Abstract
A combined UPLC-tandem mass spectrometric (UPLC-MS/MS) technique has been validated for quantitation of protein free efavirenz (EFV) as well as total concentrations of EFV in human blood and seminal plasma. The analytical method possesses capabilities for concentration measurements of EFV ranging from 0.5 ng/ml to 10,000 ng/ml with an accuracy (%dev) of −5.2% to 8.0% and precision (%CV) of <8%. Standard curves were linear with coefficients of variation (r2) > 0.98. The method employs a racemic fluorinated analog of EFV (F-EFV) as the internal standard. EFV and F-EFV were eluted from a reverse-phase UPLC column via gradient elution with detection via negative ion multiple reaction monitoring (MRM). EFV and F-EFV, respectively, were detected via the following MRM transitions: m/z 314.0 > 244.1 and m/z 298.0 > 227.9. The time required for the analysis of each sample was 8.0 minutes. The analytical technique is capable of a reliable detection limit of ~15–20 femtomoles of EFV injected on column.
Keywords: Seminal Plasma, Efavirenz, UPLC-MS/MS, Blood Plasma, Electrospray Ionization (ESI), HIV/AIDS
1. Introduction
The leading cause of HIV infection is sexual transmission through HIV-laden semen [1]. Antiretroviral (ARV) therapy has shown to effectively reduce viral replication in patients and is the main course of treatment. Many ARV drugs, however, inadequately penetrate into the male genital tract (MGT). Limited drug access to the MGT would permit viral replication and could potentially engender resistance, creating a “pharmacological sanctuary.” The mechanism of ARV drug penetration and distribution into the MGT is largely unknown [2]. Many ARV's have large blood plasma: seminal plasma ratios of total drug concentrations [3]. Protein binding has been suggested as an explanation for these large observed ratios. The extent of protein binding of an anti-retroviral drug in the blood correlates well with the observed blood: semen drug concentration ratios and may be the dominant factor influencing these ratios. It is generally assumed that only the unbound “free” drug is able to cross into the male genital tract is active against viral replication [2]. EFV is one of the most highly protein bound antiretroviral drugs existing at >99% protein bound, predominantly to albumin [4]. EFV's very high protein binding in blood makes it a unique and optimum candidate to study the distribution and penetration into the MGT. The availability of a method for reliable detection and quantitative measurement of protein-free EFV in human seminal plasma would enable investigation of the male genital tract as a “pharmacological sanctuary” for efavirenz.
The concentration of total EFV in blood plasma is approximately 20-fold higher than that observed in human seminal plasma [5,6,7,8]. There are several published LC-MS/MS methods for reliable quantitation of EFV in human blood plasma and pharmacokinetic studies of therapeutic drug monitoring [9,10,11,12]. These published methods possess adequate sensitivity for determination of concentrations of total EFV and protein free concentrations of EFV in human blood plasma, but none have examined the sensitivity necessary for quantitation of protein free EFV in seminal plasma due to the low volume limitations. In a pre-existing clinical study, blood and semen samples were obtained at simultaneous time points, from patients receiving an oral regimen of efavirenz at time intervals shown to produce steady state conditions [6]. The median interquartile range (IQR) total EFV concentration in the blood plasma was 1940 ng/ml (1593 – 4555). The median (IQR) total EFV concentration in the seminal plasma was 136 ng/ml (66 – 255). We hypothesize, that the protein-free concentrations in seminal plasma will be similar to that of blood plasma given the low binding protein concentrations, unless membrane transporters between the blood and seminal plasma are a factor. The protein-free concentrations of EFV in seminal plasma may be accurately measured via published methods if seminal plasma were obtainable from individual subjects in volumes equivalent to that for blood plasma. Human seminal plasma, however, is available in limited quantities, and protein separation procedures add an additional limitation to the volume of the matrix available for LC-MS/MS analysis. Accordingly, an analytical method with significantly lower detection limits than those stated in published methods for blood plasma is required.
Simultaneous with the assay validation of the method for quantitation of protein-free EFV in human seminal plasma, an electrospray ionization (ESI) UPLC-MS/MS technique for quantitation of total and protein-free concentrations of EFV in ultra-filtrates of blood plasma was also validated. Previous methods have been established for the separation of protein-free drug in blood plasma. The limitations of existing assays to examine protein-free efavirenz in seminal plasma are due to the limited volume availability of sample, and inadequate detection limits and sensitivity to reach the concentrations of protein-free efavirenz. This method will ultimately provide the analytical basis for comparison of the protein-free concentrations of efavirenz in both blood and seminal plasma, as well as the total concentrations in each matrix to determine the percent of binding and efavirenz distribution into the male genital tract. The remainder of this report details, demonstrates and validates a negative ion ESI-UPLC-MS/MS technique capable of reliable detection limit of ~15–20 femtomoles of EFV extracted from human seminal plasma, the range at which protein-free EFV is projected to be present in human seminal plasma.
2. Experimental
2.1 Standards, Reagents and Experimental Matrices
Efavirenz (EFV) was provided by DuPont Pharmaceuticals (Hertfordshire, UK). A racemic 6-fluorinated analog of EFV (F-EFV) for use as an internal standard was synthesized by Dr. David Meyers (Johns Hopkins University School of Medicine Synthetic Core Facility located in the Department of Pharmacology and Molecular Sciences). The compound was synthesized via modifications of procedures published in previous reports [13,14]. The chemical structures of EFV and F-EFV are depicted in Figure 1 below.
Figure 1.
Chemical structures and chemical names of EFV and the 6-fluoro-analog of EFV employed as the internal standard for UPLC-MS/MS analysis of EFV.
The chemical nomenclature for EFV is presented immediately below the structural representation of EFV. The position at which a fluorine (F) atom was substituted for a chlorine (Cl) atom during custom synthesis is noted at C6. F-EFV possesses similar physical and chemical properties to EFV and other analogs of EFV that were synthesized for evaluation of anti-retroviral and anti-tubercular properties [15,16,17].
Multiple (five) lots of blank human plasma used for calibrator, quality control preparation, and matrix effect assessments were purchased from Biological Specialty Corporation (Colmar, PA, USA). Multiple (five) lots of blank seminal plasma used for calibrator, quality control preparation, and matrix effects assessment were obtained from Bioreclamation Inc. (Westbury, NY, USA).
HPLC grade water, methanol, and hexanes were purchased from J.T. Baker (Phillipsburg, NJ, USA). HPLC grade ethyl acetate was purchased from Fischer Scientific (Pittsburgh, PA, USA). ACS grade 97% ammonium formate and ACS grade formic acid >96% were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2 Calibrators, Quality Control Standards, Standard Curves, Sample Preparation
Stock solutions of EFV analyte and F-EFV internal standard were prepared at 2.0 μg/μl in methanol. Ten-fold dilutions of each stock were prepared as working solutions from the stock solutions ranging from 200 nanograms/μl (ng/μl) to 0.2 ng/μl.
Calibrators and quality controls ranging from 0.5 ng/ml to 10,000 ng/ml were prepared from working solutions in both blood and seminal plasma. Stock analyte and internal standard solutions were prepared in methanol. All working solutions were prepared so that the added methanol was less than 1% of solution to minimize matrix alteration. These working solutions were prepared in blood and seminal plasma to dilute and prepare calibrator and quality control samples. The calibrators and quality controls were stored at −80° C.
The anticipated variations in EFV concentrations relate to the extent of protein-binding in the different matrices. The limited penetration of EFV into the male genital tract necessitated the use of multiple standard curves to be constructed for validation of UPLC-MS/MS technique. The concentration range of the low range standard curve was 0.5 −500 ng/ml with quality controls (QC's) at low (1.5 ng/ml), medium (45 ng/ml), and high concentration (450 ng/ml). This range was prepared in both blood and seminal plasma. The “seminal low curve” was validated for UPLC-MS/MS measurement of protein-free EFV concentrations in ultrafiltrates of seminal plasma (seminal ultrafiltrates) as well as protein-bound plus protein-free EFV in seminal plasma (seminal total). The total EFV concentrations are significantly less in seminal plasma than blood plasma and are therefore quantifiable on the low range curve. Blood plasma ultrafiltrates were quantified on a blood plasma low range curve. This standard curve was designated respectively as the “blood low curve”. Ultrafiltrates (protein-free EFV samples) are the result of an ultrafiltration of the matrix to separate protein-bound from protein-free EFV. The ultrafiltrates that resulted from blood or seminal plasma sample matrices, were run on the respective blood or seminal plasma low end curve. QC's prepared in ultrafiltered matrix did not show deviation in accuracy or precision to those of the matrix within the standard curve. Matrix effect assessment (further described in 3.3.2) also did not result in any matrix suppression or enhancement by subjecting the matrix to ultrafiltration. The high range standard curve determined total concentrations (protein-free and protein-bound) EFV in human blood plasma. The concentration range for this calibration curve was 100–10,000 ng/ml with QCs at low (150 ng/ml), medium (3,000 ng/ml), and high concentration (9,000 ng/ml). The respective plasma “low curves” and the “high curves” overlap in EFV concentrations from 100 ng/ml to 500 ng/ml.
Construction of these calibration curves required different aliquots of the matrices and the quantity of the internal standard employed for assay validation. A 50 μl aliquot of seminal plasma and blood plasma was used to prepare the “seminal low curve” and the “blood low curve”, and 10.0 ng (50 μl of a 0.2 ng/ml) of F-EFV, the internal standard. For preparation of the “blood high curve”, a 25 μl aliquot of the matrix was employed followed by the addition of 100.0 ng of F-EFV (50 μl of a solution of 2.0 ng/μl of internal standard) added to the biological matrix. The prepared samples were then subjected to liquid: liquid extraction of EFV and F-EFV from the biological matrix.
Clinical samples used for analysis of protein-free efavirenz were obtained using an ultrafiltration method [7]. The patient samples were the result of an archived clinical study [6], in which patients received an oral regimen of efavirenz known to achieve steady state conditions. Blood and semen samples were obtained at simultaneous time intervals. Protein-free efavirenz was separated from protein-bound efavirenz using an ultrafiltration method where the amount of free drug in a sample is determined by a linear regression model based on time [7]. The ultrafiltrates are the result of the matrix ultrafiltration, and contain the protein-free efavirenz of the sample.
The analyte and internal standard were resolved from the matrices using a liquid: liquid extraction method. The extraction solvents included a 1:1 mixture of hexane: ethyl acetate and a 50 mM buffer solution of ammonium formate in HPLC grade water. Solvents were freshly prepared every 48–72 hours. Following the addition of the internal standard to the seminal plasma and blood plasma matrices contained in a clean tube, the contents were vigorously mixed via a vortex mixer for 8 seconds. A volume of 600 μl of 50 mM ammonium formate and 900 μl of hexane:ethyl acetate (1:1 mixture) was added followed by vortex mixing for at least 10 seconds. The contents of the glass tube were then centrifuged at 1100×g for ten minutes to separate the aqueous and organic layers. The organic (top) layer containing the analyte and internal standard was carefully removed and transferred to a clean tube and evaporated to dryness using a low boiling point ThermoSavant Speedvac (GMI, Inc., Ramsey, MI, USA).
Dried residues of seminal and blood plasma samples/extracts were reconstituted in 0.5 ml of methanol and injected at a volume of 10 μl for LC-MS/MS analysis for the “seminal low curve” and the “blood low curve”. The dried residues of extracts from blood plasma were reconstituted in 2.0 ml of methanol with a sample injection volume of 2.5 μl for the “blood high curve”.
2.3 Instrumentation and UPLC-MS/MS Analysis
The instrumentation employed for UPLC-MS/MS analysis was an AB-Sciex API5000 triple quadrupole mass spectrometer (Foster City, CA) interfaced with a Waters Acquity UPLC (Milford, MA, USA). The components of the UPLC system were a Binary Solvent Manager and a Sample Manager. The dual ion source was operated in negative ion ESI mode following evaluation of analyte sensitivity and noise backgrounds observed in both positive and negative ion modes. Resolution of EFV and F-EFV employed as the internal standard was achieved via gradient elution reversed phase liquid chromatography employing a 2.1 × 50 mm Acquity UPLC BEH C18 column. The MS parameters and solvent gradient employed for the resolution of EFV and F-EFV are detailed in Table 1. The particle size of the stationary phase was 1.7 μm. The solvent gradient was from a mobile phase of 0.1% formic acid in water (mobile phase A [MPA]) to 0.1% formic acid in methanol (mobile phase B [MPB]). MPA was freshly prepared every 48–72 hours. The solvent flow rate was 0.5 ml/minute. The timed gradient elution was employed for two reasons. They were (1) to assure that lipids that co-extracted with the analyte and internal standard would be more likely to be eluted from the UPLC column during each sample analysis to minimize potential for interference for detection of EFV and F-EFV and (2) the solvent gradient provided a reliable low noise background for elution of the most narrow peaks of favorable signal to noise (S/N) ratio's for facile identification of EFV and F-EFV via multiple reaction monitoring (MRM). A valve system was employed for diverting the column eluate to waste from 0.0 to 2.6 minutes. From 2.6 to 5.0 minutes following sample injection the column eluate was then directed to the ion source of the mass spectrometer. At 5.0 minutes, the column eluate was again diverted to waste. The solvent pressure at a flow rate of 0.5 ml/minutes ranged from ~6,500 to ~10,500 pounds per square inch (psi). The UPLC system was leak checked up to ~14,000 psi to assure that solvent leaks would not occur during UPLC-MS/MS analysis.
Table 1.
| MS Parameters | Efavirenz (EFV) | F-Efavirenz (F-EFV) IS |
|---|---|---|
| MRM Transition | 314.0 > 244.1 | 298.0 > 227.9 |
| Retention Time (RT) | 3.11 min | 3.01 min |
| Declustering Potential (DP) | −95 V | −95 V |
| Collision Energy (CE) | −24 V | −22 V |
| Collision Exit Potential (CXP) | −15 V | −17 V |
| MS Parameters | |
|---|---|
| CAD gas | 5 |
| curtain gas | 25 |
| nebulizer gas | 40 |
| source gas | 40 |
| ion spray voltage | −4500 V |
| ion source temperature | 600°C |
| scan dwell time | 100 ms |
| Solvent Gradient | |
|---|---|
| Mobile Phase A (MPA) | 0.1 % Formic Acid in H20 |
| Mobile Phase B (MPB) | 0.1 % Formic Acid in Methanol |
| 0 – 3.0 minutes | 100% MPA |
| 3.0 – 3.1 minutes | 0% MPA |
| 3.0 – 5.5 minutes | 100% MPB |
| 5.5 – 5.6 minutes | 0% MPB |
| 5.6 – 8.0 minutes | 100% MPA |
2.4 Data Processing and Quantitation
A 1/x2 weighting was used to generate the calibration curves as area ratios of analyte to internal standard (IS). This weighting was chosen because it most accurately represents the lower end of the curve where most clinical sample values in seminal plasma at ultrafiltrates of seminal plasma were expected to be observed. The calibration range for the standard curve was linear over the clinically relevant ranges. The software used for data acquisition and processing was Analyst 1.4.2. The software performed integration of peak areas for each analyte and internal standard peak that presented MRM signal distinguishable and is no less than one-third the signal of that of the LLOQ calibrator.
3. RESULTS and DISCUSSION
3.1 Noise Background, Chromatography and Sensitivity of ESI-UPLC-MS/MS Analysis of EFV
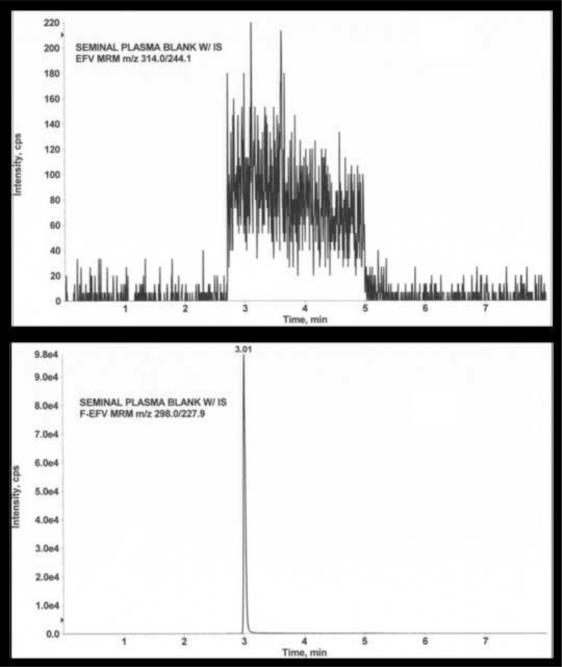
There is an approximate 20-fold difference in the total concentration of EFV in blood plasma versus that for seminal plasma [5,6,7,8]. The protein binding of EFV in the blood plasma, principally to albumin, has been reported to be >99% [2]. Because of the differences in binding protein concentrations that exist in the blood and seminal plasma, we hypothesized the protein binding of EFV to be less in the seminal plasma than the blood plasma. However, due to the low volume limitations of sample size following methods of protein-free EFV separation, it is expected the limit of detection needs to be significantly lower for seminal plasma EFV concentrations. The objective is to determine measureable concentrations of protein-free EFV in seminal and blood plasma to be able to evaluate the male genital tract as a potential pharmacological sanctuary. The assay methods presented in this report are an investigation of the combined sensitivity and selectivity of UPLC-MS/MS analysis for the detection and quantitation of protein-free EFV in ultrafiltrates of seminal plasma. For these studies, seminal plasma was employed as the matrix for such an investigation. Significant features of the analytical technique and method development are described below. Initially, the noise background of the characteristic MRM transition for EFV (m/z 314.0 > 244.1) was evaluated within the matrix of interest. Simultaneous with this evaluation is analysis of the background signal at the characteristic MRM, m/z 298.0 > 227.9 for F-EFV, the internal standard for UPLC-MS/MS analysis. Findings of these studies are presented in Figure 2.
Figure 2.
MRM signals following injection of an extract of a seminal plasma blank. Ordinate = signal intensity expressed as counts per scan (cps)/ Abscissa = time (in minutes). Upper panel, MRM = m/z 314.0 > 244.1; Lower panel, MRM = m/z 298.0 > 227.9. Refer to text for additional details.
The upper portion of figure 2 represents the signal for the MRM m/z 314.0 > 244.1, characteristic for the detection of EFV. The lower portion of the figure depicts signal for the characteristic MRM for F-EFV, m/z 298.0 > 227.9. The increase in signal at 2.6 minutes in both panels of the figure coincides in time with diversion of the column eluate from waste to the ion source of the mass spectrometer. The decrease in signal at 5.0 minutes coincides with diversion of the column eluate to waste. The range of the noise level in the upper portion of the figure is ~80 – 160 cps with a single scan “noise spike” at 3.11 minutes of ~240 cps. The noise level in the lower portion of figure 2 is the characteristic MRM for detection of F-EFV, the internal standard. The range of noise level during diversion of the column eluate to the ion source is 50 – 240 cps. The data contained in the figure represents the findings of the 5 lots of blank seminal plasma. The UPLC-MS/MS data depicted in the upper panel of the figure strongly indicate that low femtomolar concentrations of EFV may be reliably detected and accurately measured.
The UPLC-MS/MS data presented in Figure 3 was that obtained from the extraction of seminal plasma to which 10 ng of F-EFV internal standard (IS) had been added prior to extraction. The data shown in Figure 3 indicates elution of F-EFV at a retention time of 3.01 minutes (lower portion of the figure), MRM m/z 298.0 > 227.9. There is no evident signal within the IS for MRM signal m/z 314.0 > 244.1, the characteristic MRM transition for EFV. The anticipated retention of EFV, if present, is 3.11 minutes. Both compounds elute as symmetrical Gaussian-like peaks with no evidence of peak tailing. These data indicate that F-EFV employed as the internal standard contain no contaminants that generate signal that could interfere with low level measurements of EFV in extracts of seminal plasma ultrafiltrates, assuming low femtomole concentrations (<20 femtomoles). Figure 3 demonstrates that the internal standard does not present any interference to the analyte resolution. The data obtained for the noise background in the seminal plasma samples do not present significantly different from the blood plasma samples. The chromatography of EFV and F-EFV suggest that both compounds display almost ideal chromatographic properties under the conditions employed. F-EFV elutes approximately 0.1 minutes before EFV. The width of each of the peaks representing the analyte and internal standard is approximately six seconds from the leading edge to the trailing edge of each of the two peaks. At a scan dwell time of 0.1 seconds, there are more than 20 points for each signal MRM for which signal was detected after elution from the reversed phase column.
Figure 3.
MRM signals at m/z 314.0 > 244.1 (upper panel) and m/z 298.0 > 227.9 (lower panel) of extracted seminal plasma to which 10 ng of F-EFV (internal standard) was added. Ordinate = MRM signal (cps). Abscissa = time (in minutes). Refer to text for additional details.
The UPLC-MS/MS data presented in Figure 4 is a comparison of the characteristic MRM signal (m/z 314.0 > 244.1) generated from EFV LLOQ and a protein-free extract in patient seminal plasma. The concentration of EFV in the LLOQ sample is 0.5 ng/ml (or 500 picograms/1000 μl). Assuming quantitative recovery, the injection of 10 μl of this solution would result in placing 5 picograms (16 femtomoles) of EFV on column. The expected retention time of EFV is 3.11 minutes. The UPLC-MS/MS data depicted in the upper panel represents the signal obtained from the LLOQ. The peak to peak S/N ratio is 4.9. The peak to peak technique employs one or two samplings of the noise background (cps) with comparison of the peak height (cps) of the peak representing the analyte of interest. The lower portion of the figure is a representation of signal at MRM m/z 314.0 >244.1 generated from protein-free EFV extracted from patient seminal plasma via ultrafiltration. The peak to peak S/N ratio is 12.2, or approximately 2.5 times that of the signal generated from the LLOQ sample. Again, assuming quantitative extraction of EFV from the matrix, the MRM signal indicates that of ~40 femtomoles of EFV injected on column. The UPLC-MS/MS data illustrated in Figure 4 strongly indicates that the analytical technique can possesses the desired sensitivity and selectivity required for determination of concentrations of protein-free EFV in both blood and seminal plasma.
Figure 4.
MRM signal at m/z 314.0 > 244.1, the characteristic signal monitored for detection of EFV. Ordinate = signal intensity (cps). Abscissa = time (in minutes) Upper panel = extract of the lower limit of quantitation (LLOQ) calibrator. Lower panel = Protein-free EFV from volunteer subject receiving an efavirenz regimen of treatment.
3.2 Analytical Method Validation
The method format for this assay followed a partial validation, based upon recommendations of the Bioanalytical Method Validation, Guidance for Industry, and the ACTG FDA guidelines [18]. The assay was designed as a partial validation due to the matrix limitations of seminal plasma. This bioanalytical assay is designed as an extended range of the standard curve to cover the potential ranges of detection needed for both total and protein-free concentrations of patient samples.
3.2.1 Accuracy, Precision and Linearity
Each standard curve was validated a minimum of three times. Quality control samples for intra-assay accuracy and precision were prepared in replicates (n=6) for each curve at three concentrations (low, medium, high). Inter-assay accuracy and precision was determined by analysis of three individual standard curves run at three separate occasions. The low range curve employs nine calibrators within the range of concentrations specified, and the high range curve employs eight calibrators. Precision was measured as coefficient of variance (%CV), accuracy was measured as percent deviation (%Dev).
The precision of the assay method (%CV) was <8% over a range from 0.5 ng/ml – 10,000 ng/ml and the accuracy of the assay method (%Dev) was −5.2% to 8.0% for inter and intra-assay variation. Accuracy and precision of the three standard curves (seminal low, blood low, and blood high) used to measure the clinical samples are detailed in Tables 2, 3, and 4. Table 2 includes the theoretical concentrations and calculated concentrations for the seminal low curve employed for UPLC-MS/MS quantitation of EFV in seminal plasma ultrafiltrates and total seminal concentration (protein free + bound) EFV in seminal plasma. The tables also state the parameters (slope, intercept and r2 value) for each standard curve as well as the mean, SD, %CV and %Dev obtained during assay validation. Table 3 provides the summary of values for the blood low curve calibration used for measurements of protein-free EFV measurement in ultrafiltrates of blood plasma. Table 4 represents a summary of the data parameters in the blood high curve used for determination of the total (free + bound) EFV in blood plasma.
Table 2.
| Seminal Low Curve = Seminal Ultrafiltrates, Seminal Total Concentrations | |||||||
|---|---|---|---|---|---|---|---|
| Run ID | LLOQ | Low QC | Med QC | High QC | Slope | Intercept | r2 |
| 1 | 0.51 | 1.57 | 47.3 | 465 | 0.00499 | 0.00244 | 0.9989 |
| 0.5 | 1.58 | 50.3 | 476 | ||||
| 0.52 | 1.59 | 51.2 | 485 | ||||
| 2 | 0.49 | 1.55 | 46.2 | 429 | 0.00519 | 0.00197 | 0.9993 |
| 0.49 | 1.55 | 46.4 | 449 | ||||
| 0.5 | 1.56 | 47.1 | 464 | ||||
| 3 | 0.5 | 1.56 | 48.9 | 497 | 0.00389 | 0.00161 | 0.9984 |
| 0.51 | 1.56 | 49.3 | 510 | ||||
| 0.51 | 1.62 | 50.6 | 512 | ||||
| Theoretical Conc. (ng/mL) | 0.5 | 1.5 | 45 | 450 | --- | --- | --- |
| Mean | 0.5 | 1.6 | 48.6 | 476.3 | 0.00469 | 0.00201 | 0.9989 |
| SD | 0.01 | 0.02 | 1.9 | 27.78 | --- | --- | 0 |
| % CV | 1.71 | 1.44 | 3.9 | 5.83 | --- | --- | 0.05 |
| % Dev | 0.53 | 4.74 | 7.98 | 5.85 | --- | --- | --- |
| n | 9 | 9 | 9 | 9 | --- | --- | 3 |
Table 3.
| Blood Low Curve = Blood Ultrafiltrates | |||||||
|---|---|---|---|---|---|---|---|
| Run ID | LLOQ | Low QC | Med QC | High QC | Slope | Intercept | r2 |
| 1 | 0.5 | 1.48 | 45.4 | 481 | 0.00479 | 0.00189 | 0.9974 |
| 0.5 | 1.54 | 47 | 486 | ||||
| 0.51 | 1.54 | 47.8 | 502 | ||||
| 2 | 0.48 | 1.53 | 46.7 | 436 | 0.00563 | 0.00258 | 0.9978 |
| 0.51 | 1.54 | 48.6 | 450 | ||||
| 0.52 | 1.55 | 50.4 | 456 | ||||
| 3 | 0.49 | 1.48 | 45.9 | 452 | 0.00599 | 0.00224 | 0.9973 |
| 0.5 | 1.5 | 46.1 | 455 | ||||
| 0.5 | 1.54 | 47.5 | 457 | ||||
| Theoretical Conc. (ng/mL) | 0.5 | 1.5 | 45 | 450 | --- | --- | --- |
| Mean | 0.5 | 1.5 | 47.3 | 463.9 | 0.00547 | 0.00224 | 0.9975 |
| SD | 0.01 | 0.03 | 1.54 | 21.02 | --- | --- | 0 |
| % CV | 2.12 | 1.82 | 3.26 | 4.53 | --- | --- | 0.03 |
| % Dev | 0.33 | 1.48 | 5.04 | 3.09 | --- | --- | --- |
| n | 9 | 9 | 9 | 9 | --- | --- | 3 |
Table 4.
| Blood High Curve = Blood Total Concentrations | |||||||
|---|---|---|---|---|---|---|---|
| Run ID | LLOQ | Low QC | Med QC | High QC | Slope | Intercept | r2 |
| 1 | 101 | 150 | 2980 | 8710 | 0.000255 | 0.00335 | 0.9951 |
| 104 | 151 | 3000 | 9100 | ||||
| 106 | 155 | 3020 | 9800 | ||||
| 2 | 97.5 | 140 | 2760 | 8060 | 0.000273 | 0.00425 | 0.9988 |
| 97.7 | 151 | 2770 | 8070 | ||||
| 99.7 | 153 | 2900 | 8350 | ||||
| 3 | 105 | 155 | 2920 | 8040 | 0.00247 | 0.00098 | 0.9988 |
| 106 | 154 | 2940 | 8220 | ||||
| 112 | 170 | 3150 | 8470 | ||||
| Theoretical Conc. (ng/mL) | 100 | 150 | 3000 | 9000 | --- | --- | --- |
| Mean | 103.2 | 153.2 | 2937.8 | 8535.6 | 0.001 | 0.00286 | 0.9976 |
| SD | 4.70 | 7.77 | 121.94 | 588.92 | --- | --- | 0 |
| % CV | 4.56 | 5.07 | 4.15 | 6.90 | --- | --- | 0.21 |
| % Dev | 3.21 | 2.15 | −2.07 | −5.16 | --- | --- | --- |
| n | 9 | 9 | 9 | 9 | --- | --- | 3 |
A 1/x2 weighting was used to generate the calibration curve as a ratio of peak areas (analyte/internal standard) to the analyte concentration. This weighting provided the best fit of the data points within each standard curve. The correlation coefficient (r2) was greater than 0.98 for each curve. The values for each of the assay parameters are within acceptable error range of accuracy and precision, as detailed in tables 2, 3, and 4. A partial cross validation experiment showed that the quality controls of seminal and blood plasma could be read on either matrix standard curve within acceptable accuracy and precision. For the duration of the study the clinical samples were read on their respective matrix standard curves. With seminal plasma being a limited matrix, the partial cross validation showed that seminal plasma samples could be read on a blood plasma standard curve in the event it was needed. These findings indicate that the assay method possesses the needed accuracy, precision and linearity required for a valid quantitative method for EFV that includes the range of protein-free EFV in seminal plasma.
3.3.2 Method Selectivity, Matrix Effect, and Extraction Recovery
Matrix effects were evaluated for both blood and seminal plasma by analyzing five different sources of each matrix for potential interfering peaks, signal enhancement and/or signal suppression. The experiment was designed to analyze the matrix effect, extraction recovery, and process efficiency of the method for each matrix. Samples were evaluated in samples spiked containing no matrix, samples spiked to matrix post-extraction, and samples spiked to matrix pre-extraction. The analyte and internal standard have nearly identical physical and chemical properties. The halogen substitution provides near identical stability, evident by the favorable recovery, precision, and absence of signal suppression or enhancement. MRM signal from blank plasma extracts indicate that there is not spurious signal from the seminal or blood plasma matrix. The samples in the experiments were prepared at the same concentrations of the quality controls of the low end curve, 1.5 ng/ml, 45 ng/ml, and 450 ng/ml. These concentrations were used because deleterious effects would be most observed at the low end curve. It is also the detectable range of the protein-free patient samples. Matrix effect was calculated for the analyte and internal standard in both blood and seminal plasma and did not show evidence of ion suppression or enhancement. The recovery of the analyte and internal standard was favorable. In the blood plasma, the recovery was approximately 88%, and in the seminal plasma the recovery was approximately 78%. The process efficiency calculation in blood plasma was approximately 83%, and 78% in seminal plasma.
3.3.3 Stability Studies
The stability of EFV under different storage conditions is well documented [9,10,11,12]. Matrix effect assessment in seminal plasma eliminated the possibility of endogenous or interfering substances in an unknown matrix. Seminal plasma is a limited matrix so the assay was only partially validated. However, experiments showed that seminal plasma samples could be run on a blood plasma curve with acceptable accuracy and precision. Stability tests resulting from the assay have shown efavirenz to be stable in seminal plasma for as long as six months with storage at −80°C. The study has also shown efavirenz to be stable in seminal plasma for up to three freeze/thaw cycles after storage at −80°C. The stability of the stock solution of fluorinated efavirenz internal standard is ongoing and has shown no apparent reduction over time to calibrators and quality controls. The stability of efavirenz in blood plasma is well documented. Long term stability studies in human plasma storage stability at −20° C for up to 30 days were performed by Ramachandran et. al [9]. Stock solution stabilities and injection matrix stabilities were performed by Martin et. al [10]. Stock solutions in methanol were shown to be stable for a minimum of 2 months at −20°C. Freeze thaw stabilities, sample matrix stabilities, and short (6h) and long term (1 and 2 month) storage stabilities were performed by Mogatle et. al [11]. These previous studies using blood plasma provide documentation that EFV is stable in frozen biological matrix, freshly prepared matrix, and in prepared sample. Our studies have shown the long term stability and freeze thaw stability within the seminal plasma matrix. F-EFV, the internal standard used for UPLC-MS/MS analysis, shares many physicochemical properties with EFV and EFV analogs that have been synthesized for evaluation for anti-retroviral and anti-tubercular activities [15,16,17]. To date, our ongoing long term stability studies of the internal standard F-EFV have shown stability in methanol solution stored at 0°C for up to 10 months. Seminal plasma and blood plasma matrices received from clinical patient samples are subjected to ultrafiltration to isolate the protein-free EFV. The resulting ultrafiltrate is an alteration to the matrix in that proteins over 10 kDa have been removed. Cross validation of quality controls were prepared in blank ultrafiltrate matrix to show alignment with respective matrix quality controls on a standard curve assessment. Matrix effects were also assessed for the ultrafiltrates and did not show significant difference from the unaltered matrix assessments.
4. Summary
A validated UPLC-MS/MS method provides the analytical basis for determination of protein-free concentrations of EFV in ultrafiltrates of human seminal plasma comparable to those in blood plasma. The assay is capable of reliable detection limit of ~15–20 femtomoles of EFV injected on the column with a S/N ratio of 4.9. The precision of the assay method (%CV) was <8% over a range from 0.5 ng/ml – 10,000 ng/ml and the accuracy of the assay method (%Dev) was −5.2% to 8.0% for inter and intra-assay variation. The assay method was linear over a range from 0.5 ng/ml to 10,000 ng/ml with correlation coefficients (r2) of >0.98. The time required for measurement of each sample is 8.0 minutes. The analytical method is ideally suited for quantitation of protein-free EFV and total EFV in blood and seminal plasma.
Acknowledgements
This work was supported by the HIV Prevention Trials Network (HPTN) sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), National Institute on Drug Abuse (NIDA), National Institute of Mental Health (NIMH), and Office of AIDS Research, of the NIH, DHHS (U01-AI-068613). The project was also supported by Grant Number UL1 RR 025005 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research. The research was also supported by the Fight Attendant Medical Research Institute (FAMRI). The contents of the article are solely the responsibility of the authors and do not necessarily represent the official view of NIH. The authors are very grateful to Dr. Craig W. Hendrix, M.D., for the clinical guidance and helpful discussions on the clinical applications of the methods developed.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Royce R, Seña A, Cates W, Cohen M. N Engl J Med. 1997;336:1072. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- [2].Cao YJ, Hendrix C. Clin Pharmacol Ther. 2008;83:401. doi: 10.1038/sj.clpt.6100342. [DOI] [PubMed] [Google Scholar]
- [3].Kashuba A, Dyer J, Kramer L, Raasch R, Eron J, Cohen M. J Antimicrob Chemother. 1999;43:1817. doi: 10.1128/aac.43.8.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Boffito M, Back D, Blaschke T, Rowland M, Bertz R, Gerber J, Miller V. AIDS Res Hum Retroviruses. 2003;19:825. doi: 10.1089/088922203769232629. [DOI] [PubMed] [Google Scholar]
- [5].Reddy Y, Gotzkowsky K, Eron J, Kim J, Fiske W, Fiscus S, Petch L, Cohen M, Kashuba A. J Infect Dis. 2002;186:1339. doi: 10.1086/344311. [DOI] [PubMed] [Google Scholar]
- [6].Cao YJ, Ndovi T, Parsons T, Guidos A, Caffo B, Hendrix C. Clin Pharmacol Ther. 2008;83:848. doi: 10.1038/sj.clpt.6100356. [DOI] [PubMed] [Google Scholar]
- [7].Cao YJ. Doctoral Thesis. Graduate Training Program in Clinical Investigation, Johns Hopkins Univ.; Baltimore, MD: 2007. [Google Scholar]
- [8].Coombs R, Speck C, Hughes J, Lee W, Sampoleo R, Ross S. J. Infect Dis. 1998;177:320. doi: 10.1086/514213. [DOI] [PubMed] [Google Scholar]
- [9].Ramachandran G, Hemanth Kumar A, Swaminathan S, Venkatesan P, Greenblatt D. J Chromatogr B. 2006;835:131. doi: 10.1016/j.jchromb.2006.03.014. [DOI] [PubMed] [Google Scholar]
- [10].Martina J, Deslandesa G, Dailly E, Renauda C, Reliquet V, Raffic F, Jolliet P. J Chromatogr B. 2009;877:3072. doi: 10.1016/j.jchromb.2009.07.031. [DOI] [PubMed] [Google Scholar]
- [11].Mogatle S, Kanfer I. J Pharm Biomed Anal. 2009;49:1308. doi: 10.1016/j.jpba.2009.03.008. [DOI] [PubMed] [Google Scholar]
- [12].Quaranta S, Woloch C, Paccou A, Giocanti M, Solas C, Lacarelle B. Ther Drug Monit. 2009;31:695. doi: 10.1097/FTD.0b013e3181c05adf. [DOI] [PubMed] [Google Scholar]
- [13].Pierce ME, Parsons RL, Jr., Radesca LA, Lo YS, Silverman S, Moore JR, Islam Q, Choudhury A, Fortunak JMD, Nguyen D, Luo C, Morgan SJ, Davis WP, Confalone PN, Chen C, Tillyer RD, Frey L, Tan L, Xu F, Zhao D, Thompson AS, Corley EG, Grabowski EJJ, Reamer R, Reider PJ. J Org Chem. 1998;63:8536. [Google Scholar]
- [14].Radesca LA, Lo YS, Moore JR, Pierce ME. Synth Commun. 1997;27:4373. [Google Scholar]
- [15].Cocuzza AJ, Chidester DR, Cordova BC, Klabe RM, Jeffery S, Diamond S, Weigelt CA, Ko SS, Bacheler LT, Erickson-Viitanen S, Rodgers JD. Bioor Med Chem Lett. 2001;11:1389. doi: 10.1016/s0960-894x(01)00239-6. [DOI] [PubMed] [Google Scholar]
- [16].Patel M, McHugh RJ, Jr., Cordova BC, Klabe RM, Erickson-Viitanen S, Trainor GL, Ko SS. Bioor Med Chem Lett. 1999;9:3221. doi: 10.1016/s0960-894x(99)00565-x. [DOI] [PubMed] [Google Scholar]
- [17].Sriram D, Banerjee D, Yogeeswari P. J Enzyme Inhib Med Chem. 2009;24:1. doi: 10.1080/14756360902784425 . [DOI] [PubMed] [Google Scholar]
- [18].U.S. Department of Health and Human Services . FDA Guidance for Industry and Bioanalytical Method Validation. 2001. [Google Scholar]