Abstract
The ventricular zone (VZ) of the embryonic dorsal telencephalon is a major site for generating cortical projection neurons. The transcription factor Pax6 is highly expressed in apical progenitors (APs) residing in the VZ from the earliest stages of corticogenesis. Previous studies mainly focused on Pax6−/− mice have implicated Pax6 in regulating cortical progenitor proliferation, neurogenesis, and formation of superficial cortical layers. We analyzed the developing cortex of PAX77 transgenic mice that overexpress Pax6 in its normal domains of expression. We show that Pax6 overexpression increases cell cycle length of APs and drives the system toward neurogenesis. These effects are specific to late stages of corticogenesis, when superficial layer neurons are normally generated, in cortical regions that express Pax6 at the highest levels. The number of superficial layer neurons is reduced in postnatal PAX77 mice, whereas radial migration and lamina specification of cortical neurons are not affected by Pax6 overexpression. Conditional deletion of Pax6 in cortical progenitors at midstages of corticogenesis, by using a tamoxifen-inducible Emx1-CreER line, affected both numbers and specification of late-born neurons in superficial layers of the mutant cortex. Our analyses suggest that correct levels of Pax6 are essential for normal production of superficial layers of the cortex.
Keywords: cortex, lamination, neurogenesis, Pax6, proliferation
Introduction
The mammalian cerebral cortex is organized radially into 6 layers, each composed of neurons with characteristic morphologies, patterns of connectivity, and gene expression. Lamina formation in the developing cortex requires precise generation and migration of cortical neurons. Progenitors in the ventricular zone (VZ) and subventricular zone (SVZ) of the mouse dorsal telencephalon undergo 11 cell cycles over a 6-day period to generate neurons destined for the cortical plate (CP) (Takahashi et al. 1995). The cell cycle length of VZ progenitors increases with time as cortical neurogenesis proceeds, whereas it remains unchanged in SVZ progenitor cells (Takahashi et al. 1995). After cell cycle withdrawal, newborn neurons migrate to the CP successively such that layers II–VI are formed in a deep-first/superficial-last sequence (Angevine and Sidman 1961; Rakic 1974). The final laminar position and subtype of cortical projection neurons is highly dependent on the time progenitors undergo final mitosis and exit the cell cycle (McConnell and Kaznowski 1991; Takahashi et al. 1999).
Throughout corticogenesis, progenitor cell divisions generate cells that must decide whether to reenter the cell cycle or exit the cell cycle and differentiate into neurons with defined laminar fates. A number of transcription factors and cell cycle regulators have been implicated as intrinsic regulators of the decision to proliferate or differentiate. Increasing or decreasing the levels of these proteins disrupts the balance between the numbers of progenitor cells and differentiated cells and can lead to changes in the surface area and thickness of specific layers of the mature cortex (Caviness and Takahashi 1995; Rakic 1995; Kornack and Rakic 1998; Chenn and Walsh 2002, 2003; Caviness et al. 2003).
The paired-box transcription factor Pax6 is expressed in the mouse from as early as embryonic day (E) 8.5 in tissues that include the telencephalic primordium (Walther and Gruss 1991; Stoykova and Gruss 1994). Pax6−/− mutant mice exhibit severe brain defects, including an abnormally thin CP and an expanded proliferative zone (Schmahl et al. 1993; Stoykova et al. 1996; Caric et al. 1997; Stoykova et al. 2000; Tarabykin et al. 2001), lack eyes and nasal structures, and die soon after birth (Hogan et al. 1986; Hill et al. 1991). Throughout corticogenesis, Pax6 is expressed mainly in progenitors residing in the VZ, known as apical progenitors (APs) (Gotz et al. 1998; Englund et al. 2005). Several studies have indicated an essential role for Pax6 in regulating the proliferation of cortical progenitors, their commitment to a dorsal and neuronal fate, and the formation of superficial cortical layers (Schmahl et al. 1993; Gotz et al. 1998; Warren et al. 1999; Tarabykin et al. 2001; Estivill-Torrus et al. 2002; Heins et al. 2002; Talamillo et al. 2003; Hack et al. 2004; Schuurmans et al. 2004; Kroll and O'Leary 2005; Quinn et al. 2007; Manuel et al. 2007; Sansom et al. 2009; Tuoc et al. 2009). To date, the role of Pax6 in cortical development has been examined mainly through loss-of-function studies in mutant mice.
In a previous study (Manuel et al. 2007), we examined corticogenesis in PAX77 transgenic mice that carry approximately 6 additional copies of the human PAX6 gene, which produces protein identical to mouse Pax6 (Schedl et al. 1996). In these mice, overexpression of Pax6 is increased about 1.5- to 3-fold and is confined to the normal domains of Pax6 expression (Manuel et al. 2007). We found that Pax6 overexpression acts cell autonomously to impair the production of late-born cortical cells in rostral regions, where Pax6 is normally highly expressed, but the cause of this defect was not defined. In the present study, we investigated the underlying mechanisms by examining cell cycle kinetics, cell cycle exit, neuronal differentiation, and radial migration. We found that cell cycle length and cell cycle exit are increased at later stages of corticogenesis in APs in rostral cortical areas of mice overexpressing Pax6. Radial migration of late-born neurons and also laminar fate specification were unaffected in the PAX77 cortex. Taken together, these data suggest that correct levels of Pax6 are critical primarily for cell cycle regulation and control of the proportion of cells that reenter the cell cycle instead of leaving it to differentiate.
In light of the effects of Pax6 overexpression on superficial laminar development, we examined directly its function in late cortical development by studying the consequences of Pax6 deletion on layer formation. To overcome the lethality of the conventional Pax6 null mutants, we knocked out Pax6 specifically in the developing dorsal telencephalon by using a transgenic line that expressed the tamoxifen-inducible form of Cre recombinase (CreERT2) targeted to the Emx1 locus (Kessaris et al. 2006). Cre activity was induced by administering tamoxifen at E10.5. Cortex-specific mutants lacking Pax6 from midstages of corticogenesis exhibited a notable reduction in cortical tissue with respect to controls. We noted a dramatic reduction of late-born neurons in the superficial layers of mutant cortices; these late-born neurons of the mutant cortex were also not correctly specified. Collectively, our analyses of gain- and loss-of-function of Pax6 suggest that disruption of Pax6 levels leads ultimately to impaired formation of superficial layers but through different cellular and molecular mechanisms.
Materials and Methods
Animals
PAX77 hemizygous mice carry 5–7 copies of a 420-kb human genomic fragment incorporating the PAX6 gene (Schedl et al. 1996). The mice were maintained on a CD1 background. PAX77 males were mated to CD1 females to generate littermates for experiments. The date of conception was established by the presence of a vaginal plug and recorded as E0.5. The first 24 h after birth was defined as postnatal day (P) 0. To specifically inactivate Pax6 in the developing cerebral cortex, we used Pax6 floxed mutant mice (Pax6loxP/loxP) (Simpson et al. 2009) and a transgenic line that expressed the tamoxifen-inducible form of Cre recombinase (CreERT2) under the control of the Emx1 locus (Kessaris et al. 2006). The lines above were crossed with Rosa26R-YFP reporter mice (Srinivas et al. 2001) to generate triple transgenics in which the expression of the Cre transgene could be monitored. All transgenic lines were maintained by backcrossing with CD1 animals. To activate Cre activity, we administered tamoxifen (Sigma; T5648) dissolved by sonication, at a concentration of 50 mg/ml in corn oil (Sigma; C8267). Induction in embryos was performed using a single 10-mg dose of tamoxifen administered by gavage into the stomach of pregnant mothers bearing E10.5 embryos. Embryonic or postnatal brains were harvested at the times specified after tamoxifen treatment. For postnatal analysis, a caesarean section was performed on pregnant mothers in the afternoon on E18.5 and pups were fostered. Control animals were Pax6loxP/+; Emx1-CreERT2 and mutant animals were Pax6loxP/loxP; Emx1-CreERT2. In all cases, littermates were analyzed. All the experimental procedures were performed in accordance with institutional guidelines and UK Home Office regulations.
Tissue Preparation
To obtain embryos, pregnant females were deeply anesthetized by isoflurane inhalation. Pups were deeply anesthetized with sodium pentobarbitone (50 mg, intraperitoneally) and perfused transcardially with phosphate-buffered saline (PBS) followed by fixation with 4% paraformaldehyde (PFA) in PBS. Brains from embryos and pups were fixed overnight in 4% PFA in PBS at 4 °C. Tails from pups or trunks from embryos were collected for genotyping. For frozen sections, postnatal brains were cryoprotected in a series of sucrose gradients up to 30% sucrose in PBS at 4 °C before being embedded in optimal cutting temperature compound. Embryonic brains were cryoprotected in 15% sucrose in PBS at 4 °C overnight before being embedded in 15% sucrose in PBS/7.5% gelatine (Sigma). All samples were serially sectioned on a Leica cryostat in the coronal plane. Frozen samples were sectioned at 14 μm (embryonic brains) or at 20 μm (postnatal brains), whereas brains embedded in paraffin wax were sectioned at 10 μm. Paraffin sections were mounted onto poly-L-lysine–coated slides, whereas cryosections were mounted onto superfrost plus slides and stored at −20 °C until use.
Bromodeoxyuridine/Iododeoxyuridine Incorporation Studies
Timed-pregnant females bearing wild-type and PAX77 embryos were given thymidine analogues (0.2 ml of 10 mg/ml, dissolved in 0.9% NaCl) by intraperitoneal injection. The thymidine analogues, 5-bromo-2′-deoxyuridine (BrdU) and 5-iodo-2′-deoxyuridine (IdU) (Sigma-Aldrich), are incorporated into cells during S-phase of the cell cycle. For cell cycle analysis, pregnant mothers received a single injection of IdU, and after 1.5 h, BrdU was injected. Mice were sacrificed 30 min after the BrdU injection (Martynoga et al. 2005). To estimate the leaving (Q) fraction (Takahashi et al. 1994; Tarui et al. 2005), IdU was administered to E15.5 pregnant females at 9.00 AM, followed by a BrdU injection at 10.30 AM (1.5 h later). The BrdU injection was followed by a series of 7 additional BrdU injections given at 3-h intervals. Pregnant females were sacrificed 30 min after the last BrdU injection. For analysis of migration, pregnant females were given a single injection of BrdU at E15.5 or E17.5 and offspring were perfused at P7. In all cases, brains were removed, sectioned, and processed for immunohistochemistry.
Immunohistochemistry
Following deparaffinization in xylene, wax brain sections were rehydrated through a graded ethanol series and PBS and microwaved in a solution of 0.01 M sodium citrate, pH 6.0, for 20 min. Cryosections were air-dried for 30 min at room temperature (RT), washed with PBS, and boiled in 0.01 M sodium citrate, pH 6.0. Sections were washed with PBS and 0.1% Triton X-100 before applying blocking solution (20% goat or donkey serum in PBS, 0.1% Triton X-100) for 30 min at RT. Sections were then incubated overnight at 4 °C with primary antibodies diluted in blocking solution. Primary antibodies used were mouse anti-BrdU/IdU (BD Biosciences; 1:50 for immunofluorescence, 1:100 for diaminobenzidine [DAB]), rat anti-BrdU (Abcam; 1:50), mouse anti–proliferating cell nuclear antigen (PCNA) (Dako; 1:100), mouse anti-Pax6 (DSHB; 1:50 for immunofluorescence, 1:200 for DAB), rabbit anti-Tbr1 (gift from Robert Henver; 1:100), mouse anti-Satb2 (Abcam; 1:25), mouse anti-β-III-tubulin (Sigma; 1:100), goat anti-Cux1 (Santa Cruz; 1:50), and rat anti-Ctip2 (Abcam; 1:250). The tissue was then washed in PBS, 0.1% Triton X-100, and appropriate secondary antibodies were applied for 1 h at RT. For bright-field staining (BrdU birthdating experiments, PCNA and Pax6 immunohistochemistry), biotinylated goat anti-mouse antibody (Dako; 1:200) was used, followed by a standard avidin–biotin–DAB visualization procedure (Vector Laboratories). For fluorescent staining, secondary antibodies used were goat anti-mouse Alexa Fluor 488, goat anti-rat Alexa Fluor 568, goat anti-rabbit Alexa Fluor 568, donkey anti-goat Alexa Fluor 488, and donkey anti-mouse Alexa Fluor 568 (all from Molecular Probes, diluted at 1:200). For Pax6 immunofluorescence, biotinylated goat anti-mouse antibody (Dako; 1:100) was used before Alexa Fluor 488–conjugated streptavidin (Molecular Probes; 1:100). Nuclear counterstaining was performed with TOPRO-3 (1:1000 in dH2O; Molecular Probes) for immunofluorescence or 0.25% cresyl violet for light microscopy. Fluorescent images were captured using a Leica NTS confocal microscope, whereas a Leica digital camera was used for light microscopy images.
Flow Cytometry
Cortical tissue was collected from E16.5 PAX77 and wild-type embryos separately at rostral and central neocortical levels. Eight individuals of each genotype collected from 3 separate litters were used for the analysis. Cells were dissociated using papain following manufacturer's instructions (Papain Dissociation System) and fixed in ice-cold 70% ethanol at a concentration of 1 × 106 cells per ml. Dissociated cells were stained for β-tubulin isotype III (1:800) and primary antibody binding was detected using directly conjugated Alexa Fluor 488 (goat anti-mouse IgG, 1:800). To stain cellular DNA, cells were then incubated with propidium iodide (PI) at 50 μg/ml with RNAse A at 125 μg/ml. Cells were analyzed on a Beckman-Coulter XL flow cytometer; 10 000–20 000 cells were analyzed per sample.
Analysis
Cell Cycle
Age-matched wild-type and PAX77 sections were reacted to reveal IdU/BrdU labeling. To detect IdU, a mouse–anti-BrdU antibody, which cross-reacts with IdU, was used. The BrdU signal was distinguished from the IdU signal by using a rat–anti-BrdU–specific antibody. Sections were photographed at ×40 magnification on a Leica NTS confocal microscope and then imported into Adobe Photoshop for counting. The analysis at E15.5 was performed in the middle of the rostral, central, and caudal neocortex, whereas at E12.5, 4 locations were analyzed, namely the middle of the rostral neocortex, a medial, and a lateral region of the central cortex, and the middle of the caudal cortex (see Fig. 1C). This method identifies IdU-only and IdU/BrdU double-labeled cells. IdU-only labeled cells are those in S-phase at the start of the experiment that exit S-phase by the time of the BrdU injection and are therefore designated as the leaving fraction (Lcells). BrdU/IdU double-labeled cells are those still in S-phase (Scells) at the time of the BrdU injection. Proportions of Lcells and Scells were calculated in the VZ and the lengths of the S-phase and the cell cycle were calculated by using the equations shown in Figure 1A (Martynoga et al. 2005). To estimate the proportion of proliferating cells (Pcells), we counted proportions of PCNA-positive cells in the VZ of the same regions of cortex used for BrdU/IdU cell counts. Cell cycle times were also determined in a non–Pax6-expressing region of the lateral ganglionic eminence (LGE) at E15.5 following methods described above.
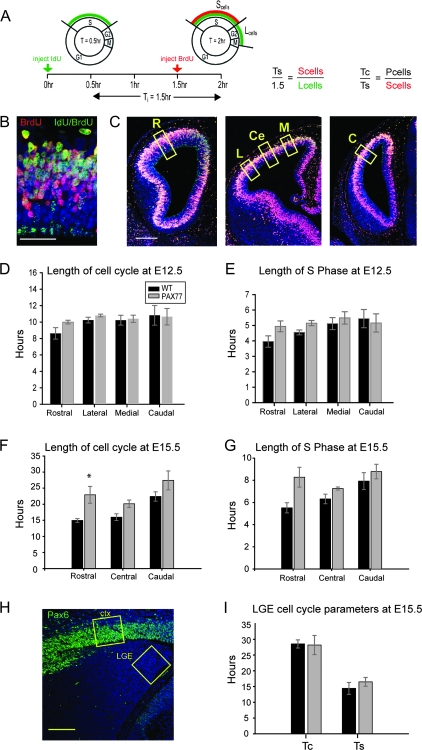
Figure 1.
Pax6 overexpression increases cell cycle length of APs at late stages of corticogenesis. (A) Schematic diagram of the timing of administration of thymidine analogues in order to label S-phase cells. The ratios shown were used to calculate cell cycle and S-phase length in wild-type and PAX77 embryos (Ts = length of S-phase; Tc = length of cell cycle; Pcells = all proliferating cells). (B) Coronal section through the cortex of an E12.5 wild-type embryo immunostained with antibodies specific for both BrdU and IdU (green) and BrdU alone (red) to identify Scells (red and green double-labeled cells) and Lcells (green-only cells). (C) Coronal sections at rostral, central, and caudal levels of the cortex of an E12.5 wild-type embryo immunostained with IdU/BrdU. Cell counts were made in 100-μm-wide sampling boxes in the VZ of the cerebral wall. At E12.5, cells were counted in the rostral (R) cortex, lateral (L) and medial (M) regions at the central level, and in the caudal (C) cortex. At E15.5, cell counts were made at the rostral (R), central (Ce), and caudal (C) levels of the cortex. (D, E) Quantitative analysis showing (D) length of cell cycle (Tc) and (E) length of S-phase (Ts) in E12.5 wild-type and PAX77 embryos. At all the cortical levels examined, Tc and Ts were not significantly altered in the PAX77 cortex compared with wild type. (F, G) Histograms showing (F) Tc and (G) Ts in the E15.5 wild-type and PAX77 cortex. Cell cycle was significantly longer in the rostral PAX77 cortex compared with wild type. (H) Example of Pax6 immunofluorescence at the central level of an E15.5 PAX77 brain. Sampling boxes (100 μm wide) indicate the regions where cell cycle kinetics were analyzed at E15.5. Tc and Ts were estimated in the Pax6-positive cortex (ctx) and a Pax6-negative region of the LGE. (I) In Pax6-negative LGE, cell cycle times were not different between wild-type and PAX77 cortex at E15.5. All sections shown were counterstained with TO-PRO-3. Scale bars: 50 μm in (B), 200 μm in (C), and 100 μm in (H). Data represent the mean ± standard error of the mean values.
Q Fraction
Following the protocol described above and summarized in Figure 2A, sections at the rostral, central, and caudal level of the cortex were processed for IdU/BrdU immunohistochemistry, photographed at ×20 magnification on a Leica NTS confocal microscope and then imported into Adobe Photoshop for analysis. The principle of the analysis follows that described in Tarui et al. (2005). As in the analysis of cell cycle, a cohort of cells exiting S-phase in the 1.5-h interval between the IdU injection and the first BrdU injection would have been labeled with IdU only (Lcells). The total duration of subsequent exposure to BrdU was longer than Tc − Ts (Tc = length of cell cycle; Ts = length of S-phase), as determined from our analysis of cell cycle times at E15.5, and so, those Lcells that reentered S-phase would have become double labeled with BrdU. The leaving fraction was estimated by dividing the number of Lcells that had not reentered S-phase at the end of the long exposure to BrdU (identified because they still contained only IdU; now designated Qcells) by the number of Lcells multiplied by 2. The number of Lcells from the cell cycle analysis was doubled because these cells would have undergone mitosis, thereby doubling the size of the Lcell cohort, before either exiting or reentering the cell cycle.
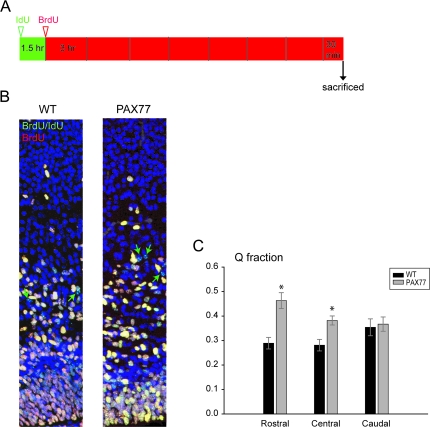
Figure 2.
Pax6 overexpression increases the fraction of late cortical progenitors leaving the cell cycle. (A) Diagrammatic representation of the timing of administration of thymidine analogues in order to label S-phase cells and calculate the leaving (Q) fraction; (B) 100-μm-wide sections at a rostral cortical level immunostained with IdU and BrdU. The IdU-only cells (green arrows show examples), representing the Q cells, were counted in the cerebral wall of wild-type and PAX77 embryos. (C) The Q fraction was significantly increased in the rostral and central PAX77 cortex compared with wild type (data represent the mean ± SEM values). Nuclei were counterstained with TO-PRO-3.
BrdU Birthdating
Anatomically matched sections from P7 wild-type and PAX77 cortex were reacted to reveal BrdU label, and camera lucida drawings were made of the laminar positions of heavily and lightly labeled BrdU-positive cells in rostral, central, and caudal cortex, following methods described previously (Gillies and Price 1993; Caric et al. 1997). Heavy labeling was defined where a cell had more than half of its nucleus stained with BrdU. Drawings were repeated on 3–5 nonconsecutive sections for each cortical region per brain. BrdU-positive cells were counted within 500-μm-wide strips through the depth of the cortex (sectioned coronally). These radial strips covered a cortical depth of 500 μm from the pial surface inward and were divided into 10 bins of equal depth (50 μm). Histograms were obtained of the average numbers of heavily and lightly BrdU labeled at each depth from the pia.
For double labeling of BrdU-positive cells with appropriate laminar markers, for example, Tbr1 or Cux1, coronal sections from P7 wild-type and PAX77 cortex or Pax6loxP/+; Emx1-CreERT2 and Pax6loxP/loxP; Emx1-CreERT2 cortex at the rostral level were reacted. The proportions of double-labeled cells over the total number of BrdU-positive cells were calculated in 200-μm-wide strips spanning the depth of the deep layers (for BrdU/Tbr1 immunostaining) or the superficial layers (for BrdU/Cux1 immunostaining) of the cortex. For BrdU/Tbr1 analysis, only heavily labeled BrdU-positive cells were counted.
Statistical Analysis
Analysis was performed on data collected from brains of at least 3 embryos/mice of each genotype. Statistical comparisons were made by Student's t-test (for single variables) and 1-way analysis of variance (ANOVA) (for more than 2-group comparisons) (Sigmastat). Asterisks above histograms indicate P < 0.05.
Results
Pax6 Overexpression Affects Cortical Cell Production by Regulating Cell Cycle Length in Late Corticogenesis
We reported previously that Pax6 overexpression causes a cell-autonomous reduction in the generation of cells at late stages of corticogenesis, specifically in rostral cortical regions where Pax6 is highly expressed (Manuel et al. 2007). To investigate the cause of this defect, we analyzed cell cycle parameters in the VZ of PAX77 and wild-type cortex using the method illustrated in Figure 1A–C (Martynoga et al. 2005; Quinn et al. 2007; see Materials and Methods). Because Pax6 is expressed in a gradient throughout the cortex, the analysis was performed at different levels across the rostral–caudal and medial–lateral axes (Fig. 1C). To calculate cell cycle lengths, proportions of proliferating cells (Pcells) in the VZ needed to be estimated. Because it has been shown previously that all VZ cells proliferate at E12.5 (Caviness et al. 1995; Estivill-Torrus et al. 2002; Martynoga et al. 2005), Pcells was estimated by counting all VZ cells at E12.5. To determine the proportion of Pcells at E15.5, PCNA immunohistochemistry was performed in PAX77 and wild-type cortex and the proportions of PCNA-positive cells were calculated at rostral, central, and caudal levels of the cortex (Supplementary Fig. 1): values were around 90% in all cases.
In E12.5 wild-type embryos, we observed a tendency for cell cycle and S-phase to lengthen slightly (by about 2 and 1.5 h, respectively) from rostral to caudal cortex (Fig. 1D,E). These rostral-to-caudal trends were less obvious in E12.5 PAX77 embryos; in mutant rostral cortex, the cell cycle and S phase were about 1 h longer than in wild types, whereas in mutant caudal cortex, they were similar to wild-type values (Fig. 1D,E). None of the differences at E12.5 were statistically significant. At E15.5, however, there were similar trends and differences were significant. In wild-type embryos, average cell cycle times lengthened progressively by about 7 h from rostral to caudal cortex (Fig. 1F), and these regional differences were significant (ANOVA P < 0.01; n = 3). In PAX77 embryos, neither cell cycle nor S-phase length varied regionally (Fig. 1F,G); ANOVA failed to show significant differences. Average cell cycle time was significantly longer in PAX77 cortex than in wild-type cortex in rostral regions (Student's t-test P < 0.05; n = 3), but not in central and caudal regions (Fig. 1F).
We also compared cell cycle parameters between wild-type and PAX77 E15.5 embryos in a region of the developing telencephalon that does not express Pax6 at this age, the LGE (Fig. 1H), to verify that the abnormal cell cycle parameters are not general effects on forebrain development. We did not detect any difference in cell cycle parameters in this region between PAX77 and wild-type embryos (Fig. 1I). These findings suggest that at late stages of corticogenesis, the cell cycle parameters of APs in rostral cortical regions, where expression levels of Pax6 are normally highest, are modulated by the level of Pax6.
Pax6 Overexpression Causes Increased Cell Cycle Exit at Late Stages of Corticogenesis
To determine whether Pax6 overexpression causes a shift in the proportion of dividing cells that leave the cell cycle instead of remaining as progenitors, we examined cell cycle exit at E15.5 by determining the leaving (Q) fraction. This was accomplished by using an IdU/BrdU labeling protocol (Fig. 2A; see Materials and Methods). The approach depends on counts of numbers of IdU-positive cells: examples of these cells are shown with green arrows in Figure 2B. We found that the Q fraction was significantly increased in the PAX77 cortex at rostral (Student's t-test P < 0.01; n = 5) and central levels (Student's t-test P < 0.05; n = 5) but was not altered at caudal levels compared with wild types (Fig. 2C). These results indicate that at late stages of corticogenesis increased levels of Pax6 lead to increased proportions of proliferating cells exiting the cell cycle.
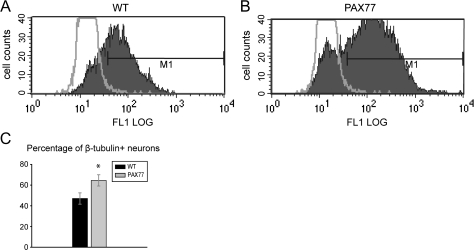
We predicted that an increased rate of cell cycle exit before E16.5 should produce a corresponding increase in the proportion of neurons in the mutant cortex by E16.5. To investigate this, we used the early neuronal marker β-III-tubulin and assessed the proportion of positive postmitotic neurons by flow cytometry. Samples of mutant and control cortices covering rostral and central levels were dissociated at E16.5, and cells were stained with β-III-tubulin before analysis (Fig. 3A–C). We detected a significant increase in the proportion of β-III-tubulin–positive neurons in the PAX77 cortex compared with the wild-type cortex (Fig. 3D; Student's t-test P < 0.05; n = 8).
Figure 3.
Increased proportion of neurons in the PAX77 cortex at E16.5. (A, B) Expression of β-III-tubulin quantified with flow cytometry in dissociated (A) wild-type and (B) PAX77 E16.5 cortical cells. Gating (M1) was established by a control reaction in cells stained with PI and secondary antibody only (no primary antibody). Gray line in histograms represents the plot obtained from control reaction; cells whose fluorescence (FL1LOG) fell within M1 considered to be positive for β-III-tubulin. Plots shown are from a single representative experiment for each genotype. (C) Histogram showing the mean proportions of cells (±standard error of the means) classified as β-III-tubulin–positive in wild-type and PAX77 cortex. The percentage of β-III-tubulin–expressing cells was significantly higher in PAX77 cortex than in wild-type cortex.
These findings on cell cycle exit, together with those on cell cycle times, provide an explanation for the reduced production of cortical cells in PAX77 embryos reported previously (Manuel et al. 2007): at E15.5, Pax6 overexpression slows the cell cycle and increases the proportions of cells exiting the cell cycle, thereby reducing the size of the proliferative pool.
Pax6 Overexpression Reduces the Radial Extent of the Superficial Layers
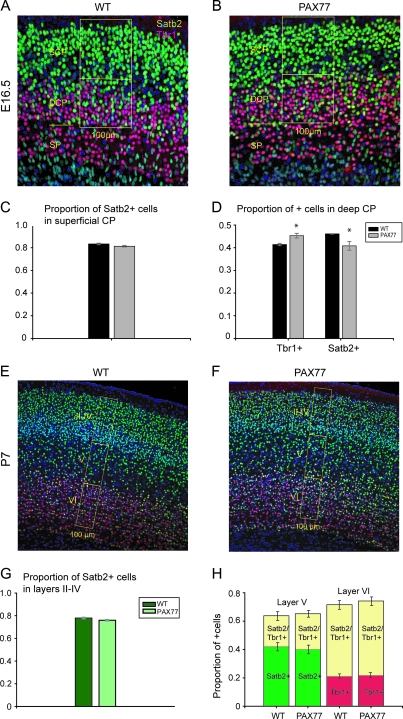
To explore laminar formation in PAX77 mice, we analyzed the expression of cell type–specific markers known to be required for the specification of distinct subsets of neurons. Double immunohistochemistry for Satb2, which is expressed largely by superficial layer neurons but also by some deep-layer neurons and is required for the specification of callosal neurons (Alcamo et al. 2008; Britanova et al. 2008), and Tbr1, known to be involved in the specification of deep-layer neurons (Hevner et al. 2001), was performed in rostral PAX77 and wild-type cortices at E16.5 (Fig. 4A,B). As expected, Satb2-positive neurons were detected in both superficial and deep positions of the CP, whereas Tbr1 was expressed at high levels in the subplate and layer 6 neurons (Fig. 4A,B). For our quantitative analysis, the CP was divided into 2 domains: a superficial Satb2-positive/Tbr1-negative domain and an inferior Satb2-positive/Tbr1-positive domain. The proportion of Satb2-positive cells was determined in the superficial CP; the proportions of Tbr1-positive and of Satb2-positive neurons were calculated in the lower CP (proportions are the numbers of positive cells divided by the total number of all cells in the counting area). Interestingly, in the PAX77 cortex, we found a significant increase in the proportion of Tbr1-positive cells in the lower CP (Student's t-test P < 0.05; n = 3) accompanied by a significant decrease in the proportion of Satb2-positive cells (Student's t-test P < 0.05; n = 3) (Fig. 4D). The proportion of Satb2-positive neurons in the superficial CP was not significantly changed in the PAX77 cortex (Fig. 4C).
Figure 4.
Neurons in the PAX77 cortex are correctly specified according to their laminar position. (A, B) Immunohistochemistry for Satb2 (green) and Tbr1 (red) on coronal sections of E16.5 wild-type and PAX77 cortex at a rostral level. Cells were counted in the 100-μm-wide sampling box placed in the CP of wild-type and PAX77 cortex. For our quantitative analysis, we divided the CP into a superficial Satb2-positive/Tbr1-negative domain and a lower Satb2-/Tbr1-positive domain by drawing a line at the border delineated by the band of Tbr1-positive cells in the upper part of the deep CP. (C, D) Quantification of the proportions of Satb2-positive neurons in the superficial CP and Satb2-positive and Tbr1-positive neurons in the deep CP of the E16.5 wild-type and PAX77 cortex. The proportion of Satb2-positive neurons in the superficial CP was not significantly altered in PAX77 embryos compared with wild type. The proportion of Tbr1-positive neurons was significantly increased and the proportion of Satb2-positive neurons was significantly decreased in the deep CP of PAX77 mice compared with wild types. (E, F) Examples of Satb2/Tbr1 labeling in coronal sections through the rostral cortex of P7 wild-type and PAX77 mice: cells were counted in the 100-μm-wide sampling boxes. Sampling boxes are the same size in (E) and (F); the gap in the superficial domain of the sampling box in (F) highlights the reduced thickness of the PAX77 cortex compared with wild type. In both wild-type and PAX77 cortex, Satb2-positive neurons were primarily detected in layers II–IV and also layer V, whereas Tbr1-positive neurons were mainly located in layer VI but were also detected in layer V where they appear as Tbr1/Satb2 double labeled. (G) The proportion of Satb2-positive neurons was not significantly altered in the superficial layers (II–IV) of PAX77 cortex compared with wild type. (H) Histograms show the proportions of Satb2-positive and Satb2-positive/Tbr1-positive neurons in layer V, as well as the proportions of Tbr1-positive and Tbr1-positive/Satb2-positive neurons in layer VI, of P7 wild-type and PAX77 cortex. No difference in the radial distribution of these neuronal populations in the lower cortical layers V and VI was detected between PAX77 and wild-type mice. DCP, deep cortical plate; SCP, superficial cortical plate; SP, subplate.
The altered proportions of Tbr1-positive cells and Satb2-positive cells in the deep CP of E16.5 PAX77 mice suggested that an alteration in the specification of deep-layer neurons might be developing, and to test this, we performed Tbr1/Satb2 double immunohistochemistry at P7 (Fig. 4E,F). Coronal sections from PAX77 and wild-type cortex at a rostral level were reacted and Tbr1-positive, Satb2-positive, and Tbr1/Satb2 double-labeled neurons were counted according to their laminar position. The proportion of Satb2-positive neurons in the superficial layers II-IV was not significantly altered in the PAX77 cortex (Fig. 4G). Neither the proportions of Satb2-positive and Satb2-positive/Tbr1-positive neurons in layer V nor the proportions of Tbr1-positive and Tbr1-positive/Satb2-positive neurons in layer VI were significantly different in the PAX77 cortex compared with wild type (Fig. 4H). These results indicate that cortical neurons are correctly specified in correct proportions in PAX77 mice and express appropriate markers depending on their laminar position.
We reported previously that the thickness of superficial layers (II–IV combined) is significantly reduced in the PAX77 compared with wild-type cortex at P7 (Manuel et al. 2007). In line with this, we found here that, at P7, the total number of Satb2-positive neurons in the 100-μm-wide strips was significantly decreased in the superficial cortical layers of the PAX77 mice (183 ± 6 in wild type, 153 ± 8 in PAX77; Student's t-test P < 0.05; n = 3), whereas cell numbers in layers V and VI were not significantly different between mutants and wild types. This result indicates that the defects of cell cycle parameters in PAX77 embryos, which reduce the size of the proliferative pool by E15.5, feed through into reductions in superficial layer cell numbers postnatally.
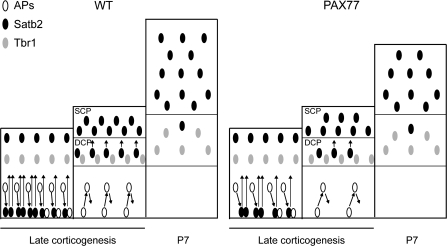
The most likely explanation for the reduced proportion of Satb2-positive cells in the deep CP of E16.5 PAX77 embryos (Fig. 4D) is a reduction in the size of the population of late-generated Satb2-positive cells. Because superficial layers are only just starting to form at E16.5, many Satb2-positive neurons that will eventually contribute to the superficial layers might still be intermingled with deep-layer Tbr1-expressing cells at E16.5. The presence of reduced numbers of Satb2-positive cells in the deep CP at E16.5 would result in a corresponding increase in the proportions of Tbr1-positive deep-layer cells, as was seen at E16.5 (Fig. 4D). These would be transient changes and would disappear postnatally once all Satb2-expressing cells reach their final destinations, leaving both superficial and deep layers with normal “proportions” of Satb2- and Tbr1-expressing cells, although superficial layers would have reduced “absolute numbers” of Satb2-expressing cells (summarized in Fig. 7).
Figure 7.
Model of cortical lamination in mice overexpressing Pax6. At late stages of corticogenesis, progenitors in the proliferative zone generate superficial layer fate carried by Satb2-positive cells. These cells migrate through the deep CP, where earlier-born Tbr1-positive cells reside, to reach their final superficial laminar positions. In the PAX77 cortex, the number of deep-layer Tbr1-positive cells is normal but, at late stages of corticogenesis, the progenitor pool is depleted due to lengthened cell cycle times and increased fractions of cells exiting the cell cycle. There is, therefore, a reduced production of late-born Satb2-positive cells and less Satb2-positive cells transit though the deep layers to the superficial layers of the CP in PAX77 mice. Postnatally, superficial layers are thinned in the PAX77 cortex compared with wild type due to a reduction in the number of Satb2-positive cells. DCP, deep cortical plate; SCP, superficial cortical plate.
Radial Migration and Layer Specification of Cortical Neurons Appear Normal in Postnatal Mice Overexpressing Pax6
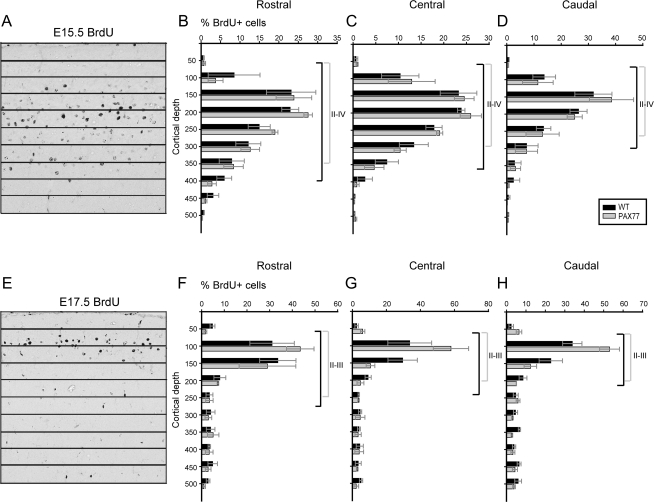
To address whether radial migration defects might contribute to the formation of thinner superficial layers in the PAX77 cortex, BrdU birthdating experiments were performed. Pregnant females were injected with a single dose of BrdU at E15.5 or E17.5, and the laminar distribution of BrdU-positive cells was examined at P7, when neuronal migration is largely completed. Although we counted densely and lightly labeled cells (Del Rio and Soriano 1989; Gillies and Price 1993) separately, the radial distributions and numbers of both cell populations were similar in PAX77 and wild-type littermates, and so data from the 2 sets were combined for presentation here.
Most cells born from E15.5 onward were observed in superficial positions corresponding to cortical layers IV and III (Fig. 5A), whereas cells born from E17.5 onward occupied an area corresponding to layers III and II (Fig. 5E). The radial distributions of E15.5- or E17.5-labeled cells were very similar in the PAX77 and wild-type P7 cortex (Fig. 5B–D,F–H). These findings indicate that migration defects do not make a significant contribution to cortical abnormalities in PAX77 mice.
Figure 5.
BrdU birthdating reveals that laminar positioning of late-born neurons is unaffected in the PAX77 cortex. (A, E) Coronal sections through the P7 rostral PAX77 cortex labeled with BrdU at the indicated age. BrdU-positive nuclei were quantified in each 50-μm-deep bin. (B–D, F–H) Graphical representations of data from birthdating studies show the mean percentages (±standard error of the means) of BrdU-positive neurons in each bin at 3 rostrocaudal levels. Layer thickness is marked on the graphs for all cortical regions analyzed (black for wild-type, gray for PAX77). Labeling at E15.5 revealed accumulation of BrdU-positive nuclei in layers II–IV in both wild-type and PAX77 cortices. A BrdU pulse at E17.5 primarily marked the genesis of layer II neurons in both wild type and PAX77. No differences in the radial distribution of late-born neurons were detected between PAX77 and wild-type cortex.
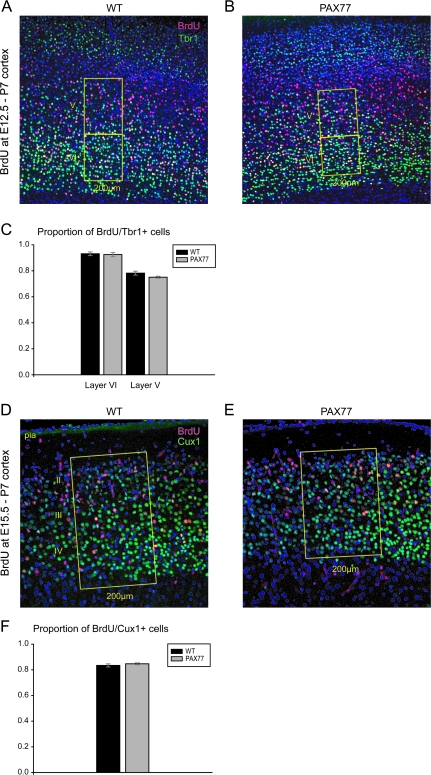
To investigate directly whether cortical neurons acquire their correct laminar fate according to their birth date, we injected BrdU at E12.5 or E15.5 and double labeled the birth-dated cells with layer-specific markers at P7. BrdU-positive neurons born at E12.5 were double labeled with Tbr1 (Fig. 6A,B); BrdU-positive neurons born at E15.5 were double labeled with Cux1 (Fig. 6D,E) that normally identifies pyramidal neurons of superficial layers IV–II of the cortex (Nieto et al. 2004). Coronal sections from PAX77 and wild-type cortex at a rostral level were reacted and BrdU/Tbr1 double-labeled neurons were counted in layers VI and V separately (Fig. 6A,B). The proportions of BrdU/Tbr1 double-labeled neurons over total BrdU-positive neurons were not significantly altered in either layer VI or layer V of the PAX77 cortex (Fig. 6C), indicating that early-born neurons are correctly specified in the mutant cortex. The proportions of BrdU/Cux1 double-labeled neurons in layers IV–II were also not significantly different in the PAX77 cortex compared with wild type (Fig. 6F). These results further confirm that Pax6 overexpression does not affect the specification of deep or superficial layer neurons born at early or late stages of corticogenesis, respectively.
Figure 6.
Cortical neurons in the PAX77 cortex are correctly specified according to their birth date. (A, B) Double immunostaining for BrdU (red) and Tbr1 (green) on coronal sections through the cortex of P7 wild-type and PAX77 mice given BrdU at E12.5. The numbers of heavily labeled BrdU cells and BrdU (heavily labeled)/Tbr1 double-labeled cells were counted in 200-μm-wide radial stripes in layers VI and V of wild-type and PAX77 cortices. (C) The proportions of BrdU-labeled cells that were Tbr1 double labeled were not significantly altered in either layer VI or V of the PAX77 cortex compared with wild type. (D, E) High-power views of the superficial layers of the rostral cortex double immunostained for Cux1 (green), a marker of superficial layer (IV–II) neurons, and BrdU (red) in P7 wild-type and PAX77 mice injected with BrdU at E15.5. The numbers of BrdU-positive and BrdU/Cux1 double-labeled cells were counted in 200-μm-wide radial stripes spanning through the superficial layers of wild-type and PAX77 cortices. (F) The proportions of BrdU/Cux1 double-labeled neurons were not significantly different in the superficial layers of the PAX77 cortex compared with wild type.
Cortex-Specific Pax6 Loss-of-Function Mutants Display a Reduced Number and Disturbed Differentiation of Late-Born Neurons in Superficial Cortical Layers
To test further the function of Pax6 in modulating the development of superficial layers during late corticogenesis, we took advantage of a Pax6 conditional allele described previously (Simpson et al. 2009). We generated Pax6loxP/loxP; Emx1-CreERT2 mice to ablate Pax6 specifically in the dorsal telencephalon after tamoxifen administration. Deletion of Pax6 from these genetically modified animals did not result in perinatal death and so, unlike in full Pax6−/− mutants that die at birth, we were able to examine the impact of loss of Pax6 on superficial layer development, which is not complete until about a week after birth.
To determine the recombination efficiency induced by tamoxifen and the consequent loss of Pax6, tamoxifen was administered into pregnant females at E10.5 and embryos were analyzed at E13.5 (72-h posttamoxifen administration). There was a clear loss of Pax6 protein from dorsal telencephalic progenitors that express Emx1 (cells around the pallial–subpallial boundary are spared) in the E13.5 mutant (Pax6loxP/loxP; Emx1-CreERT2) cortex (Supplementary Fig. 2A,B). As expected, in other brain regions where Pax6 is normally expressed but Emx1 is not (e.g., thalamus), Pax6 expression in mutants was comparable to controls (Supplementary Fig. 2A,B).
To determine the earliest developmental stage at which Pax6 protein is removed from our model system, we examined the expression of Pax6 at E12.5, 48-h posttamoxifen injection. Tamoxifen-induced recombination peaks at 24- to 48-h postinjection in Emx1-CreERT2 embryos (Kessaris et al. 2006), and Pax6 protein was still detected with an apparently normal pattern in E12.5 mutant Pax6loxP/loxP; Emx1-CreERT2 embryos (data not shown). Therefore, Pax6 is lost from mutant embryos between E12.5 and E13.5, before the onset of superficial layer generation from E14.5 onward. Successful recombination and faithful expression of the Cre transgene was also monitored with the Rosa26R-YFP reporter allele (Srinivas et al. 2001) (data not shown).
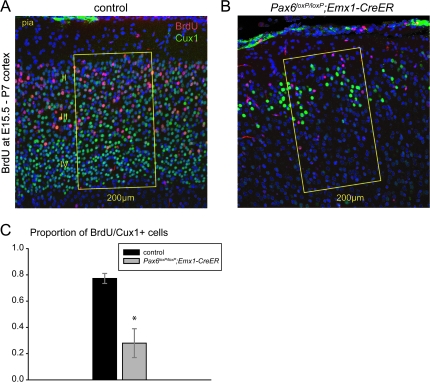
Mutant brains showed a markedly reduced size of the cerebral cortex at P7 (Supplementary Fig. 2C,D). Histological examination revealed a severe reduction in cortical thickness. Layer-specific immunostaining of P7 cortices with Ctip2, which is known to be required for specification of corticospinal motor neurons located in layer V (Arlotta et al. 2005), showed strongly reduced thickness of Ctip2-negative superficial layers in the mutant cortex (Supplementary Fig. 2E,F). To examine further the production of superficial layers of the conditional mutant cortex, we birth-dated late-born neurons by injecting BrdU at E15.5 and double-labeling BrdU-positive cells with Cux1 at P7 (Fig. 8A,B). As judged by correct laminar positioning of the majority of BrdU-labeled cells, neuronal migration was not greatly affected in the mutant cortex. The number of E15.5-born neurons was, however, significantly reduced in the superficial layers of the P7 mutant cortex (69.15 ± 6.67 standard error of the mean in control, 14.45 ± 2.43 in mutant; Student's t-test P < 0.002; n = 3 of each genotype). In controls, nearly 80% of BrdU-labeled cells were double labeled for Cux1; in mutants, there was a large significant reduction to about 30% in the proportion of BrdU-labeled cells that were Cux1 double labeled (Fig. 8C). These findings indicate that both generation and specification of superficial layer neurons are severely affected by loss of Pax6 from midstages of corticogenesis.
Figure 8.
Late-born neurons are severely reduced in number and are not correctly specified in superficial cortical layers of Pax6 conditional knockout mice. (A, B) High-power views of superficial cortical layers (IV–II) of P7 control (Pax6loxP/+; Emx1-CreERT2) and mutant (Pax6loxP/loxP; Emx1-CreERT2) mice injected with tamoxifen at E10.5 followed by BrdU administration at E15.5; images show double immunostaining for BrdU (red) and Cux1 (green). Note the severe reduction in the number of both BrdU-labeled cells and Cux1-labeled cells in the superficial layers of the mutant cortex. The numbers of BrdU-positive and BrdU/Cux1 double-labeled cells were counted in 200-μm-wide radial stripes through the cortex of control and mutant mice. (C) The proportions of BrdU-positive cells that were Cux1/BrdU double labeled were significantly reduced in the mutant cortex compared with control (Student's t-test P < 0.01; n = 3 of each genotype).
Discussion
We showed in Manuel et al. (2007) that, at late stages of corticogenesis, overexpression of Pax6 acts cell autonomously to reduce the production of cortical cells, resulting in the formation of thinner superficial layers in the postnatal cortex. In this study, we examined possible mechanisms by which Pax6 controls the production of late-born neurons and superficial layers of the cortex. We found that Pax6 overexpression lengthens the cell cycle of cortical VZ progenitors and promotes cell cycle exit at late stages of corticogenesis. Both these abnormalities would reduce the size of the progenitor pool as corticogenesis proceeds and therefore reduce cortical cell production. Consistent with this, we report here that Pax6 overexpression resulted in reduced numbers of superficial layer neurons, without affecting the laminar specification of this depleted population (summarized in Fig. 7). These findings indicate that correct levels of Pax6 are required by cortical progenitors at late stages of corticogenesis to maintain their cell cycle times and moderate their exit from the cell cycle, thereby regulating the size of the progenitor pool. Conditional inactivation of Pax6 from midstages of corticogenesis also resulted in a significant decrease in the number of superficial layer neurons born at late stages of corticogenesis. Interestingly, superficial layer neurons were not correctly specified in the loss-of-function mutant cortex. Our findings suggest that increased or decreased Pax6 levels lead ultimately to defects in superficial laminar formation but through different mechanisms.
Dysregulation of Pax6 Levels Influences Cortical Progenitor Proliferation
During cortical neurogenesis, cell divisions in the cortical VZ generate additional progenitors as well as postmitotic neurons. The total number of mitotic cycles progenitors undergo is limited throughout corticogenesis and is determined in part by cell cycle length and in part by the fraction of cells leaving the cell cycle to differentiate. The balance between these 2 processes is a critical determinant of the final neuronal number and therefore of the size of the cortex (Takahashi et al. 1994).
In APs, which normally express high levels of Pax6 (Gotz et al. 1998; Englund et al. 2005), both cell cycle length and proportions of cells exiting the cell cycle increase as corticogenesis advances (Takahashi et al. 1994; Estivill-Torrus et al. 2002; present study). Here, we also observed regional differences in the lengths of the cell cycles, which lengthened progressively from rostral to caudal cortex at E15.5, indicating that cell cycle parameters are regulated both temporally and spatially during corticogenesis in wild-type embryos. Pax6 is also differentially regulated spatially and temporally: its levels are higher in rostral than in caudal APs and its levels decline as corticogenesis progresses (Stoykova and Gruss 1994; Manuel et al. 2007). We showed previously that a rostral (high) to caudal (low) gradient of Pax6 expression is maintained in PAX77 embryos, with levels of expression increased proportionately along the gradient (i.e., with the largest absolute increases in Pax6 levels occurring rostrally; Manuel et al. 2007). Our present results show that the consequence of this increase is to abolish the rostral–caudal difference in cell cycle lengths by increasing lengths rostrally. It appears, therefore, that the relationship between Pax6 expression level and cell cycle length across the cortex is not linear. It is possible that for normal cell cycling, Pax6 levels must be within a band and if its upper limit is exceeded cell cycles lengthen.
Loss of Pax6 also leads to a reduced number of apically dividing cortical progenitors (Estivill-Torrus et al. 2002; Tamai et al. 2007). Our previous detailed analyses of cell cycle kinetics have suggested that, although absence of Pax6 does not lengthen cell cycle times at early stages of corticogenesis, it does lengthen them at E15.5 (Estivill-Torrus et al. 2002; Quinn et al. 2007). Furthermore, recent analyses showed that Pax6−/− mutant cells exit the cell cycle in abnormally large numbers, suggesting a primary cell-autonomous role for Pax6 in control of cell cycle exit (Quinn et al. 2007). Both gain and loss of Pax6 have similar effects on cortical progenitors, indicating that not just the presence of Pax6 but also maintenance of correct Pax6 levels is critical for spatially and temporally appropriate cell cycle parameters. This finding is reminiscent of the effects of both lowering and raising Pax6 levels on eye development, both of which generate small eyes (Schedl et al. 1996).
Our new results provide an explanation for previous observations that the number of late APs in S-phase and M-phase is reduced in the PAX77 cortex (Manuel et al. 2007) and are in line with in vitro work where Pax6 transduction, which is likely to cause very large increases in Pax6 levels, inhibits progenitor proliferation (Heins et al. 2002; Hack et al. 2004; Cartier et al. 2006). Recent analyses have suggested that Pax6 has the ability to both promote and inhibit cortical progenitor proliferation by regulating the expression of cell cycle regulators (Sansom et al. 2009). Its cell cycle commitment functions are, however, counterbalanced by its positive regulation of genes that promote cell cycle exit (Sansom et al. 2009). Taking these findings together with results reported here suggests that when Pax6 is overexpressed its ability to drive progenitors to exit the cell cycle is dominant over its function in cell cycle commitment.
Lengthening of the cell cycle and increased cell cycle exit during superficial layer formation would be predicted to reduce numbers of superficial layer neurons, and indeed, results presented here confirm this prediction. Reduction in the size of the progenitor pool as a result of disruption of normal levels of Pax6, either up or down, provides an explanation for the underproduction of superficial cortical layer neurons in both Pax6−/− mutant and Pax6-overexpressing mice (Tarabykin et al. 2001; Quinn et al. 2007; Manuel et al. 2007; Sansom et al. 2009; Tuoc et al. 2009; present study).
Roles of Pax6 in Neurogenesis and Laminar Specification of Cortical Neurons
Cell cycle exit and neuronal differentiation are tightly regulated during cortical development. Loss of Pax6 function increases the proportions of neurons in the developing cortex at early stages of corticogenesis (Estivill-Torrus et al. 2002; Quinn et al. 2007), as well as in other parts of the CNS such as the eye and the spinal cord (Philips et al. 2005; Bel-Vialar et al. 2007). The increased proportions of neurons in the Pax6−/− mutant cortex or retina during early development seem best explained by an increased cell cycle exit (Philips et al. 2005; Quinn et al. 2007; Tuoc et al. 2009). Our finding here, that increased levels of Pax6 in vivo enhance cell cycle exit and lead to increased proportions of neurons at late stages of corticogenesis, is in accord with previous in vitro studies showing that Pax6 overexpression promotes neuronal differentiation under culture conditions (Heins et al. 2002; Hack et al. 2004; Haubst et al. 2004). Recent analyses have also suggested that increasing Pax6 levels in vivo results in advanced neurogenesis in the developing cortex (Sansom et al. 2009).
Cell cycle exit is also closely associated with laminar positioning and laminar fate of cortical neurons. Both cell cycle properties of cortical progenitors and laminar fates of cortical neurons are determined when they are early postmitotic cortical precursors, with potential laminar fates becoming progressively restricted as development advances (McConnell and Kaznowski 1991; Frantz and McConnell 1996; Desai and McConnell 2000; Britanova et al. 2008). Some previous studies have suggested that Pax6 might be involved in regulating the laminar properties of cortical neurons (Schuurmans et al. 2004; Osumi et al. 2008). Our finding that cell cycle parameters are disrupted in the PAX77 cortex motivated us to investigate whether laminar fate is altered in these mice. They provide a good model to explore the role of altered Pax6 levels on laminar specification because, unlike Pax6−/− mutants, they do not die perinatally, before laminar formation is complete, their cortical cells are not fundamentally respecified to ventral telencephalic fates (Kroll and O'Leary 2005; Quinn et al. 2007; Manuel et al. 2007), and late-generated neurons do not fail to migrate appropriately (Caric et al. 1997; Fukuda et al. 2000). Our BrdU birthdating experiments demonstrated that late-born neurons are properly positioned in the superficial layers of the PAX77 cortex. We found that cortical neurons acquire their correct laminar identities at correct proportions in both deep and superficial cortical layers in the postnatal PAX77 cortex, indicating that increased levels of Pax6 do not affect laminar phenotypes.
To gain more insight into the role of Pax6 in regulating the formation of superficial layers, we generated Pax6 conditional knockout mice that are viable postnatally. We provide evidence that the generation of superficial layers is severely affected when Pax6 is inactivated in cortical progenitors from midstages of corticogenesis. Both the numbers and specification of late-born neurons were markedly affected in the superficial layers of mutant cortex. This laminar phenotype is in agreement with recent data from Tuoc et al. (2009) where Pax6 was conditionally removed from the onset of corticogenesis by using a conventional Emx1-Cre. Interestingly, the presence of Pax6 also appears to be required for correct specification of deep-layer neurons (Tuoc et al. 2009).
Transplantation studies have indicated that the inability of late-born neurons to migrate to superficial positions of the Pax6−/− cortex is an indirect consequence of the altered environment present in mutant animals (Caric et al. 1997). Conditional inactivation of Pax6 starting from the onset of corticogenesis significantly affects the migratory ability of late-born cortical neurons resulting in an enlarged germinal zone postnatally (Tuoc et al. 2009). Here, we find that the majority of late-born cortical neurons retain the ability to migrate to appropriate laminar positions of the mutant cortex, suggesting that removal of Pax6 from cortical progenitors at later stages of corticogenesis might produce a more normal cortical environment where neurons born at later stages have the potential to migrate superficially.
How do Pax6 levels regulate the development of superficial layer neurons? Increased Pax6 levels control specific aspects of laminar development related more to cell cycle commitment and cell cycle length regulation of late cortical progenitors than to cell fate determination. However, conditional inactivation of Pax6 in cortical progenitors starting from midstages of corticogenesis resulted in a decreased number of late-born neurons together with a significant failure of late-born neurons to adopt an appropriate superficial layer identity. Collectively, our findings indicate that both gain- and loss-of-function of Pax6 affect the formation of superficial layers of the cortex, but we suggest that the final laminar phenotype is achieved through different mechanisms. Precise levels of Pax6 are required primarily to regulate cell cycle properties, thereby ensuring the production of appropriate numbers of superficial layer neurons. The presence of Pax6, but not its precise levels, is required for specification of superficial layer neurons.
Supplementary Material
Supplementary Figures 1 and 2 can be found at: http://www.cercor.oxfordjournals.org/
Funding
The Medical Research Council (G0800429); The Welcome Trust (082456).
Supplementary Material
Acknowledgments
We are grateful to Drs John Mason and Jane Quinn for helpful discussions and insight. We are indebted to Dr Nicoletta Kessaris for providing the Emx1-CreER mice. We thank Ms Katy Gillies and Mr Mike Molinek for excellent technical assistance. We thank Drs Tom Pratt, Peter Kind, Chris Conway, Vassiliki Fotaki, and Victor Tarabykin for thoughtful discussions. We thank Robert Hevner for providing the Tbr1 antibody. Conflict of Interest: None declared.
References
- Alcamo EA, Chirivella L, Dautzenberg M, Dobreva G, Fariñas I, Grosschedl R, McConnell SK. Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron. 2008;57(3):364–377. doi: 10.1016/j.neuron.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Angevine JB, Sidman RL. Autoradiographic study of cell migration during histogenesis of the cerebral cortex in the mouse. Nature. 1961;192:766–768. doi: 10.1038/192766b0. [DOI] [PubMed] [Google Scholar]
- Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45(2):207–221. doi: 10.1016/j.neuron.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Bel-Vialar S, Medevielle F, Pituello F. The on/off of Pax6 controls the tempo of neuronal differentiation in the developing spinal cord. Dev Biol. 2007;305(2):659–673. doi: 10.1016/j.ydbio.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Britanova O, de Juan Romero C, Cheung A, Kwan KY, Schwark M, Gyorgy A, Vogel T, Akopov S, Mitkovski M, Agoston D, et al. Satb2 is a postmitotic determinant for upper-layer neuron specification in the neocortex. Neuron. 2008;57(3):378–392. doi: 10.1016/j.neuron.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Caric D, Gooday D, Hill RE, McConnell SK, Price DJ. Determination of the migratory capacity of embryonic cortical cells lacking the transcription factor Pax-6. Development. 1997;124:5087–5096. doi: 10.1242/dev.124.24.5087. [DOI] [PubMed] [Google Scholar]
- Cartier L, Laforge T, Feki A, Arnaudeau S, Dubois-Dauphin M, Krause KH. Pax6-induced alteration of cell fate: shape changes, expression of neuronal alpha tubulin, postmitotic phenotype, and cell migration. J Neurobiol. 2006;66(5):421–436. doi: 10.1002/neu.20225. [DOI] [PubMed] [Google Scholar]
- Caviness V, Takahashi T. Proliferative events in the cerebral ventricular zone. Brain Dev. 1995;17(3):159–163. doi: 10.1016/0387-7604(95)00029-b. [DOI] [PubMed] [Google Scholar]
- Caviness V, Takahashi T, Nowakowski R. Numbers, time and neocortical neuronogenesis: a general developmental and evolutionary model. Trends Neurosci. 1995;18:379–383. doi: 10.1016/0166-2236(95)93933-o. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Goto T, Tarui T, Takahashi T, Bhide PG, Nowakowski RS. Cell output, cell cycle duration and neuronal specification: a model of integrated mechanisms of the neocortical proliferative process. Cereb Cortex. 2003;13:592–598. doi: 10.1093/cercor/13.6.592. [DOI] [PubMed] [Google Scholar]
- Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297(5580):328. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- Chenn A, Walsh CA. Increased neuronal production, enlarged forebrains and cytoarchitectural distortions in beta-catenin overexpressing transgenic mice. Cereb Cortex. 2003;13(6):599–606. doi: 10.1093/cercor/13.6.599. [DOI] [PubMed] [Google Scholar]
- Del Rio JA, Soriano E. Immunocytochemical detection of 5'-bromodeoxyuridine incorporation in the central nervous system of the mouse. Brain Res Dev Brain Res. 1989;49(2):311–317. doi: 10.1016/0165-3806(89)90033-3. [DOI] [PubMed] [Google Scholar]
- Desai AR, McConnell SK. Progressive restriction in fate potential by neural progenitors during cerebral cortical development. Development. 2000;127:2863–2872. doi: 10.1242/dev.127.13.2863. [DOI] [PubMed] [Google Scholar]
- Englund C, Fink A, Lau C, Pham D, Daza RA, Bulfone A, Kowalczyk T, Hevner RF. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci. 2005;25:247–251. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estivill-Torrus G, Pearson H, van Heyningen V, Price DJ, Rashbass P. Pax6 is required to regulate the cell cycle and the rate of progression from symmetrical to asymmetrical division in mammalian cortical progenitors. Development. 2002;129(2):455–466. doi: 10.1242/dev.129.2.455. [DOI] [PubMed] [Google Scholar]
- Frantz GD, McConnell SK. Restriction of late cerebral cortical progenitors to an upper-layer fate. Neuron. 1996;17(1):55–61. doi: 10.1016/s0896-6273(00)80280-9. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Kawano H, Osumi N, Eto K, Kawamura K. Histogenesis of the cerebral cortex in rat fetuses with a mutation in the Pax-6 gene. Dev Brain Res. 2000;120:65–75. doi: 10.1016/s0165-3806(99)00187-x. [DOI] [PubMed] [Google Scholar]
- Gillies K, Price DJ. The fates of cells in the developing cerebral cortex of normal and methylazoxymethanol acetate-lesioned mice. Eur J Neurosci. 1993;5:73–84. doi: 10.1111/j.1460-9568.1993.tb00207.x. [DOI] [PubMed] [Google Scholar]
- Gotz M, Stoykova A, Gruss P. Pax6 controls radial glia differentiation in the cerebral cortex. Neuron. 1998;21:1031–1044. doi: 10.1016/s0896-6273(00)80621-2. [DOI] [PubMed] [Google Scholar]
- Hack MA, Sugimori M, Lundberg C, Nakafuku M, Götz M. Regionalization and fate specification in neurospheres: the role of Olig2 and Pax6. Mol Cell Neurosci. 2004;25(4):664–678. doi: 10.1016/j.mcn.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Haubst N, Berger J, Radjendirane V, Graw J, Favor J, Saunders GF, Stoykova A, Gotz M. Molecular dissection of Pax6 function: the specific roles of the paired domain and homeodomain in brain development. Development. 2004;131:6131–6140. doi: 10.1242/dev.01524. [DOI] [PubMed] [Google Scholar]
- Heins N, Malatesta P, Cecconi F, Nakafuku M, Tucker KL, Hack MA, Chapouton P, Barde YA, Gotz M. Glial cells generate neurons: the role of the transcription factor Pax6. Nat Neurosci. 2002;5:308–315. doi: 10.1038/nn828. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Shi L, Justice N, Hsueh Y, Sheng M, Smiga S, Bulfone A, Goffinet AM, Campagnoni AT, Rubenstein JL. Tbr1 regulates differentiation of the preplate and layer 6. Neuron. 2001;29:353–366. doi: 10.1016/s0896-6273(01)00211-2. [DOI] [PubMed] [Google Scholar]
- Hill RE, Favor J, Hogan BL, Ton CC, Saunders GF, Hanson IM, Prosser J, Jordan T, Hastie ND, van Heyningen V. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature. 1991;354:522–525. doi: 10.1038/354522a0. [DOI] [PubMed] [Google Scholar]
- Hogan BLM, Horsburgh G, Cohen J, Hetherington CM, Fischer G, Lyon MF. Small eye (Sey): a homozygous lethal mutation on chromosome 2 which affects the differentiation of both lens and nasal placodes in the mouse. J Embryol Exp Morphol. 1986;97:95–110. [PubMed] [Google Scholar]
- Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 2006;9(2):173–179. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Changes in cell-cycle kinetics during the development and evolution of primate neocortex. Proc Natl Acad Sci U S A. 1998;95(3):1242–1246. doi: 10.1073/pnas.95.3.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll TT, O'Leary DD. Ventralized dorsal telencephalic progenitors in Pax6 mutant mice generate GABA interneurons of a lateral ganglionic eminence fate. Proc Natl Acad Sci U S A. 2005;102(20):7374–7379. doi: 10.1073/pnas.0500819102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuel M, Georgala PA, Carr CB, Chanas S, Kleinjan DA, Martynoga B, Mason JO, Molinek M, Pinson J, Pratt T, et al. Controlled overexpression of Pax6 in vivo negatively autoregulates the Pax6 locus, causing cell-autonomous defects of late cortical progenitor proliferation with little effect on cortical arealization. Development. 2007;134:545–555. doi: 10.1242/dev.02764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martynoga B, Morrison H, Price DJ, Mason JO. Foxg1 is required for specification of ventral telencephalon and region-specific regulation of dorsal telencephalic precursor proliferation and apoptosis. Dev Biol. 2005;283(1):113–127. doi: 10.1016/j.ydbio.2005.04.005. [DOI] [PubMed] [Google Scholar]
- McConnell SK, Kaznowski CE. Cell cycle dependence of laminar determination in developing neocortex. Science. 1991;254:282–285. doi: 10.1126/science.254.5029.282. [DOI] [PubMed] [Google Scholar]
- Nieto M, Monuki ES, Tang H, Imitola J, Haubst N, Khoury SJ, Cunningham J, Gotz M, Walsh CA. Expression of Cux-1 and Cux-2 in the subventricular zone and upper layers II-IV of the cerebral cortex. J Comp Neurol. 2004;479(2):168–180. doi: 10.1002/cne.20322. [DOI] [PubMed] [Google Scholar]
- Osumi N, Shinohara H, Numayama-Tsuruta K, Maekawa M. Concise review: Pax6 transcription factor contributes to both embryonic and adult neurogenesis as a multifunctional regulator. Stem Cells. 2008;26(7):1663–1672. doi: 10.1634/stemcells.2007-0884. [DOI] [PubMed] [Google Scholar]
- Philips GT, Stair CN, Young Lee H, Wroblewski E, Berberoglu MA, Brown NL, Mastick GS. Precocious retinal neurons: Pax6 controls timing of differentiation and determination of cell type. Dev Biol. 2005;279:308–321. doi: 10.1016/j.ydbio.2004.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JC, Molinek M, Martynoga BS, Zaki PA, Faedo A, Bulfone A, Henver RF, West JD, Price DJ. Pax6 controls cerebral cortical cell number by regulating exit from the cell cycle and specifies cortical cell identity by a cell autonomous mechanism. Dev Biol. 2007;302:50–65. doi: 10.1016/j.ydbio.2006.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science. 1974;183:425–427. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- Rakic P. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 1995;18(9):383–388. doi: 10.1016/0166-2236(95)93934-p. [DOI] [PubMed] [Google Scholar]
- Sansom SN, Griffiths DS, Faedo A, Kleinjan DJ, Ruan Y, Smith J, van Heyningen V, Rubenstein JL, Livesey FJ. The level of the transcription factor Pax6 is essential for controlling the balance between neural stem cell self-renewal and neurogenesis. PloS Genet. 2009;5(6):e1000511. doi: 10.1371/journal.pgen.1000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedl A, Ross A, Lee M, Engelkamp D, Rashbass P, van Heyningen V, Hastie ND. Influence of PAX6 gene dosage on development: overexpression causes severe eye abnormalities. Cell. 1996;86(1):71–82. doi: 10.1016/s0092-8674(00)80078-1. [DOI] [PubMed] [Google Scholar]
- Schmahl W, Knoedlseder M, Favor J, Davidson D. Defects of neuronal migration and the pathogenesis of cortical malformations are associated with Small eye (Sey) in the mouse, a point mutation at the Pax-6-locus. Acta Neuropathol. 1993;86:126–135. doi: 10.1007/BF00334879. [DOI] [PubMed] [Google Scholar]
- Schuurmans C, Armant O, Nieto M, Stenman JM, Britz O, Klenin N, Brown C, Langevin LM, Seibt J, Tang H, et al. Sequential phases of cortical specification involve Neurogenin-dependent and -independent pathways. EMBO J. 2004;23:2892–2902. doi: 10.1038/sj.emboj.7600278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson TI, Pratt T, Mason JO, Price DJ. Normal ventral telencephalic expression of Pax6 is required for normal development of thalamocortical axons in embryonic mice. Neural Dev. 2009;4:19. doi: 10.1186/1749-8104-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoykova A, Fritsch R, Walther C, Gruss P. Forebrain patterning defects in Small eye mutant mice. Development. 1996;122:3453–3465. doi: 10.1242/dev.122.11.3453. [DOI] [PubMed] [Google Scholar]
- Stoykova A, Gruss P. Roles of Pax-genes in developing and adult brain as suggested by expression patterns. J. Neurosci. 1994;14:1395–1412. doi: 10.1523/JNEUROSCI.14-03-01395.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoykova A, Treichel D, Hallonet M, Gruss P. Pax6 modulates the dorsoventral patterning of the mammalian telencephalon. J Neurosci. 2000;20(21):8042–8050. doi: 10.1523/JNEUROSCI.20-21-08042.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Goto T, Miyama S, Nowakowski RS, Caviness VS., Jr Sequence of neuron origin and neocortical laminar fate: relation to cell cycle of origin in the developing murine cerebral wall. J Neurosci. 1999;19(23):10357–10371. doi: 10.1523/JNEUROSCI.19-23-10357.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Nowakowski R, Caviness VS., Jr The cell cycle of the pseudostratified ventricular epithelium of the murine cerebral wall. J Neurosci. 1995;15:6046–6057. doi: 10.1523/JNEUROSCI.15-09-06046.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Nowakowski RS, Caviness VS., Jr Mode of cell proliferation in the developing mouse neocortex. Proc Natl Acad Sci U S A. 1994;91(1):375–379. doi: 10.1073/pnas.91.1.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talamillo A, Quinn JC, Collinson JM, Caric D, Price DJ, West JD, Hill RE. Pax6 regulates regional development and neuronal migration in the cerebral cortex. Dev Biol. 2003;255:151–163. doi: 10.1016/s0012-1606(02)00046-5. [DOI] [PubMed] [Google Scholar]
- Tamai H, Shinohara H, Miyata T, Saito K, Nishizawa Y, Nomura T, Osumi N. Pax6 transcription factor is required for the interkinetic nuclear movement of neuroepithelial cells. Genes Cells. 2007;12(9):983–996. doi: 10.1111/j.1365-2443.2007.01113.x. [DOI] [PubMed] [Google Scholar]
- Tarabykin V, Stoykova A, Usman N, Gruss P. Cortical upper layer neurons derive from the subventricular zone as indicated by Svet1 gene expression. Development. 2001;128:1983–1993. doi: 10.1242/dev.128.11.1983. [DOI] [PubMed] [Google Scholar]
- Tarui T, Takahashi T, Nowakowski RS, Hayes NL, Bhide PG, Caviness VS. Overexpression of p27 Kip 1, probability of cell cycle exit, and laminar destination of neocortical neurons. Cereb Cortex. 2005;15(9):1343–1355. doi: 10.1093/cercor/bhi017. [DOI] [PubMed] [Google Scholar]
- Tuoc TC, Radyushkin K, Tonchev AB, Pinon MC, Ashery-Padan R, Molnar Z, Davidoff MS, Stoykova A. Selective cortical layering abnormalities and behavioral deficits in cortex-specific Pax6 knock-out mice. J Neurosci. 2009;29(26):8335–8349. doi: 10.1523/JNEUROSCI.5669-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther C, Gruss P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development. 1991;113:1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- Warren N, Caric D, Pratt T, Clausen JA, Asavaritikrai P, Mason JO, Hill RE, Price DJ. The transcription factor, Pax6, is required for cell proliferation and differentiation in the developing cerebral cortex. Cereb Cortex. 1999;9:627–635. doi: 10.1093/cercor/9.6.627. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.