Abstract
The syntheses of 4-C-Me-DAB [1,4-dideoxy-1,4-imino-4-C-methyl-d-arabinitol] from l-erythronolactone and of 4-C-Me-LAB [from d-erythronolactone] require only a single acetonide protecting group. The effect of pH on the NMR spectra of 4-C-Me-DAB [pKa of the salt around 8.4] is discussed and illustrates the need for care in analysis of both coupling constants and chemical shift. 4-C-Me-DAB (for rat intestinal sucrase Ki 0.89 μM, IC50 0.41 μM) is a competitive - whereas 4-C-Me-LAB (for rat intestinal sucrase Ki 0.95 μM, IC50 0.66 μM) is a non-competitive - specific and potent α-glucosidase inhibitor. A rationale for the α-glucosidase inhibition by DAB, LAB, 4-C-Me-DAB, 4-C-Me-LAB, and isoDAB – but not isoLAB – is provided. Both are inhibitors of endoplasmic reticulum (ER) resident α-glucosidase I and II.
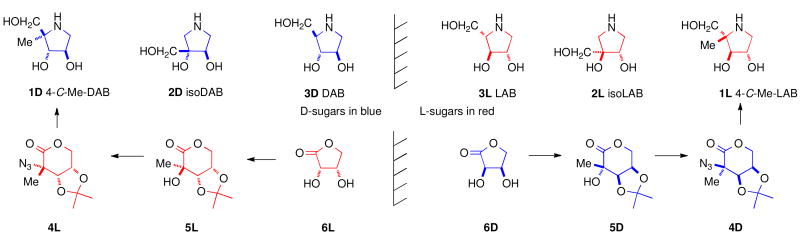
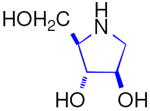
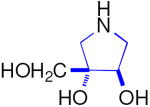
This paper describes the synthesis of the enantiomers 4-C-Me-DAB 1D [1,4-dideoxy-1,4-imino-4-C-methyl-d-arabinitol] and 4-C-Me-LAB 1L with only a single acetonide needed as a protecting group, both of which are micromolar inhibitors of some α-glucosidases; in accord with Asano's hypothesis,i the d-iminosugar 1D is a competitive inhibitor, whereas the enantiomer 1L is a non-competitive inhibitor. Synthetic enantiomers of natural iminosugars are frequently powerful glycosidase inhibitors.ii Natural and synthetic iminosugars comprise a major family of glycosidase inhibitors.iii The introduction of an alkyl substituent into a sugar mimic usually removes any significant glycosidase inhibition,iv however, introduction of a C6 methyl branch into the piperidine ring of l-swainsonine increases the inhibition of naringinase by an order of magnitudev in comparison to the parent indolizidine, l-swainsonine.vi The natural product DAB 3D is a good – but the enantiomer LAB 3L is a more potent and more specific – inhibitor of α-glucosidases.vii The isomer isoDAB 2D is also a very good inhibitor of α-glucosidases but the enantiomer 2L shows no inhibition of any glycosidase. This paper provides a rationale for isoLAB 2L being the only one of the six simple pyrrolidines 1, 2, and 3 which does not inhibit α-glucosidases; isoLAB 2L is the only one of the sugar mimics which partially rescues the defective F508del-CFTR function in cystic fibrosis.viii N-Alkylation of monocyclic iminosugars can enhance glycosidase inhibition by several orders of magnitude;ix such modification of the alkyl-branched parent structures may access a series of new bioactive compounds.
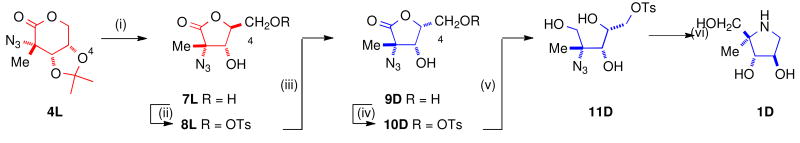
The enantiomeric azidolactones 4L and 4D are key intermediates in the synthesis of 4-C-methyl pyrrolidines 1L and 1D [Scheme 1]. The protected 2-C-methyl arabinono-lactone 5D can be prepared by a Kiliani reactionx on a protected 1-deoxyribulosexi derived from d-erythronolactone 6D; 5D is a useful chiron for the preparation of 2′-C-methyl nucleosidesxii and carbon-branched ketoses.xiii Reaction of 5D with triflic anhydride, followed by treatment with sodium azide, results in nucleophilic displacement at a tertiary center with inversion to give the azidolactone 4D.xiv The enantiomeric azido-2-C-methyl-l-arabinonolactone 4L was prepared from l-erythronolactone 6L by similar procedures.
Scheme 1. Structural relationship and synthesis of 4-C-methyl branched pyrrolidines.
For the synthesis of 4-C-Me-DAB 1D, it was necessary to invert the configuration at C4 of the azidolactone 4L [Scheme 2]. Hydrolysis of the isopropylidene protecting group in the 1,5-lactone 4L by trifluoroacetic acid in aqueous 1,4-dioxane gave the 1,4-lactone 7L, {m.p. 76-78 °C; [α]D25 -132.9 (c, 1.06 in CH3OH)} in 87% yield. Selective esterification of the primary hydroxy group in 7L with tosyl chloride in pyridine afforded the tosylate 8L {m.p. 110-112 °C; [α]D21 -107.7 (c, 1.07 in CH3CN)} in 57% yield. Reaction of l-ribonolactone 8L with potassium hydroxide in aqueous 1,4-dioxane, followed by treatment with an acidic ion exchange resin, resulted in inversion of configuration at C4 to form the d-lyxonolactone 9D {oil; [α]D21 +48.4 (c, 0.74 in MeOH)} in 87% yield. Esterification of 9D with tosyl chloride in pyridine yielded the primary tosylate 10D {oil; [α]D20 +13.2 (c, 0.85 in CH3CN)} (66% yield) which on reduction with sodium borohydride in methanol formed the azidotriol 11D, (oil, 66% yield); it was thus possible to reduce the hindered lactone 10D to the triol 11D without any competing formation of epoxide. Hydrogenation of the unprotected azide 11D in the presence of palladium on carbon in 1,4-dioxane gave the corresponding amine which spontaneously cyclized to form 4-C-Me-DAB 1D, in quantitative yield. The tosylate salt of 1D could be converted into the free base 1D by ion exchange chromatography with Dowex 1-X2 (OH- form) with water as eluent {free base: oil, [α]D25 -20.1 (c, 0.57 in H2O); hydrochloride salt: [α]D25 -5.22 (c, 1.07 in H2O)}. The enantiomer 4-C-Me-LAB 1L was prepared by an identical route from d-erythronolactone {free base: oil, [α]D25 +21.3 (c, 0.71 in H2O); hydrochloride salt: [α]D25 +4.10 (c, 1.0 in H2O)}.xv It is noteworthy that only one isopropylidene group is used throughout this sequence.
Scheme 2. (i) CF3COOH:H2O:1,4-dioxane, 4:1:1, 87% (ii) TsCl, pyridine, 57% (iii) KOH, 1,4-dioxane; then Dowex, 87% (iv) TsCl, pyridine, 66% (v) NaBH4, MeOH, 66% (vi) H2, Pd/C, 1,4-dioxane: H2O (2:1), 100%.
Since the pKa of the salts of imino sugars is around 7-8, the 1H NMR spectrum is strongly dependent on the pH. This is illustrated for 4-C-Me-DAB 1D at six different pH values with CH3CN as the internal standard [Figure 1]. The pKa of the hydrochloride salt of 1D was determined as 8.38 from the change in the chemical shift of the C-methyl group with pH [Figure 2]. The full resonance assignments of the salt at pH 2.4 and of the free base at pH 11.1 of 1D are given in Tables 1 and 2, respectively.
Figure 1.
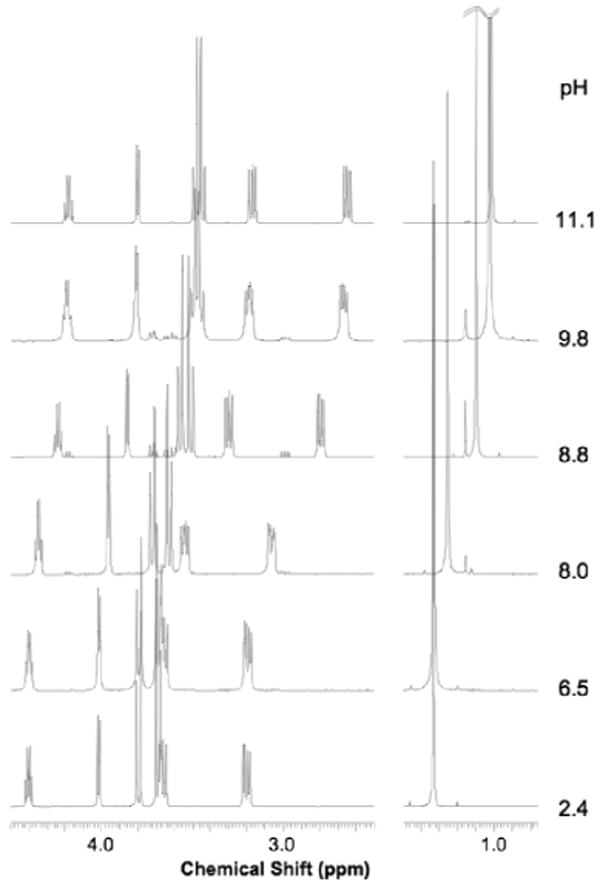
1H NMR (500 MHz) of 4-C-Me-DAB 1D in D2O at different pHs.
Figure 2.
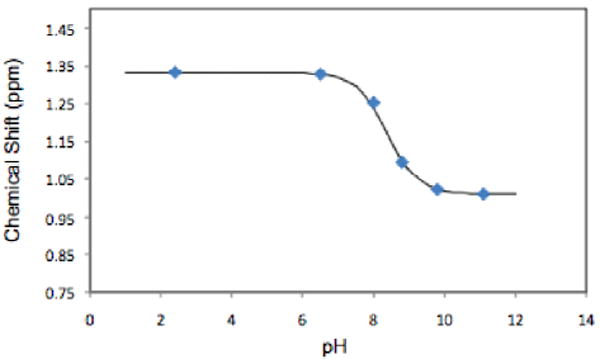
Chemical shift of the methyl peak in 1D versus pH. The points are the experimental values; the line is the fitted curve, pKa of 8.38.
Table 1.
NMR [1H (500 MHz) 13C (125 MHz) D2O] assignments of the HCl salt of 1D, pH 2.4.
| Label | 1H | 13C | ||
|---|---|---|---|---|
| pH 2.24 |
δ (ppm) |
mult | 3JHH (Hz) | δ (ppm) |
| C1 | 3.664 3.202 |
dd dd |
12.7 / 6.8 12.7 / 5.0 |
47.83 |
| C2 | 4.401 | ddd | 6.8 / 5.0 / 5.0 | 74.26 |
| C3 | 4.014 | d | 5.0 | 77.36 |
| C4 | -- | -- | -- | 69.48 |
| C5 | 3.797 3.688 |
d d |
12.3 12.3 |
63.75 |
| CH3 | 1.332 | s | na | 15.12 |
Table 2.
NMR [1H (500 MHz) 13C (125 MHz) D2O] assignments of the free base of 1D, pH 11.1.
| Label | 1H | 13C | ||
|---|---|---|---|---|
| pH 11.1 |
δ (ppm) |
mult | 3JHH (Hz) | δ (ppm) |
| C1 | 3.170 2.648 |
dd dd |
12.1 / 7.3 12.1 / 6.2 |
48.39 |
| C2 | 4.181 | ddd | 7.3 / 6.2 / 6.0 | 77.61 |
| C3 | 3.801 | D | 6.0 | 80.67 |
| C4 | -- | -- | -- | 63.41 |
| C5 | 3.485 3.443 |
D d |
11.6 11.6 |
66.98 |
| CH3 | 1.010 | S | na | 17.60 |
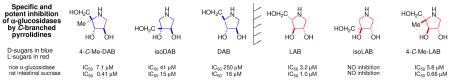
LAB 3L is a more potent and specific inhibitor of α-glucosidases than its naturally occurring enantiomer DAB 3D [Table 3],xvi whilst isoDAB 2D is a more potent inhibitor of α-glucosidases than DAB 3D and is completely specific; in contrast, isoLAB 2L does not show any inhibition of these enzymes.viii 4-C-Me-DAB 1D and 4-C-Me-LAB 1L were both found to be specific and potent (μM) inhibitors of α-glucosidases. In particular, these compounds showed strong inhibition of rat intestinal sucrase with IC50 values of 0.41 and 0.66 μM, respectively. The kinetic analysis showed that 4-C-Me-DAB 1D is a competitive inhibitor of rat intestinal sucrase with a Ki value of 0.89 μM, whereas the enantiomer 4-C-Me-LAB 1L is a non-competitive inhibitor (Ki value of 0.95 μM) of the enzyme. Although DAB 3D has been reported to be a good inhibitor of glycogen phosphorylase and has been investigated as a potential therapeutic agent for the treatment of diabetes,xvii LAB 3L, isoDAB 2D, isoLAB 2L, 4-C-Me-DAB 1D, and 4-C-Me-LAB 1L showed no significant inhibition of this enzyme.
Table 3.
Concentration of iminosugars giving 50% inhibition of various glycosidases and glycogen phosphorylase.
| IC50 (μM) | ||||||
|---|---|---|---|---|---|---|
 |
 |
 |
 |
 |
 |
|
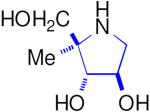
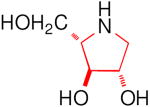
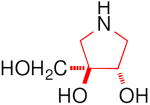
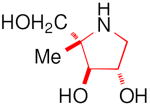
| Enzyme | DAB 3D | isoDAB 2D | 4-C-Me-DAB 1D | LAB 3L | isoLAB 2L | 4-C-Me-LAB 1L |
| α-Glucosidase | ||||||
| Rice | 250 | 41 | 7.1 | 3.2 | NI | 5.8 |
| Yeast | 0.15 | NI | 1.9 | 70 | NI | NI |
| Rat intestinal maltase | 55 | 24 | 0.74 | 0.93 | NI | 2.4 |
| Rat intestinal isomaltase | 5.8 | 20 | 3.4 | 0.36 | NI | 5.1 |
| Rat intestinal sucrase | 16 | 15 | 0.41 | 1.0 | NI | 0.66 |
| β-Glucosidase | ||||||
| Almond | 250 | NI | NI | NI | NI | NI |
| Bovine liver | 638 | NI | NI | NI | NI | NI |
| Rat intestinal cellobiase | 756 | NI | NI | NI | NI | NI |
| α-Galactosidase | ||||||
| Coffee beans | NIa | NI | NI | NI | NI | NI |
| Human lysosome | NI | NI | NI | NI | NI | NI |
| β-Galactosidase | ||||||
| Bovine liver | NI | NI | NI | NI | NI | NI |
| Rat intestinal lactase | 323 | NI | NI | 415 | NI | NI |
| α-Mannosidase | ||||||
| Jack beans | 320 | NI | NI | NI | NI | NI |
| β-Mannosidase | ||||||
| Snail | NI | NI | NI | NI | NI | NI |
| α-l-Rhamnosidase | ||||||
| P. decumbens | NI | NI | NI | NI | NI | NI |
| α-l-Fucosidase | ||||||
| Bovine epididymis | NI | NI | NI | NI | NI | NI |
| Trehalase | ||||||
| Rat intestinal trehalase | 61 | NI | 38 | 75 | NI | NI |
| Glycogen phosphorylase | ||||||
| Rabbit muscle | 0.33 | NI | NI | NI | NI | NI |
NI: No inhibition (less than 50% inhibition at 1000 μM).
In order to rationalize the glucosidase inhibition results, it was assumed that these inhibitors mimic the glucoside substrate. The six pyrrolidines were overlayed with glucose to: (i) position the ring nitrogen close to the ring oxygen of glucose so that, in the protonated form, the positive charge can mimic the partial positive charge of the transition state during hydrolysis, and (ii) match as many hydroxy positions as possible, to retain any specific binding interactions in the active sites.xviii The different glucosidases will recognise the terminal glucose residue of the substrates in different ways. Thus it is not surprising that these inhibitors show slightly different patterns of inhibition for the different glucosidases, however, some general principles can be suggested.
The best match between DAB 3D and glucose placed the C2 and C3 hydroxy (OH) groups of 3D to overlay the C3 and C4 OHs of glucose, respectively [Figure 3(a)]. This positioned the CH2OH group of 3D close to the C6 OH of glucose and the ring nitrogen of 3D close to the ring oxygen of glucose. In this orientation the inhibitor mimicked three of the glucose OH groups. 4-C-Me-DAB 1D was overlayed with glucose in the same way as 3D, with the inhibitor methyl group in the position of the glucose C5 axial proton [Figure 3(b)]. IsoDAB 2D could be overlayed with glucose in the same way, with the inhibitor CH2OH group in the position of the glucose C4 axial proton [Figure 3(c)]. This CH2OH group in 2D might be able to interact with groups which normally bind to the glucose C6 OH, depending on the glucose C5-C6 torsion angle in the bound form. In contrast, the best match between LAB 3L and glucose places the C2 and C3 OHs of 3L overlaying the C2 and C3 OHs of glucose respectively [Figure 3(d)]. This placed the CH2OH group of 3L close to the C4 OH of glucose and the ring nitrogen of 3L close to the ring oxygen of glucose. Again, in this orientation 3L mimicked three of the glucose OH groups. 4-C-Methyl-LAB 1L was overlayed with glucose in the same way as 3L, with the methyl group of 1L in the position of the glucose C4 axial proton [Figure 3(e)]. If the inactive isoLAB 2L is overlayed in the same manner as 3L, then the CH2OH group in 2L would be in the position of the glucose C3 axial proton, on the C2OH/C4OH face of glucose. For a terminal α-linked glucose, the rest of the glycan was on the C2OH/C4OH face of the glucose residue (indicated by the large spheres in Figure 3). Alternatively, 2L could be overlayed with the C2 and C3 OHs of 2L overlaying the C3 and C2 OHs of glucose respectively, with the CH2OH group in 2L in the position of the glucose C2 axial proton, on the opposite face [Figure 3(f)]. In either case, the inhibitor could only mimic two of the four glucose OH groups.
Figure 3.
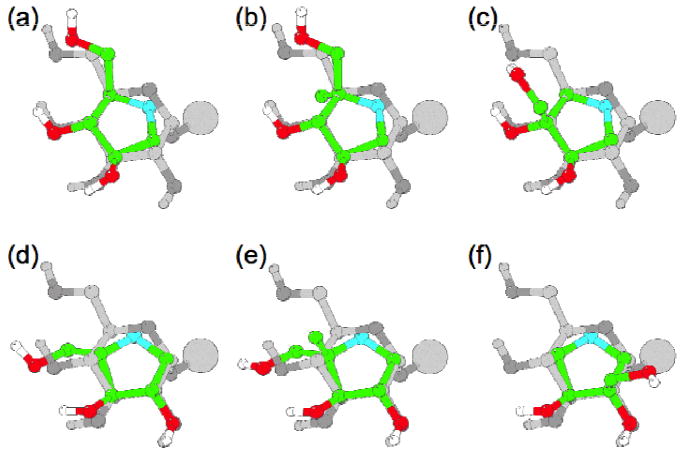
Overlay of the six inhibitors, (a) DAB 3D, (b) 4-C-Me-DAB 1D, (c) isoDAB 2D, (d) LAB 3L, (e) 4-C-Me-LAB 1L and (f) isoLAB 2L, with an α-glucoside. The glucose residues are in grey, with the oxygens in slightly darker grey, and the inhibitors are in colour. Only the hydroxy protons are shown, for clarity. The large sphere shows the position of the rest of the glycan.
There are several possible explanations for the absence of activity of isoLAB 2L compared to the other five pyrrolidines, including: (i) the inhibitor must mimic at least three of the glucose hydroxy groups for effective binding, or (ii) a hydroxy group near the position of the glucose C4 hydroxy is required for binding, or (iii) an additional axial group at the positions of C2 or C3 in glucose cannot be tolerated, whereas it can at positions C4 or C5. The differential inhibition of different α-glucosidases by the various pyrrolidines showed that the remainder of the substrates can significantly affect the potency of inhibition. Whatever the explanation, it is remarkable to find five such structurally simple pyrrolidines with potent and specific α-glucosidase inhibition.
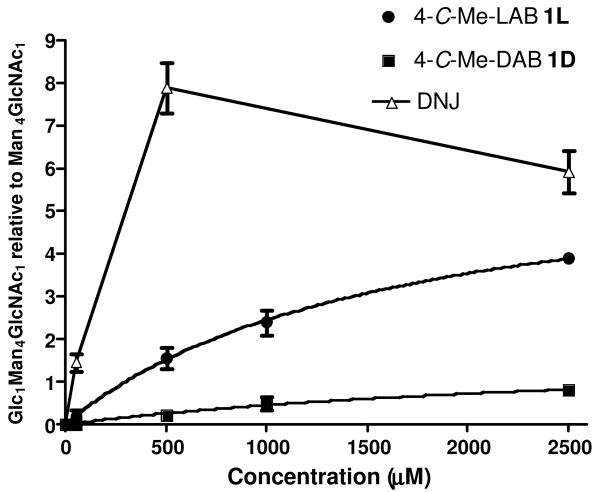
The effect of 1D and 1L on cellular endoplasmic reticulum (ER) resident α-glucosidase I and II activity in cells was studied using a free oligosaccharide assay.xix HL-60 cells were treated at concentrations of 1D and 1L as indicated for 24 hours [Figure 4] and were compared with the effect of 1-deoxynojirimycin (DNJ); free oligosaccharides (FOS) were isolated from cells, fluorescently labelled, and followed by separation using NP-HPLC. Peak areas for Glc1Man4GlcNAc1 and Man4GlcNAc1 (non-glucosylated control species) were determined and expressed as a ratio. The presence of Glc1Man4GlcNAc1 indicated inhibition of α-glucosidase II; at 500 μM DNJ is five times a more potent inhibitor than 1L and forty times more potent than 1D. The decline in Glc1Man4GlcNAc1 at concentrations of DNJ higher than 500 μM is due to inhibition of α-glucosidase I and therefore production of Glc3Man5GlcNAc1 species at the expense of Glc1Man4GlcNAc1 species. In the presence of 2.5 mM branched imino sugars, cells do not produce any Glc3Man5GlcNAc1 indicating that compounds are either unable to inhibit this enzyme, i.e. are specific α-glucosidase II inhibitors, or most likely, insufficient concentrations have been reached to inhibit the α-glucosidase I step in the pathway. In summary, both 4-C-methyl branched imino sugars of 1D and 1L are weak inhibitors of α-glucosidase II in cells; this is in contrast to the behaviour of the hydroxymethyl branched pyrrolidines 2D and 2L, neither of which showed any inhibition of ER glucosidases.8 Both DAB 3D and LAB 3L are presumed inhibitors of processing glucosidases; their N-butyl analogues are weak inhibitors (IC50, 319 μM and 769 μM, respectively) of α-glucosidase I using an in vitro assay and are consequently ineffective at inhibiting glucosidase activity in cellular assays at concentrations of 1 mM or less.xx
Figure 4.
Glc1Man4GlcNAc1 following α-glucosidase inhibition in FOS assay.
DNJxxi [an α-glucosidase inhibitor] and isoLAB 2L8 [which has no α-glucosidase inhibition] both show significant rescue of the defective F508del-CFTR function and thus might have potential in the treatment of cystic fibrosis.xxii Neither of the 4-C-methyl pyrrolidines 1D and 1L had any corrector effect on CFTR function in CF-KM4 cells,xxiii as assessed by single-cell fluorescence imaging;xxiv thus, isoLAB 2L – the only one of the DAB analogues that is not an α-glucosidase inhibitor – is the sole pyrrolidine to have chloride channel rescue properties.
In summary, only a single acetonide protecting group is used in the synthesis of the first examples of potent glycosidase inhibitors by C-alkyl branching of the carbon chain of iminosugars. Of the three pairs of enantiomers 1, 2 and 3, five structurally simple pyrrolidines are potent (μM) and specific α-glucosidase inhibitors; isoLAB 2L does not inhibit any glycosidase but is the only one to exhibit significant rescue of the defective F508del-CFTR function. Only DAB 3D shows any inhibition of glycogen phosphorylase.
Acknowledgments
Financial support [to F.P.C.] provided by the Fundação para a Ciência e Tecnologia, Portugal is gratefully acknowledged. Part of this work was supported by Grant Number R01CA125642 from the National Cancer Institute (T.D.B., D.S.A.) and by grants from the French association “Vaincre la Mucoviscidose” C.N., F.B.). We thank Dextra Laboratories Limited, Reading, UK for generous gifts of d-erythronolactone and l-erythronolactone.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- i.Asano N, Ikeda K, Yu L, Kato A, Takebayashi K, Adachi I, Kato I, Ouchi H, Takahata H, Fleet GWJ. Tetrahedron: Asymmetry. 2005;16:223–229. [Google Scholar]
- ii.(a) d'Alonzo D, Guaragna A, Palumbo G. Curr Med Chem. 2009;16:473–505. doi: 10.2174/092986709787315540. [DOI] [PubMed] [Google Scholar]; (b) Blériot Y, Gretzke D, Krülle TM, Butters TD, Dwek RA, Nash RJ, Asano N, Fleet GWJ. Carbohydr Res. 2005;340:2713–2718. doi: 10.1016/j.carres.2005.10.002. [DOI] [PubMed] [Google Scholar]; (c) Kato A, Kato N, Kano E, Adachi I, Ikeda K, Yu L, Okamoto T, Banba Y, Ouchi H, Takahata H, Asano N. J Med Chem. 2005;48:2036–2044. doi: 10.1021/jm0495881. [DOI] [PubMed] [Google Scholar]; (d) Asano N, Ikeda K, Yu L, Kato A, Takebayashi K, Adachi I, Kato I, Ouchi H, Takahata H, Fleet GWJ. Tetrahedron: Asymmetry. 2005;16:223–229. [Google Scholar]; (e) Macchi B, Minutolo A, Grelli S, Cardona F, Cordero FM, Mastino A, Brandi A. Glycobiology. 2010;21:500–506. doi: 10.1093/glycob/cwp202. [DOI] [PubMed] [Google Scholar]
- iii.(a) Asano N. Cell Mol Life Sci. 2009;66:1479–1492. doi: 10.1007/s00018-008-8522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Asano N, Nash RJ, Molyneux RJ, Fleet GWJ. Tetrahedron: Asymmetry. 2000;11:1645–1680. [Google Scholar]; (c) Watson AA, Fleet GWJ, Asano N, Molyneux RJ, Nash RJ. Phytochemistry. 2001;56:265–295. doi: 10.1016/s0031-9422(00)00451-9. [DOI] [PubMed] [Google Scholar]
- iv.(a) Blanco MJ, Sardina FJ. J Org Chem. 1998;63:3411–3416. [Google Scholar]; (b) Burley I, Hewson AT. Tetrahedron Lett. 1994;35:7099–7102. [Google Scholar]; (c) Hotchkiss DJ, Kato A, Odell B, Claridge TDW, Fleet GWJ. Tetrahedron: Asymmetry. 2007;18:500–512. [Google Scholar]
- v.Hakansson AE, van Ameijde J, Horne G, Nash RJ, Wormald MR, Kato A, Besra GS, Gurcha S, Fleet GWJ. Tetrahedron Lett. 2008;49:179–184. [Google Scholar]
- vi.(a) Hakansson AE, van Ameijde J, Guglielmini L, Horne G, Nash RJ, Evinson EL, Kato A, Fleet GWJ. Tetrahedron: Asymmetry. 2007;18:282–289. [Google Scholar]; (b) Davis B, Bell AA, Nash RJ, Watson AA, Griffiths RC, Jones MG, Smith C, Fleet GWJ. Tetrahedron Lett. 1996;37:8565–8568. [Google Scholar]; (c) Bell AA, Pickering L, Watson AA, Nash RJ, Griffiths RC, Jones MG, Fleet GWJ. Tetrahedron Lett. 1996;37:8561–8564. [Google Scholar]
- vii.(a) Fleet GWJ, Nicholas SJ, Smith PW, Evans SV, Fellows LE, Nash RJ. Tetrahedron Lett. 1985;26:3127–3130. [Google Scholar]; (b) Scofield AM, Fellows LE, Nash RJ, Fleet GWJ. Life Sci. 1986;39:645–650. doi: 10.1016/0024-3205(86)90046-9. [DOI] [PubMed] [Google Scholar]; (c) Fleet GWJ, Smith PW. Tetrahedron. 1986;42:5685–5692. [Google Scholar]; (d) Behling JR, Campbell AL, Babiak KA, Ng JS, Medich J, Farid P, Fleet GWJ. Tetrahedron. 1993;49:3359–3366. [Google Scholar]
- viii.Best D, Jenkinson SF, Saville AW, Alonzi DS, Wormald MR, Butters TD, Norez C, Becq F, Bleriot Y, Adachi I, Kato A, Fleet GWJ. Tetrahedron Lett. 2010;51:4170–4174. [Google Scholar]
- ix.(a) Chang CF, Ho CW, Wu CY, Chao TA, Wong CH, Lin CH. Chem Biol. 2004;11:1301–1306. doi: 10.1016/j.chembiol.2004.07.009. [DOI] [PubMed] [Google Scholar]; (b) Wu CY, Chang CF, Chen JSY, Wong CH, Lin CH. Angew Chem Int Ed. 2003;42:4661–4664. doi: 10.1002/anie.200351823. [DOI] [PubMed] [Google Scholar]; (c) Ho CW, Popat SD, Liu TA, Tsai KC, Ho MJ, Chen WH, Yang AS, Lin CH. ACS Chem Biol. 2010;5:489–497. doi: 10.1021/cb100011u. [DOI] [PubMed] [Google Scholar]; (d) Rawlings AJ, Lomas H, Pilling AW, Lee MJR, Alonzi DS, Rountree JSS, Jenkinson SF, Fleet GWJ, Dwek RA, Jones JH, Butters TD. ChemBioChem. 2009;10:1101–1105. doi: 10.1002/cbic.200900025. [DOI] [PubMed] [Google Scholar]
- x.(a) Hotchkiss DJ, Soengas R, Simone MI, van Ameijde J, Hunter S, Cowley AR, Fleet GWJ. Tetrahedron Lett. 2004;45:9461–9464. [Google Scholar]; (b) Soengas R, Izumori K, Simone MI, Watkin DJ, Skytte UP, Soetaert W, Fleet GWJ. Tetrahedron Lett. 2005;46:5755–5759. [Google Scholar]
- xi.(a) Hotchkiss DJ, Jenkinson SF, Storer R, Heinz T, Fleet GWJ. Tetrahedron Lett. 2006;47:315–318. [Google Scholar]; (b) Booth KV, da Cruz FP, Hotchkiss DJ, Jenkinson SF, Jones NA, Weymouth-Wilson AC, Clarkson R, Heinz T, Fleet GWJ. Tetrahedron: Asymmetry. 2008;19:2417–2424. [Google Scholar]
- xii.Jenkinson SF, Jones NA, Moussa A, Stewart AJ, Heinz T, Fleet GWJ. Tetrahedron Lett. 2007;48:4441–4445. [Google Scholar]
- xii.Rao D, Yoshihara A, Gullapalli P, Morimoto K, Takata G, da Cruz FP, Jenkinson SF, Wormald MR, Dwek RA, Fleet GWJ, Izumori K. Tetrahedron Lett. 2008;49:3316–3321. [Google Scholar]
- xiv.da Cruz FP, Horne G, Fleet GWJ. Tetrahedron Lett. 2008;49:6812–6815. [Google Scholar]
- xv.Data for free base 1D: [α]D25 -20.1 (c 0.57, H2O); δH (400 MHz, D2O) 1.01 (3H, s, CH3), 2.65 (1H, dd, H1, J1,1′ 12.1, J1,2 6.2), 3.17 (1H, dd, H1′, J1′,1 12.1, J1′,2 7.3), 3.44 (1H, d, H5, J5,5′ 11.6), 3.49 (1H, d, H5′, J5′,5 11.6), 3.80 (1H, d, H3, J3,4 6.0), 4.18 (1H, ddd, H2, J2,1′ 7.3, J2,1 6.2, J2,3 6.0); δC (100.6 MHz, D2O) 17.6 (C4′), 48.4 (C1), 63.4 (C4), 67.0 (C5), 77.6 (C2), 80.7 (C3). Data for HCl salt of 1D: [α]D25 -5.22 (c 1.07, H2O); νmax (thin film, Ge): 3356 (br s, OH, NH); δH (400 MHz, D2O) 1.33 (3H, s, CH3), 3.20 (1H, dd, H1, J1,1′ 12.7, J1,2 5.0), 3.66 (1H, dd, H1′, J1′,1 12.7, J1′,2 6.8), 3.69 (1H, d, H5, J5,5′ 12.3), 3.80 (1H, d, H5′, J5′,5 12.3), 4.01 (1H, d, H3, J3,4 5.0), 4.40 (1H, ddd, H2, J2,1′ 6.8, J2,1 5.0, J2,3 5.0); δC (100.6 MHz, D2O) 15.1 (C4′), 47.8 (C1), 63.7 (C5), 69.5 (C4), 74.3 (C2), 77.4 (C3); HRMS (FI+) Calcd. for C6H13NO3 [M•]: 147.0895. Found: 147.0895.
- xvi.For details of assays, see: Mercer TB, Jenkinson SF, Nash RJ, Miyauchi S, Kato A, Fleet GWJ. Tetrahedron: Asymmetry. 2009;20:2368–2373.Best D, Wang C, Weymouth-Wilson AC, Clarkson RA, Wilson FX, Nash RJ, Miyauchi S, Kato A, Fleet GWJ. Tetrahedron: Asymmetry. 2010;21:311–319.
- xvii.(a) Andersen B, Rassov A, Westergaard N, Lundgren K. Biochem J. 1999;342:545–550. [PMC free article] [PubMed] [Google Scholar]; (b) Fosgerau K, Westergaard N, Quistorff B, Grunnet N, Kristiansen M, Lundgren K. Arch Biochem Biophys. 2000;15:274–284. doi: 10.1006/abbi.2000.1930. [DOI] [PubMed] [Google Scholar]; (c) Minami Y, Kuriyama C, Ikeda K, Kato A, Takebayashi K, Adachi I, Fleet GWJ, Kettawan Q, Okamoto T, Asano N. Bioorg Med Chem. 2008;16:2734–2740. doi: 10.1016/j.bmc.2008.01.032. [DOI] [PubMed] [Google Scholar]
- xviii.Molecular modelling was performed on a Silicon Graphics Fuel workstation, using the programs InsightII and Discover (Accelrys Inc., San Diego, USA).
- xix.Alonzi DS, Neville DC, Lachmann RH, Dwek RA, Butters TD. Biochem J. 2008;409:571–580. doi: 10.1042/BJ20070748. [DOI] [PubMed] [Google Scholar]
- xx.Butters TD, van den Broek LAGM, Fleet GWJ, Krulle TM, Wormald MR, Dwek RA, Platt FM. Tetrahedron: Asymmetry. 2000;11:113–124. [Google Scholar]
- xxi.(a) Norez C, Antigny F, Noel S, Vandebrouck C, Becq F. Am J Respir Cell Mol Biol. 2009;41:217–225. doi: 10.1165/rcmb.2008-0285OC. [DOI] [PubMed] [Google Scholar]; (b) Noel S, Faveau C, Norez C, Rogier C, Mettey Y, Becq F. J Pharmacol Expt Therapeut. 2006;319:349–359. doi: 10.1124/jpet.106.104521. [DOI] [PubMed] [Google Scholar]
- xxii.Becq F. Drugs. 2010;70:241–259. doi: 10.2165/11316160-000000000-00000. [DOI] [PubMed] [Google Scholar]
- xxiii.For details of the human tracheal gland serous epithelial cell line CF-KM4 derived from a CF patient homozygous for the F508del mutation, see: Kammouni W, Moreau B, Becq F, Saleh R, Pavirani A, Figarella C, Merten MD. Am J Respir Cell Mol Biol. 1999;20:684–691. doi: 10.1165/ajrcmb.20.4.3341.
- xxiv.CFTR Cl- channel activity was assayed by single-cell fluorescence imaging using the potential-sensitive probe, bis-(1,3-diethylthiobarbituric acid)trimethine oxonol. For experimental details, see reference 21 (a).