Abstract
Background
Chronic opioid therapy for chronic non-cancer pain (CNCP) is increasingly common in community practice. Concomitant with this practice change, rates of fatal opioid overdose have increased. It is not known to what extent overdose risks are elevated among patients receiving medically prescribed chronic opioid therapy.
Objective
To estimate rates of opioid overdose and their association with average prescribed daily opioid dose among patients receiving medically prescribed chronic opioid therapy.
Design
Cox proportional hazards models were used to estimate overdose risk as a function of average daily opioid dose (morphine equivalents) received at time of overdose.
Setting
Health maintenance organization.
Patients
Individuals (n=9940) who received 3+ opioid prescriptions within 90-days for CNCP between 1997 and 2005.
Measurements
Average daily opioid dose over the previous 90 days from automated pharmacy data. Primary outcomes, non-fatal and fatal overdoses, were identified through diagnostic codes from inpatient and outpatient care and death certificates and confirmed by medical record review.
Results
Fifty-one opioid-related overdoses were identified, including six deaths. Compared to patients receiving 1-20mg of opioids per day (0.2% annual overdose rate), patients receiving 50-99 mg had a 3.7 fold increase in overdose risk (95% C.I. 1.5, 9.5) and a 0.7% annual overdose rate. Patients receiving 100mg or more per day had an 8.9 fold increase in overdose risk (95% C.I. 4.0, 19.7) and a 1.8% annual overdose rate.
Limitations
Increased overdose risk among patients on higher dose regimens may be due to confounding by patient differences and by use of opioids in ways not intended by prescribing physicians. The small number of overdoses in the study cohort is also a limitation.
Conclusions
Patients receiving higher doses of prescribed opioids are at increased risk of opioid overdose, underscoring the need for close supervision of these patients.
In response to growing awareness of chronic pain as a significant patient concern, chronic opioid therapy is prescribed with increased frequency,(1-3) with over 3% of adults now receiving chronic opioid therapy for chronic non-cancer pain (CNCP).(2) Concurrently, death rates from opioid analgesic poisoning have increased.(4-8). From 1995-2004, opioid-related poisoning hospitalizations doubled in Washington State.(9) A recent study in West Virginia reported that less than half (44%) of autopsy-identified unintentional prescription drug overdose fatalities had received opioids from a physician, suggesting that overdose typically resulted from drug diversion.(10;11) However, overdose risks in patients receiving medically prescribed opioids have not been studied.
Some hold that the rise in poisonings is related to excessive use of opioid analgesics in community practice.(12) Others express concern that such interpretations may lead to under-prescribing of opioids for CNCP patients.(13) The association of prescription opioid exposure and overdose risk has been inferred from: uncontrolled case series of autopsies subject to selection biases or ecological time series studies where individual-level associations cannot be examined. While opioids provide partial pain relief for chronic pain.(14-15), the balance of long-term risks and benefits is poorly understood.(16-21) Large-scale epidemiologic studies assessing patient use of prescribed opioids are needed to assess whether there is a relationship between receiving medically prescribed opioids and opioid-related poisonings. A key unanswered question is whether overdose risk differs by opioid dose among patients receiving chronic opioid therapy.
The objectives of this report are: 1) to estimate overall overdose rates (non-fatal and fatal) among persons receiving opioids long-term for CNCP from medical sources; and, 2) to compare risks of opioid overdose among patients recently receiving chronic opioid therapy at differing dosage levels.
Methods
This paper reports findings from the CONSORT study - CONsortium to Study Opioid Risks and Trends.(22) The study setting was Group Health Cooperative (GHC) which provides comprehensive care on a pre-paid basis to about 500,000 persons in Washington State.(23) The study was approved by the GHC Institutional Review Board.
Sample
The study cohort consisted of individuals who initiated repeated use of opioid analgesic prescriptions for a pain problem. Specific inclusion criteria were (i) adults aged 18 or over initiating a new episode of opioid use (no opioid prescriptions filled in the previous 6 months) between 1997 and 2005 (inclusive), (ii) 3 or more prescriptions for opioid analgesics in the first 90 days of the episode, and (iii) a diagnosis of a non-cancer pain problem from the prescribing physician in the two weeks prior to the initial opioid prescription. Eligible pain diagnoses were back or neck pain, osteoarthritis, headache, extremity pain, abdominal pain / hernia, menstrual pain, temporomandibular disorder pain and fractures / contusions / injuries. Individuals entered the study cohort on the 90th day of their episode, once eligibility was established, and continued to be included in the cohort whether or not they continued to receive prescriptions for opioid analgesics.
Exclusion criteria were (i) individuals with a cancer diagnosis (except non-melanoma skin cancer) in the Cancer Surveillance and End Results Registry up to the end of 2006, (ii) two or more cancer diagnoses (excluding non-melanoma skin cancer) from visit or hospital data between the episode start date and the date of censoring, (iii) individuals not enrolled for at least 270 days in the one-year period prior to study cohort entry. Persons who disenrolled from GHC after baseline were censored on their date of disenrollment, otherwise they were censored on December 31, 2006, the end of the study observation period.
Classification of opioids
Medication data were obtained from GHC automated pharmacy files. These data have been shown to cover over 90% of the prescription medications used by GHC enrollees.(23) Total morphine equivalents dispensed were calculated for each opioid prescription filled during the follow-up period, defined by the quantity of pills dispensed multiplied by their strength (in milligrams (mg)), multiplied by a conversion factor.(22) The average daily morphine equivalent dose dispensed was then calculated for 90 day exposure windows (see Analysis section) by summing the morphine equivalents for the prescriptions dispensed for the 90-day period and dividing by 90. For each 90 day exposure window and person, the average daily opioid dose dispensed was calculated and categorized into none, 1 to 19 mg, 20 to 49 mg, 50 to 99 mg and 100 mg or more.
Covariate data collection
Information on baseline covariates was obtained from automated health care data. These included age, sex, tobacco use, and depression diagnosis and substance abuse diagnosis in the two years prior to entry into the cohort. The type of pain diagnosis at the index visit was identified. Chronic disease comorbidity adjustors were calculated at the time of the index visit: RxRISK risk (24) and the Romano version of the Charlson Score.(25) The days supply of sedative-hypnotics dispensed (based on prescriptions of benzodiazepines, barbiturates and muscle relaxants from automated pharmacy files) was calculated for 90-day exposure windows. Sedative-hypnotic use was classified into 80% of days or more (72+ days), 50-79% of days (45-71 days), 25-49% of days (23-44 days), 1-24% of days (1-22 days), and none.
Definition of overdose
Potential opioid-related overdoses were identified from electronic medical records, and medical-record reviews were conducted to classify and validate overdose events. Potential cases were identified from the electronic medical records using the following two definitions: 1. International Classification of Disease (ICD) code indicating opioid-related poisoning (see case definition 1 in the Appendix provided in www.annals.org), or 2. ICD code indicating an opioid-related adverse event (a), plus a diagnosis code on the same date considered to be identifying an overdose (b) (see case definition 2 in the Appendix). Fatal overdoses were identified from the Washington State mortality registry, which is linked to the GHC enrollment file annually (23), using the ICD codes listed in the Appendix.
The medical records for all potential cases identified were examined and classified according to the available evidence for an opioid-related overdose (categories: definite / probable / uncertain / probably not / definitely not) (see Appendix Table providing operational definitions of overdose categories in www.annals.org). Further information was extracted from the medical records on the severity of consequences [death, serious (e.g. requiring hospitalization, unconsciousness, respiratory failure), not serious (e.g. dizziness)]. This record review was carried out without knowledge of opioid exposure status.
Overdose status (present / absent) was ascertained for each participant on a daily basis. For each individual we modeled the time to the first overdose event during the study period at which the full case criteria were met (i.e. after medical record review). Subsequent overdose events, if they occurred, were not included in the analyses. Separate analyses examined risk for any opioid related overdoses, and serious opioid related overdoses. In analysis of serious overdoses, individuals who had an initial overdose that was not serious were included in analyses until they had a subsequent serious overdose or were censored.
Analyses
We used a Cox proportional hazards model (SAS’s PROC PHREG) to estimate the risk of overdose across individuals as a function of their average daily opioid dose. (26,27) Opioid dose was included as a time varying covariate, estimated for continuously updated 90-day exposure windows. Participants could be classified as either exposed to opioids (at any of four dosage levels) or unexposed on any given day, based on their average daily opioid dose during the prior 90 days including the event date. Estimated hazard ratios for opioid dose were based on comparing the opioid dose for an individual who had an overdose (evaluated at the time of event), relative to the opioid dose for all other individuals at risk for overdose at the time of the event (i.e. at the same number of days since entering the study cohort). Whether each person had initiated (started or restarted) opioid use in the prior 90 days was included in the model as a time-varying covariate. Persons were classified as initiating opioid use for the first 30 days of the study period, and subsequently for any 30 day period following the date of receiving an opioid prescription when no opioids had been received in the prior 90 days.
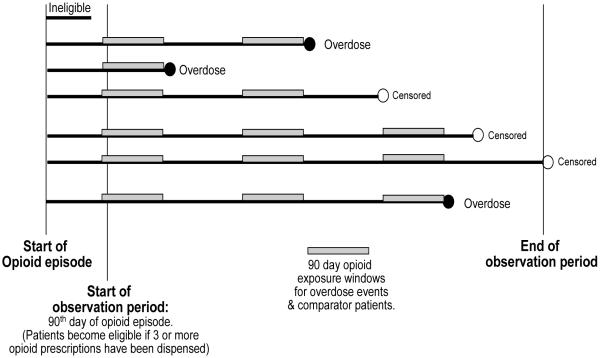
Figure 1 is a schematic that depicts the observation period starting at cohort entry (i.e. 90 days after the start of a new episode of opioid use if 3 or more opioid prescriptions were received) and demonstrates how the 90 day opioid exposure windows were used to compare patients who experienced an overdose to comparator patients who remained at risk for overdose. Opioid dose was compared for patients with an overdose and all eligible comparator patients evaluated at the same number of days since cohort entry for each patient.
Figure 1. Cohort entry, overdose events, and 90 day opioid exposure windows for overdose events and comparators.
Average opioid dose was compared for each patient who overdosed for the preceding 90 days to all patients remaining eligible as of the same number of elapsed days since the start of the observation period. Patients were followed until their first opioid overdose, or until censored due to health plan disenrollment, death or reaching the end of the observation period.
Sedative hypnotic use was included as a time varying covariate, estimated for continuously updated 90-day exposure windows. Participants were classified as either exposed to sedative hypnotics (at any of the 4 levels of days supply dispensed) or unexposed on any given day. Hazard ratios were also adjusted for the following covariates not treated as time-varying: age (included as a continuous variable), sex, tobacco use, depression diagnosis, substance abuse diagnosis, index pain diagnosis, and chronic disease comorbidity adjustors (included as continuous variables). We assessed the validity of the proportional hazards assumption using Schoenfeld residuals.(28)
Analysis focused on the increased risk of overdose associated with recent receipt of opioids at higher dosage levels relative to the risk among individuals receiving opioids at the lowest dosage level (1 to 19mg). We also compared differences in overdose risk between patients not currently receiving prescribed opioids to patients receiving opioids at the lowest dosage level. Exploratory analyses examined potential interactions between opioid use and baseline covariates.
Results
A total of 9940 people initiating chronic opioid therapy were included in the cohort. They were followed for a mean of 42 months (range <1 to 119 months) from their initial 90-day exposure window. Of the total cohort, 61% had complete follow-up (from entry into the cohort until the end of the study period, or until an event), 32% left GHC during the study period and 7% died. The characteristics of the cohort are shown in Table 1. Around 60% was female, with a mean age of 54 years. Two thirds of the cohort received a diagnosis of back pain or extremity pain at the index visit (38% and 30% respectively). The mean daily dose of opioids prescribed was 13.3 mg (morphine equivalents). Among 46% of the cohort, hydrocodone was the opioid they were most commonly prescribed, and 10% of the cohort received predominately long-acting opioids. Cohort patients were using opioids during 51.2% of the follow-up time: with 40.1% of observation time at the lowest dosage level (1 to <20 mg. morphine equivalents); 6.7% at 20 to <50 mg.; 2.6% at 50 to <100 mg., and 1.8% was at 100 mg. or greater. Three quarters (74%) of the cohort was also prescribed sedative-hypnotics at some point.
Table 1. Characteristics of sample.
| Baseline characteristics | |
| Female (%) | 59.6% |
| Age (Mean, s.d., range) | 54 (16.8) 18 to 99 |
| Tobacco use (%) | 29.4% |
| Depression diagnosis (%) | 26.9% |
| Substance abuse diagnosis (%) | 6.2% |
| Comorbidity | |
| Rxrisk (Mean, s.d., range) | 3057 (2434) 70.7 to 20802 |
| Charlson Index (Mean, s.d., range) | 0.71 (1.48) 0 to 14 |
| Pain diagnosis at the index visit (% of cohort) | |
| Back pain | 37.9% |
| Extremity pain | 30.3% |
| Osteoarthritis | 12.7% |
| Injury / contusion / fracture | 12.3% |
| Neck pain | 8.9% |
| Abdominal pain | 6.4% |
| Headache | 4.9% |
| Menstrual pain | 2.1% |
| Temporomandibular pain | 0.4% |
| Characteristics of follow-up | |
| Person months of follow-up (Mean, s.d., range) | 42.1 (30.5) 0.1 to 118.7 |
| Daily dose of opioids (milligrams of morphine equivalent)a | |
| Mean daily dose | 13.3mg |
| Median daily dose | 6.0mg |
| Sedative-hypnotic use (% of cohort) | |
| Prescribed any sedative-hypnotic during follow-up | 74.7% |
| Prescribed muscle relaxants during follow-up | 52.3% |
| Prescribed benzodiazepines during follow-up | 42.7% |
| At least 45 days of sedative-hypnotics prescribed in one or more 90 day period |
31.9% |
| Most commonb opioids prescribed during follow-up (% of cohort) | |
| Hydrocodone | 46.3% |
| Oxycodone | 24.5% |
| Codeine combination | 11.6% |
| LA morphine (long-acting) | 6.2% |
| Propoxyphene | 4.9% |
| Oxycodone CR (controlled release) | 2.5% |
| Tramadol | 1.7% |
| Hydromorphone | 0.9% |
| Methadone | 0.7% |
| Fentanyl patch | 0.6% |
| Type of opioids received most frequently | |
| Any short-acting opioid | 90.4% |
| Any long-acting opioid | 9.6% |
Abbreviation: s.d., standard deviation
Daily dose among those prescribed opioids.
Top 10 shown. Based on number of days of opioids prescribed during follow-up.
Clinical description of identified opioid overdoses
During the study period, 6 fatal opioid-related overdoses and 74 non-fatal events were identified, of which 13 were classified as definite non-fatal opioid overdoses and 32 as probable non-fatal opioid overdoses (10 were uncertain, 17 probably not, and 2 definitely not opioid overdoses). Using a definition of death, definite or probable non-fatal overdose for an opioid-related overdose, we identified 51 patients who experienced one or more overdose events. Of these, 40 (78.4%) experienced a fatal or otherwise serious overdose, and 11 (21.6%) had only non-serious overdose events. Common clinical contexts for overdose were varied and included accidental excess ingestion of opioids (n=8) and suicide attempt (n=6). Additional opioids obtained from non-medical sources were noted for three people and drug abuse was mentioned for four. Four patients had notes indicating overdoses associated with applying extra Fentanyl patches or sucking on a patch. The largest category of noted clinical effects of the overdose was delirium, loss of consciousness or confusion (n=23), followed by respiratory problems (n=15) and falls (n=4). The most common initial care settings identified for overdose events were the emergency room (n=23), inpatient care (n=14), urgent care (n=2) or other ambulatory care (n=6).
Overdose rates
The annual rate of overdose for the total sample was 148 per 100,000 person years overall, and 116 per 100,000 for serious overdose (Table 2). The overdose rates were somewhat higher among persons age 65+ than among persons in the two younger age groups, and were similar in men and women. Overdose rates were elevated among those with a history of depression or substance abuse treatment (Table 2). The overall rate of overdose mortality (n=6 deaths) was 17 per 100,000 person years, so there were over seven times as many non-fatal overdoses as fatal overdoses in this cohort. When stratified by recent receipt of opioids, the annual overdose rate was 256 per 100,000 person years among patients recently receiving medically prescribed opioids, compared to 36 per 100,000 person years among the sub-sample not recently receiving medically prescribed opioids (Table 3). We examined overdose events by clinic and did not observe notable clustering of overdose within any of the 29 clinics included in this study (data not shown).
Table 2. Rates of overdose.
| No. of overdoses | Person- years |
Overdose rate (and 95% confidence intervals) per 100,000 person years |
|||
|---|---|---|---|---|---|
|
|
|||||
| All a | Serious b | All a | Serious b | ||
| Total sample | 51 | 40 | 34362 | 148 (111-192) |
116 (83-155) |
| Age group (years) | |||||
| 18-44 | 15 | 11 | 9208 | 163 (91-255) |
119 (60-200) |
| 45-64 | 18 | 14 | 15219 | 118 (70-179) |
92 (50-146) |
| 65+ | 18 | 15 | 9935 | 181 (107-274) |
151 (85-236) |
| Gender | |||||
| Male | 21 | 17 | 13822 | 152 (94-223) |
123 (72-188) |
| Female | 30 | 23 | 20540 | 146 (99-203) |
112 (71-162) |
| History of depression diagnosis | |||||
| No | 25 | 20 | 25994 | 96 (62-137) |
77 (47-114) |
| Yes | 26 | 20 | 8368 | 311 (203-441) |
239 (146-354) |
| History of substance abuse diagnosis | |||||
| No | 45 | 35 | 32541 | 138 (101-182) |
107 (75-146) |
| Yes | 6 | 5 | 1821 | 329 (121-641) |
274 (89-562) |
Overdose defined as opioid-related overdose death, or non-fatal event defined as definite or probable opioid-related overdose (n=51).
Overdose defined as opioid-related overdose death, or serious non-fatal event (n=40).
Table 3. Hazard ratios for the relationship between recent opioid dosage level and overdose.
| Opioid dosage level |
Number of overdosesa |
Person- years |
Overdose ratea (95% C.I.) per 100,000 person years |
All overdose events Hazard ratio (95% C.I.) ab |
Serious overdose events Hazard ratio (95% C.I.) bc |
|---|---|---|---|---|---|
| 0 | 6 | 16,780 | 36 (13-70) |
0.31 (0.12, 0.80) |
0.19 (0.05, 0.68) |
| 1 - <20 | 22 | 13,770 | 160 (100-233) |
1.00 | 1.00 |
| 20 - <50 | 6 | 2,311 | 260 (95-505) |
1.44 (0.57, 3.62) |
1.19 (0.40, 3.60) |
| 50 - < 100 | 6 | 886 | 677 (249-1317) |
3.73 (1.47, 9.50) |
3.11 (1.01, 9.51) |
| 100+ | 11 | 614 | 1791 (894-2995) |
8.87 (3.99, 19.72) |
11.18 (4.80, 26.03) |
| Any opioid use |
45 | 17,582 | 256 (187-336) |
5.16 (2.14, 12.48) |
8.39 (2.52, 27.98) |
Abbreviation: C.I., confidence interval
Overdose defined as opioid-related overdose death, or non-fatal event defined as definite or probable opioid-related overdose.
Adjusted for smoking, depression, substance abuse, comorbidity, pain site, age, gender, recent sedative-hypnotic prescription and recent initiation of opioid use.
Overdose defined as opioid-related overdose death, or serious non-fatal event (n=40).
Relationship between opioid dose dispensed and overdose
Hazard ratios for the relationship between recently prescribed opioid dosage level and opioid-related overdose, adjusted for potential confounders, are shown in Table 3. People receiving the lowest opioid dosage levels (<20mg per day) had an annual overdose rate of 160 per 100,000 person years. The risk of overdose increased with increasing opioid dosage level. Among persons receiving an opioid dose of 100mg or more per day, the annual overdose rate was 1791 per 100,000 person years, a nine-fold increase in overdose risk [8.87 (95% C.I. 3.99, 19.72)] compared to those receiving the lowest doses. When analysis was restricted to serious events, the hazard ratios were of a similar magnitude, and demonstrated a comparable difference by dose (Table 3). Persons recently receiving sedative-hypnotic medications were also at increased risk of opioid overdose, but risk did not increase with the frequency of receiving sedative-hypnotic medications. Relative to persons not receiving any sedative-hypnotic medications in the 90 days prior to opioid overdose, the overdose hazard ratios were as follows: 3.4 [1.6–7.2] for 1-22 days supply; 0.9 [0.2-4.0] for 23-44 days supply; 3.7 [1.6-8.9] for 45-71 days supply; and 2.7 [1.2-6.0] for 72+ days supply. In multivariate analyses, recent initiation of opioid use (starting or restarting) was not associated with either increased or reduced risk of opioid overdose (data not shown).
We assessed patient differences by the maximum opioid dose received over the follow-up period. Patients receiving the highest opioid doses (relative to those in the lowest dose group) more often: were male (48.4% vs. 39.5%); were current smokers (40.0% vs. 28.0%); had a history of depression treatment (32.0% vs. 25.9%); had a history of substance abuse treatment (13.7% vs. 5.3%); and had higher Charlson comorbidity scores (mean =0.93 SD=1.61 vs. mean=0.63 SD=1.40), but did not differ in age. The intermediate dosage groups were generally similar to the lowest dose group on these variables.
Persons who had not recently received opioids had lower risk of overdose than patients receiving opioids at low dosage levels (Table 3). In covariate stratified analyses, the consistency of differences in overdose risk was compared for persons recently receiving opioids and persons not recently receiving opioids. Elevated overdose risk was observed among those recently receiving prescribed opioids in all sub-groups (data not shown).
Discussion
In this study, patients receiving higher doses of medically prescribed opioids for CNCP were at increased risk of overdose relative to patients receiving lower doses. Based on a Medline search in September 2009, we believe this study provides the first estimates of the relationship of prescribed opioid dose and overdose risk in a chronic pain population. This increased risk remained after controlling for demographic and clinical variables. Patients who received high opioid dosage levels were somewhat higher risk patients (e.g. somewhat more likely to smoke, somewhat greater medical comorbidity) than patients who received the lowest opioid dosage levels. At low opioid dosages, the absolute risk of overdose was small. In contrast, the unadjusted overdose rate was 1.8% per annum among patients receiving 100 mg. or more morphine equivalents per day. While opioid overdose risk was highest among those receiving higher opioid dosage, the majority of overdoses occurred among patients receiving low to moderate dose regimens, since the large majority of patients received lower opioid dosages. There were over seven non-fatal opioid overdose events for each fatal opioid overdose identified in the study cohort.
Previous studies have indicated that the rise in opioid-related overdoses is paralleled by increased prescribing of opioids for non-cancer pain,(7) but it has been believed that opioid overdose occurs predominately among persons obtaining prescription opioids from non-medical sources.(10) This study provides the first estimates that directly link receipt of medically prescribed opioids to overdose risk. This study was not designed to identify mechanisms, but information from medical records reviews suggests that accidental ingestion of excess opioids, suicide attempt, obtaining additional opioids from non-medical sources, using more opioid than prescribed, and use of opioids in the context of drug abuse were noted clinical contexts, but no one of these explanations was predominant.
Limitations
This observational study cannot establish whether overdose risk differences reflect direct effects of opioid dose differences or patient differences. Patients receiving high opioid dosage levels tended to be higher risk patients, but differences in risk profile were controlled in multivariate analyses. Because opioid events were uncommon, it was not possible to account for potential correlation of observations within physician or clinic due to the small number of events within provider and setting. We examined the clustering of overdose events by clinic and did not observe notable clustering.
It is possible that patients receiving higher dose regimens were more likely to deviate from medically prescribed use (e.g. increasing dose above prescribed levels, using opioids not prescribed, using other substances that influence overdose risks). Some study patients used prescribed opioids in dangerous ways, such as applying multiple Fentanyl patches or substituting a opioid obtained from a friend for a prescribed medication. Further research is needed to understand the specific determinants of overdose risks among patients receiving chronic opioid therapy. However, the results of this research suggest that patients using opioids long-term require close supervision and careful instruction in appropriate use of opioids, as recommended by expert guidelines,(29;30) particularly those receiving higher dose regimens. Due to the small number of events observed in the sample it was not possible to assess overdose risk for specific opioids, or risk differences for long-versus short-acting opioids. Further research is needed to assess these risks.
The comparison group was persons who recently received prescribed opioids at low dosage levels. This comparison group was employed (rather than the group not receiving opioids) to minimize the possibility of overdose ascertainment bias, for example, if physician awareness of patient opioid use influenced identification of overdose. While we adjusted for a number of potential confounders, the possibility of residual confounding cannot be excluded. Substance abuse and depression history based only on diagnostic codes is likely to be selective, and adjustment for medical comorbidities with the Charlson Score and RxRISK is imperfect.
The inclusion of non-fatal overdoses adds to understanding of the problem, as most previous work has examined only fatal drug overdoses. The overall overdose rate in the sample was 148 per 100,000 person years, indicating that fatal overdose represents only the tip of the iceberg (88% of identified overdose events were non-fatal). Most of the non-fatal overdoses were clinically serious. A significant limitation is that only overdoses brought to medical attention and identified by study procedures were counted. Hence, the overdose rates reported here may be conservative. The diagnostic algorithm for identifying overdose was highly specific. A more sensitive search algorithm based on text data from the electronic medical record might identify additional events.
Overdose occurs in patients prescribed opioids for chronic non-cancer pain at increased rates, and the risk of overdose appears to increase markedly with average daily dose prescribed. Over the past 20 years, there have been substantial increases in prescribing rates of opioid analgesics for chronic non-cancer pain.(1;2) However, large-scale, controlled studies evaluating the effectiveness and safety of long-term opioid therapy are not available.(17;32) Observational studies suggest that many patients receiving opioids for chronic non-cancer pain often continue to experience significant pain and activity limitations.(33) Given that millions of adults now receive opioids long-term, with an uncertain risk-benefit profile, large-scale controlled studies evaluating the effectiveness and safety of long-term use of opioids in community practice are urgently needed.
Conclusion
Increased risk of overdose was observed among patients receiving medically prescribed opioids at higher dosage levels. Most opioid overdoses were medically serious, and 12% were fatal. This study cannot conclusively establish whether dose-related differences in overdose were due to patient differences, direct effects of higher opioid dose, or indirect effects. Given uncertainties regarding effectiveness and risks of chronic opioid therapy (32), it should be undertaken with awareness of risks and close patient monitoring. (29;30) Close monitoring may not be happening consistently at present.(34) Further research on overdose risks of chronic opioid therapy, and risk reduction, is needed.
Acknowledgements
This research was supported by a grant to Dr. Michael Von Korff from the National Institute of Drug Abuse [DA022557]. Dr. Dunn participated in this work with Dr. Von Korff at the Group Health Center for Health Studies through a grant from the Wellcome Trust [083572]. Dr. Michael Von Korff had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Primary funding source
National Institute of Drug Abuse [DA022557].
Appendix
Codes used to identify potential opioid-related overdoses
| ICD version |
ICD code |
Description |
|---|---|---|
|
Opioid-related poisoning codes (case definition 1) | ||
| ICD-9 | 9650a | Poisoning by opiates and related narcotics |
| E850.1 | Accidental poisoning by Methadone | |
| E950.0 | Suicide and self-inflicted poisoning by analgesics, antipyretics, and antirheumatics |
|
| E980.0 | Undetermined poisoning by analgesics, antipyretics, and antirheumatics |
|
| ICD-10 | T40.0 | Poisoning by Opium |
| T40.2 | Poisoning by Other opioids | |
| T40.3 | Poisoning by Methadone | |
| T40.4 | Poisoning by Other synthetic narcotics | |
| X42 | Accidental poisoning by and exposure to narcotics and psychodysleptics, not elsewhere classified |
|
| X62 | Intentional self-poisoning by and exposure to narcotics and psychodysleptics, not elsewhere classified |
|
| Y12 | Undetermined poisoning by and exposure to narcotics and psychodysleptics, not elsewhere classified |
|
|
Opioid specific adverse event codes (Case definition 2a) | ||
| ICD-9 | E935.0 | Adverse effects of Heroin |
| E935.1 | Adverse effects of Methadone | |
| E935.2 | Adverse effects of other opiates and related narcotics | |
| ICD-10 | Y45.0 | Adverse effects of opioids and related analgesics |
|
Overdose diagnostic codes (Case definition 2b) | ||
| ICD-9 | 276.4 | Mixed acid-base balance disorder |
| 292.1 | Drug-induced psychotic disorders (including 292.11, 292.12) | |
| 292.81 | Drug induced delirium | |
| 292.8 a | Drug-induced mental disorder (excluding 292.81) | |
| 486 | Pneumonia, organism unspecified | |
| 496 | Chronic airway obstruction not elsewhere classified | |
| 518.81 | Acute respiratory failure | |
| 518.82 | Other pulmonary insufficiency, not elsewhere classified | |
| 780.0 a | Alteration of consciousness | |
| 780.97 | Altered mental state | |
| 786.03 | Apnea | |
| 786.05 | Shortness of breath | |
| 786.09 | Dyspnea and respiratory abnormalities - Other | |
| 786.52 | Painful respiration | |
| 799.0 a | Asphyxia & hypoxemia | |
| E950- E959 |
Suicide and self-inflicted injury | |
Includes all sub-codes beginning with this code.
Case definition 2 is met when participants have a diagnostic code from 2a plus one from 2b on the same date.
Appendix Table
Criteria for classifying events to their likelihood of being an opioid-related overdose, based on medical record review
| Categories | Criteria | Examples |
|---|---|---|
| Definite | Clearly stated as opioid overdose. | Accidental methadone overdose. |
| Probable | Mention of overdose with involvement of opioids, or stated as probable opioid overdose. Or, mention of overdose, and mention of opioids, but not explicitly stated as opioid-related overdose. |
Acute alteration in level of consciousness presumed due to narcotic excess. Respiratory depression due to narcotics or Obstructive Sleep Apnea. |
| Uncertain | Records not clear. | In hospital but no specific mention of overdose. |
| Probably not | Event with no mention of opioids. Or, mention of opioids but not stated as operation. |
Adverse effect in context of overdose |
| Definitely not | Clearly not opioid-related overdose. | Opiate withdrawal. |
Footnotes
Publisher's Disclaimer: “This is the prepublication, author-produced version of a manuscript accepted for publication in Annals of Internal Medicine. This version does not include post-acceptance editing and formatting. The American College of Physicians, the publisher of Annals of Internal Medicine, is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to www.annals.org or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record.”
Disclosures: Dr. Von Korff has a grant pending from Johnson and Johnson. Dr. Sullivan has received grant support from Wyeth, Lilly, Aetna, Johnson & Johnson, and Ortho McNeil and has been a consultant for Eli Lilly.
Protocol: Not available
Statistical code: Available to interested readers by contacting Dr. Von Korff at vonkorff.m@ghc.org.
Data: Not available
Reference List
- (1).Caudill-Slosberg MA, Schwartz LM, Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in US: 1980 vs. 2000. Pain. 2004;109:514–19. doi: 10.1016/j.pain.2004.03.006. [DOI] [PubMed] [Google Scholar]
- (2).Boudreau D, Von Korff M, Rutter CM, Saunders K, Ray TG, Sullivan MD, et al. Trends in Long-term Opioid Therapy for Chronic Non-Cancer Pain”. Pharmacoepidemiology and Drug Safety. doi: 10.1002/pds.1833. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Franklin GM, Mai J, Wickizer T, Turner JA, Fulton-Kehoe D, Grant L. Opioid dosing trends and mortality in Washington State workers’ compensation, 1996-2002. Am J Ind Med. 2005;48:91–99. doi: 10.1002/ajim.20191. [DOI] [PubMed] [Google Scholar]
- (4).Paulozzi LJ, Budnitz DS, Xi Y. Increasing deaths from opioid analgesics in the United States. Pharmacoepidemiology and Drug Safety. 2006;15:618–27. doi: 10.1002/pds.1276. [DOI] [PubMed] [Google Scholar]
- (5).Shah NG, Lathrop SL, Reichard RR, Landen MG. Unintentional drug overdose death trends in New Mexico, USA, 1990-2005: combinations of heroin, cocaine, prescription opioids and alcohol. Addiction. 2008;103:126–36. doi: 10.1111/j.1360-0443.2007.02054.x. [DOI] [PubMed] [Google Scholar]
- (6).Wysowski DK. Surveillance of prescription drug-related mortality using death certificate data. Drug Saf. 2007;30:533–40. doi: 10.2165/00002018-200730060-00007. [DOI] [PubMed] [Google Scholar]
- (7).Paulozzi LJ, Ryan GW. Opioid analgesics and rates of fatal drug poisoning in the United States. Am J Prev Med. 2006;31:506–11. doi: 10.1016/j.amepre.2006.08.017. [DOI] [PubMed] [Google Scholar]
- (8).Fernandez W, Hackman H, McKeown L, Anderson T, Hume B. Trends in opioid-related fatal overdoses in Massachusetts, 1990-2003. J Subst Abuse Treat. 2006;31:151–56. doi: 10.1016/j.jsat.2006.04.008. [DOI] [PubMed] [Google Scholar]
- (9).Washington State Department of Health . The Health of Washington State, 2007. Washington State Department of Health; Olympia, WA: 2007. Ref Type: Report. [Google Scholar]
- (10).Hall AJ, Logan JE, Toblin RL, Kaplan JA, Kraner JC, Bixler D, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300:2613–20. doi: 10.1001/jama.2008.802. [DOI] [PubMed] [Google Scholar]
- (11).Kuehn BM. Efforts aim to curb opioid deaths, injuries. JAMA. 2009;301:1213–15. doi: 10.1001/jama.2009.367. [DOI] [PubMed] [Google Scholar]
- (12).Fishman SF. prescription drug abuse and safe pain management. Pharmacoepidemiology and Drug Safety. 2006;15:628–31. doi: 10.1002/pds.1292. Commentary in Response To Paulozzi et al. [DOI] [PubMed] [Google Scholar]
- (13).Reidenberg MM, Willis O. Prosecution of Physicians for Prescribing Opioids to Patients. Clin Pharmacol Ther. 2007;81:903–6. doi: 10.1038/sj.clpt.6100127. [DOI] [PubMed] [Google Scholar]
- (14).Won A, Lapane KL, Vallow S, Schein J, Morris JN, Lipsitz LA. Long-Term Effects of Analgesics in a Population of Elderly Nursing Home Residents With Persistent Nonmalignant Pain. J Gerontol A Biol Sci Med Sci. 2006;61:165–69. doi: 10.1093/gerona/61.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Ballantyne JC. Opioid analgesia: perspectives on right use and utility. Pain Physician. 2007;10:479–91. [PubMed] [Google Scholar]
- (16).Hartung DM, Middleton L, Haxby DG, Koder M, Ketchum KL, Chou R. Rates of Adverse Events of Long-Acting Opioids in a State Medicaid Program. Ann Pharmacother. 2007;41:921–28. doi: 10.1345/aph.1K066. [DOI] [PubMed] [Google Scholar]
- (17).Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med. 2003;349:1943–53. doi: 10.1056/NEJMra025411. [DOI] [PubMed] [Google Scholar]
- (18).Martell BA, O’Connor PG, Kerns RD, Becker WC, Morales KH, Kosten TR, et al. Systematic review: opioid treatment for chronic back pain: prevalence, efficacy, and association with addiction. Ann Intern Med. 2007;146:116–27. doi: 10.7326/0003-4819-146-2-200701160-00006. [DOI] [PubMed] [Google Scholar]
- (19).Furlan AD, Sandoval JA, Mailis-Gagnon A, Tunks E. Opioids for chronic noncancer pain: a meta-analysis of effectiveness and side effects. CMAJ. 2006;174:1589–94. doi: 10.1503/cmaj.051528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Breivik H. Opioids in chronic non-cancer pain, indications and controversies. European Journal of Pain. 2005;9:127–30. doi: 10.1016/j.ejpain.2004.05.013. [DOI] [PubMed] [Google Scholar]
- (21).Ballantyne JC, Shin NS. Efficacy of opioids for chronic pain: a review of the evidence. Clin J Pain. 2008;24:469–78. doi: 10.1097/AJP.0b013e31816b2f26. [DOI] [PubMed] [Google Scholar]
- (22).Von Korff M, Saunders K, Thomas Ray G, Boudreau D, Campbell C, Merrill J, et al. De facto long-term opioid therapy for noncancer pain. Clin J Pain. 2008;24:521–27. doi: 10.1097/AJP.0b013e318169d03b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Saunders KW, Davis RL, Stergachis A. Group Health Cooperative. In: Strom B, editor. Pharmacoepidemiology. John Wiley and Sons; West Sussex, England: 2005. pp. 223–39. [Google Scholar]
- (24).Fishman P, Goodman M, Hornbrook M, Meenan RT, Bachman DJ, O’Keefe Rosetti MC. Risk adjustment using automated pharmacy data: the Rx Risk Model. Med Care. 2003;41:84–99. doi: 10.1097/00005650-200301000-00011. [DOI] [PubMed] [Google Scholar]
- (25).Romano PS, Roos LL, Jollis JG. Further evidence concerning the use of a clinical comorbidity index with ICD-9-CM administrative data. Journal of Clinical Epidemiology. 1993;46:1085–90. doi: 10.1016/0895-4356(93)90103-8. [DOI] [PubMed] [Google Scholar]
- (26).Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. Springer-Verlag; New York: 2000. [Google Scholar]
- (27).SAS/STAT 9.2 User’s Guide. SAS Publishing; Mar, 2008. [Google Scholar]
- (28).Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–26. [Google Scholar]
- (29).Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113–30. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Trescot AM, Helm S, Hansen H, Benyamin R, Glaser SE, Adlaka R, et al. Opioids in the management of chronic non-cancer pain: an update of American Society of the Interventional Pain Physicians’ (ASIPP) Guidelines. Pain Physician. 2008;11:S5–S62. [PubMed] [Google Scholar]
- (31).Webster BS, Verma SK, Gatchel RJ. Relationship between early opioid prescribing for acute occupational low back pain and disability duration, medical costs, subsequent surgery and late opioid use. Spine. 2007;32:2127–32. doi: 10.1097/BRS.0b013e318145a731. [DOI] [PubMed] [Google Scholar]
- (32).Von Korff M, Deyo RA. Potent opioids for chronic musculoskeletal pain: flying blind? Pain. 2004;109:207–9. doi: 10.1016/j.pain.2004.02.019. [DOI] [PubMed] [Google Scholar]
- (33).Eriksen J, Sjogren P, Bruera E, Ekholm O, Rasmussen NK. Critical issues on opioids in chronic non-cancer pain: an epidemiological study. Pain. 2006;125:172–79. doi: 10.1016/j.pain.2006.06.009. [DOI] [PubMed] [Google Scholar]
- (34).McLellan AT, Turner B. Prescription opioids, overdose deaths, and physician responsibility. JAMA. 2008;300:2672–73. doi: 10.1001/jama.2008.793. [DOI] [PubMed] [Google Scholar]