Summary
A great deal of concern has recently arisen regarding the safety of anaesthesia in infants and children. There is mounting and convincing preclinical evidence in rodents and non-human primates that anaesthetics in common clinical use are neurotoxic to the developing brain in vitro and cause long-term neurobehavioural abnormalities in vivo. An estimated 6 million children (including 1.5 million infants) undergo surgery and anaesthesia each year in the USA alone, so the clinical relevance of anaesthetic neurotoxicity is an urgent matter of public health. Clinical studies that have been conducted on the long-term neurodevelopmental effects of anaesthetic agents in infants and children are retrospective analyses of existing data. Two large-scale clinical studies are currently underway to further address this issue. The PANDA study is a large-scale, multisite, ambi-directional sibling-matched cohort study in the USA. The aim of this study is to examine the neurodevelopmental effects of exposure to general anaesthesia during inguinal hernia surgery before 36 months of age. Another large-scale study is the GAS study, which will compare the neurodevelopmental outcome between two anaesthetic techniques, general sevoflurane anaesthesia and regional anaesthesia, in infants undergoing inguinal hernia repair. These study results should contribute significant information related to anaesthetic neurotoxicity in children.
Keywords: anaesthesia, paediatric; children; neurocognitive outcome; neurotoxicity; risk
Key points.
Preclinical evidence indicates anaesthetic exposure in neonatal animals leads to neurotoxicity and neurobehavioural deficits.
Available clinical studies related to anaesthetic neurotoxicity are retrospective and inconclusive.
Two multicentre clinical studies are currently underway to address anaesthetic neurotoxicity in children.
An estimated 6 million children receive anaesthesia annually in the USA.1 Among infants, defined as those under 12 months of age, the Nationwide Inpatient Sample data indicate that 1.5 million undergo surgery as inpatients each year in the USA. Surgical anaesthesia provides amnesia, analgesia, immobility, and control of autonomic responses during surgical procedures. In the non-surgical setting, anaesthesia in children provides safe and appropriate conditions for interventional procedures, imaging studies, and diagnostic procedures. The benefits of anaesthesia in children include alleviation of pain, anxiety, maintaining stable vital signs, and providing adequate conditions for surgery or the procedures in question. These benefits have accounted for the exponential increase in the number of anaesthetics administered to children in many different settings, for many different procedures, and to children of increasingly younger age.
The widespread and growing use of anaesthesia in infants and young children thus makes its safety a major public health issue of interest to the public, government agencies, and the anaesthesia community. This issue has become a matter of great concern with the evidence that anaesthetics are neurotoxic in animal studies.
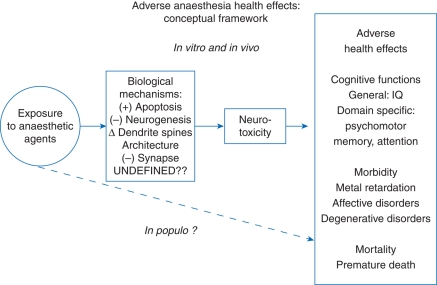
A conceptual framework for research related to the adverse health effects of anaesthesia exposure in vivo, in vitro, and in populo is presented in Figure 1. The goal of this review is to provide an overview of available clinical studies related to neurocognitive development and early childhood exposure to anaesthesia, including the outline of two large-scale ongoing clinical studies. The review only briefly summarizes the preclinical studies that have reported functional outcomes, which serve as important background information to the clinical studies. For a more comprehensive review of all of the preclinical studies on anaesthetic neurotoxicity, the reader is referred to other recent reviews.2–6
Fig 1.
A conceptual framework in studying anaesthetic neurotoxicity (originally conceived by Dr Guohua Li, MD, DrPH, Columbia University).
Preclinical studies of anaesthetic neurotoxicity in the developing brain
Experimental studies in animals (rats, mice, guinea pigs, piglets, and non-human primates) have shown that exposure of the developing mammalian brain to a variety of commonly used anaesthetic agents during critical developmental periods can lead to neuronal apoptosis or neurodegeneration in vitro and measureable neurobehavioural and functional deficits in vivo.2–4,7–53
Ikonomidou and colleagues22 first made the observation that N-methyl-d-aspartate glutamate receptor (NMDAR) antagonists induced extensive neuronal apoptosis in the developing rat brain. This has potentially important implications for clinical paediatric and obstetric anaesthesia since certain clinically used anaesthetic agents have similar mechanisms of action as NMDAR antagonists.22 The concern was thus raised with respect to the potential neurotoxic effects of other anaesthetic agents. Subsequently, studies by Jevtovic-Todorovic and colleagues7 and Fredriksson and colleagues20 found the same pattern of in vitro neuronal apoptosis after exposure of the developing rat brain to anaesthetic agents that act as NMDAR antagonists and γ-aminobutyric acid type A receptor (GABAR) agonists. Jevtovic-Todorovic and colleagues7 exposed rats at postnatal day 7 to a ‘cocktail’ of clinically used anaesthetic agents (midazolam, isoflurane, and nitrous oxide) and demonstrated not only neuronal apoptosis in the infant rat brain, but also persistent functional deficits in memory and learning in juvenile rats, with impairment of both spatial reference and working memory in adult rats.
Dose-dependent neuronal apoptosis in response to anaesthetics has been documented in both rodent and non-human primate studies. Ketamine induces neuronal apoptosis and neurodegeneration in both rats and monkeys with high doses, prolonged exposure, or repeated doses.2,3,49,50,52 Similar dose-dependent effects have been documented for propofol and isoflurane.3,20,37 Neurotoxic effects were more prominent when the exposure was to a combination of anaesthetic agents with both NMDAR and GABAR actions than from exposure to an agent with either NMDAR or GABAR actions alone.
Another important feature of anaesthetic neurotoxicity is that there is a critical period of vulnerability for exposure. Studies in rodents found neuronal apoptosis to be the greatest if exposure occurred at postnatal day 7, the period of peak synaptogenesis.10,50 In non-human primates, exposure occurring at postnatal day 5, but not at postnatal day 35, was found to cause neurotoxicity.
These preclinical studies indicate that neurotoxicity of anaesthetic agents in the developing brain, as evidenced by neuronal apoptosis and necrosis, is greatest if the exposure occurs during periods of peak synaptogenesis, with high doses or with a combination of anaesthetic agents. However, anaesthetic-induced neurotoxic effects involve more than neuronal apoptosis and necrosis during synaptogenesis. Several recent studies suggest that anaesthetics also inhibit neurogenesis and alter the development of dendritic spine architecture, important developmental processes in synapse formation 0.17,53 Although much work remains to be done to elucidate the specific mechanisms of anaesthetic neurotoxicity, much progress has been made. One proposed mechanism is the inhibition of brain-derived neurotrophic factor (BDNF) signalling pathways by GABAergic and NMDAR-acting anaesthetic agents.25,53 BDNF has also been shown to be involved in the developmental neurotoxicity observed with lead exposure.54
Most preclinical studies have examined the effects of various anaesthetics on histopathological changes in vitro. The focus of the present review is on those studies that also examined functional outcomes (Table 1).7,9–13,20,24,45 To date, functional outcome studies have only been performed in rats and mice. Preliminary findings in non-human primates have been reported at meetings, but are not yet published. Although studies have consistently demonstrated neuronal apoptosis after anaesthesia, not all studies showing histopathological neurotoxicity have found evidence of abnormal neurobehavioural outcome.10,11
Table 1.
Summary of preclinical studies related to anaesthetic neurotoxicity with functional outcomes
| Agents | Species | In vivo effects | Tests |
|---|---|---|---|
| Isoflurane7,10–12,24 | Rats | Learning | Open field maze |
| Memory | Radial arm maze | ||
| Spatial memory | Fear conditioning | ||
| Spontaneous motor activity | Object recognition | ||
| Attention | IntelliCage | ||
| Balance beam | |||
| Water maze | |||
| Sevoflurane32,45 | Mice | Learning | Open field |
| Social memory | Elevated plus maze, Y-maze | ||
| Fear conditioning | |||
| Social recognition, social interaction | |||
| Olfactory and novelty tests | |||
| Propofol20,32 | Mice | Spontaneous activity | Spontaneous motor activity |
| Learning | Radial arm maze | ||
| Spatial memory | Elevated plus maze | ||
| Water maze | |||
| Hole board | |||
| Phenobarbital/thiopental9,11 | Rats | Reduced hippocampus-dependent behaviour performance | Balanced beam |
| Open field maze | |||
| Elevated plus maze | |||
| N2O7,24 | Rats | Learning | Radial arm maze |
| Memory | Water maze | ||
| Spontaneous motor activity | |||
| Attention | |||
| Midazolam7,24 | Rats | Learning | Radial arm maze |
| Memory | Water maze | ||
| Spontaneous motor activity | |||
| Attention | |||
| Ketamine7,20 | Mice | Reduced hippocampus-dependent behaviour performance | Spontaneous motor activity |
| Radial arm maze | |||
| Elevated plus maze |
Is general anaesthesia neurotoxic to infants and children?
This alarming preclinical evidence of anaesthetic neurotoxicity from in vitro and in vivo animal studies raises serious concern that the use of anaesthetic agents in children might lead to long-term adverse neurodevelopmental outcomes. Findings of abnormal attention, learning, and memory tasks and social behaviour in adult animals that had neonatal anaesthesia exposure generated a great deal of interest from the media, public, and parents. The US Food and Drug Agency (FDA) responded to these concerns and convened a scientific advisory committee meeting in 2007 to discuss whether specific recommendations for changes in the use of anaesthetic agents in infants and children are needed.2 The consensus from the advisory committee was that without data from clinical studies, the question of whether anaesthetic agents are neurotoxic in children cannot be answered.
Clinical studies of neurodevelopmental effects of anaesthesia exposure during early childhood
Since 2007, a total of five clinical studies have been published.55–59 All of these studies are retrospective cohort studies and are summarized in Table 2. Two of these studies derived their data from the Olmstead County Birth Cohort.58,59 In both of these studies, the outcome used was learning disability (in maths, language, and reading).58,59 Sprung and colleagues58 studied a cohort of 5320 children to specifically determine the neurocognitive effects of prenatal/fetal exposure during labour and delivery. There were 4823 children who were born by vaginal delivery, 197 who were born by Caesarean delivery under general anaesthesia, and 304 who were born by Caesarean delivery under regional anaesthesia. Fetal exposure to general anaesthesia during Caesarean delivery did not increase the risk for developing a learning disability compared with vaginal delivery without anaesthesia. Using the same cohort, Wilder and colleagues59 examined the effects of postnatal anaesthesia before age 4 and found that learning disability (maths, language, or reading) was higher in those children with multiple anaesthesia exposure and surgery before age 4. The Olmstead County Birth Cohort provides a large sample of study subjects. In addition, the investigators were able to obtain medical records to abstract information on anaesthesia exposure.
Table 2.
Summary of clinical studies related to anaesthetic neurotoxicity
| Neurocognitive outcome | Assessment tools | Study details |
|---|---|---|
| Learning disability (in language or math or reading) | Learning Disability criteria (based on IQ and achievement test results) | Olmstead County Birth Cohort22,29 |
| Findings: multiple exposure increases risk for learning disability | ||
| Findings: there is no difference in incidence of learning disability between children whose mother had Caesarean section under general anaesthesia and regional anaesthesia | ||
| Behaviour | Child Behavior Checklist (CBCL) | Retrospective ‘pilot’ study in urology patients aged 0–6 yr.21 Underpowered |
| Findings: trend towards more atypical behaviour with exposure | ||
| Developmental delay | ICD-9 diagnosis codes | NYS Medicaid data set20 |
| Behavioural problems | Findings: hernia under 36 months of age increases risks for developmental disorder | |
| Autism spectrum disorder | ||
| Education achievement | Dutch CITO-elementary test-cognitive problems/inattention subscale of Conner's Teacher Rating Scale: short form | Young-Netherlands Twin Register19 |
| Teacher rating of behaviour | Findings: no differences in twins discordant for anaesthesia exposure before 36 months |
These are significant strengths in both studies,58,59 but there are also limitations with respect to the outcomes examined. The authors were careful to adjust for age, sex, birth weight, and maternal education, known confounders in evaluating neurocognitive function. However, the study cohort does not reflect the patient characteristic and cultural and racial/ethnic diversity of the overall US population. In addition, the retrospective cohort had exposure to anaesthesia from January 1976 to December 1982, a period during which the most commonly used anaesthetic agents were halothane and nitrous oxide. Since the early 1980s, anaesthesia practice has evolved and halothane is no longer in clinical use in the USA, and the use of nitrous oxide has also declined. Therefore, based on the relative lack of population diversity and significant changes in anaesthesia practice, the results of these two well-designed studies are not generalizable to paediatric anaesthesia practice in the USA today. Another limitation of these two studies is the use of learning disability as the outcome measure. Learning disability is a categorical determination based on a discrepancy between a child's IQ and his/her actual achievement; it is not a specific neuropsychological outcome. In the state of Minnesota where the study was conducted, learning disability can be identified using three different approaches, so the endpoints were not standardized. Furthermore, the authors combined the three different types of learning disability in language, maths, and reading into a single outcome measure. Because language, reading, and maths are subserved by discrete brain regions with distinct developmental trajectories, a unitary outcome of ‘learning disability’ would be non-specific.
DiMaggio and colleagues56 used the New York State Medicaid data set and constructed a birth cohort of 383 children who underwent inguinal hernia repair during the first 3 yr of life as the exposed group. The unexposed cohort for comparison was a sample of 5050 children who were frequency-matched on age with no history of hernia repair before age 3. Using ICD-9 diagnostic codes for developmental delay or behavioural problems, the authors found the exposed cohort to have a 2.3-fold (95% CI 1.3–4.1) increased risk for such diagnosis compared with the unexposed cohort.56 As with the Sprung and Wilder studies,56,58,59 a significant limitation of this study was the outcome measure used. It was non-standardized and therefore could easily be subject to variations due to local practice patterns and misclassification from diagnostic coding. In addition, the study did not have an explicit variable of anaesthesia exposure. Although hernia surgery is not known to be associated with any specific conditions that give rise to abnormal neurocognitive function, it is still possible that there was bias from confounding due to indications for surgery. Unlike the Olmsted County population which was too homogeneous, the Medicaid population in the DiMaggio study was a much higher risk cohort than the general population due to the criteria for eligibility to enrol in the Medicaid system (low socioeconomic status).
Kalkman and colleagues57 performed a survey of long-term behaviour after childhood surgery using Child Behavior Checklist (CBCL) parental reports. Their study sample consisted of 243 individuals who were currently 12.5–15.8 yr of age and who had general anaesthesia for urological surgery from 0 to 6 yr of age. Those who had anaesthesia and surgery before 24 months of age appeared to be more likely to have ‘deviant’ behaviour than those who had surgery and anaesthesia at an older age.57 However, the study sample size was inadequate to provide sufficient power to yield statistically significant results, thus making this more of a pilot study for a larger study in the future.
Although these retrospective cohort studies suggest that early anaesthesia exposure might be associated with late adverse neurodevelopmental outcome, a more recent study from the Netherlands failed to show any effects of anaesthesia exposure on long-term neurocognitive function using the Young Netherlands Twin Registry.55 In a study of 1143 monozygotic twin pairs, exposure to anaesthesia before age 3 correlated with reduced educational achievement. But there were no differences between twin pairs when they were discordant for anaesthesia exposure. They therefore concluded that there was no causal relationship between exposure to anaesthesia and cognitive performance later in life. Limitations in this study include the lack of comparative exposure data for the twins and data on indications for surgery. The outcome measures used were education achievement scores at age 12 and reports of behaviour problems by teachers. Although the authors presented data that there was a demonstrable correlation between IQ and educational achievement scores (CITO scores), academic achievement scores cannot be considered as an objective neuropsychological outcome measure as they can be influenced by many factors.60
Important issues and consideration in clinical studies of anaesthetic neurotoxicity
Key issues that need to be addressed by clinical studies include what should be considered as adverse neurodevelopmental outcome and the age at risk. Neurodevelopmental outcome could be assessed as the presence of neurodevelopmental disorders, such as autism, mental retardation, language delay, learning disability, or attention deficit hyperactivity disorder.61–63 To date, studies examining the association of anaesthesia exposure with neurodevelopmental outcome have adopted this approach.55–59 However, objective evaluation of neurodevelopmental abnormality requires direct assessment of neurocognitive functions using standardized neuropsychological instruments. Neuropsychological assessment in a developing child is very different than in adults. First, neuropsychological assessment instruments in children are age-specific and those available for the very young do not always predict later functions.64 Secondly, many of the neurocognitive functions are not yet fully developed at the time of exposure.65,66 Therefore, pre-exposure ‘baseline’ neuropsychological assessment of young children would not even be possible as in adults.
The age of vulnerability in children cannot be extrapolated easily from the clinical studies because cross-species translation of brain development is still an area of ongoing study. The vulnerable period of injury has been consistently demonstrated to be during peak synaptogenesis.7,10,30,50 Therefore, our current understanding of human brain development may be informative in choosing the age most likely to be at risk for anaesthetic neurotoxicity. In the human brain, there are significant regional differences in the timing for peak synaptogenesis. The earliest is in the primary sensorimotor cortex, occurring around birth. This is followed by the parietal and temporal association cortex, important in language and spatial attention, where peak synaptogenesis occurs at around 9 months. The last region to peak in synaptogenesis is in the prefrontal cortex, which occurs at age 2–3 yr. The prefrontal cortex is key in executive function and integrative and modulatory brain function. Since peak synaptogenesis occurs between birth and 2–3 yr of age,65,67,68 the vulnerability period for anaesthetic-induced neurotoxicity might be up to 36 months of age in the developing human brain.
Currently, there are two large-scale studies underway that attempt to address the issue of anaesthetic neurotoxicity in children. The GAS study is an international randomized trial comparing general sevoflurane anaesthesia with regional anaesthesia in 600 infants undergoing inguinal hernia repair. The follow-up period will be 5 yr, with evaluation performed at ages 2 and 5 yr. The evaluation at age 2 will be performed using the Bayley Scales for Infant Development-III, and the evaluation at age 5 will include the Wechsler Preschool and Primary Scale of Intelligence-III and additional neuropsychological tests within NEPSY II (second edition of the neuropsychological test battery for children and adolescents).
The PANDA (Pediatric Anesthesia and NeuroDevelopment Assessment) study is a multisite study that will involve eight US study sites. It is an ambi-directional, sibling-matched cohort study that will enrol a total of 1000 children or 500 sibling pairs. The anaesthesia exposure will be limited to a single episode of general anaesthesia for inguinal hernia repair in ASA I and II patients before 36 months of age. The study will perform an extensive neuropsychological battery in children between age 8 and 15 yr (Table 3).
Table 3.
Proposed neurocognitive outcomes and assessment instruments in the PANDA study
| Neurocognitive outcome | Assessment instrument | Cognitive domain |
|---|---|---|
| 1. Visual memory | 1. NEPSY II (faces, delayed faces) | Memory/learning |
| 2. Verbal memory | 2. CVLT-C | |
| 3. Motor speed and dexterity | 3. Grooved Pegboard | Motor/processing speed |
| 4. Processing speed | 4. Coding from WISC-IV | |
| 5. Visuospatial function | 5. Block design, matrix reasoning from WASI | Visuospatial |
| 6. Executive function components | 6. BRIEF | Attention/executive function |
| 7. Verbal working memory | 7. Digit Span from WISC-IV | |
| 8. Sustained and selective attention, impulsivity | 8. CPT II | |
| 9. Visual scanning, visual-motor skills, speed, and cognitive flexibility | 9. DKEFS Trails Making | |
| 10. Verbal concept formation | 10. NEPSY II (word generation) | |
| 11. Verbal reasoning | 11. Vocabulary from WASI | Language |
| 12. Expressive vocabulary | 12. Similarities from WASI | |
| 13. Receptive language | 13. NEPSY II (comprehension of instructions) | |
| 14. Language fluency | 14. NEPSY II (speed naming) | |
| 15. Global cognitive function | 15. WASI | IQ |
| 16. Behaviour | 16. CBCL | Behaviour |
Results from the PANDA study will be applicable to children undergoing elective procedures who are otherwise healthy, which constitute the great majority of patients in the USA. If anaesthetic exposure is found to be without effects in the study patients, reassurance could be offered to millions of parents. However, other patients who have significant co-morbidities or with more prolonged or frequent exposure to anaesthesia would still need to be examined. Should the study find anaesthesia exposure to have deleterious neurocognitive effects, we must urgently consider alternative strategies in the timing and delivery of anaesthesia care to young children and the development of novel anaesthetic agents with different mechanisms of action with minimal neurotoxic effects. Additional studies would be needed to more specifically determine the age of vulnerability and examine the specific exposure variables related to types of anaesthetic agents, drug doses, and exposure duration. Although additional studies would still be needed depending on the findings from these studies, these large-scale studies should produce data that will contribute significantly in addressing whether the preclinical studies on anaesthetic neurotoxicity are clinically relevant and thus inform clinical decision-making.
Conclusions
The difficulty in the interpretation of the clinical studies that have been completed to date is related to the retrospective nature of the studies, the lack of precise information in terms of age, agent, duration, and dose of anaesthetics, specific agents used, the variable outcome endpoints used, and the way these outcomes were assessed. These studies used variable neurocognitive outcomes including learning disability, diagnosis of developmental delay, and parental reports of behaviour. These outcome measures lack specificity and standardization in most cases. These study populations do not reflect a sampling of the population at large and therefore the findings might not be generalizable. At present, results from in populo studies remain too sparse and too inconsistent to allow any recommendations for specific practice guidelines or changes in paediatric anaesthesia practice. However, these studies do underscore the need for definitive studies in which outcome endpoints are specific and comprehensive, assessments are prospective and direct, and neuropsychological instruments used for assessment are validated and standardized.
Conflict of interest
None declared.
Funding
NIH-R34 HD060741 FDA SAFEKIDS Initiative.
Acknowledgements
The author would like to acknowledge the contributions of the PANDA group in the preparation of this review: Drs Guohua Li, Mary Byrne, Charles DiMaggio, Caleb Ing, Kimberly Noble, Jeanne Brooks-Gunn, Virginia Rauh, Frank McGowan, Tonya Miller, David Bellinger, Lynne Maxwell, Ari Weintraub, Ronald Litman, Jerilynn Radcliffe, Cynthia Salorio, Andreas Loepke, Dean Beebe, Suresh Santhanam, Frank Zelko, Jayant Deshpande, Timothy Cooper, Alan Tait, Shobha Malviya, Peter Davis, Edmund Jooste, Robert Noll, Alan Moskowitz, and Ron Levitan. The author wishes to gratefully acknowledge Ms Allana Forde, Barbara Lang, Amy Garwood, Mary Huang, and Sena Han for their administrative contributions to the PANDA study.
References
- 1.DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat. 2007;13:1–209. [PubMed] [Google Scholar]
- 2.Mellon RD, Simone AF, Rappaport BA. Use of anesthetic agents in neonates and young children. Anesth Analg. 2007;104:509–20. doi: 10.1213/01.ane.0000255729.96438.b0. doi:10.1213/01.ane.0000255729.96438.b0. [DOI] [PubMed] [Google Scholar]
- 3.Loepke AW, Soriano SG. An assessment of the effects of general anesthetics on developing brain structure and neurocognitive function. Anesth Analg. 2008;106:1681–707. doi: 10.1213/ane.0b013e318167ad77. doi:10.1213/ane.0b013e318167ad77. [DOI] [PubMed] [Google Scholar]
- 4.Istaphanous GK, Loepke AW. General anesthetics and the developing brain. Curr Opin Anaesthesiol. 2009;22:368–73. doi: 10.1097/aco.0b013e3283294c9e. doi:10.1097/ACO.0b013e3283294c9e. [DOI] [PubMed] [Google Scholar]
- 5.Loepke AW. Developmental neurotoxicity of sedatives and anesthetics: a concern for neonatal and pediatric critical care medicine? Pediatr Crit Care Med. 2010;11:217–26. doi: 10.1097/PCC.0b013e3181b80383. [DOI] [PubMed] [Google Scholar]
- 6.Wilder RT. Is there any relationship between long-term behavior disturbance and early exposure to anesthesia? Curr Opin Anaesthesiol. 2010;23:332–6. doi: 10.1097/ACO.0b013e3283391f94. doi:10.1097/ACO.0b013e3283391f94. [DOI] [PubMed] [Google Scholar]
- 7.Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–82. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jevtovic-Todorovic V, Olney JW. PRO: anesthesia-induced developmental neuroapoptosis: status of the evidence. Anesth Analg. 2008;106:1659–63. doi: 10.1213/ane.0b013e3181731ff2. doi:10.1213/ane.0b013e3181731ff2. [DOI] [PubMed] [Google Scholar]
- 9.Sanders RD, Xu J, Shu Y, et al. Dexmedetomidine attenuates isoflurane-induced neurocognitive impairment in neonatal rats. Anesthesiology. 2009;110:1077–85. doi: 10.1097/ALN.0b013e31819daedd. doi:10.1097/ALN.0b013e31819daedd. [DOI] [PubMed] [Google Scholar]
- 10.Stratmann G, Sall JW, May LD, et al. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology. 2009;110:834–48. doi: 10.1097/ALN.0b013e31819c463d. doi:10.1097/ALN.0b013e31819c463d. [DOI] [PubMed] [Google Scholar]
- 11.Stratmann G, Sall JW, May LD, Loepke AW, Lee MT. Beyond anesthetic properties: the effects of isoflurane on brain cell death, neurogenesis, and long-term neurocognitive function. Anesth Analg. 2010;110:431–7. doi: 10.1213/ANE.0b013e3181af8015. [DOI] [PubMed] [Google Scholar]
- 12.Zhu C, Gao J, Karlsson N, et al. Isoflurane anesthesia induced persistent, progressive memory impairment, caused a loss of neural stem cells, and reduced neurogenesis in young, but not adult, rodents. J Cereb Blood Flow Metab. 2010;30:1017–30. doi: 10.1038/jcbfm.2009.274. doi:10.1038/jcbfm.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rothstein S, Simkins T, Nunez JL. Response to neonatal anesthesia: effect of sex on anatomical and behavioral outcome. Neuroscience. 2008;152:959–69. doi: 10.1016/j.neuroscience.2008.01.027. doi:10.1016/j.neuroscience.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perouansky M. Neurotoxicity of general anesthetics: cause for concern? Anesthesiology. 2009;111:1365–7. doi: 10.1097/ALN.0b013e3181bf1d61. doi:10.1097/ALN.0b013e3181bf1d61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brambrink AM, Evers AS, Avidan MS, et al. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834–41. doi: 10.1097/ALN.0b013e3181d049cd. doi:10.1097/ALN.0b013e3181d049cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bree B, Gourdin M, De Kock M. Anesthesia and cerebral apoptosis. Acta Anaesthesiol Belg. 2008;59:127–37. [PubMed] [Google Scholar]
- 17.Briner A, De Roo M, Dayer A, Muller D, Habre W, Vutskits L. Volatile anesthetics rapidly increase dendritic spine density in the rat medial prefrontal cortex during synaptogenesis. Anesthesiology. 2010;112:546–56. doi: 10.1097/ALN.0b013e3181cd7942. doi:10.1097/ALN.0b013e3181cd7942. [DOI] [PubMed] [Google Scholar]
- 18.Cattano D, Williamson P, Fukui K, et al. Potential of xenon to induce or to protect against neuroapoptosis in the developing mouse brain. Can J Anaesth. 2008;55:429–36. doi: 10.1007/BF03016309. doi:10.1007/BF03016309. [DOI] [PubMed] [Google Scholar]
- 19.Creeley CE, Olney JW. The young: neuroapoptosis induced by anesthetics and what to do about it. Anesth Analg. 2009;110:442–8. doi: 10.1213/ANE.0b013e3181c6b9ca. doi:10.1213/ANE.0b013e3181c6b9ca. [DOI] [PubMed] [Google Scholar]
- 20.Fredriksson A, Ponten E, Gordh T, Eriksson P. Neonatal exposure to a combination of N-methyl-d-aspartate and gamma-aminobutyric acid type A receptor anesthetic agents potentiates apoptotic neurodegeneration and persistent behavioral deficits. Anesthesiology. 2007;107:427–36. doi: 10.1097/01.anes.0000278892.62305.9c. doi:10.1097/01.anes.0000278892.62305.9c. [DOI] [PubMed] [Google Scholar]
- 21.Gascon E, Klauser P, Kiss JZ, Vutskits L. Potentially toxic effects of anaesthetics on the developing central nervous system. Eur J Anaesthesiol. 2007;24:213–24. doi: 10.1017/S0265021506002365. doi:10.1017/S0265021506002365. [DOI] [PubMed] [Google Scholar]
- 22.Ikonomidou C, Bosch F, Miksa M, et al. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–4. doi: 10.1126/science.283.5398.70. doi:10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 23.Johnson SA, Young C, Olney JW. Isoflurane-induced neuroapoptosis in the developing brain of nonhypoglycemic mice. J Neurosurg Anesthesiol. 2008;20:21–8. doi: 10.1097/ANA.0b013e3181271850. doi:10.1097/ANA.0b013e3181271850. [DOI] [PubMed] [Google Scholar]
- 24.Loepke AW, Istaphanous GK, McAuliffe JJ, 3rd, et al. The effects of neonatal isoflurane exposure in mice on brain cell viability, adult behavior, learning, and memory. Anesth Analg. 2009;108:90–104. doi: 10.1213/ane.0b013e31818cdb29. doi:10.1213/ane.0b013e31818cdb29. [DOI] [PubMed] [Google Scholar]
- 25.Lu LX, Yon JH, Carter LB, Jevtovic-Todorovic V. General anesthesia activates BDNF-dependent neuroapoptosis in the developing rat brain. Apoptosis. 2006;11:1603–15. doi: 10.1007/s10495-006-8762-3. doi:10.1007/s10495-006-8762-3. [DOI] [PubMed] [Google Scholar]
- 26.Ma D, Williamson P, Januszewski A, et al. Xenon mitigates isoflurane-induced neuronal apoptosis in the developing rodent brain. Anesthesiology. 2007;106:746–53. doi: 10.1097/01.anes.0000264762.48920.80. doi:10.1097/01.anes.0000264762.48920.80. [DOI] [PubMed] [Google Scholar]
- 27.Nikizad H, Yon JH, Carter LB, Jevtovic-Todorovic V. Early exposure to general anesthesia causes significant neuronal deletion in the developing rat brain. Ann N Y Acad Sci. 2007;1122:69–82. doi: 10.1196/annals.1403.005. doi:10.1196/annals.1403.005. [DOI] [PubMed] [Google Scholar]
- 28.Olney JW, Farber NB, Wozniak DF, Jevtovic-Todorovic V, Ikonomidou C. Environmental agents that have the potential to trigger massive apoptotic neurodegeneration in the developing brain. Environ Health Perspect. 2000;108((Suppl. 3)):383–8. doi: 10.1289/ehp.00108s3383. doi:10.2307/3454524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rizzi S, Carter LB, Ori C, Jevtovic-Todorovic V. Clinical anesthesia causes permanent damage to the fetal guinea pig brain. Brain Pathol. 2008;18:198–210. doi: 10.1111/j.1750-3639.2007.00116.x. doi:10.1111/j.1750-3639.2007.00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rizzi S, Ori C, Jevtovic-Todorovic V. Timing versus duration: determinants of anesthesia-induced developmental apoptosis in the young mammalian brain. Ann N Y Acad Sci. 2010;1199:43–51. doi: 10.1111/j.1749-6632.2009.05173.x. doi:10.1111/j.1749-6632.2009.05173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rudin M, Ben-Abraham R, Gazit V, Tendler Y, Tashlykov V, Katz Y. Single-dose ketamine administration induces apoptosis in neonatal mouse brain. J Basic Clin Physiol Pharmacol. 2005;16:231–43. doi: 10.1515/jbcpp.2005.16.4.231. [DOI] [PubMed] [Google Scholar]
- 32.Satomoto M, Satoh Y, Terui K, et al. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology. 2009;110:628–37. doi: 10.1097/ALN.0b013e3181974fa2. doi:10.1097/ALN.0b013e3181974fa2. [DOI] [PubMed] [Google Scholar]
- 33.Scallet AC, Schmued LC, Slikker W, Jr, et al. Developmental neurotoxicity of ketamine: morphometric confirmation, exposure parameters, and multiple fluorescent labeling of apoptotic neurons. Toxicol Sci. 2004;81:364–70. doi: 10.1093/toxsci/kfh224. doi:10.1093/toxsci/kfh224. [DOI] [PubMed] [Google Scholar]
- 34.Blusse Van Oud-Alblas HJ, Bosenberg AT, Tibboel D. Awareness in children: another two cases. Paediatr Anaesth. 2008;18:654–7. doi: 10.1111/j.1460-9592.2008.02576.x. doi:10.1111/j.1460-9592.2008.02576.x. [DOI] [PubMed] [Google Scholar]
- 35.Straiko MM, Young C, Cattano D, et al. Lithium protects against anesthesia-induced developmental neuroapoptosis. Anesthesiology. 2009;110:862–8. doi: 10.1097/ALN.0b013e31819b5eab. doi:10.1097/ALN.0b013e31819b5eab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang C, Sadovova N, Fu X, et al. The role of the N-methyl-d-aspartate receptor in ketamine-induced apoptosis in rat forebrain culture. Neuroscience. 2005;132:967–77. doi: 10.1016/j.neuroscience.2005.01.053. doi:10.1016/j.neuroscience.2005.01.053. [DOI] [PubMed] [Google Scholar]
- 37.Yon JH, Carter LB, Reiter RJ, Jevtovic-Todorovic V. Melatonin reduces the severity of anesthesia-induced apoptotic neurodegeneration in the developing rat brain. Neurobiol Dis. 2006;21:522–30. doi: 10.1016/j.nbd.2005.08.011. doi:10.1016/j.nbd.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 38.Yon JH, Daniel-Johnson J, Carter LB, Jevtovic-Todorovic V. Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience. 2005;135:815–27. doi: 10.1016/j.neuroscience.2005.03.064. doi:10.1016/j.neuroscience.2005.03.064. [DOI] [PubMed] [Google Scholar]
- 39.Young C, Jevtovic-Todorovic V, Qin YQ, et al. Potential of ketamine and midazolam, individually or in combination, to induce apoptotic neurodegeneration in the infant mouse brain. Br J Pharmacol. 2005;146:189–97. doi: 10.1038/sj.bjp.0706301. doi:10.1038/sj.bjp.0706301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zou X, Patterson TA, Sadovova N, et al. Potential neurotoxicity of ketamine in the developing rat brain. Toxicol Sci. 2009;108:149–58. doi: 10.1093/toxsci/kfn270. doi:10.1093/toxsci/kfn270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stratmann G, May LD, Sall JW, et al. Effect of hypercarbia and isoflurane on brain cell death and neurocognitive dysfunction in 7-day-old rats. Anesthesiology. 2009;110:849–61. doi: 10.1097/ALN.0b013e31819c7140. doi:10.1097/ALN.0b013e31819c7140. [DOI] [PubMed] [Google Scholar]
- 42.Lunardi N, Ori C, Erisir A, Jevtovic-Todorovic V. General anesthesia causes long-lasting disturbances in the ultrastructural properties of developing synapses in young rats. Neurotox Res. 2010;17:179–88. doi: 10.1007/s12640-009-9088-z. doi:10.1007/s12640-009-9088-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Culley DJ, Xie Z, Crosby G. General anesthetic-induced neurotoxicity: an emerging problem for the young and old? Curr Opin Anaesthesiol. 2007;20:408–13. doi: 10.1097/ACO.0b013e3282efd18b. doi:10.1097/ACO.0b013e3282efd18b. [DOI] [PubMed] [Google Scholar]
- 44.Sall JW, Stratmann G, Leong J, et al. Isoflurane inhibits growth but does not cause cell death in hippocampal neural precursor cells grown in culture. Anesthesiology. 2009;110:826–33. doi: 10.1097/ALN.0b013e31819b62e2. doi:10.1097/ALN.0b013e31819b62e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bercker S, Bert B, Bittigau P, et al. Neurodegeneration in newborn rats following propofol and sevoflurane anesthesia. Neurotox Res. 2009;16:140–7. doi: 10.1007/s12640-009-9063-8. doi:10.1007/s12640-009-9063-8. [DOI] [PubMed] [Google Scholar]
- 46.Jevtovic-Todorovic V, Carter LB. The anesthetics nitrous oxide and ketamine are more neurotoxic to old than to young rat brain. Neurobiol Aging. 2005;26:947–56. doi: 10.1016/j.neurobiolaging.2004.07.009. doi:10.1016/j.neurobiolaging.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 47.Wang C, Sadovova N, Hotchkiss C, et al. Blockade of N-methyl-d-aspartate receptors by ketamine produces loss of postnatal day 3 monkey frontal cortical neurons in culture. Toxicol Sci. 2006;91:192–201. doi: 10.1093/toxsci/kfj144. doi:10.1093/toxsci/kfj144. [DOI] [PubMed] [Google Scholar]
- 48.Vutskits L, Gascon E, Tassonyi E, Kiss JZ. Effect of ketamine on dendritic arbor development and survival of immature GABAergic neurons in vitro. Toxicol Sci. 2006;91:540–9. doi: 10.1093/toxsci/kfj180. doi:10.1093/toxsci/kfj180. [DOI] [PubMed] [Google Scholar]
- 49.Vutskits L, Gascon E, Kiss JZ. Effects of ketamine on the developing central nervous system. Ideggyogy Sz. 2007;60:109–12. [PubMed] [Google Scholar]
- 50.Slikker W, Jr, Zou X, Hotchkiss CE, et al. Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol Sci. 2007;98:145–58. doi: 10.1093/toxsci/kfm084. doi:10.1093/toxsci/kfm084. [DOI] [PubMed] [Google Scholar]
- 51.Wang C, Slikker W., Jr Strategies and experimental models for evaluating anesthetics: effects on the developing nervous system. Anesth Analg. 2008;106:1643–58. doi: 10.1213/ane.ob013e3181732c01. doi:10.1213/ane.ob013e3181732c01. [DOI] [PubMed] [Google Scholar]
- 52.Hayashi H, Dikkes P, Soriano SG. Repeated administration of ketamine may lead to neuronal degeneration in the developing rat brain. Paediatr Anaesth. 2002;12:770–4. doi: 10.1046/j.1460-9592.2002.00883.x. doi:10.1046/j.1460-9592.2002.00883.x. [DOI] [PubMed] [Google Scholar]
- 53.Head BP, Patel HH, Niesman IR, Drummond JC, Roth DM, Patel PM. Inhibition of p75 neurotrophin receptor attenuates isoflurane-mediated neuronal apoptosis in the neonatal central nervous system. Anesthesiology. 2009;110:813–25. doi: 10.1097/ALN.0b013e31819b602b. doi:10.1097/ALN.0b013e31819b602b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neal AP, Stansfield KH, Worley PF, Thompson RE, Guilarte TR. Lead exposure during synaptogenesis alters vesicular proteins and impairs vesicular release: potential role of NMDA receptor-dependent BDNF signaling. Toxicol Sci. 2010;116:249–63. doi: 10.1093/toxsci/kfq111. doi:10.1093/toxsci/kfq111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bartels M, Althoff RR, Boomsma DI. Anesthesia and cognitive performance in children: no evidence for a causal relationship. Twin Res Hum Genet. 2009;12:246–53. doi: 10.1375/twin.12.3.246. doi:10.1375/twin.12.3.246. [DOI] [PubMed] [Google Scholar]
- 56.DiMaggio C, Sun LS, Kakavouli A, Byrne MW, Li G. A retrospective cohort study of the association of anesthesia and hernia repair surgery with behavioral and developmental disorders in young children. J Neurosurg Anesthesiol. 2009;21:286–91. doi: 10.1097/ANA.0b013e3181a71f11. doi:10.1097/ANA.0b013e3181a71f11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kalkman CJ, Peelen L, Moons KG, et al. Behavior and development in children and age at the time of first anesthetic exposure. Anesthesiology. 2009;110:805–12. doi: 10.1097/ALN.0b013e31819c7124. doi:10.1097/ALN.0b013e31819c7124. [DOI] [PubMed] [Google Scholar]
- 58.Sprung J, Flick RP, Wilder RT, et al. Anesthesia for cesarean delivery and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;111:302–10. doi: 10.1097/ALN.0b013e3181adf481. doi:10.1097/ALN.0b013e3181adf481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilder RT, Flick RP, Sprung J, et al. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. doi:10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bartels M, Rietveld MJ, Van Baal GC, Boomsma DI. Heritability of educational achievement in 12-year-olds and the overlap with cognitive ability. Twin Res. 2002;5:544–53. doi: 10.1375/136905202762342017. doi:10.1375/136905202762342017. [DOI] [PubMed] [Google Scholar]
- 61.Jacobson JL, Jacobson SW. Methodological issues in research on developmental exposure to neurotoxic agents. Neurotoxicol Teratol. 2005;27:395–406. doi: 10.1016/j.ntt.2005.01.009. doi:10.1016/j.ntt.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 62.Susser E, Bresnahan M. Epidemiologic approaches to neurodevelopmental disorders. Mol Psychiatry. 2002;7(Suppl. 2)):S2–3. doi: 10.1038/sj.mp.4001161. doi:10.1038/sj.mp.4001161. [DOI] [PubMed] [Google Scholar]
- 63.Winneke G. Appraisal of neurobehavioral methods in environmental health research: the developing brain as a target for neurotoxic chemicals. Int J Hyg Environ Health. 2007;210:601–9. doi: 10.1016/j.ijheh.2007.07.015. doi:10.1016/j.ijheh.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 64.Hack M, Taylor HG, Drotar D, et al. Poor predictive validity of the Bayley Scales of Infant Development for cognitive function of extremely low birth weight children at school age. Pediatrics. 2005;116:333–41. doi: 10.1542/peds.2005-0173. doi:10.1542/peds.2005-0173. [DOI] [PubMed] [Google Scholar]
- 65.Adeniran JO, Abdur-Rahman L. One-stage correction of intermediate imperforate anus in males. Pediatr Surg Int. 2005;21:88–90. doi: 10.1007/s00383-004-1211-x. doi:10.1007/s00383-004-1211-x. [DOI] [PubMed] [Google Scholar]
- 66.Korkman M, Kemp SL, Kirk U. Effects of age on neurocognitive measures of children ages 5 to 12: a cross-sectional study on 800 children from the United States. Dev Neuropsychol. 2001;20:331–54. doi: 10.1207/S15326942DN2001_2. doi:10.1207/S15326942DN2001_2. [DOI] [PubMed] [Google Scholar]
- 67.Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–15. doi: 10.1038/nn1008. doi:10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- 68.Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54:241–57. doi: 10.1016/s0301-0511(00)00058-2. doi:10.1016/S0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]