt to a phosphate buffer at pH 7 regardless of cation (NaCl, KCl or NH4Cl). This effect was further enhanced at pH 5 where the addition of 1 M KCl led to a sevenfold increase in modulus. A number of important differences between the two experiments can explain the discrepancy, one of which is loading geometry. Because dry collagen fibrils swell markedly upon hydration (11,16,36–38), the mechanical properties in the radial direction on hydrated fibrils probably involves a large extent of water displacement between collagen molecules. This would account for the three-orders-of-magnitude reduction in the reported indentation modulus (11). In contrast, fibrils do not swell significantly along their axial direction, and the difference in tensile mechanical properties between dry and hydrated fibrils also appear to be significantly smaller.
Yang et al. (18) found bending moduli in the range 1–3.9 GPa for dry and 0.07–0.17 GPa for hydrated fibrils suspended across a gap, which is a ∼10–20-fold decrease upon hydration. Using a tensile method similar to ours, van der Rijt et al. (16) reported a 10-fold decrease from ∼5 GPa to ∼0.5 GPa, whereas we have observed an ∼2–3-fold decrease in fibril tensile modulus upon hydration in pilot studies. The lesser axial sensitivity to hydration would imply that modulation of the collagen molecule interactions by salts would also have a lesser impact on the axial properties. The reported effect on the compressive modulus was greatly enhanced at pH 5. We therefore performed a similar measurement on a single fibril, and found that the tensile modulus also appeared to be more sensitive to salts at pH 5 than pH 7 (Fig. S1 in the Supporting Material), which suggests that the same mechanisms are in play. However, the effect was much smaller on our sample under tension (∼12%) than reported for compression (∼600%), and the effect was reduced at greater stress levels (Fig. S2).
Another major difference is the biochemical integrity of the collagen fibrils under investigation. In this work, we used native collagen fibrils from the patellar tendon of a 28-year-old male human, which likely contains a high concentration of mature cross-links. In contrast, Grant et al. (15) used reconstituted Bovine Achilles tendon, which, due to the reconstitution process, would be expected to contain few-to-no mature cross-links; however, immature cross-links may be present (41). The presence and type of cross-links may significantly affect the tensile properties of the fibril (42) by transmitting force between collagen molecules through covalent bonds rather than the noncovalent interactions responsible for fibril self-assembly. Any disruption or strengthening of the noncovalent interactions would therefore be shielded by the cross-links during tensile testing.
This type of reconstituted bovine collagen has previously been used in single fibril tensile experiments by van der Rijt et al. (16) who were able to attain large moduli (∼5 GPa) and stresses (∼90 MPa) on dry fibrils. However, upon hydration, plastic deformation was reported already at 15 MPa stress, which appears to be in the toe region (Fig. 4 in (16)). This is far less than the ∼90 MPa stress reached in this work, which is limited by technical constraints and not plastic deformation of the fibril. Those results indicate that the lack of cross-links in the reconstituted fibrils may lead to reduced yield strength and possibly reduced moduli, although the low value of 0.5 GPa is likely caused by being in the toe and not the linear region. This is in agreement with recent modeling results showing that the primary effect of cross-linking is on the failure mechanics rather than the modulus (43).
It is possible that performing our experiment on reconstituted fibrils would lead to greater salt sensitivity than observed in this work. A small pilot study on the compressive modulus of a few mature human fibrils (Fig. S3) did not demonstrate any significant effect of salts, indicating that they are indeed less sensitive to their saline environment than the reconstituted fibrils. This suggests that fibril cross-linking is the primary cause of the observed differences in response to salts.
Another concern related to cross-linking is the drying of the fibrils, which is necessary for the glue to cure. Drying the fibrils could very well alter their response; however, in the work by Grant et al. (15) the fibrils were also dried before being rehydrated and tested, indicating that the drying, per se, does not make the fibril insensitive to the ionic environment. It is possible, though, that increased cross-linking of the fibril when it dries (44) could shield the tensile response as mentioned previously.
Conclusions
Herein we investigated the tensile mechanical properties of individual collagen fibrils and found that they were largely insensitive to environmental salt concentration (20, 150, and 1000 mM PBS), choice of buffer (phosphate versus HEPES) and the presence of calcium. The only systematic effect was a an increase of ∼4% in the relative energy dissipation of collagen fibrils in the 1000 mM PBS solution, indicating a stabilizing effect of the high salt concentration on the deformed state of the fibril. For comparison, equivalent measurements were performed on collagen fascicles, which were also largely insensitive to the environmental conditions. The disagreement of this finding with previous findings on the radial compressive mechanics of fibrils underlines the anisotropic nature of collagen and the need to also investigate fibril mechanics under axial tensile load.
Acknowledgments
We thank Michael Krogsgaard, MD, PhD, Orthopedic Department, Bispebjerg Hospital, Copenhagen, Denmark, for obtaining biopsy tissue during routine anterior cruciate ligament construction.
This work was supported by the Danish Medical Research Council, the Lundbeck Foundation, and the Novo Nordisk Foundation. The funding bodies had no influence on the project.
Supporting Material
References
- 1.Fogh-Andersen N., Altura B.M., Siggaard-Andersen O. Composition of interstitial fluid. Clin. Chem. 1995;41:1522–1525. [PubMed] [Google Scholar]
- 2.Hodgson T.H. The chloride content of blood serum and aqueous humor. Its relation to glaucoma and to the formation of intra-ocular fluid. J. Physiol. 1938;94:118–123. doi: 10.1113/jphysiol.1938.sp003666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Åstrand P.-O., Rodahl K. 3rd Ed. McGraw-Hill; Singapore: 1986. Textbook of Work Physiology: Physiological Bases of Exercise. [Google Scholar]
- 4.Good N.E., Winget G.D., Singh R.M. Hydrogen ion buffers for biological research. Biochemistry. 1966;5:467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- 5.Lujan T.J., Underwood C.J., Weiss J.A. Contribution of glycosaminoglycans to viscoelastic tensile behavior of human ligament. J. Appl. Physiol. 2009;106:423–431. doi: 10.1152/japplphysiol.90748.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chimich D., Shrive N., Bray R. Water content alters viscoelastic behavior of the normal adolescent rabbit medial collateral ligament. J. Biomech. 1992;25:831–837. doi: 10.1016/0021-9290(92)90223-n. [DOI] [PubMed] [Google Scholar]
- 7.Screen H.R., Shelton J.C., Lee D.A. The influence of noncollagenous matrix components on the micromechanical environment of tendon fascicles. Ann. Biomed. Eng. 2005;33:1090–1099. doi: 10.1007/s10439-005-5777-9. [DOI] [PubMed] [Google Scholar]
- 8.Haut R.C., Powlison A.C. The effects of test environment and cyclic stretching on the failure properties of human patellar tendons. J. Orthop. Res. 1990;8:532–540. doi: 10.1002/jor.1100080409. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman A.H., Robichaud D.R., 2nd, Grigg P. Determining the effect of hydration upon the properties of ligaments using pseudo Gaussian stress stimuli. J. Biomech. 2005;38:1636–1642. doi: 10.1016/j.jbiomech.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 10.Thornton G.M., Shrive N.G., Frank C.B. Altering ligament water content affects ligament pre-stress and creep behavior. J. Orthop. Res. 2001;19:845–851. doi: 10.1016/S0736-0266(01)00005-5. [DOI] [PubMed] [Google Scholar]
- 11.Grant C.A., Brockwell D.J., Thomson N.H. Effects of hydration on the mechanical response of individual collagen fibrils. Appl. Phys. Lett. 2008;92:3902. [Google Scholar]
- 12.Atkinson T.S., Ewers B.J., Haut R.C. The tensile and stress relaxation responses of human patellar tendon varies with specimen cross-sectional area. J. Biomech. 1999;32:907–914. doi: 10.1016/s0021-9290(99)00089-5. [DOI] [PubMed] [Google Scholar]
- 13.Haut T.L., Haut R.C. The state of tissue hydration determines the strain-rate-sensitive stiffness of human patellar tendon. J. Biomech. 1997;30:79–81. doi: 10.1016/s0021-9290(96)00108-x. [DOI] [PubMed] [Google Scholar]
- 14.Först P., Werner F., Delgado A. On the pressure dependence of the viscosity of aqueous sugar solutions. Rheol. Acta. 2002;41:369–374. [Google Scholar]
- 15.Grant C.A., Brockwell D.J., Thomson N.H. Tuning the elastic modulus of hydrated collagen fibrils. Biophys. J. 2009;97:2985–2992. doi: 10.1016/j.bpj.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Rijt J.A.J., van der Werf K.O., Feijen J. Micromechanical testing of individual collagen fibrils. Macromol. Biosci. 2006;6:697–702. doi: 10.1002/mabi.200600063. [DOI] [PubMed] [Google Scholar]
- 17.Shen Z.L., Dodge M.R., Eppell S.J. Stress-strain experiments on individual collagen fibrils. Biophys. J. 2008;95:3956–3963. doi: 10.1529/biophysj.107.124602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang L., van der Werf K.O., Feijen J. Mechanical properties of native and cross-linked type I collagen fibrils. Biophys. J. 2008;94:2204–2211. doi: 10.1529/biophysj.107.111013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Screen H.R.C., Lee D.A., Shelton J.C. An investigation into the effects of the hierarchical structure of tendon fascicles on micromechanical properties. Proc. Inst. Mech. Eng. H–J. Eng. Med. 2004;218:109–119. doi: 10.1243/095441104322984004. [DOI] [PubMed] [Google Scholar]
- 20.Screen H.R.C. Investigating load relaxation mechanics in tendon. J. Mech. Behav. Biomed. Mater. 2008;1:51–58. doi: 10.1016/j.jmbbm.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Craig A.S., Birtles M.J., Parry D.A. An estimate of the mean length of collagen fibrils in rat tail-tendon as a function of age. Connect. Tissue Res. 1989;19:51–62. doi: 10.3109/03008208909016814. [DOI] [PubMed] [Google Scholar]
- 22.Provenzano P.P., Vanderby R., Jr. Collagen fibril morphology and organization: implications for force transmission in ligament and tendon. Matrix Biol. 2006;25:71–84. doi: 10.1016/j.matbio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Birk D.E., Nurminskaya M.V., Zycband E.I. Collagen fibrillogenesis in situ: fibril segments undergo post-depositional modifications resulting in linear and lateral growth during matrix development. Dev. Dyn. 1995;202:229–243. doi: 10.1002/aja.1002020303. [DOI] [PubMed] [Google Scholar]
- 24.Fantner G.E., Hassenkam T., Hansma P.K. Sacrificial bonds and hidden length dissipate energy as mineralized fibrils separate during bone fracture. Nat. Mater. 2005;4:612–616. doi: 10.1038/nmat1428. [DOI] [PubMed] [Google Scholar]
- 25.Thompson J.B., Kindt J.H., Hansma P.K. Bone indentation recovery time correlates with bond reforming time. Nature. 2001;414:773–776. doi: 10.1038/414773a. [DOI] [PubMed] [Google Scholar]
- 26.Matthews L.S., Ellis D. Viscoelastic properties of cat tendon: effects of time after death and preservation by freezing. J. Biomech. 1968;1:65–71. doi: 10.1016/0021-9290(68)90008-0. [DOI] [PubMed] [Google Scholar]
- 27.Giannini S., Buda R., Ruggeri A. Effects of freezing on the biomechanical and structural properties of human posterior tibial tendons. Int. Orthop. 2008;32:145–151. doi: 10.1007/s00264-006-0297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moon D.K., Woo S.L.Y., Abramowitch S.D. The effects of refreezing on the viscoelastic and tensile properties of ligaments. J. Biomech. 2006;39:1153–1157. doi: 10.1016/j.jbiomech.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Ng B.H., Chou S.M., Chong A. The changes in the tensile properties of tendons after freeze storage in saline solution. Proc. Inst. Mech. Eng. H–J. Eng. Med. 2005;219:387–392. doi: 10.1243/095441105X63309. [DOI] [PubMed] [Google Scholar]
- 30.Woo S.L.Y., Orlando C.A., Akeson W.H. Effects of postmortem storage by freezing on ligament tensile behavior. J. Biomech. 1986;19:399–404. doi: 10.1016/0021-9290(86)90016-3. [DOI] [PubMed] [Google Scholar]
- 31.Hutter J.L., Bechhoefer J. Calibration of atomic-force microscope tips. Rev. Sci. Instrum. 1993;64:1868–1873. [Google Scholar]
- 32.Yamamoto E., Hayashi K., Yamamoto N. Mechanical properties of collagen fascicles from the rabbit patellar tendon. J. Biomech. Eng. 1999;121:124–131. doi: 10.1115/1.2798033. [DOI] [PubMed] [Google Scholar]
- 33.Svensson R.B., Hassenkam T., Peter Magnusson S. Viscoelastic behavior of discrete human collagen fibrils. J. Mech. Behav. Biomed. Mater. 2010;3:112–115. doi: 10.1016/j.jmbbm.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Kongsgaard M., Qvortrup K., Magnusson S.P. Fibril morphology and tendon mechanical properties in patellar tendinopathy: effects of heavy slow resistance training. Am. J. Sports Med. 2010;38:749–756. doi: 10.1177/0363546509350915. [DOI] [PubMed] [Google Scholar]
- 35.Gutsmann T., Hassenkam T., Hansma P.K. Sacrificial bonds in polymer brushes from rat tail tendon functioning as nanoscale Velcro. Biophys. J. 2005;89:536–542. doi: 10.1529/biophysj.104.056747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang Y.F., Meek K.M. Swelling studies on the cornea and sclera: the effects of pH and ionic strength. Biophys. J. 1999;77:1655–1665. doi: 10.1016/S0006-3495(99)77013-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meek K.M., Fullwood N.J., Worthington C.R. Synchrotron x-ray diffraction studies of the cornea, with implications for stromal hydration. Biophys. J. 1991;60:467–474. doi: 10.1016/S0006-3495(91)82073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sasaki N., Shiwa S., Hikichi K. X-ray diffraction studies on the structure of hydrated collagen. Biopolymers. 1983;22:2539–2547. doi: 10.1002/bip.360221208. [DOI] [PubMed] [Google Scholar]
- 39.Hansen P., Haraldsson B.T., Peter Magnusson S. Lower strength of the human posterior patellar tendon seems unrelated to mature collagen cross-linking and fibril morphology. J. Appl. Physiol. 2010;108:47–52. doi: 10.1152/japplphysiol.00944.2009. [DOI] [PubMed] [Google Scholar]
- 40.Haut R.C. The influence of specimen length on the tensile failure properties of tendon collagen. J. Biomech. 1986;19:951–955. doi: 10.1016/0021-9290(86)90190-9. [DOI] [PubMed] [Google Scholar]
- 41.Tanzer M.L. Intermolecular cross-links in reconstituted collagen fibrils. Evidence for the nature of the covalent bonds. J. Biol. Chem. 1968;243:4045–4054. [PubMed] [Google Scholar]
- 42.Bailey A.J., Paul R.G., Knott L. Mechanisms of maturation and ageing of collagen. Mech. Ageing Dev. 1998;106:1–56. doi: 10.1016/s0047-6374(98)00119-5. [DOI] [PubMed] [Google Scholar]
- 43.Buehler M.J. Nanomechanics of collagen fibrils under varying cross-link densities: atomistic and continuum studies. J. Mech. Behav. Biomed. Mater. 2008;1:59–67. doi: 10.1016/j.jmbbm.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 44.Silver F.H., Christiansen D.L., Chen Y. Role of storage on changes in the mechanical properties of tendon and self-assembled collagen fibers. Connect. Tissue Res. 2000;41:155–164. doi: 10.3109/03008200009067667. [DOI] [PubMed] [Google Scholar]
- 45.Magnusson S.P., Langberg H., Kjaer M. The pathogenesis of tendinopathy: balancing the response to loading. Nat. Rev. Rheumatol. 2010;6:262–268. doi: 10.1038/nrrheum.2010.43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


