Abstract
Objective
The objective was to determine if physiological hyperglycemia induces a proatherogenic inflammatory response in mononuclear cells (MNCs) in obese reproductiveage women.
Research Methods and Procedures
Seven obese and 6 age-matched lean women (20 to 39 years of age) underwent a 2-hour 75-g oral glucose tolerance test. The release of interleukin-6 (IL-6) and interleukin-1β (IL-1β) from MNCs cultured in the presence of lipopolysaccharide (LPS) was measured after isolation from blood samples drawn fasting and 2 hours after glucose ingestion. Reactive oxygen species (ROS) generation and intra-nuclear nuclear factor κB (NFκB) from MNCs were quantified from the same blood samples. Insulin resistance was estimated by homeostasis model assessment of insulin resistance (HOMA-IR). Total body fat and truncal fat were determined by DXA.
Results
Obese women had a higher (p < 0.03) total body fat (42.2 ± 1.1 vs. 27.7 ± 2.0%), truncal fat (42.1 ± 1.2 vs. 22.3 ± 2.4%), and HOMA-IR (3.3 ± 0.5 vs. 1.8 ± 0.2). LPS-stimulated IL-6 release from MNCs was suppressed during hyperglycemia in lean subjects (1884 ± 495 vs. 638 ± 435 pg/mL, p < 0.05) but not in obese women (1184 ± 387 vs. 1403 ± 498 pg/mL). There was a difference (p < 0.05) between groups in the hyperglycemia-induced MNC-mediated release of IL-6 (−1196 ± 475 vs. 219 ± 175 pg/mL) and IL-1β (−79 ± 43 vs. 17 ± 12 pg/mL). In addition, the obese group exhibited increased (p < 0.05) MNC-derived ROS generation (39.3 ± 9.9 vs. −1.0 ± 12.8%) and intra-nuclear NFκB (9.4 ± 7.3 vs. −23.5 ± 13.5%). Truncal fat was positively correlated with the MNC-derived IL-6 response (ρ = 0.58, p < 0.05) and intra-nuclear NFκB (ρ = 0.64, p < 0.05).
Discussion
These data suggest that obese reproductive-age women are unable to suppress proatherogenic inflammation during physiological hyperglycemia. Increased adiposity may be a significant contributor to this pro-inflammatory susceptibility.
Keywords: inflammation, atherosclerosis, hyperglycemia, abdominal adiposity
Introduction
Obesity is a major risk factor for developing atherosclerosis and hyperglycemia (1,2). Obesity is also a pro-inflammatory state as evidenced by elevated plasma concentrations of interleukin-6 (IL-6),1 interleukin-1β (IL-1β), and C-reactive protein (CRP) (3-6). Circulating IL-6 and IL-1β promote atherosclerosis by inducing endothelial expression of adhesion molecules and chemokines that cause peripheral mononuclear cells (MNCs) to attach to the endothelial layer of the blood vessel wall, and to subsequently migrate into the vascular interstitium (7-10). Several studies have documented overexpression of IL-6 and IL-1β in adipose tissue when obesity or type 2 diabetes mellitus is present, with roughly 30% of circulating IL-6 originating from fat depots (3,6,11,12). IL-6 and, to a lesser extent, IL-1β also stimulate CRP synthesis (13-15). CRP is a major predictor of atherosclerosis in asymptomatic individuals and may also play a functional role by promoting the uptake of lipids into MNC-derived foamy macrophages within atherosclerotic plaques (16-18). Although the majority of circulating CRP is produced by the liver, recent studies have shown that adipose tissue is an additional source (19).
It has recently been shown that MNCs of the obese are activated in a pro-inflammatory state (20). This is important because MNCs are known to migrate into adipose tissue to activate adipocyte cytokine production (21,22). However, it is now clear that roughly one half of IL-6 produced in adipose tissue of the obese comes from MNC-derived macrophages present in the stromal-vascular compartment (22,23). MNCs exhibit increased reactive oxygen species (ROS) generation in response to hyperglycemia. This induces oxidative stress, which, in turn, activates nuclear factor κB (NFκB), a pro-inflammatory transcription factor that promotes both IL-6 and IL-1β gene transcription (24-26).
In our previous studies of obesity-related insulin resistance, we evaluated tumor necrosis factor-alpha (TNF-α), another pro-inflammatory cytokine that is a known mediator of insulin resistance. We found that in older men and in obese young women, lipopolysaccharide (LPS)-stimulated TNF-α release from MNC was altered in response to hyperglycemia, and that the increased abdominal adiposity of these individuals was related to the altered TNF-α response (27,28). However, the hyperglycemic response of MNC-derived proatherogenic cytokines has never been explored in obese young women.
Thus, we embarked on a study to determine the status of IL-6 and IL-1β release from MNC in response to hyperglycemia in asymptomatic obese women of reproductive age. We also examined plasma IL-6 and CRP levels, as well as MNC-derived ROS generation and activated intra-nuclear NFκB. We hypothesized that LPS-stimulated release of IL-6 and IL-1β, ROS generation, and intra-nuclear NFκB from MNC of obese reproductive-age women are altered in response to an oral glucose challenge, as compared with age-matched lean controls.
Research Methods and Procedures
Subjects
Thirteen women (7 obese and 6 lean) between 20 and 39 years of age participated in the study. Obesity was defined as a BMI between 30 and 40 kg/m2. Lean subjects had a BMI between 18 and 25 kg/m2. All subjects were ovulatory, as evidenced by regular menses, and had a luteal phase serum progesterone level >5 ng/mL. All subjects were screened for diabetes, inflammatory illnesses, or endocrinopathies, and none was taking medications that would affect carbohydrate metabolism or immune function. Based on Adult Treatment Panel III guidelines to diagnose the metabolic syndrome, none of the subjects exhibited 3 or more of the following features: waist circumference >88 cm, plasma triglyceride ≥150 mg/dL, plasma high-density lipoprotein cholesterol <50 mg/dL, blood pressure ≥130 mm Hg systolic/≥ 85 mm Hg diastolic, and fasting glucose ≥110 mg/dL (29). None of the subjects was involved in any regular exercise program for at least 6 months before the time of testing. All of the subjects provided written informed consent in accordance with Institutional Review Board guidelines for the protection of human subjects.
Study Design
All study subjects underwent an oral glucose tolerance test (OGTT) between days 5 and 8 after the onset of menses. Before the OGTT, they were provided with a healthy diet consisting of 50% carbohydrate, 35% fat, and 15% protein for 3 consecutive days (Days 1 to 3). The test was performed on the morning of Day 4 after an overnight fast of ~12 hours. All subjects also underwent body composition assessment on the same day the OGTT was performed.
OGTT
Baseline blood samples were drawn for glucose and insulin determination. A 75-g glucose beverage was subsequently ingested over 10 minutes. Blood samples were again drawn for glucose and insulin determination 2 hours after glucose ingestion. On completion of the test, subjects were fed a high carbohydrate snack. Plasma glucose concentrations were assayed immediately from the blood samples collected. Additional plasma was isolated from the same blood samples and stored at −80 °C until assayed for CRP, IL-6, and lipids. Glucose tolerance was assessed by the World Health Organization criteria with normal glucose tolerance defined as a 2-hour glucose-stimulated value <140 mg/dL (30). Insulin resistance was estimated by homeostasis model assessment of insulin resistance (HOMA-IR) using the following formula: fasting glucose (mM) × fasting insulin /22.5 (31).
Body Composition Assessment
Height without shoes was measured to the nearest 1.0 cm. Body weight was measured to the nearest 0.1 kg. Waist circumference was measured at the level of the umbilicus and used to estimate abdominal adiposity (32). In addition, all subjects underwent DXA to determine percentage of total body fat and percentage of truncal fat using the QDR 4500 Elite model scanner (Hologic, Inc., Waltham, MA). Truncal fat content was defined as the area between the dome of the diaphragm (cephalad limit) and the top of the greater trochanter (caudal limit) (33).
MNC Culture
MNCs were isolated by Histopaque-1077 density gradient centrifugation from blood samples drawn while fasting and 2 hours after glucose ingestion (34). The cells were washed twice in pyrogen-free saline, resuspended in RPMI (0.3 mg/mL l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin) with TCH Serum Replacement (MD Biomedicals, Inc., Irvine, CA), and seeded in coated culture plates (VWR International, West Chester, PA) at a concentration of 2.5 × 106 cells/mL. The cells were then incubated (95% humidity, 5% CO2, 37 °C) for 24 hours with LPS endotoxin (1 ng/mL). Cell supernatants were subsequently collected by centrifugation (10,000g for 2 minutes) and stored at −80 °C until analysis.
ROS Generation
MNCs were isolated by density gradient centrifugation using polymorphonuclear cell isolation medium (Robbins Scientific Corp., Sunnyvale, CA) from blood samples drawn while fasting and 2 hours after glucose ingestion. The cells were washed twice with Hank’s buffered saline solution, and reconstituted to a concentration of 4 × 105 cells/mL in Hank’s buffered saline solution. Respiratory burst activity of MNCs was measured by detection of superoxide radical via chemiluminescence. Duplicate cuvettes containing 500 μL of MNC (400 cells/μL) were placed into a two-channel lumi-aggregometer (Chronolog Corporation, Haverton, PA). Fifteen microliters of 10 mM luminol followed by 1 μL of 10 mM formylmethionyl leucine phenylalanine were added to each cuvette. Chemilumunescence was recorded in mV by computer software (Chronolog Aggrolink). This method was developed by Thusu and Dandona (35) and is similar to that published by Tosi and Hamedani (36). In this assay system, measurement of superoxide radical release has been shown to be linearly correlated with the ferricytochrome C method (37). In addition, superoxide dismutase, catalase, and diphenylene, a specific inhibitor of nicotinamide adenine dinucleotide phosphate oxidase, have been shown to inhibit chemiluminescence in a dose-dependent fashion. The specific inhibitory effect of diphenylene iodonium on nicotinamide adenine dinucleotide phosphate oxidase has been established by Hancock and Jones (38). As previously validated, the variation of ROS generation by MNCs in humans using this method varies by <8% over a 2-week period (39).
NFκB Electrophoretic Mobility Shift Assay
Nuclear extracts of DNA-binding protein were prepared from MNCs using the method described by Andrews and Faller (40). Total protein concentrations were determined using the bicinchoninic acid protein assay (Pierce Chemical Co., Rockville, IL). An NFκB gel retardation assay was performed using the NFKB-Binding Protein Detection Kit (Life Technologies, Inc., Long Island, NY). A double-stranded oligonucleotide containing a tandem repeat of the consensus sequence for the NFκB-binding site (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) was radiolabeled with γ-P32 (GE Healthcare Bio-Sciences Corporation, Piscataway, NJ) using T4 kinase (Invitrogen, Carlsbad, CA). Nuclear extract (7.5 μg) was mixed with the incubation buffer, and the mixture was pre-incubated at 4 °C for 15 minutes. Labeled oligonucleotide (60,000 cpm) was added, and the mixture was incubated at room temperature for 20 minutes. The samples were electrophoresed on 6% non-denaturing polyacrylamide gels. The gels were dried under vacuum and exposed to X-ray film. Densitometry was performed using Kodak 1-Day Image Analysis software version 3.6 (Rochester, NY).
Plasma Measurements
Plasma glucose concentrations were measured by the glucose oxidase method (YSI, Yellow Springs, OH) while plasma insulin concentrations were measured by a double antibody radioimmunoassay (Linco Research, Inc., St. Charles, MO). Plasma CRP concentrations were measured by a high sensitivity enzyme-linked immunosorbent assay (Alpha Diagnostics International, San Antonio, TX). The concentrations of IL-6 and IL-1β were also measured by enzyme-linked immunosorbent assay (eBioscience, San Diego, CA). Levels of total cholesterol, triglyceride, and high-density lipoprotein cholesterol were measured by enzymatic methods (SYNCHRON LX20 PRO automatic analyzer; Beckman Coulter, Inc., Fullerton, CA). Low-density lipoprotein cholesterol was calculated using Friedewald’s formula (41). All samples from each subject were measured in duplicate in the same assay at the end of the study. The inter-assay and intra-assay coefficients of variation for all assays were 7% and 12%, respectively.
Statistics
The StatView statistical package (SAS Institute, Inc., Cary, NC) was used for data analysis. The difference between the pre- and post-glucose challenge values for primary dependent variables, such as the release of IL-6 and IL-1β from MNCs, was calculated to represent the incremental change. The percentage change between the pre- and post-glucose challenge values was used to express alterations in ROS generation and nuclear-bound NFκB from MNCs due to the inter-individual variability. Descriptive data and the change of variables were compared between-groups using the unpaired Student’s t test. Differences between pre- and post-glucose challenge variables within-groups were analyzed using the paired Student’s t test. Regression analyses were performed using Pearson (r) correlation for parametric data and Spearman rank order (ρ) correlation for non-parametric data. All values are expressed as means ± standard error. An α level of 0.05 was used to determine statistical significance.
Results
Body Composition, Glycemic Status, and Insulin Resistance
Age, height, and lipid levels were similar between groups, and all subjects were normotensive. The obese group had significantly higher (p < 0.003) weight, BMI, percentage of total body fat, percentage of truncal fat, and waist circumference (Table 1).
Table 1.
Body composition, fasting lipid levels, and insulin resistance of subjects
| Lean (n = 6) |
Obese (n = 7) |
|
|---|---|---|
| Age (yrs) | 33 ± 2 | 29 ± 3 |
| Systolic blood pressure (mm Hg) |
104 ± 3 | 117 ± 6 |
| Diastolic blood pressure (mm Hg) |
56 ± 3 | 76 ± 4* |
| Height (cm) | 166 ± 1.0 | 163 ± 2.8 |
| Body weight (kg) | 59.1 ± 2.2 | 92.1 ± 4.0* |
| BMI (kg/m2) | 21.6 ± 0.8 | 34.8 ± 1.2* |
| Total body fat (%) | 27.7 ± 2.0 | 42.2 ± 1.1* |
| Truncal fat (%) | 22.3 ± 2.4 | 42.1 ± 1.0* |
| Waist circumference (cm) | 71.3 ± 2.0 | 101.6 ± 3.5* |
| Total cholesterol (mg/dL) | 164 ± 7 | 201 ± 23 |
| Triglycerides (mg/dL) | 55 ± 6 | 121 ± 44 |
| HDL-cholesterol (mg/dL) | 52 ± 3 | 50 ± 3 |
| LDL-cholesterol (mg/dL) | 105 ± 6 | 121 ± 19 |
HDL, high-density lipoprotein; LDL, low-density lipoprotein. Values are mean ± standard error.
Significantly different from lean group (p < 0.003).
Levels of glucose while fasting and 2 hours post-glucose ingestion were similar in both groups (Table 2). All subjects had a normal glucose response during the OGTT with 2-hour glucose levels ranging between 62 and 138 mg/dL. However, the 2-hour glucose level in the obese group and the 2-hour insulin level in both groups were significantly increased (p < 0.02) compared with fasting levels. HOMA-IR was significantly greater (p < 0.05) in the obese group compared with the lean control group. HOMA-IR was positively correlated with percentage of total body fat (r = 0.67, p < 0.02), percentage of truncal fat (r = 0.67, p < 0.02), and waist circumference (r = 0.82, p < 0.004) for the combined groups (data not shown).
Table 2.
Plasma glucose, insulin, IL-6, and C-reactive protein levels while fasting and in response to the oral glucose challenge, and HOMA-IR in lean and obese women
| Fasting | 2 hours post-glucose |
|
|---|---|---|
| Glucose (mg/dL) | ||
| Lean | 85.3 ± 2.0 | 99.0 ± 11.8 |
| Obese | 82.9 ± 4.4 | 119.4 ± 5.5* |
| Insulin (μU/mL) | ||
| Lean | 8.5 ± 1.2 | 33.1 ± 8.8* |
| Obese | 14.0 ± 2.3 | 88.2 ± 24.2* |
| HOMA-IR (mM) × fasting insulin (μU/mL) | ||
| Lean | 1.8 ± 0.02 | |
| Obese | 3.3 ± 0.05† | |
| Plasma IL-6 (pg/mL) | ||
| Lean | 0.68 ± 0.06 | 0.72 ± 0.11 |
| Obese | 2.26 ± 0.61† | 2.99 ± 0.61† |
| CRP (ng/mL) | ||
| Lean | 219 ± 92 | 221 ± 99 |
| Obese | 7215 ± 941† | 6701 ± 1001† |
IL-6, interleukin-6; CRP, C-reactive protein; HOMA-IR, homeostasis model assessment of insulin resistance. Values are mean ± standard error.
Two hours post-glucose significantly higher than fasting (p < 0.02).
Significantly higher than the lean group (p < 0.05).
IL-6 and IL-1β Release
LPS-stimulated IL-6 release from MNC in the fasting state was similar in both groups. However, fasting LPS-stimulated IL-1β release was significantly (p < 0.02) lower in the obese group. Hyperglycemia resulted in significant (p < 0.05) suppression of LPS-stimulated IL-6 release and modest suppression of IL-1β release from MNC of lean controls, but no change in either of these cytokine responses in obese women (Figure 1). In addition, the incremental change in the MNC-derived IL-6 and IL-1β response between the two groups was significantly different (p < 0.05).
Figure 1.
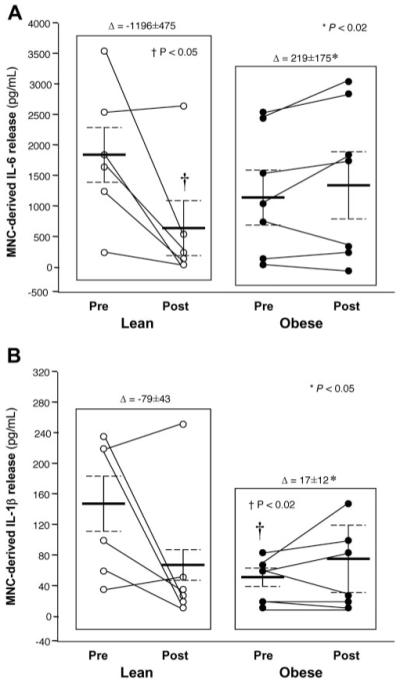
(A) Interleukin-6 (IL-6) release (pg/mL) from mononuclear cells cultured with lipopolysaccharide for 24 hours when fasting samples (pre) were compared with the samples collected 2 hours after glucose ingestion (post). * Incremental change (Δ) in IL-6 release during oral glucose challenge in the obese group was significantly different from that of lean controls (p < 0.02). † Two hours post-glucose was significantly lower than fasting in the lean group (p < 0.05). (B) Interleukin-1β (IL-1β) release (pg/mL) from mononuclear cells cultured with lipopolysaccharide for 24 hours when fasting samples (pre) were compared with the samples collected 2 hours after glucose ingestion (post). * Incremental change in IL-1β release during oral glucose challenge in the obese group was significantly different from that of lean controls (p < 0.05). † Fasting was significantly lower than in the lean group (p < 0.02).
There was a direct correlation between the incremental change in IL-6 release from MNC and that of IL-1β (ρ = 0.64, p < 0.04). There was also a direct relationship between the MNC-derived incremental change in IL-6 release and percentage of total body fat, percentage of truncal fat, and waist circumference for the combined groups (Table 3). There was no correlation between the incremental change in IL-1β release from MNC and any of these body composition parameters.
Table 3.
Spearman rank correlations for the combined groups
| Plasma IL-6 (pg/mL) |
Plasma CRP (ng/mL) |
ROS (% change) |
NFκB (% change) |
MNC IL-6 (pg/mL) |
MNC IL-1β (pg/mL) |
||
|---|---|---|---|---|---|---|---|
| HOMA-IR (mM) × fasting insulin (μU/mL) |
ρ | 0.700 | 0.622 | 0.164 | 0.673 | 0.302 | 0.336 |
| p | 0.027* | 0.039* | 0.605 | 0.044* | 0.295 | 0.266 | |
| BMI (kg/m2) | ρ | 0.811 | 0.665 | 0.655 | 0.164 | 0.516 | 0.238 |
| p | 0.007* | 0.021* | 0.039* | 0.624 | 0.074 | 0.430 | |
| Total body fat (%) | ρ | 0.699 | 0.791 | 0.418 | 0.588 | 0.582 | 0.189 |
| p | 0.020* | 0.006* | 0.186 | 0.078 | 0.044* | 0.531 | |
| Truncal fat (%) | ρ | 0.727 | 0.797 | 0.455 | 0.636 | 0.567 | 0.217 |
| p | 0.016* | 0.006* | 0.151 | 0.049* | 0.049* | 0.472 | |
| Waist circumference (cm) | ρ | 0.733 | 0.618 | 0.217 | 0.333 | 0.700 | 0.491 |
| p | 0.028* | 0.049* | 0.540 | 0.378 | 0.027* | 0.141 | |
| Plasma IL-6 (pg/mL) | ρ | — | 0.790 | 0.552 | 0.552 | 0.476 | 0.154 |
| p | — | 0.009* | 0.098 | 0.098 | 0.115 | 0.610 | |
| Plasma CRP (ng/mL) | ρ | 0.790 | — | 0.627 | 0.758 | 0.495 | 0.126 |
| p | 0.009* | — | 0.047* | 0.023* | 0.087 | 0.676 |
IL-6, interleukin-6; CRP, C-reactive protein; ROS, reactive oxygen species; NFκB, nuclear factor κB; MNC, mononuclear cell; IL-1β, interleukin-1β; HOMA-IR, homeostasis model assessment of insulin resistance.
p < 0.05.
Plasma CRP and IL-6, ROS Generation, and Intra-nuclear NFκB
Fasting plasma concentrations of CRP and IL-6 were significantly (p < 0.05) higher in the obese group but remained unchanged after glucose ingestion in both groups (Table 2). The percentage change in MNC-derived ROS generation and intra-nuclear NFκB in response to the oral glucose load was significantly (p < 0.05) higher in the obese group compared with that of lean controls (Figure 2). For the combined groups, there was a positive correlation between fasting plasma IL-6 and CRP. Each of these levels was positively correlated with BMI, percentage of total body fat, percentage of truncal fat and waist circumference. Plasma CRP was also positively correlated with ROS generation and intra-nuclear NFκB. HOMA-IR was positively correlated with fasting plasma IL-6 and CRP, and with intra-nuclear NFκB. There was also a positive correlation between ROS generation and BMI, and between intra-nuclear NFκB and percentage of truncal fat (Table 3).
Figure 2.
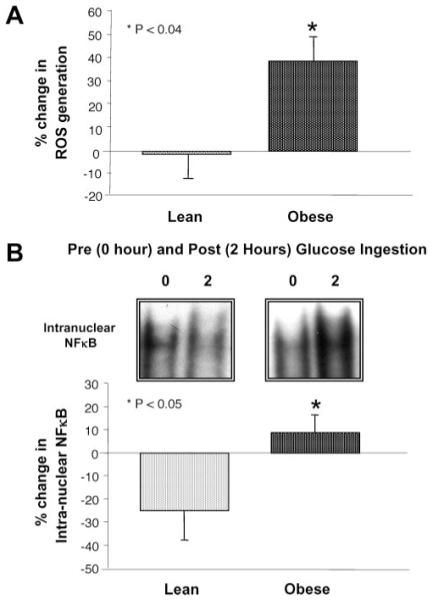
(A) The percentage change in ROS generation from mononuclear cells (MNC) when fasting samples (pre) were compared with the samples collected 2 hours after glucose ingestion (post). * ROS generation in the obese group was significantly greater compared with lean controls (p < 0.04). (B) Representative bands from the electrophoretic mobility shift assay showing the change in quantity of NFκB in nuclear extracts, and densitometric analysis of intra-nuclear NFκB protein content from MNC when fasting samples (pre) were compared with the samples collected 2 hours after glucose ingestion (post). * The percentage change in intra-nuclear NFκB was significantly greater in the obese group compared with lean controls (p < 0.05).
Discussion
Our data clearly show that obese reproductive-age women have impaired suppression of IL-6 and IL-1β release from MNC in response to physiological hyperglycemia. In contrast, the release of these cytokines from MNC was suppressed in lean controls of similar age under postprandial-like conditions. ROS generation, a promoter of oxidative stress; intra-nuclear NFκB, the cardinal signal of inflammation; and plasma IL-6 and CRP levels were also elevated in the obese group compared with lean controls. These findings in our study population provide further support for the role of inflammation in the development of atherosclerosis, and that in obese women, MNCs are likely to be involved in atherogenesis at an early age. Furthermore, the independent associations between multiple biomarkers of inflammation and abdominal adiposity suggest that the accumulation of abdominal fat is an important contributing factor in promoting atherogenesis in obese reproductive-age women.
Suppression of MNC-derived inflammation may be the normal in vivo response to physiological hyperglycemia. Lean controls in the present study showed decreases in LPS-stimulated release of IL-6 and IL-1β from MNC in response to the oral glucose load. These results are similar to the hyperglycemia-induced TNF-α response that we have previously reported in lean young healthy men and women (27,28). Modest declines in MNC-derived ROS generation and intra-nuclear NFκB were also observed in lean controls. Our findings differ from those of previous studies that reported pro-inflammatory effects after an oral glucose challenge in normal subjects (42-44). The majority of subjects in these studies were men, which may account for the different results. Because insulin is an anti-inflammatory hormone (39,45), the postprandial insulin release in the more insulin sensitive lean controls may reverse the hyperglycemia-induced pro-inflammatory response. In this scenario, the suppressed inflammatory response may serve as a protective mechanism to preserve blood vessel integrity in the presence of hyperglycemia. Increased release of IL-6 and IL-1β after NFκB activation by ROS-induced oxidative stress may trigger MNCs to adhere to the endothelium and migrate into the vascular interstitium (10,24-26). Thus, young lean individuals may be capable of retarding atherogenesis by controlling the release of IL-6 and IL-1β in the postprandial state.
In contrast, the MNCs of obese reproductive-age women may have an impaired ability to down-regulate the inflammatory response to physiological hyperglycemia. Indeed, the elevations in plasma IL-6 and CRP observed in this group confirm previous reports demonstrating that obesity is a pro-inflammatory state (5,6,46). The increases in ROS generation and intra-nuclear NFκB, and the failed suppression of MNC-derived IL-6 and IL-1β release in the obese group provide further corroboration, and is consistent with our previous report of failed suppression of TNF-α from MNCs in obese young women (28). It is paradoxical that IL-1β release in the fasting state is low in the obese group, raising the possibility that the study of MNC in vitro may not reflect physiology in vivo. The direct relationship of plasma CRP with ROS generation, intra-nuclear NFκB, and plasma IL-6 are consistent with previous investigations demonstrating that CRP production in the obese is the culmination of oxidative stress-induced ROS generation that activates NFκB to alter the transcription of IL-6 and IL-1β (24-26). Furthermore, obese reproductive-age women also exhibited evidence of insulin resistance based on an increase in HOMA-IR, a feature highly associated with atherosclerosis (47). This is confirmed in the present study by the direct relationship of HOMA-IR with intranuclear NFκB and plasma IL-6 and CRP levels. Further corroboration is provided by recent evidence that, in the obese, tyrosine phosphorylation of the insulin receptor is inversely related to pro-inflammatory mediators and HOMA-IR (48). Thus, our data suggest that chronically increased macronutrient intake leading to obesity at an early age results in an increase in oxidative stress and inflammation. A subsequent failure to suppress MNC-derived inflammation in the postprandial state may promote atherosclerosis. This concept is further supported by previous reports of a reduction in oxidative stress and inflammatory mediators after caloric restriction in the obese, and after a 2-day fast in normal subjects (49-51).
Our data suggest that there may be a link between obesity and MNC-derived inflammation that promotes atherosclerosis. There was a direct relationship between MNC-derived ROS generation, intra-nuclear NFκB and IL-6 release, as well as plasma IL-6 and CRP in response to physiological hyperglycemia, and measures of adiposity, particularly abdominal adiposity. Obese reproductive-age women with insulin resistance exhibited increased BMI, percentage of total body fat, percentage of truncal fat, and waist circumference. Because activated MNC-derived macrophages produce roughly one half of the IL-6 in the expanded adipose mass of obese individuals, it is possible that the inflamed adipose tissue of the obese, especially in the abdominal region, perpetuates the inability to suppress inflammation after hyperglycemia (22,25). It remains to be established whether failed suppression of inflammation is also evident in MNC-derived macrophages within the adipose tissue stromal-vascular compartment of obese individuals. Nevertheless, these data are striking because they suggest that obesity-related glucose or macronutrient-induced inflammation may initiate a proatherogenic milieu in women at an early age.
In summary, obese reproductive-age women are unable to suppress inflammation during physiological hyperglycemia. The resultant increases in MNC-derived ROS generation and intra-nuclear NFκB, failed suppression of LPS-stimulated IL-6 and IL-1β release from MNC, and increased plasma IL-6 and CRP levels compared with a lean control group demonstrates that inflammation can be a factor that places these individuals at an increased risk of developing atherosclerosis. The association between mediators of inflammation and both total and abdominal fat suggests that increased adiposity is a significant contributor to the proinflammatory state in obese young women. Thus, it is intriguing to consider the possibility that when glucose increases during the postprandial period in lean individuals, inflammation is suppressed to protect against atherogenesis. Conversely, the loss of this postprandial response may be one of the factors that contribute to the promotion of atherogenesis in obese women at an early age.
Acknowledgments
The authors thank Husam Ghanim of the State University of New York at Buffalo for providing expert advice on laboratory techniques. This research was supported by NIH Grant HD-048,535 (to F.G.).
Footnotes
- IL-6
- interleukin-6
- IL-1β
- interleukin-1β
- CRP
- C-reactive protein
- MNC
- mononuclear cell
- ROS
- reactive oxygen species
- NFκB
- nuclear factor κB
- TNF-α
- tumor necrosis factor-alpha
- LPS
- lipopolysaccharide
- OGTT
- oral glucose tolerance test
- HOMA-IR
- homeostasis model assessment of insulin resistance
References
- 1.National Institutes of Health, NHLBI Clinical guidelines on the identification, evaluation and treatment of overweight and obesity in adults: the evidence report. Obes Res. 1998;6(Suppl 2):51–209. [PubMed] [Google Scholar]
- 2.Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122:481–6. doi: 10.7326/0003-4819-122-7-199504010-00001. [DOI] [PubMed] [Google Scholar]
- 3.Mohamed-Ali V, Goodrick S, Rawesh A, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-α, in vivo. J Clin Endocrinol Metab. 1997;82:4196–200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 4.Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab. 1997;82:1313–6. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- 5.Bastard JP, Jardel C, Bruckert E, et al. Elevated levels of interleukin-6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J Clin Endocrinol Metab. 2000;85:3338–42. doi: 10.1210/jcem.85.9.6839. [DOI] [PubMed] [Google Scholar]
- 6.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–8. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 7.Marui N, Offermann MK, Swerlick R, et al. Vascular cell adhesion molecule-1 (VCAM-1) gene transcription and expression are regulated through an antioxidant-sensitive mechanism in human vascular endothelial cells. J Clin Invest. 1993;92:1866–74. doi: 10.1172/JCI116778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romano M, Sironi M, Toniatti C, et al. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity. 1997;6:315–25. doi: 10.1016/s1074-7613(00)80334-9. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Feuerstein GZ, Gu JL, Lysko PG, Yue TL. Interleukin–1 beta induces expression of adhesion molecules in human vascular smooth muscle cells and enhances adhesion of leukocytes to smooth muscle cells. Atherosclerosis. 1995;115:89–98. doi: 10.1016/0021-9150(94)05503-b. [DOI] [PubMed] [Google Scholar]
- 10.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 11.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissue of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–50. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 12.Puroht A, Ghilchik MW, Duncan L, et al. Aromatase activity and interleukin-6 production by normal and malignant breast tissues. J Clin Endocrinol Metab. 1995;80:3052–8. doi: 10.1210/jcem.80.10.7559896. [DOI] [PubMed] [Google Scholar]
- 13.Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265:621–36. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bataille R, Klein B. C-reactive protein levels as a direct indicator of interleukin-6 levels in humans in vivo. Arthritis Rheum. 1992;35:982–3. doi: 10.1002/art.1780350824. [DOI] [PubMed] [Google Scholar]
- 15.Calabro P, Wilerson JT, Yeh ET. Inflammatory cytokines stimulated C-reactive protein production by human coronary artery smooth muscle cells. Circulation. 2003;108:1930–2. doi: 10.1161/01.CIR.0000096055.62724.C5. [DOI] [PubMed] [Google Scholar]
- 16.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 17.Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein (a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001;285:2481–5. doi: 10.1001/jama.285.19.2481. [DOI] [PubMed] [Google Scholar]
- 18.Zwaka TP, Hombach V, Torzewski J. C-reactive protein-mediated low density lipoprotein uptake by macrophages: implications for atherosclerosis. Circulation. 2001;103:1194–7. doi: 10.1161/01.cir.103.9.1194. [DOI] [PubMed] [Google Scholar]
- 19.Ouchi N, Kihara S, Fanahashi T, et al. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation. 2003;107:671–4. doi: 10.1161/01.cir.0000055188.83694.b3. [DOI] [PubMed] [Google Scholar]
- 20.Ghanim H, Aljada A, Hoffmeyer D, Syed T, Mohanty P, Dandona P. Circulating mononuclear cells in the obese are in a proinflammatory state. Circulation. 2004;110:1564–71. doi: 10.1161/01.CIR.0000142055.53122.FA. [DOI] [PubMed] [Google Scholar]
- 21.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–8. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fain JN, Madan AK, Hyler ML, Cheema P, Bahouth SM. Comparison of the release of adiponectin by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–82. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 24.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 25.Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H, Dandona P. Glucose challenge stimulates reactive oxygen species generation by leucocytes. J Clin Endocrinol Metab. 2000;85:2970–3. doi: 10.1210/jcem.85.8.6854. [DOI] [PubMed] [Google Scholar]
- 26.Barnes PJ, Karin M. Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–71. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 27.Kirwan JP, Krishnan RK, Weaver JA, Del Aguila LF, Evans WJ. Human aging is associated with altered TNF-α production during hyperglycemia and hyperinsulinemia. Am J Physiol Endocrinol Metab. 2001;281:E1137–43. doi: 10.1152/ajpendo.2001.281.6.E1137. [DOI] [PubMed] [Google Scholar]
- 28.González F, Minium J, Rote NS, Kirwan JP. Altered tumor necrosis factor α release from mononuclear cells of obese reproductive age women during hyperglycemia. Metabolism. 2006;55:271–6. doi: 10.1016/j.metabol.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 29.Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 30.Modan M, Harris MI, Halkin H. Evaluation of WHO and NDDG criteria for impaired glucose tolerance: results from two national samples. Diabetes. 1989;38:1630–5. doi: 10.2337/diab.38.12.1630. [DOI] [PubMed] [Google Scholar]
- 31.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 32.Kohrt WM, Kirwan JP, King DS, Staten MA, Holloszy JO. Insulin resistance of aging is related to abdominal obesity. Diabetes. 1993;42:273–81. [PubMed] [Google Scholar]
- 33.Taylor RW, Keil D, Gold EJ, Williams SM, Goulding A. Body mass index, waist girth, and waist to hip ratio as indexes of total and regional adiposity in women: evaluation using receiver operating characteristic curves. Am J Clin Nutr. 1988;67:44–9. doi: 10.1093/ajcn/67.1.44. [DOI] [PubMed] [Google Scholar]
- 34.Boyam A. Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Invest. 1968;21(suppl):77–89. [PubMed] [Google Scholar]
- 35.Thusu K, Abdel-Rahman E, Dandona P. Measurement of reactive oxygen species in whole blood and mononuclear cells using chemiluminescence. Methods Mol Biol. 1998;196:99–103. doi: 10.1385/0-89603-472-0:57. [DOI] [PubMed] [Google Scholar]
- 36.Tosi MF, Hamedani A. A rapid specific assay for superoxide release from phagocytes in small volumes of whole blood. Am J Clin Pathol. 1992;97:566–73. doi: 10.1093/ajcp/97.4.566. [DOI] [PubMed] [Google Scholar]
- 37.Land EJ, Swallow AJ. One-electron reactions in biochemical systems as studied by pulse radiolysis: V. Cytochrome c. Arch Biochem Biophys. 1971;145:365–72. doi: 10.1016/0003-9861(71)90049-x. [DOI] [PubMed] [Google Scholar]
- 38.Hancock JT, Jones OT. The inhibition by diphenyleneiodonium and its analogues of superoxide generation by macrophages. Biochem J. 1987;242:103–7. doi: 10.1042/bj2420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dandona P, Aljada A, Mohanty P, et al. Insulin inhibits intranuclear factor κB and stimulates IκB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? J Clin Endocrinol Metab. 2001;86:3257–65. doi: 10.1210/jcem.86.7.7623. [DOI] [PubMed] [Google Scholar]
- 40.Andrews NC, Faller DV. A rapid micropreparation technique for extraction of DNA-binding proteins from limited numbers of mammalian cells. Nucleic Acids Res. 1991;19:2499. doi: 10.1093/nar/19.9.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 42.Aljada A, Friedman J, Ghanim H, et al. Glucose ingestion induces an increase in intranuclear nuclear factor kappaB, a fall in cellular inhibitor kappaB, and in increase in tumor necrosis factor alpha messenger RNA by mononuclear cells in healthy human subjects. Metabolism. 2004;55:1177–85. doi: 10.1016/j.metabol.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 43.Aljada A, Mohanty P, Ghanim H, et al. Increase in intranuclear nuclear factor kappaB and decrease in inhibitor kappaB in mononuclear cells after a mixed meal: evidence for a proinflammatory effect. Am J Clin Nutr. 2004;79:682–90. doi: 10.1093/ajcn/79.4.682. [DOI] [PubMed] [Google Scholar]
- 44.Aljada A, Ghanim H, Mohanty P, Syed T, Bandyopadhyay A, Dandona P. Glucose intake induces an increase in activator protein 1 and early growth response 1 binding activities, in the expression of tissue factor and matrix metalloproteinase concentrations. Am J Clin Nutr. 2004;80:51–7. doi: 10.1093/ajcn/80.1.51. [DOI] [PubMed] [Google Scholar]
- 45.Chaudhuri A, Janicke D, Wilson MF, et al. Anti-inflammatory and profibrinolytic effect of insulin in acute ST-segment-elevation myocardial infarction. Circulation. 2004;109:849–54. doi: 10.1161/01.CIR.0000116762.77804.FC. [DOI] [PubMed] [Google Scholar]
- 46.Bastard JP, Jardel C, Delattre J, Hainque B, Bruckert E, Oberlin F. Evidence for a link between adipose tissue interleukin-6 content and serum C-reactive protein concentrations in obese subjects. Circulation. 1999;99:2221–2. [PubMed] [Google Scholar]
- 47.Nigro J, Osman N, Dart AM, Little PJ. Insulin resistance and atherosclerosis. Endocr Rev. 2006;27:242–59. doi: 10.1210/er.2005-0007. [DOI] [PubMed] [Google Scholar]
- 48.Ghanim H, Aljada A, Daoud N, Deopurkar R, Chaudhura A, Dandona P. Role of inflammatory mediators in the suppression of insulin receptor phosphorylation in circulating mononuclear cells of obese subjects. Diabetologia. 2007;50:278–85. doi: 10.1007/s00125-006-0508-9. [DOI] [PubMed] [Google Scholar]
- 49.Dandona P, Weinstock R, Thusu K, Abdel-Rahman E, Aljada A, Wadden T. Tumor necrosis factor-α in sera of obese patients: fall with weight loss. J Clin Endocrinol Metab. 1998;83:2907–10. doi: 10.1210/jcem.83.8.5026. [DOI] [PubMed] [Google Scholar]
- 50.Dandona P, Mohanty P, Ghanim H, et al. The suppressive effect of dietary restriction and weight loss in the obese on the generation of reactive oxygen species by leukocytes, lipid peroxidation, and protein carboxylation. J Clin Endocrinol Metab. 2001;86:355–62. doi: 10.1210/jcem.86.1.7150. [DOI] [PubMed] [Google Scholar]
- 51.Dandona P, Mohanty P, Hamouda W, et al. Inhibitory effect of a two day fast on reactive oxygen species (ROS) generation by leucocytes and plasma ortho-tyrosine and metatyrosine concentrations. J Clin Endocrinol Metab. 2001;86:2899–902. doi: 10.1210/jcem.86.6.7745. [DOI] [PubMed] [Google Scholar]


