Abstract
Overexpression of the human MYCN oncogene driven by a tyrosine hydroxylase promoter causes tumours in transgenic mice that recapitulate the childhood cancer neuroblastoma. To establish an in vitro model to study this process, a series of isogenic cell lines were developed from these MYCN-driven murine tumours. Lines were established from tumours arising in homozygous and hemizygous MYCN transgenic mice. Hemizygous tumours gave rise to cell lines growing only in suspension. Homozygous tumours gave rise to similar suspension lines as well as morphologically distinct substrate-adherent lines characteristic of human S-type neuroblastoma cells. FISH analysis demonstrated selective MYCN transgene amplification in cell lines derived from hemizygous mice. Comparative genomic hybridisation (CGH) and fluorescence in situ hybridisation (FISH) analysis confirmed a range of neuroblastoma-associated genetic changes in the various lines, in particular, gain of regions syntenic with human 17q. These isogenic lines together with the transgenic mice thus represent valuable models for investigating the biological characteristics of aggressive neuroblastoma.
Keywords: Neuroblastoma, MYCN oncogene, Transgenic mice, Murine cell lines
1. Introduction
Neuroblastoma, a tumour consisting of undifferentiated neuroectodermal cells of the sympathetic nervous system, is the most common extracranial tumour of childhood and accounts for 15% of cancer-related deaths in children.1,2 MYCN gene amplification is the best characterised genetic aberration described in neuroblastoma to date, occurring in 25–30% of neuroblastomas.3 Amplification of MYCN is a strong prognostic indicator of poor clinical outcome and is associated with advanced-stage disease, rapid tumour progression and a survival rate of less than 15%.2,3 A murine model of neuroblastoma, established by targeted expression of the human MYCN oncogene in neuroectodermal cells of transgenic mice, has provided definitive evidence for the role of MYCN in neuroblastoma tumourigenesis.4 This model closely mirrors human neuroblastoma with respect to location, histology, expression of neuronal markers and syntenic chromosomal alterations in murine tumours.5,6
The MYCN gene encodes a nuclear phosphoprotein that functions as a transcriptional regulator of genes that may be involved in neuroblastoma pathogenesis.7 Established MYCN target genes include ornithine decarboxylase (ODC)7,8 and multidrug resistance-associated protein 1 (MRP1).9,10 ODC is the rate-limiting enzyme in the production of polyamines and its over-expression has been associated with a variety of malignancies.11,12 MRP1 is a membrane-bound glycoprotein that confers resistance to many of the cytotoxic drugs used in the treatment of neuroblastoma.13 We have previously shown a strong correlation between both MYCN and MRP1 expression,10,14-16 as well as MYCN and ODC gene expression.17
Human neuroblastoma cell lines have been shown to consist of a mix of different cell types including neuroblastic (N-type) cells, substrate-adherent (S-type) cells and morphologically intermediate (I-type) stem cells that display a capacity for phenotypic interconversion between both N-type and S-type lineages.18,19 N-type cells express high levels of neuronal markers including MYCN and tyrosine hydroxylase (TH), while S-type cells appear to be non-tumourigenic cells expressing genetic markers including S100A6 and vimentin.
In this study, we have developed and characterised genetic changes in a series of isogenic cell lines from the TH-MYCN-driven murine tumours. The tumours used to derive the cell lines were obtained from mice that were either homozygous or hemizygous for the MYCN transgene. The cell lines exhibited many of the molecular and biological features characteristic of both the primary murine tumours and clinical neuroblastoma.
2. Methods
2.1. Derivation of cell lines from TH-MYCN transgenic murine tumours
Transgenic murine neuroblastomas were passed through a stainless-steel sieve to obtain a cell suspension. The cells were maintained in RPMI-1640 medium (Invitrogen) supplemented with 2 mM l-glutamine, 10−5 mM, 2-mercaptoethanol, 1 mM sodium pyruvate, 1× non-essential amino acids and 20% v/v heat-inactivated foetal calf serum (Trace Scientific). All suspension cells were cultured in 24-well plates and passaged every second day, while the adherent cells were cultured in T75 flasks. All cell lines have been in continuous culture for at least 12 months, and all results reported here have been obtained from cells cultured for 3–12 months.
2.2. Cell ploidy
Splenocytes from transgenic mice were purified and 106 cells resuspended in PBS and fixed on ice by the addition of an equal volume of 60% ice-cold ethanol. Tumour cells were also resuspended in PBS and fixed as described. Fixed cells (2.5 × 105) were incubated in PBS containing 50 μM propidium iodide (Sigma) and 2 μg/ml RNase (Boehringer Mannheim) for 30 min on ice. Samples were run on a FACSCalibur flow cytometer (Becton Dickinson) and FL2-A was acquired for each cell population. The DNA index of the tumour cell lines was calculated as the ratio of the tumour cell peak channel/splenocyte peak channel.
2.3. Fluorescent immunocytochemistry
All cell lines were centrifuged onto glass slides and fixed prior to immunostaining for MYCN, odc and mrp1 as previously described.17 An identical immunostaining protocol was used to detect S100A6. The immunodetection of TH was modified by fixing the cytocentrifuged cells in 4% v/v paraformaldehyde/PBS for 10 min at room temperature. S100A6 protein was detected with the use of a rabbit anti-human antibody (1/25 dilution; DakoCytomation) followed by incubation with a Cy3-conjugated goat anti-rabbit antibody (1/2000 dilution; Amersham). TH was detected with the use of a rabbit anti-rat polyclonal antibody (1/200 dilution; Chemicon International Inc.) followed by incubation with a Cy3-conjugated goat anti-rabbit antibody (1/2000 dilution; Amersham).
2.4. RNA isolation and gene expression analysis
Total cellular RNA was extracted and cDNA synthesised as previously described.15 The mouse ACTB (beta-actin) gene was used as an internal control for all reverse transcription PCRs. Human MYCN, murine odc and murine mrp1 gene expression was determined by real-time PCR analysis using the ΔΔCt method as previously described.17 The primer and probe sequences for Human MYCN, murine odc, murine mrp1 and murine ACTB have been previously described.17 Expression of the TH and S100A6 genes relative to the ACTB control was determined by 35 cycles of conventional RT-PCR with the following TH and S100A6 primer sequences: MTH43S 5′-GCTTCAGAAGAGCCGTCTCAGA-3′, MTH149A 5′-CTCCTTGCGGGCATCCT-3′; S100A6F 5′-TGGCTCCAAGCTGCAGG-3′, S100A6R 5′-CCCAGGAAGGCGACATACTC-3′. All analyses of gene expression were performed on at least three separate occasions.
2.5. Chromosome preparations
Cell cultures were treated with colchicine (5 μg/ml) at 37 °C (30 min), harvested and pelleted. Pellets were resuspended in 75 mM KCl and incubated at 37 °C for 10 min. Carnoy fixative was added to the suspension prior to centrifugation at 2500 rpm (5 min). The pellets were resuspended in fixative and incubated at room temperature (20 min), followed by pelleting and two further washes with the fixative.
2.6. Fluorescence in situ hybridisation (FISH)
A fresh cut surface from the tissue was touched gently on to the glass slides, fixed in cold methanol (20 min) followed by Carnoy fixative at room temperature, then dehydrated in an ethanol series and air-dried. The transgene plasmid pTHMYCN was labelled with digoxigenin-11-dUTP using nick-translation (Roche). Slides were denatured in 70% v/v formamide at 72 °C, dehydrated and air-dried. Probe (100 ng/slide) was denatured in hybridisation solution (10 min) at 72 °C and incubated overnight on slides sealed with coverslips. The slides were washed twice in 2× SSC (10 min, 64 °C), once in 0.1× SSC (10 min, 64 °C), once in 0.1× SSC (10 min, RT) and equilibrated in 4× SSC, 0.1% v/v Tween. FISH signal detection and washes were carried out with a rhodamine conjugated anti-digoxigenin antibody (Roche) according to the manufacturer’s protocol. The slides were counterstained with DAPI and analysed by fluorescence microscopy. In addition, double-colour FISH was also used to analyse the murine neuroblastoma cell lines for the presence of chromosome 11 gain as we have previously described.20
2.7. Microarray-based comparative genomic hybridisation (CGH)
Array CGH analysis was performed as described previously.6 Briefly, genomic DNA (cell line or tumour) and reference DNA (normal mouse spleen) were labelled with Cy3 or Cy5, respectively. The labelled probes were combined and hybridised to slides containing arrays of murine BACs providing 5-Mb average resolution across the genome. The slides were imaged using a CCD camera and spot statistics were analysed as described.6
2.8. Engraftment of cell lines into nude mice
Nude mice (nu/nu) of BALB/c background were housed in ventirack cages, given sterile water and fed ad libidum. Mice were five weeks old at the time of injection and all experimental procedures were approved by the UNSW Animal Care and Ethics Committee and conducted under the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (1997). For nude mouse inoculations, cells were washed in RPMI media and viable cell number was determined using trypan blue exclusion. For suspension lines, cell number was estimated by comparing the pellet size to that of the adherent lines, since an accurate cell count could not be determined due to clumping of cell aggregates. Cells (5 × 106) were mixed with 1 mL ice-cold Matrigel Base Membrane matrix (BD Sciences) and injected subcutaneously into both flanks of anaesthetised mice. Mice were monitored daily for 1 week following injection and measured every second day thereafter for tumour growth using vernier calipers. Tumour mass in milligrams was calculated using the formula L × W2/2 with L being the length of the longest side and W being the length (width) of the smallest side of the tumour.21
3. Results
3.1. Morphological characterisation of murine tumourderived cell lines
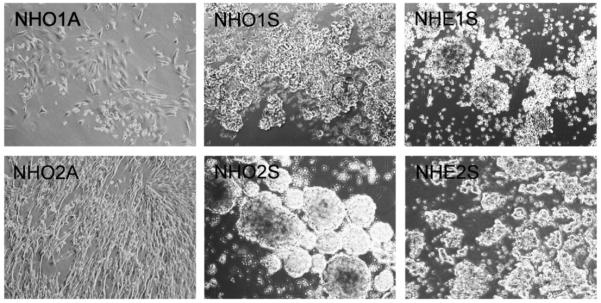
A total of six cell lines were derived from neuroblastoma tumours arising in four transgenic mice, two of which were homozygous and two hemizygous for the TH-MYCN transgene. Interestingly, the tumours derived from homozygous mice each gave rise to a suspension (NHO1S and NHO2S) and an adherent (NHO1A and NHO2A) cell line, while the two hemizygous tumours only gave rise to suspension cell cultures (NHE1S and NHE2S) (Fig. 1). The suspension cultures had similar growths rates with the exception of the more slowly growing NHO2S cells, and all were morphologically similar, comprising mostly large cellular aggregates of small round cells. A persistent population of adherent cells was observed in NHO1S cell cultures, despite the repeated selective passage of only the suspension cell population. All suspension cell lines had higher nuclear:cytoplasmic volume ratios than both adherent cell lines. The adherent cell line NHO1A had an epitheloid-like morphology, while the faster growing NHO2A cell line displayed a fibroblast-like appearance (Fig. 1).
Fig. 1.
Cellular morphology of murine neuroblastoma cell lines. Two tumours homozygous (HO) for the MYCN transgene each gave rise to a suspension (S) and an adherent (A) cell line, while two hemizygous (HE) tumours only gave rise to suspension lines. Suspension cell lines grew as dense cell aggregates, whereas adherent cell lines grew as monolayers with variable morphology, ranging from epithelioid (NHO1A) to fibroblast-like (NHO2A). Cells were photographed at 100× magnification.
3.2. Analysis of cytogenetic aberrations
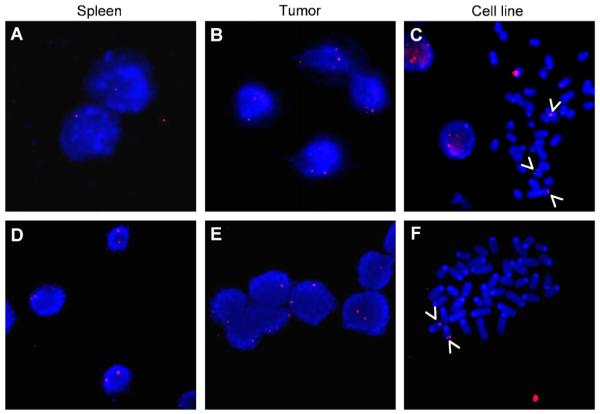
We have previously demonstrated that tumours of mice hemizygous for the MYCN transgene, but not those homozygous, display increased numbers of copies of the transgene in the tumour cells.6,16,17 FISH analysis was performed to quantify MYCN transgene copy number in tissues and cell lines from the TH-MYCN mice (Fig. 2). Increased transgene dosage was evident in both tumours and cell lines derived from mice hemizygous for the transgene compared to normal tissues (Fig. 2a–c). Thus, spleen showed one MYCN transgene signal at interphase FISH (Fig. 2a), while tumours and cell lines showed two or more transgene hybridisation signals (Fig. 2b and c). As anticipated, mice homozygous for the transgene showed two MYCN signals in normal spleen, and this copy number was evident both in tumours and in derived cell lines (Fig. 2d–f).
Fig. 2.
FISH analysis of the MYCN transgene copies in spleen (a and d), tumour (b and e) and cell line (c and f) derived from the TH-MYCN transgenic mice. Examples shown are from the hemizygous mouse NHE1 (a–c) and the homozygous NH01 (d–f) mouse. Similar findings were observed for the other hemizygous (NHE2) and homozygous (NH02) mice (Table 1). Results from spleens and tumours are shown for interphase hybridisations. The cell line results from hybridisation on metaphase spreads. Arrows indicate the MYCN transgene signals on the murine chromosomes.
Gain of chromosome 17q is the most common cytogenetic alteration found in human neuroblastoma. Human chromosome 17q is syntenic to regions on murine chromosome 11. We therefore analysed the murine neuroblastoma cell lines by FISH with a chromosome 11 painting probe. Cells derived from the hemizygous tumour NHE1S and cells derived from the homozygous tumour NHO2A showed gain of murine chromosome 11 and included a region syntenic to human chromosome 17q (Fig. 3). Interestingly, in NHO2, this gain is limited to the adherent cell line and is absent in the suspension cell line.
Fig. 3.
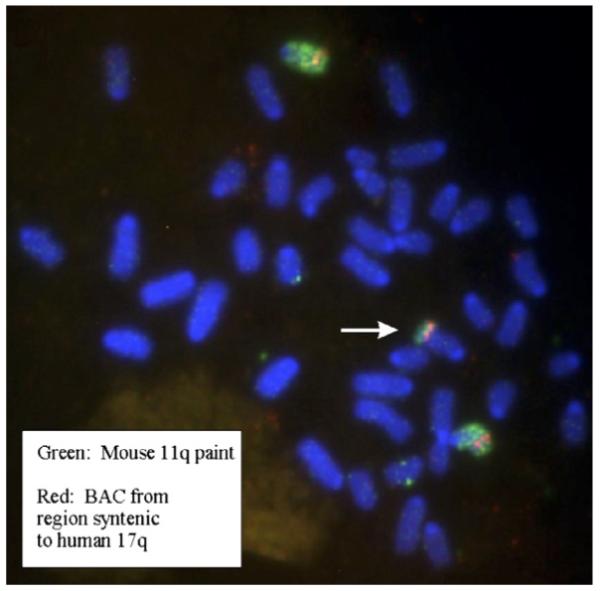
FISH analysis of murine chromosome 11 gain in NHE1S neuroblastoma cells. The white arrow indicates an additional fragment of the distal part of chromosome 11 translocated onto a marker chromosome. The BAC probe maps to a region of mouse chromosome 11 syntenic to human 17q and is labelled in red, while the murine chromosome 11 paint is labelled in green. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
To investigate the presence of other cytogenetic abnormalities in the murine cell lines, we undertook both karyotype and microarray-based CGH analyses (Table 1). Karytope analysis demonstrated that each of the cell lines was diploid or near diploid with the exception of NHO2A, which at early culture passages displayed an approximately equal ratio of diploid and tetraploid cells. After a further three months in culture, the tetraploid population gradually disappeared. The chromosomal content of the cell lines was confirmed by DNA ploidy analysis (Table 1).
Table 1.
Genetic and cytogenetic analysis of TH-MYCN transgenic murine tumour-derived cell lines
| Cell line | MYCN FISH | Ploidy | Chr11 FISH | Karyotype | CGH microarray |
|
|---|---|---|---|---|---|---|
| Gain | Loss | |||||
| NHE1S+/−a | 3 | 1.23 | Gain | 41–42 | 1, 8, 15, 18, Part 3 Part 11 |
X |
| NHE2S+/− | 2 | 1.18 | Normal | Normal, 3+ | Part 3 | |
| NHO1S+/+b | 2 | 1.13 | Normal | Normal, 5+ | Part 15 | Part 1 Part 6 |
| NHO1A+/+ | 2 | 1.18 | Normal | Normal, 5+ | Part 10 Part 15 |
Part 6 |
| NHO2S+/+ | 2 | 1.15 | Normal | 41, +1, +6 | 1, 6 | |
| NHO2A+/+ | 2–3 | 1.41 (2.35)c |
Gain | 42–43, (76–80)c |
Part 2, part 8, part 10, part 11, part 19 | Part 14 |
Hemizygous for the MYCN transgene.
Homozygous for the MYCN transgene.
For the cell line at early passage, where approximately 50% of the population was tetraploid.
Both karyotype and CGH microarray analyses demonstrated multiple chromosomal abnormalities in the cell lines. In most cases, there was consistency between the two analyses. While the chromosomal abnormalities observed in the suspension and adherent cell lines derived from tumour NH01 were quite similar, consistent with their common origin, there were dramatic differences between the chromosomal abnormalities observed in the suspension and adherent cell lines derived from tumour NH02. Thus NH02S demonstrated trisomy of chromosomes 1 and 6, however, neither of these changes was observed in NH02A. Instead, the latter line demonstrated multiple, variable chromosomal changes on karyotypic analysis, which were subsequently shown by CGH microarray to involve gain of several chromosomal regions including the 11q region previously demonstrated by FISH analysis, as well as partial loss of chromosome 14.
The karyotypes of the two hemizygous cell lines were both distinct from each other, and from the patterns observed in the homozygous cell lines. The karyotype of NHE2S was relatively normal, with the exception of a proportion of cells having an extended chromosome 3. This change was reflected on CGH microarray where the profile similarly suggested gain in the middle portion of this chromosome. In contrast, NHE1S displayed numerous karyotypic abnormalities and many of these changes were confirmed by CGH microarray including whole chromosome gain of chromosomes 1, 15 and 18, as well as chromosome X loss. CGH microarray also demonstrated gain on chromosome 11.
A number of the chromosomal changes observed in the cell lines are in common with those that we have previously described in murine neuroblastoma tumours.4-6 A detailed comparison of the chromosomal changes observed in the murine transgenic cell lines and the corresponding human chromosomal positions is shown in Table 2.
Table 2.
Comparison of murine and syntenic human chromosomal gains and losses in the murine neuroblastoma cell lines
| Cell line | Chromosome | Chromosomal location | Human position |
|---|---|---|---|
| NHE1S | 1 gain a | Whole chromosome | 8q, 2p,q, 6p,q, 5q, 13q, 1q, 4q, 18q |
| 3 gain a | 67 to end | 3q, 4q, 1p,q, 7p | |
| 8 gain a | Whole chromosome | 19p, 13q, 4q, 10p, 8p, 16q, 1q | |
| 11 gain a | 97 to end | 17p,q | |
| 15 gain | Whole chromosome | 22q, 12q, 8q, 5p | |
| 18 gain a | Whole chromosome | 18p,q, 5q, 10p, 2q | |
| NHE2S | 3 gain | 95–140 | 1p, 4q |
| NHO1S | 1 loss | 94–122 | 5q, 18q |
| 6 loss | 12.9–34 | 7p,q | |
| 15 gain | 77 to end | 22q, 12q | |
| NHO1A | 6 loss | 12.9–34 | 7p,q |
| 10 gain | 92.3–122 | 12q | |
| 15 gain | 77.5 to end | 22q, 12q | |
| NHO2A | 2 gain | 77.6 to end | 2q, 11p,q, 15q, 20p,q |
| 8 gain a | 19–74 | 19p, 13q, 4q, 8p | |
| 10 gain | 147 to end | 12q | |
| 11 gain a | Beginning to 76 | 22q, 7p, 2p, 5q, 16p, 17p,q | |
| 14 loss | 46 to end | 14q, 13q, 8p | |
| 19 gain | Beginning to 34 | 11q, 9p,q |
Changes also observed in the TH-MYCN neuroblastoma by Hackett et al. (2003).
3.3. Neuroblastoma-associated genes expression
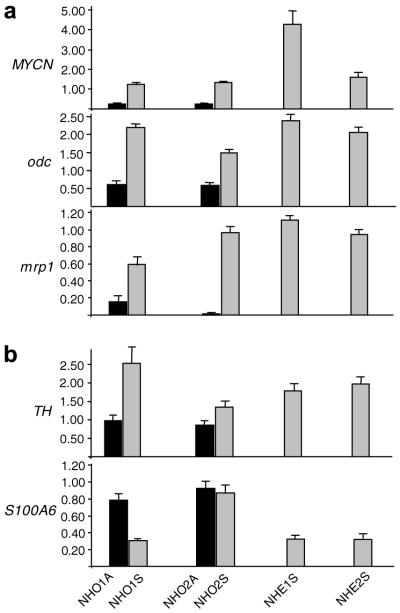
To investigate whether any differences in expression patterns of neuroblastoma-associated genes would be observed between the cell lines, we examined the expression of a number of genes at both the RNA (Fig. 4) and protein (Fig. 5) levels. As anticipated, all six cell lines displayed MYCN oncogene expression; however, both NHO1A and NHO2A adherent cell lines displayed considerably lower levels of MYCN compared to the four suspension lines. The highest level of MYCN expression was observed in NHE1S suspension cells. The expression levels of murine odc and mrp1, that have previously been shown to be MYCN target genes, were found to parallel MYCN expression in the panel of six cell lines (Fig. 4a). These findings were confirmed at the protein level, with staining for MYCN, odc and mrp1 being much more pronounced in the suspension than the adherent cultures (Fig. 5a).
Fig. 4.
Relative expression of neuroblastoma-associated genes in murine transgenic cell lines. RT-PCR analysis of MYCN, odc, mrp1 (a), TH and S100A6 (b) gene expression in adherent (dark bars) and suspension (shaded bars) cell lines. Expression of each of the genes was determined relative to the internal ACTB control gene as described in Section 2. Data represent the means ± SE from triplicate assays
Fig. 5.
Immunohistochemical detection of MYCN and MYCN-associated targets. Positive staining of MYCN, odc, mrp1 (a) and TH (b) in the suspension cell line (NHO1S) is indicative of an N-type (neuronal) phenotypic variant, whereas positive staining of S100A6 (b) in the adherent cell line (NHO1A) suggests that this cell line has an S-type (substrate adherent) or I-type (intermediate) phenotype. Slides were counterstained with DAPI to indicate location of cells.
Clear expression of the neuronal marker, TH, was observed in all four suspension cell lines, while the level of expression of this gene was markedly reduced in both the adherent cell lines (Figs. 4b and 5b). In contrast to all the other genes examined, high level expression of the S-type marker, S100A6, was observed in the two adherent cell lines NH01A and NH02A (Fig. 4b), a result confirmed by strong cytoplasmic staining for this protein in these lines (Fig. 5b). The NH02S cell line was the only one of the suspension cultures to display similarly high levels of S100A6. Co-expression of high levels of S100A6 together with TH in the NHO2S cell line is consistent with the I-type phenotype previously described in cultured human neuroblastoma lines.18,19
3.4. Engraftment of murine tumour-derived cell lines into nude mice
To investigate the tumourigenic potential of the murine tumour-derived cell lines, cells from each of the six lines were injected into the flanks of nude mice and monitored for tumour formation. All four cell lines derived from tumours homozygous for the MYCN transgene resulted in reliable tumour formation within a fortnight of allotransplantation (Fig. 6). Interestingly, no significant differences were observed between the suspension and adherent cell lines derived from the tumours homozygous for the transgene, in terms of either tumourigenicity or latency. Of the two cell lines derived from tumours hemizygous for the transgene, NHE2S failed to result in tumour development in nude mice. The other line, NHE1S, consistently led to tumour formation; however, the latency was substantially increased by comparison with the homozygous cell lines (Fig. 6).
Fig. 6.
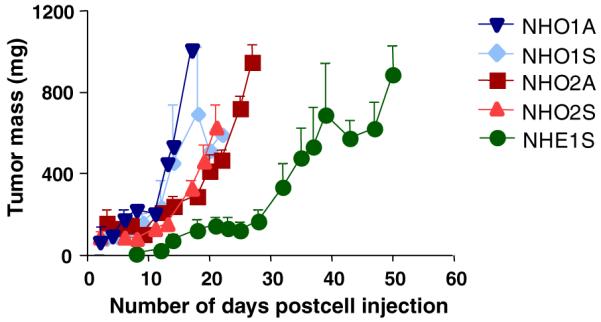
Engraftment of murine neuroblastoma cell lines into nude mice. Single cell suspensions from NHO1A (▼), NHO1S (◆), NHO2A (■), NHO2S (▲) and NHE1S (●) at 5 × 106 cells per graft were inoculated into both flanks of nude mice at day 0. The tumour development was monitored for up to 50 days by measuring with vernier calipers, and tumour mass (mg) was calculated using the formula shown in Section 2. Data shown here are means ± SE of three grafts on two separate mice.
4. Discussion
The targeting of the human MYCN gene to mouse neural crest cells has provided direct evidence of the critical contribution of this oncogene to the malignant phenotype of neuroblastoma. Neuroblastoma tumourigenesis in these mice correlates with transgene dosage, and similarly to human neuroblastoma, we have also demonstrated amplification of the MYCN transgene.6,16,17 The development and characterisation of unique isogenic cell lines from tumours arising in these transgenic mice further extends the utility of this model. Importantly, these cell lines displayed molecular and biological features characteristic of clinical neuroblastoma and in conjunction with the transgenic mice, the cell lines should prove valuable for basic and pre-clinical studies of neuroblastoma biology and treatment.
In addition to MYCN amplification, a number of genetic alterations have been implicated in the pathogenesis of neuroblastoma. In particular, gain of human chromosome 17q is frequently observed in human neuroblastomas,22 and available evidence suggests that it is associated with aggressive, advanced stage disease and poor clinical outcome.23 We have previously demonstrated that gain of chromosome 11, corresponding to 17q gain in humans, occurs in up to 30% of MYCN transgenic murine tumours and is one of the most common genetic changes observed in these tumours.4-6 Furthermore, this region of gain is conserved in neuroblastomas occurring in man, mouse and rats.20 The finding of gain on distal chromosome 11 in two of the six murine neuroblastoma cell lines reduces the smallest region of overlap of chromosome 11 gain to 15Mb.
Karyotype and array CGH analyses of the murine cell lines identified a range of additional cytogenetic abnormalities, with many consistent with those described by us and others in both primary TH-MYCN tumours and human neuroblastoma. For example, mouse chromosome 6 gained in NHO2S has significant homology with human chromosomes 7 and 12, which are gained in 50% and 15% of human cases, respectively. In addition, of the six chromosomal alterations observed in the NHE1S cell line, five were identical to the genetic changes most frequently observed in a previous study of primary TH-MYCN tumours.6 These changes, including the gain of parts or all of the chromosomes 1, 3, 8 and 18, in addition to 11q gain, correspond to frequently gained chromosomes 1q and 18q in human neuroblastoma. The two hemizygous-derived cell lines, NHE1S and NHE2S, demonstrated increased copies of the MYCN transgene, consistent with our previous findings in the tumours of MYCN transgenic mice.6,16,17 Increase in copy number of the MYCN transgene in cell lines and tumours hemizygous for the transgene appears to be a reliable phenomenon, since we have recently derived two further cell lines from tumours of mice hemizygous for the MYCN transgene, both of which demonstrate increased MYCN copy number. Thus, the results of our chromosomal analyses indicate that the murine tumour-derived cell lines share molecular profiles in common with those of childhood neuroblastoma, and suggest that these lines represent clinically relevant models of this disease.
Gene expression analysis in our panel of murine cell lines showed MYCN expression correlated with specific cell phenotypes. Murine suspension cell lines that were phenotypically similar to N-type human neuroblastoma cells expressed high levels of MYCN, while the substrate-adherent cell lines had markedly reduced expression. High expression of S100A6in the adherent cell lines, together with their non-neuronal morphology, suggested that these lines represent the murine equivalent of S-type human neuroblastoma cells. Previous studies in human neuroblastoma cell lines have shown that MYCN expression, regulated by differentiation state, directly modulates the malignant potential of neuroblastoma cells.24 Thus, N-type human neuroblastoma cells preferentially expressing MYCN are tumourigenic when xenografted into nude mice, while S-type cells are non-tumourigenic. The ability of the S-type adherent murine cell lines, derived from tumours homozygous for the MYCN transgene, to form tumours more aggressively than the N-type murine cell lines, derived from tumours hemizygous for the MYCN transgene, appears counterintuitive. This is particularly the case since the MYCN transgene selectively undergoes amplification in both tumours 17 and tumour cell lines derived from hemizygous mice, and hence, it might have been anticipated that these cells would be associated with increased tumourigenicity and malignant potential. The present findings, however, demonstrating reduced tumourigenicity of the cell lines derived from mice hemizygous for the MYCN transgene, are in fact entirely consistent with our previous observations of spontaneous tumour formation in MYCN transgenic mice, where MYCN hemizygous mice were found to have both decreased tumour incidence and increased tumour latency by comparison with homozygous mice.2-4 The results suggest the existence of intrinsic biological differences between tumour cells arising from mice either homozygous or hemizygous for the transgene and warrant further investigation.
In conclusion, we have demonstrated in this study that many of the molecular and biological features of our murine TH-MYCN tumour-derived cell lines are similar to those found in TH-MYCN primary tumours and clinical neuroblastomas. The conservation of characteristic neuroblastoma-specific cytogenetic alterations and biological features between human neuroblastoma and our murine cell lines suggests that core pathways contribute to the malignant phenotype. Our murine cell lines thus represent a reliable model for studying neuroblastoma pathogenesis, and should provide a valuable tool for the preclinical testing of novel therapies for this disease.
Acknowledgements
Supported by research grants from the National Health and Medical Research Council, Australia (G.M.M., M.D.N., M.H.), the Cancer Council New South Wales, Australia (G.M.M., M.D.N., M.H.), by Newcastle upon Tyne Hospitals NHS Charity and the Neuroblastoma Society, UK (M.L., M.S.J.), and by NIH Grants K02NS02226-05 and RO1CAA102321 (W.A.W.).
Footnotes
Conflict of interest statement
None of the authors have any financial or personal relationships with other people or organisations that could inappropriately influence this work.
REFERENCES
- 1.Brodeur GM, Seeger RC, Schwab M, Varmus HE, Bishop JM. Amplification of N-myc in untreated human neuroblstomas correlates with advanced disease stage. Science. 1984;224:1121–4. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- 2.Seeger RC, Brodeur GM, Sather H, et al. Association of multiple copies of the N-myc oncogene with rapid disease progression of neuroblastomas. N Engl J Med. 1985;313:1111–6. doi: 10.1056/NEJM198510313131802. [DOI] [PubMed] [Google Scholar]
- 3.Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–16. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 4.Weiss W, Aldape K, Mohapatra G, Feuerstein B, Bishop J. Targeted expression of MYCN causes neuroblastoma in transgenic mice. EMBO J. 1997;16:2985–95. doi: 10.1093/emboj/16.11.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiss WA, Godfrey T, Francisco C, Bishop JM. Genome-wide screen for allelic imbalance in a mouse model for neuroblastoma. Cancer Res. 2000;60:2483–7. [PubMed] [Google Scholar]
- 6.Hackett CS, Hodgson JG, Law ME, et al. Genome-wide array CGH analysis of murine neuroblastoma reveals distinct genomic aberrations which parallel those in human tumors. Cancer Res. 2003;63:5266–73. [PubMed] [Google Scholar]
- 7.Ben-Yosef T, Yanuka O, Halle D, Benvenisty N. Involvement of Myc targets in c-myc and N-myc induced human tumors. Oncogene. 1998;17:165–71. doi: 10.1038/sj.onc.1201939. [DOI] [PubMed] [Google Scholar]
- 8.Lutz W. Conditional expression of N-myc in human neuroblastoma cells increases expression of alpha-prothymosin and ODC and accelerates progression into S-phase. Oncogene. 1996;13:803–12. [PubMed] [Google Scholar]
- 9.Manohar CF, Bray JA, Salwen HR, et al. MYCN-mediated regulation of the MRP1 promoter in human neuroblastoma. Oncogene. 2004;23:753–62. doi: 10.1038/sj.onc.1207151. [DOI] [PubMed] [Google Scholar]
- 10.Haber M, Bordow S, Gilbert J, et al. Altered expression of the MYCN oncogene modulates MRP gene expression and response to cytotoxic drugs in neuroblastoma cells. Oncogene. 1999;18:2777–82. doi: 10.1038/sj.onc.1202859. [DOI] [PubMed] [Google Scholar]
- 11.Auvinen M, Passinen A, Andersson LC, Holtta E. Ornithine decarboxylase activity is critical for cell transformation. Nature. 1992;360:355–8. doi: 10.1038/360355a0. [DOI] [PubMed] [Google Scholar]
- 12.Mohan RR, Challa A, Gupta S, et al. Overexpression of ornithine decarboxylase in prostate cancer and prostate fluid in humans. Clin Cancer Res. 1999;5:143–7. [PubMed] [Google Scholar]
- 13.Kavallaris M. The role of multidrug resistance-associated protein (MRP) expression in multidrug resistance. Anti-Cancer Drugs. 1997;8:17–25. doi: 10.1097/00001813-199701000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Bordow S, Haber M, Madafiglio J, Chueng B, Marshall GM, Norris MD. Expression of the multidrug resistance-associated protein (MRP) gene correlates with amplification and overexpression of the N-myc oncogene in childhood neuroblastoma. Cancer Res. 1994;54:5036–40. [PubMed] [Google Scholar]
- 15.Norris M, Bordow S, Marshall G, Haber P, Cohn S, Haber M. Expression of the gene for multidrug-resistance-associated protein and outcome in patients with neuroblastoma. N Engl J Med. 1996;334:231–8. doi: 10.1056/NEJM199601253340405. [DOI] [PubMed] [Google Scholar]
- 16.Norris MD, Burkhart CA, Marshall GM, Weiss WA, Haber M. Expression of N-myc and MRP genes and their relationship to N-myc gene dosage and tumor formation in a murine neuroblastoma model. Med Ped Oncol. 2000;35:585–9. doi: 10.1002/1096-911x(20001201)35:6<585::aid-mpo20>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 17.Burkhart CA, Cheng AJ, Madafiglio J, et al. Effects of MYCN antisense oligonucleotide administration on tumorigenesis in a murine model of neuroblastoma. J Natl Cancer Inst. 2003;95:1394–403. doi: 10.1093/jnci/djg045. [DOI] [PubMed] [Google Scholar]
- 18.Ross RA, Biedler JL, Spengler BA. A role for distinct cell types in determining malignancy in human neuroblastoma cell lines and tumors. Cancer Lett. 2003;197:35–9. doi: 10.1016/s0304-3835(03)00079-x. [DOI] [PubMed] [Google Scholar]
- 19.Ciccarone V, Spengler BA, Meyers MB, Biedler JL, Ross RA. Phenotypic diversification in human neuroblastoma cells: expression of distinct neural crest lineages. Cancer Res. 1989;49:219–25. [PubMed] [Google Scholar]
- 20.Lastowska M, Chung YJ, Cheng Ching N, et al. Regions syntenic to human 17q are gained in mouse and rat neuroblastoma. Genes Chrom Cancer. 2004;40:158–63. doi: 10.1002/gcc.20031. [DOI] [PubMed] [Google Scholar]
- 21.Geran RI, Greenberg NH, Macdonald MM, Schumacher AM, Abbott BJ. Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemother Rep. 1972;3:47–51. [Google Scholar]
- 22.Lastowska M, Cotterill S, Pearson AD, et al. Gain of chromosome arm 17q predicts unfavourable outcome in neuroblastoma patients. U.K. Children’s Cancer Study Group and the U.K. Cancer Cytogenetics Group. Eur J Cancer. 1997;33:1627–33. doi: 10.1016/s0959-8049(97)00282-7. [DOI] [PubMed] [Google Scholar]
- 23.Bown N, Cotterill S, Lastowska M, et al. Gain of chromosome arm 17q and adverse outcome in patients with neuroblastoma. N Engl J Med. 1999;340:1954–61. doi: 10.1056/NEJM199906243402504. [DOI] [PubMed] [Google Scholar]
- 24.Spengler BA, Lazarova DL, Ross RA, Biedler JL. Cell lineage and differentiation state are primary determinants of MYCN gene expression and malignant potential in human neuroblastoma cells. Oncol Res. 1997;9:467–76. [PubMed] [Google Scholar]