Abstract
Reasoning is a key component of adaptable “executive” behavior and is known to depend on a network of frontal and parietal brain regions. However, the mechanisms by which this network supports reasoning and adaptable behavior remain poorly defined. Here, we examine the relationship between reasoning, executive control, and frontoparietal function in a series of nonverbal reasoning experiments. Our results demonstrate that, in accordance with previous studies, a network of frontal and parietal brain regions is recruited during reasoning. Our results also reveal that this network can be fractionated according to how different subregions respond when distinct reasoning demands are manipulated. While increased rule complexity modulates activity within a right lateralized network including the middle frontal gyrus and the superior parietal cortex, analogical reasoning demand—or the requirement to remap rules on to novel features—recruits the left inferior rostrolateral prefrontal cortex and the lateral occipital complex. In contrast, the posterior extent of the inferior frontal gyrus, associated with simpler executive demands, is not differentially sensitive to rule complexity or analogical demand. These findings accord well with the hypothesis that different reasoning demands are supported by different frontal and parietal subregions.
Keywords: analogical reasoning, fMRI, frontal lobe, rostrolateral prefrontal cortex, rule integration
Introduction
When faced with a novel problem, the search for a suitable response is often reasoned, being guided by predictions based on prior experience of situations that, while not identical, are in some respect comparable. Hence, reasoning can be considered to be crucial to adaptable behavior. It has been known for many years that the human frontal lobes play a particularly important role in supporting adaptability, with frontal lobe damage leading to poorly adapted or “dysexecutive” behaviors (Luria et al. 1966; Stuss and Benson 1986; Fuster 1997). More recently, neuroimaging research has demonstrated that a network distributed across the frontal and parietal lobes is commonly recruited when difficulty is increased across a broad range of task contexts (Duncan and Owen 2000; Nyberg et al. 2003; Dosenbach et al. 2006; Duncan 2006). This network is also recruited by novel nonverbal reasoning problems similar to those found in archetypal tests of fluid intelligence such as Cattell's Culture Fair and Raven's Progressive Matrices (Duncan et al. 2000). Furthermore, it has been demonstrated that patients with damage to this network are impaired on tests of fluid intelligence (Duncan et al. 1995) and that the volume of damage within this network is correlated with the size of the observed deficit (Duncan 2005).
While this evidence supports the hypothesis that a frontoparietal network contributes to adaptive behavior, at least in part, by supporting reasoning, the mechanisms by which this is achieved remain poorly understood. A growing number of authors have argued that although the frontoparietal network tends to corecruit when the demand for executive control increases, different components of that network may preferentially support different aspects of executive function, with higher-order executive functions being preferentially supported by dorsal and anterior frontal lobe subregions (Koechlin et al. 1999, 2003; Owen et al. 2000; Fletcher and Henson 2001; Corbetta and Shulman 2002; Badre and Wagner 2004; Ramnani and Owen 2004; Petrides 2005; Hampshire and Owen 2006; Hampshire et al. 2007). Less is known about the localization of functions underlying reasoning and fluid intelligence as a consequence of which a number of key questions remain unaddressed. For example, which frontoparietal brain regions are recruited when reasoning demands are manipulated? Do different regions support different aspects of reasoning? Do frontoparietal brain regions that are sensitive to reasoning demands differ from those that have been associated with other aspects of adaptable behavior, for example, the orienting of attention toward task-relevant stimuli (Linden et al. 1999; Corbetta and Shulman 2002; Hampshire and Owen 2006; Hampshire et al. 2007, 2009; Hampshire, Thompson, et al. 2008), the processing of environmental feedback (O'Doherty et al. 2001), and the suppression of habitual responses (Aron et al. 2004).
Here, we use functional magnetic resonance imaging (fMRI) to examine the complex relationship between reasoning, executive control, and frontoparietal function in 2 novel nonverbal reasoning tasks. First, we identify the neural network that is recruited when participants are solving a series of nonverbal reasoning problems. Then, we identify and contrast directly between those subregions of the frontoparietal network that are affected when 2 factors that contribute to problem difficulty are orthogonally manipulated: 1) rule complexity, or the number of subrules from which a problem is composed, and 2) analogical distance, or the extent to which the surface features differ between the contexts in which the rule is derived and applied. In a second experiment, we replicate our findings in a different population sample and using a modified version of the task that examines the rule derivation and rule application stages of the reasoning process separately.
Materials and Methods
Experiment 1
Task Design
Participants were required to solve a series of novel nonverbal reasoning problems (Fig. 1). Each problem consisted of 2 panels, a rule derivation panel and a rule application panel. These were presented simultaneously at the top and the bottom of the screen. The rule derivation panel contained 3 objects that differed according to a stepwise rule running from left to right across the screen. For example, if the objects were a triangle, a square, and a pentagon, then the rule would be an increase in the number of sides that form the objects. Rules could be either simple with just 1 component (e.g., an increase in the number of sides) or compound with 2 components (e.g., an increase in the number of sides and a decrease in the overall size). The rule application panel contained 4 objects, 1 at the top and 3 at the bottom (Fig. 1). Participants were required to choose which of the 3 objects at the bottom followed the one at the top when applying the rule extracted from the derivation panel. Each rule derivation panel was included in 2 types of problem. 1) A near-analogy problem, in which surface features were drawn from the same category in the derivation and the application panels, for example, if number of dots incremented by 2 in each step of the derivation panel, then the application panel would also consist of objects with a variable number of dots (Fig. 1, left). 2) A far analogical problem in which the surface features were drawn from visually distinct categories in the derivation and application panels, for example, if the number of dots incremented by 2 in each step of the derivation panel, then the application panel could contain shapes with a variable numbers of sides (Fig. 1, right). Near and far analogical problems were presented in a predefined pseudorandomized sequence in order to control for any effects of rule or task familiarity. The features that were relevant in the application portion of the problems were balanced across the near and far analogical panels, that is, color, number, position, etc., were relevant in an equal number of near and far analogical problems. Completion of the task was self-paced, and all problems were displayed on the screen until a response was made by pressing left, down, or right with the right thumb on a custom-made response dial. In order to motivate and guide behavior, feedback consisting of either the word “CORRECT” in green or “INCORRECT” in red was presented in the center of the screen for 600 ms immediately after the response. Subsequently, there was a 4-s blank screen prior to the presentation of the next problem.
Figure 1.
Nonverbal reasoning problems. The figure shows typical examples of the different types of problem used in the reasoning task. Problems consisted of a rule derivation panel and a rule application panel that were presented simultaneously at the top and the bottom of the screen. Participants first worked out the stepwise rule in the top (rule derivation) panel. This rule could either be simple, consisting of 1 rule, or compound, consisting of 2 rules. They then had to apply the rule to work out which of the 3 objects at the bottom of the application panel followed the one at the top. If the rule was applied to surface features of the same type, for example, the number of small circles (top left) or the positions of colored squares (bottom left), then the problem was near analogical. By contrast, if the rules had to be remapped from one type of surface feature to another, for example, from the number of small circles to the number of sides (top right) or from the position of colored squares to the number of dots on a die (bottom right), then the problem was far analogical.
Data Acquisition
Sixteen right-handed volunteers between the ages of 20 and 40 undertook the fMRI task. All volunteers had normal or corrected-to-normal vision and no history of neurological or psychiatric illness. Data were collected in one continuous block of scanning acquisition containing a total of 24 problems. Each of the 4 possible combinations of rule complexity (simple vs. compound rules) and application context (near vs. far analogical) were repeated 6 times. The task ran until all problems were completed or a maximum time of 15 min was reached, with all but one participant completing all problems within the allocated time. Scanning was carried out at the Medical Research Council Cognition and Brain Sciences Unit using a 3 Tesla Siemens Trim Trio scanner; 32 3-mm slices (0.75 mm interslice gap) were acquired using a time repetition of 2 s and in-plane resolution of 3 × 3 mm; 480 -weighted echo-planar images depicting blood oxygen level–dependent (BOLD) contrast were acquired in the task, with the first 10 discarded to avoid T1 equilibrium effects. The experiment was programmed in Visual Basic 6. The stimulus display was projected onto a screen located behind the bore of the magnet and viewed via a mirror mounted to the headcoil. Each display subtended a visual angle of approximately 9 degrees.
Images were preprocessed and analyzed using SPM5 (Wellcome Department of Cognitive Neurology). Prior to analysis, images were slice time corrected, reoriented to correct for subject motion, spatially normalized to the standard Montreal Neurological Institute template, and smoothed with an 8-mm full-width at half-maximum Gaussian kernel. Data were also high-pass filtered prior to analysis (cutoff period 128 s). Separate fixed-effects analyses were carried out on each volunteer's data using general linear models. Regressor functions were created by convolving timing functions, indicating the onset and duration of each event with a basis function representing the canonical hemodynamic response. Explicitly modeling the duration of each event ensured that the resultant beta values represented an estimate of the neural response per unit time spent solving the problem. In this way, the model controlled for any systematic differences in the time taken to solve different types of problem with any activation differences observed in harder problems being due to a heightened as opposed to prolonged neural response. In the current task, each block of trials was modeled by 6 regressors. In addition to one regressor for each of the 4 reasoning conditions (simple near analogical, simple far analogical, compound near analogical, and compound far analogical), 2 regressors were used to model any confounding effects of positive and negative feedback events. Activity relating to the generation of the button response was also accounted for by the feedback regressors as feedback occurred at the time of response. Six further regressors were included representing the translational and rotational movement parameters within the x, y, and z planes.
Images depicting beta weights for the 4 reasoning conditions were examined at the group level using random-effects analyses in SPM5 in order to identify brain regions that 1) were recruited during the reasoning task, 2) showed increased BOLD activation when rule complexity was manipulated, 3) showed increased BOLD activation when analogical distance was manipulated, and 4) showed activation increases that were “significantly higher” for either one of these reasoning demands when contrasted directly with the other.
Experiment 2
Task Design
Experiment 2 was designed to replicate the functional dissociations observed in experiment 1, while investigating whether regional activations observed during increased rule complexity were specific to the rule derivation or application stages of the reasoning process. The design was identical to that used in experiment 1 except that the rule derivation and rule application panels were displayed successively rather than simultaneously. Separating the rule derivation and rule application panels in this way also controlled for the diversity of the visual display when applying rules during far-analogy problems. Each trial began with a rule derivation panel displayed on its own in the center of the screen. Participants pressed a button to indicate when they had derived the rule, and after a delay of 3.6 s, the application panel was presented. This was visible until participants indicated which of the 3 objects in the bottom of the panel followed the one at the top when they applied the rule they had just derived. Immediately after response, feedback was displayed for 600 ms, subsequent to which there was a 3-s blank screen prior to the start of the next problem. Rules could be either simple or compound and in order to manipulate analogical distance could be applied to surface features of either the same or a different category. Each rule derivation panel was presented twice, once prior to a near analogical panel and once prior to a far analogical panel. As in the first experiment, problems were presented in a predefined pseudorandomized sequence that was designed to balance rule and task familiarity across the 4 reasoning conditions.
Data Acquisition
Twenty-one right-handed volunteers between the ages of 20 and 40 undertook the fMRI task. Data were collected in one continuous block containing 24 problems (6 of each type). The task ran until all problems were completed or a maximum time of 15 min had elapsed. Scanning acquisition and preprocessing parameters were identical to those described above for experiment 1. Analysis of each participant's data was carried out using similar general linear models, with the exception that data were modeled using 10 experimental event types. Presentations of the rule derivation panels were divided into 4 event types depending on whether they depicted simple or compound rules and whether it was the first or the second exposure of the rule derivation slide. Since all rule derivation panels were displayed twice (once prior to a near analogical and once prior to a far analogical application panel), explicitly modeling presentation number in this way allowed us to examine derivation of rules free from any potential confounds related to rule familiarity. In keeping with experiment 1, application panels were broken down into 4 event types, simple near analogical, simple far analogical, compound near analogical, and compound far analogical. Two regressors were used to model positive and negative feedback events, and 6 further regressors were included representing the translational and rotational movement parameters within the x, y, and z planes.
Data from the application stage of the task were examined at the group level using a focused test–retest approach. Activation clusters from experiment 1 for the direct contrast between high rule complexity and high analogical distance were defined as regions of interest (ROIs) using the MARSBAR ROI toolbox (Brett et al. 2002). Mean beta weights for all voxels within these ROIs were extracted separately for each participant for each of the 4 application event types, and these data were exported for group-level analysis in SPSS. The results of the ROI analyses were backed up with whole-brain analyses. Whole-brain maps depicting beta weights for the 4-rule application regressors were examined at the group level in a 2 × 2 factorial design (rule complexity × analogical distance).
Data from the rule derivation stage of the task were also examined using whole-brain analysis. Simple rules were subtracted from complex rules and the resultant contrast images were examined at the group level using a one-sample t-test in SPM5.
Results
Experiment 1: Behavioral Results
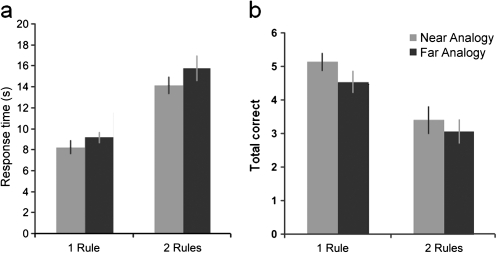
One outlier was removed from the behavioral data due to particularly slow response times that were over 2.5 standard deviations from the mean. For the remaining 15 participants, the effects of rule complexity and analogical distance on response times (Fig. 2a) were examined in a 2 × 2 repeated-measures analysis of variance (ANOVA). The results showed that both manipulations caused an increase in response time (rule complexity F1,14 = 40.26, P < 0.001; analogical distance F1,14 = 8.41, P = 0.01) and can therefore be considered to increase the overall difficulty of the reasoning process. There was no interaction between the 2 main effects. The mean effects of both of these difficulty manipulations on the total number of correctly solved problems (Fig. 2b) were also examined using a similar ANOVA. The results revealed a significant decrease in the number of correctly solved problems when complexity was increased (F1,14 = 36.82, P < 0.001) and when analogical distance was increased (F1,14 = 5.91, P < 0.05) with no significant interaction.
Figure 2.
Behavioral results from experiment 1. The figure shows behavioral data for the different task conditions. (a) Mean response times with the standard error of the mean. Rule complexity and analogical distance both caused an increase in response time. (b) Mean number correct (out of a total of 6). Rule complexity and analogical distance both caused a decrease in the number of correctly solved problems.
Overall scores for the task (mean total correct = 16.1 out of 24, standard error = 1.13) were also compared with IQ as measured by the Cattell Culture Fair test (average score = 123, standard error = 3.17) using linear regression. As expected, given the similarity between this task and classic tests of IQ, the overall score on the reasoning task showed a significant correlation with IQ as measured on the Cattell (r = 0.619, P = 0.018). The relationship between different types of task demand and IQ was also investigated by calculating a difference score representing the number correct on the easy levels for each difficulty factor minus the hard levels for that factor (i.e., simple rules minus compound rules and near analogical application minus far analogical application). Taken individually, neither of these scores showed a significant correlation with IQ. The average effect of general difficulty was also examined by averaging the success rates for 2-rule near analogical, 2-rule far analogical, and 1-rule far analogical problems and subtracting this value from the success rate for the one-move near analogical problems. A significant correlation was observed with IQ for this average effect of difficulty (r = −0.61, P = 0.02). These results demonstrate that while higher IQ individuals were better at the reasoning task in general and were better able to cope with more difficult reasoning problems, this ability was not specific to dealing with either increased rule complexity or increased analogical distance.
Experiment 1: Imaging Results
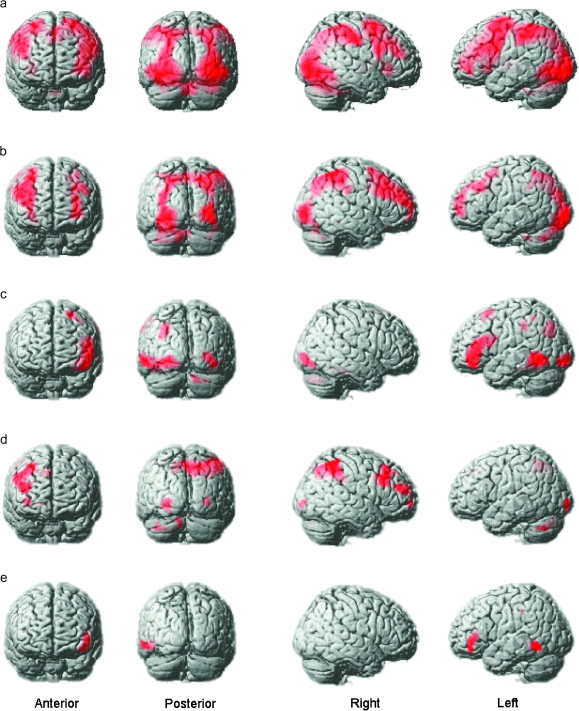
In order to identify brain regions that were recruited during performance of the reasoning task, the beta images for the 4 types of reasoning problem were averaged at the individual participant level. The resultant contrast images were examined at the group level using a one-sample t-test in SPM5 and evaluated at a threshold corrected for false discovery rate (FDR) at P < 0.05. As expected, this contrast identified an extensive network of brain regions, including frontal, parietal, and higher visual areas (Fig. 3a and Table 1). Within the frontoparietal network, activity was observed bilaterally in the posterior inferior frontal gyrus (pIFG), the middle frontal gyrus (MFG), the posterior parietal cortex (PPC) in both hemispheres, and medially in the presupplementary motor area. Activity was also observed in subcortical areas including the caudate head.
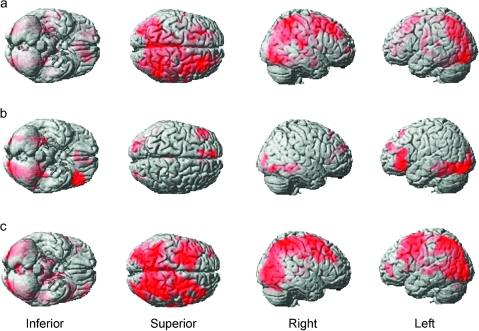
Figure 3.
Whole-brain analyses from experiment 1. The figure depicts whole-brain maps from the group-level analyses of experiment 1 with FDR correction at P < 0.05 for the whole-brain mass. (a) In accordance with previous findings, an extensive network of brain regions was recruited during problem solving including a broad swathe of the MFG, the pIFG, the PPC, the ventral and the dorsal visual processing streams, and the caudate in both hemispheres. (b) When the complexity of the rules increased, the MFG and the PPC showed an increase in activation. (c) When the analogical distance between the rule derivation and application slides increased activation within the iRLPFC, left aLOC, and the parahippocampal gyrus increased. (d) Activation in much of the right MFG, more focally in the left MFG, and in the PPC, was elevated when directly contrasting increased rule complexity minus increased analogical distance. (e) The left iRLPFC and the left aLOC were activated more when directly contrasting increased analogical distance with increased rule complexity.
Table 1.
Peak activation coordinates during reasoning in experiment 1
| x | y | z | t | Region | Approximate BA |
| −30 | 24 | −2 | 9.97 | pIFG left | BA47/44 |
| 30 | 28 | 0 | 9.69 | pIFG right | BA47/44 |
| −28 | −2 | 60 | 11.37 | Premotor left | BA6 |
| −44 | 48 | 16 | 4.34 | MFG left | BA10 |
| 26 | 4 | 54 | 8.82 | Premotor right | BA6 |
| 48 | 44 | 22 | 7.37 | MFG right | BA46 |
| −4 | 16 | 48 | 10.17 | preSMA | BA6 |
| −28 | −48 | 48 | 11.96 | PPC left | BA7 |
| 30 | −68 | 36 | 8.71 | PPC right | BA7 |
| −10 | 4 | 2 | 4.25 | Caudate head | — |
| 12 | 4 | 0 | 4.54 | Caudate head | — |
Note: BA, Brodmann area; preSMA, presupplementary motor area.
In order to identify brain regions that were more active when reasoning demands increased, the data were examined using a 2 × 2 full factorial design in SPM5 in which the factors were rule complexity (1- vs. 2-rule problems) and analogical distance (near vs. far analogical problems). The results revealed distinctive patterns of activation for the 2 main effects (Table 2). More specifically, the positive main effect of rule complexity (Fig. 3b) rendered a right lateralized dorsal network including the superior parietal cortex and precuneus (PPC) and the MFG. By contrast, the positive main effect of analogical distance (Fig. 3c) rendered activation in the left inferior rostrolateral prefrontal cortex (iRLPFC) and the left anterior lateral occipital complex (aLOC). Interestingly, the pIFG, which was strongly activated in both hemispheres during the reasoning task, did not respond significantly at the whole-brain corrected threshold to either of the positive main effects. Focused ROI analysis using 10-mm radius spheres based on the peak coordinates for the pIFG in the contrast of task to baseline (left x = −30, y = 24, z = −2; right x = 30, y = 28, z = 0) confirmed this result with no significant increase in activation in the pIFG ROIs when rule complexity (left t = 1.20, P = 0.12; right t = 1.15, P = 0.13) and analogical distance increased (left t = 0.56, P = 0.29; right t = −0.14, P = 0.55).
Table 2.
Peak activation coordinates for rule complexity and analogical distance in experiment 1
| x | y | z | t | Region | Approximate BA |
| Main effect of rule complexity | |||||
| −30 | 52 | −2 | 4.28 | FPC left | BA10 |
| 36 | 60 | 16 | 4.38 | FPC right | BA10 |
| −32 | 54 | 20 | 4.93 | MFG left | BA10 |
| −44 | 28 | 38 | 4.08 | MFG left | BA9 |
| 44 | 46 | 20 | 5.04 | MFG right | BA10 |
| 50 | 24 | 32 | 4.35 | MFG right | BA46 |
| 8 | −56 | 58 | 5.97 | PPC center | BA7 |
| −26 | −56 | 56 | 4.27 | PPC left | BA7 |
| 22 | −60 | 46 | 6.83 | PPC right | BA7 |
| Main effect of analogical distance | |||||
| −46 | 40 | 0 | 6.55 | iRLPFC left | BA47 |
| −46 | 30 | 16 | 5.35 | iRLPFC left | BA46 |
| −40 | 40 | −16 | 6.50 | iRLPFC left | BA11 |
| −44 | −50 | −8 | 7.26 | aLOC left | BA37 |
| −28 | −66 | 32 | 4.64 | PPC left | BA7 |
| −22 | 18 | 60 | 4.94 | Premotor left | BA6 |
| Rule complexity—analogical distance | |||||
| −32 | 60 | 16 | 3.26 | FPC left | BA10 |
| 16 | 64 | −2 | 3.76 | FPC right | BA10 |
| −26 | 44 | 36 | 3.26 | MFG left | BA9 |
| 36 | 58 | −2 | 3.38 | MFG right | BA10 |
| 42 | 22 | 38 | 3.71 | MFG right | BA9 |
| 44 | 46 | 20 | 4.41 | MFG right | BA10 |
| 14 | −68 | 44 | 5.32 | PPC center | BA7 |
| −16 | −54 | 44 | 4.37 | PPC left | BA7 |
| 44 | −48 | 48 | 4.92 | PPC right | BA40 |
| Analogical distance—rule complexity | |||||
| −46 | 40 | 0 | 5.42 | iRLPFC left | BA47 |
| −40 | 40 | −10 | 4.27 | iRLPFC left | BA47 |
| −62 | −52 | −8 | 4.64 | aLOC left | BA37 |
Note: BA, Brodmann area.
A common issue in neuroimaging studies relates to the fact that while the patterns of activation that are observed under 2 experimental conditions may appear to differ visually, those differences may not be statistically significant. More precisely, within a given brain region, activation under 2 different conditions may fall just above and below the threshold for rejecting the null hypothesis. Thus, in order to confirm that the patterns of activation observed for rule complexity and analogical distance were significantly different to each other, 2-rule near analogical problems were contrasted directly with 2-rule far analogical problems using FDR correction for the whole-brain mass at P < 0.05. This contrast is mathematically equivalent to contrasting directly between the main effects. The results confirmed that the patterns of activation observed for the main effects of rule complexity and analogical distance were indeed significantly different to each other. Significantly greater activation was observed for increased rule complexity (Fig. 3d and Table 2) across a broad swathe of the right MFG, more focally in the left MFG and spanning the PPC and precuneus. Conversely, there was significantly greater activation when analogical distance increased (Fig. 3e and Table 2) in the left iRLPFC and in the left aLOC—an area that is implicated in the higher-level processing of visual objects (Malach et al. 2002). These activation clusters were defined as ROIs using the MARSBAR ROI toolbox for retest analysis in experiment 2.
Experiment 2: Behavioral Results
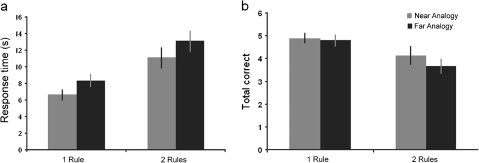
Response time data from the rule application stage of experiment 2 (Fig. 4a) were examined using a 2 × 2 repeated-measures ANOVA in which rule complexity (simple vs. compound rules) and analogical distance (near analogical vs. far analogical application) were the factors. The results revealed significant increases in response time associated with both difficulty factors (rule complexity F1,20 = 36.65, P < 0.001; analogical distance F1,20 = 18.49, P < 0.001) and no significant interaction (F1,20 = 0.034, P = 0.855). The total number of correctly solved problems (Fig. 4b) was also examined using a similar design. The results revealed a significant main effect of rule complexity (F1,20 = 14.69, P < 0.001), but in contrast to experiment 1, there was no effect of analogical distance (F1,20 = 0.92, P = 0.349). There was no significant interaction between rule complexity and analogical distance (F1,20 = 0.836, P = 0.372).
Figure 4.
Behavioral results from experiment 2. The figure shows behavioral data from experiment 2. (a) Both rule complexity and analogical distance caused a significant increase in response time. (b) In contrast to experiment 1, only rule complexity caused a significant decrease in the total number of correctly solved problems.
Response time data were also examined for the rule derivation stage of the task using a 2 × 2 repeated-measures ANOVA with rule complexity (simple vs. compound rules) and rule familiarity (first vs. second presentation) as factors. There were significant effects of both rule complexity (F1,20 = 27.23, P < 0.001) and rule familiarity (F1,20 = 17.40, P < 0.001) with no significant interaction (F1,20 = 0.183, P = 0.673). IQ data were not collected for experiment 2 participants.
Experiment 2: Imaging Results
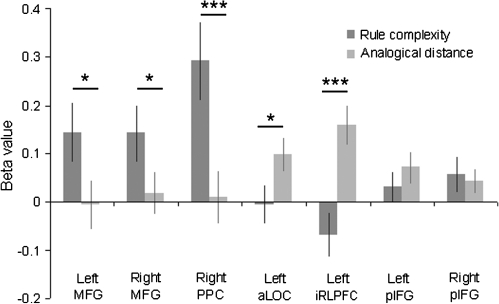
In experiment 2, the difference between the rule complexity and analogical distance manipulations was examined statistically using a focused test–retest approach. Thus, ROI analyses were carried out using the regions derived from the contrast between 2-rule near analogical and 1-rule far analogical problems in experiment 1. Results from the application stage showed a direct replication of the results from experiment 1 with rule complexity modulating activity in the right MFG and PPC ROIs, and analogical distance modulating activation within the left iRLPFC and left aLOC (Fig. 5). Activity in the pIFG was also examined in more detail using the ROIs generated in experiment 1. Significant increases in BOLD response were observed when reasoning difficulty was manipulated (left t = 1.83, P < 0.05 one tailed; right t = 2.14, P < 0.05). In contrast to the other regions examined (Fig. 5), a direct contrast between the 2 difficulty manipulations showed that this increase was not specific to either type of demand (rule complexity vs. analogical distance left pIFG F1,20 = 0.83, P = 0.37; right pIFG F1,20 = 0.21, P = 0.65). Furthermore, the increase in the pIFG BOLD response was subtle, being significantly lower for analogical reasoning than that observed in the left iRLPFC (iRLPFC vs. left pIFG t = 3.50, P < 0.005; iRLPFC vs. right pIFG t = 4.71, P < 0.001) and significantly lower than that observed in the right MFG and PPC for rule complexity MFG (right MFG vs. left pIFG t = 3.12, P = 0.005; right MFG vs. right pIFG t = 2.56, P < 0.05; right PPC vs. left pIFG t = 4.26, P < 0.001; right PPC vs. right pIFG t = 4.20, P < 0.001).
Figure 5.
ROI analyses of experiment 2. The figure illustrates results from the analysis of data extracted from the rule application phase of experiment 2 using the experiment 1 ROIs. The results confirmed the findings from experiment 1 with the MFG and PPC showing increased activation when rule complexity was increased, and the iRLPFC and aLOC showing increased activation when the analogical distance was increased. By contrast, the pIFG bilaterally was sensitive to a lesser extent to both manipulations. *P < 0.05, **P < 0.01, **P < 0.001.
Supplementary whole-brain analyses were also carried out on the experiment 2 data to confirm that the ROIs examined in the test–retest analyses accurately described the activation patterns observed for increased rule complexity and increased analogical distance. Contrast maps comparing each of the experimental conditions to baseline were generated for individual participants and entered into a series of group-level random-effects analyses. Data from the application stage were analyzed using a 2 × 2 factorial design, with rule complexity and analogical distance as factors. Contrasts examining the main effects of both factors (thresholded at P < 0.05 using an FDR correction for multiple comparisons) showed a high degree of consistency with the results of experiment 1: increased rule complexity was associated with increased activity in a predominantly dorsal network (Fig. 6a and Table 3), while increasing analogical distance recruited left iRLPFC and left aLOC (Fig. 6b and Table 3). In addition, a region of the superior frontal gyrus situated in the left frontal polar cortex was more active during far analogical problems. Once again, neither difficulty manipulation produced significantly increased activity in the pIFG at a whole-brain corrected threshold.
Figure 6.
Whole-brain analyses from experiment 2. The figure shows whole-brain analyses of the experiment 2 data thresholded with an FDR correction of P < 0.05 for the whole-brain mass. (a) In common with experiment 1, a predominantly dorsal network was recruited by increased rule complexity during the rule application phase of the task. (b) The left iRLPFC and the left aLOC were recruited when analogical distance was manipulated. (c) The recruitment of a dorsal network during increased rule complexity was also apparent at the rule derivation stage of the task.
Table 3.
Peak activation coordinates for the main effects of rule complexity and analogical distance in experiment 2
| x | y | z | t | Region | Approximate BA |
| Main effect of rule complexity | |||||
| 26 | −54 | 50 | 5.19 | PPC left | BA7 |
| −28 | −54 | 48 | 3.81 | PPC right | BA7 |
| −16 | 48 | 4 | 3.95 | MFG left | BA10 |
| 30 | 30 | 42 | 4.16 | MFG right | BA46 |
| 26 | 60 | 2 | 3.41 | FPC right | BA10 |
| Main effect of analogical distance | |||||
| −28 | −46 | −18 | 5.80 | aLOC left | BA37 |
| −40 | 36 | −12 | 5.01 | iRLPFC left | BA47 |
| −48 | 36 | 8 | 3.68 | iRLPFC left | BA45 |
| −48 | 46 | 4 | 3.40 | iRLPFC left | BA10 |
| −10 | 50 | 22 | 4.17 | FPC left | BA10 |
Note: BA, Brodmann area.
Results from the rule derivation stage of the task were also analyzed using a 2 × 2 factorial design with rule complexity and rule familiarity as factors. Using a threshold of P < 0.05 FDR corrected for the whole-brain mass (Fig. 6c), the main effect of rule complexity once again identified a predominantly dorsal network including the MFG and PPC.
Discussion
It has previously been proposed that the deliberate and effortful control of thoughts and actions is dependent on an executive network that is distributed across the frontal and parietal cortices (Duncan 2001, 2005, 2006; Miller and Cohen 2001). This executive network is of particular importance when habitual responses are either unavailable or insufficient and a new behavior must be acquired and applied; for example, when faced with novel problems or changes in the relationship between environmental cues and behavioral outcomes (Norman and Shallice 1980). The findings presented here accord well with the concept of a global executive network as, in line with previous findings (Duncan et al. 2000), increased BOLD responses were observed in a broad swathe of frontal and parietal cortex when solving novel reasoning problems. Our results also extend those of previous studies by demonstrating that the BOLD response within this network is not homogeneous but rather that the different anatomical components from which it is composed respond preferentially when different reasoning demands are manipulated. These results add to the growing body of evidence supporting the hypothesis that the frontal lobes are functionally heterogeneous, with different components supporting different aspects of executive function (Koechlin et al. 1999, 2003; Owen et al. 2000; Fletcher and Henson 2001; Corbetta and Shulman 2002; Badre and Wagner 2004; Ramnani and Owen 2004; Petrides 2005; Hampshire and Owen 2006; Hampshire et al. 2007). While much research is still required to understand the exact nature of this heterogeneity, a consensus is beginning to emerge.
Functional Dissociations between the MFG and the IFG
Historically, it has been proposed that a ventral/dorsal axis exists within the frontal lobes, with more posterior and inferior regions of the lateral prefrontal cortex supporting simple first-order executive demands, while more dorsal and anterior portions are involved in higher-order executive processes (Petrides 1994, 1995, 2005; Petrides and Pandya 2002). For example, whereas the pIFG is recruited during the active maintenance of information in working memory, when that information is manipulated in some way the MFG is also recruited (Owen et al. 1996). More recently, it has been proposed that more dorsal regions of the lateral prefrontal cortex are involved in goal-directed attention (Corbetta and Shulman 2002; Shulman et al. 2009) and that a hierarchy exists in which dorsal and anterior portions of the frontal lobes support increasingly abstract representations (Badre 2008; Badre et al. 2009). It has also been suggested that the lateral PFC is organized as a cascade of executive processes from a representation of simple stimulus–response mappings in premotor cortex to a representation of the overarching task context in the anterior PFC (Koechlin et al. 1999, 2000, 2001, 2003). It has been argued that the frontopolar cortex forms the apex of a frontal lobe hierarchy, integrating the outcomes of separate cognitive operations in the pursuit of long-term or more global behavioral goals (Ramnani and Owen 2004).
Data from our own laboratory are broadly consistent with these hierarchical perspectives on frontal lobe function. For example, when using a trial and error process to determine the current target stimulus from a set of candidate objects, the pIFG responds transiently when attention switches between different visual dimensions and objects, whereas the MFG stays active throughout the search phase of the task (Hampshire and Owen 2006), suggestive of a general role in guiding the search. Similarly, when identifying a pre-learnt target object in a sequence of distractors, the pIFG shows a response that is tightly tuned to the individual target stimuli while the response in MFG tends to be either weaker (Hampshire, Thompson, et al. 2008; Hampshire et al. 2009) or more widely tuned, responding to distractors that are from the same category as the current target object (Hampshire et al. 2007). Furthermore, in the normal ageing population, abnormal activation in the pIFG is associated with inefficient strategy application during trial and error target detection (Hampshire, Gruszka, et al. 2008), even though the strategy itself is still clearly apparent. In contrast, decreased activation in the MFG and PPC in PD patients carrying out the same task is associated with a loss of overall strategy (Williams-Gray et al. 2008) and a deficit in spatial planning (Williams-Gray et al. 2007). Taken together, these findings converge on the hypothesis that while the pIFG controls attention and behavior at a relatively concrete level, more dorsal and anterior frontal lobe subregions support the higher-level rules and relationships that make up the overarching task schema.
The results presented here accord well with this hypothesis. Thus, while the pIFG was strongly recruited during the reasoning task, the increase in the BOLD response when higher-order reasoning demands were manipulated was significantly weaker and less functionally specific than that observed in the MFG, the PPC, and the left iRLPFC. When taken in conjunction with the previous literature, it seems sensible to suggest, therefore, that the role played by the pIFG during reasoning is most likely to facilitate attention to the task at a concrete level, with the abstract rules and higher-level relationships that form the overarching task schema being preferentially processed in more dorsal/anterior frontal lobe subregions. Thus, a right lateralized dorsal network including much of the MFG, spanning from the most posterior extent up to the frontal pole, along with the PPC, was specifically sensitive to increased rule complexity. While this dorsal network was active during reasoning in general, the level of BOLD activation showed a strong increase when rule complexity was manipulated, both during the period of time when the rule was being derived and when it was being applied. This effect was not related to general difficulty as increased analogical distance did not cause a significant increase in activation within this dorsal network.
The Role of the Left Inferior Rostrolateral Prefrontal Cortex in Analogical Reasoning
Of particular interest here is the double dissociation between the MFG and the left iRLPFC. The left iRLPFC cluster, located at the most anterior extent of the IFG, was recruited when analogical distance was increased, that is, when the currently relevant rule had to be mapped onto a novel set of surface features. Activation in the iRLPFC did not increase when rule complexity was manipulated and so could not have been related to an increase in general difficulty. Furthermore, unlike the pIFG and the posterior MFG, the iRLPFC activation cluster lies completely outside the previously identified “multiple demand” network (Duncan 2006). Thus, in contrast to the pIFG, the MFG, and the PPC, the left iRLPFC may form part of a more specialized frontal lobe system. In support of this view, activation within the same activation coordinates (variously labeled as the frontal pole, the rostrolateral prefrontal cortex, Brodmann area 10, or anterior prefrontal cortex within the reasoning literature) has been consistently reported during tasks that involve analogical reasoning. For example, iRLPFC activation has been reported when pairs of words are compared for a valid analogical relationship (Bunge et al. 2005; Wendelken et al. 2008). Furthermore, the level of activation within the iRLPFC has been reported to increase when the number of concurrent relations to be evaluated increases (Cho et al. 2010). The data presented here extend those from the language domain by demonstrating that the iRLPFC also plays a role in nonverbal reasoning. The precise contribution that the iRLPFC makes during analogical reasoning remains to be defined; however, it is interesting to note that the peak activation coordinates from studies of analogical reasoning cluster just anterior to the area that has been reported to play a role in the effortful retrieval of semantic information (Table 4) (Thompson-Schill 2003; Badre and Wagner 2005; Badre et al. 2005; Dobbins and Wagner 2005; Gold et al. 2006). Of particular relevance is the suggestion by Wagner and colleagues (Wagner et al. 2001; Badre and Wagner 2007) that the anterior IFG is responsible for retrieving semantic information via weak associations using top-down biasing signals. One possibility is that a similar mechanism to that proposed by Wagner and colleagues underlies the contribution made by the iRLPFC to analogical reasoning, that is, the effortful retrieval of weakly associated representations, in this instance the higher-level object features that are thought to be represented within the aLOC (Kourtzi and DiCarlo 2006). Another possibility is that the most anterior extent of the left iRLPFC supports abstract mind states (Christoff et al. 2009)—representing the context within which abstract associations are identified and processed. Thus, it may be the case that a similar hierarchical processing cascade exists within the left IFG to that proposed by Koechlin et al. (2003) for more dorsal prefrontal regions but tending to operate on semantic as opposed to action-related information. A recent (Green et al. 2010) reported activation within the left IFG during analogical reasoning. In that study, analysis focused on a previously identified (Green et al. 2006) region of the left superior frontal gyrus (x = −12, y = 58, z = 31 converted from Talairach space). This more medial frontopolar region was also recruited when applying rules to far analogies in experiment 2. Thus, it seems likely that the iRLPFC is not the only anterior frontal lobe brain region that is sensitive to analogical reasoning demands.
Table 4.
Previously published peak activation coordinates
| Study | x | y | z | Stimulus type |
| Relational complexity | ||||
| Christoff et al. (2001) | −34 | 50 | 9 | Objects |
| 38 | 26 | 13 | ||
| −44 | 4 | 33 | ||
| 28 | 8 | 36 | ||
| Kroger et al. (2002) | 46 | 23 | 29a | Objects |
| 40 | 23 | 43a | ||
| −8 | 43 | 48a | ||
| −32 | 40 | 26a | ||
| −4 | 36 | 24a | ||
| Near analogical integration | ||||
| Smith et al. (2007) | −33 | 53 | 9b | Objects |
| 35 | 54 | 5b | ||
| Bunge et al. (2009) | −36 | 57 | 9 | Objects |
| 39 | 54 | 14 | ||
| Far analogical integration | ||||
| Luo et al. (2003) | −42 | 25 | 15a | Words |
| 39 | 31 | −14a | ||
| 16 | 34 | −15a | ||
| Bunge et al. (2005) | −42 | 48 | −12 | Words |
| −42 | 48 | −15 | ||
| Wendelken et al. (2008) | −45 | 42 | −3 | Words |
| Green et al. (2010) | −53 | 19 | 18a | Words |
| −12 | 58 | 31a | ||
| Cho et al. (2010) | −50 | 42 | −10 | Objects |
| Abstract reasoning | ||||
| Christoff et al. (2009) | −38 | 48 | 0 | Words |
| Effortful semantic retrieval | ||||
| Wagner et al. (2001) | −48 | 27 | −12 | Words |
| −42 | 33 | −12 | ||
| Badre et al. (2005) | −54 | 27 | −9 | Words |
| Dobbins and Wagner (2005) | −48 | 33 | −9 | Objects |
| Badre and Wagner (2007) | −54 | 27 | −9 | Words |
Converted to MNI from Talairach space.
Averaged coordinates.
Relevance to Theories of Rule Integration
Perhaps the most important finding from the current study relates to the neural basis of rule integration. It has been suggested in a number of studies that the more anterior portions of the frontal lobes are specialized for rule integration, that is, the processing of the outputs of other frontal lobe subregions (Ramnani and Owen 2004). Rule integration is common to both analogical reasoning, where a rule must be integrated with novel surface features and to reasoning using compound rules, where the products of the individual rules must be integrated in order to derive the solution. Both these reasoning demands have previously been reported to recruit the rostrolateral prefrontal (Christoff and Gabrieli 2000; Christoff et al. 2001; Kroger et al. 2002; Luo et al. 2003; Bunge et al. 2005, 2009; Smith et al. 2007; Wendelken et al. 2008; Christoff et al. 2009). However, closer examination of previously published activation coordinates associated with these different forms of rule integration suggests that they may recruit different subregions of the rostrolateral prefrontal cortex (Table 4), and the choice of the manipulations used in the current study was in part motivated by this statistically untested observation. More specifically, while abstract analogical reasoning appears to activate the iRLPFC as discussed above, integrating rules on a more concrete level tends to activate the more dorsal portion of the rostrolateral prefrontal that is situated within the anterior MFG (Christoff and Gabrieli 2000; Christoff et al. 2001; Kroger et al. 2002; Smith et al. 2007; Bunge et al. 2009). The findings presented here demonstrate that this dissociation is a statistically significant and strongly replicable phenomenon within the nonverbal reasoning domain. However, in neither case was the pattern of activation restricted to just the most anterior portions of the frontal lobes; rather, corecruitment was observed with more posterior brain regions. In the case of analogical reasoning, the iRLPFC corecruited with the left aLOC, while in the case of rule complexity, the anterior MFG corecruited with much of the rest of the MFG and the PPC (indeed the PPC showed the greatest response). These results demonstrate that the requirement to integrate rules with surface features and the requirement to integrate rules with other rules recruits distinctive frontal and posterior brain circuits.
Relevance to Theories of IQ
The problems used in this study were similar to those used in classic tests of fluid intelligence such as the Cattell Culture Fair Test and Raven's Matrices, and it is unsurprising, therefore, that the behavioral performance showed a high correlation with Cattell score. A fundamental theoretical question with respect to fluid intelligence is whether it represents a single general (g) factor (Spearman 1904) or whether it is an emergent property of a range of independent factors (Thomson 1951; Mackintosh 1998). The task presented here independently manipulated a number of factors that are typically used to modulate difficulty in nonverbal tests of fluid IQ. When examining the effects of the 2 difficulty manipulations in experiment 1, the best correlation to Cattell IQ score was when both manipulations were averaged. On the surface, it could be argued that the evidence presented here supports the idea of multiple components to “g” as different frontoparietal subregions are associated with distinct reasoning demands, both of which contribute to the correlation with IQ score. However, one should not rule out the possibility that some common genetic factors can affect neural function in general, regardless of which modules those neurons belong to at the macro level (Plomin and Spinath 2004).
Funding
Medical Research Council Grant (U1055.01.002.00001.01).
Acknowledgments
Conflict of Interest: None declared.
References
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Badre D. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends Cogn Sci. 2008;12:193–200. doi: 10.1016/j.tics.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Badre D, Hoffman J, Cooney JW, D'Esposito M. Hierarchical cognitive control deficits following damage to the human frontal lobe. Nat Neurosci. 2009;12:515–522. doi: 10.1038/nn.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Poldrack RA, Pare-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Selection, integration, and conflict monitoring; assessing the nature and generality of prefrontal cognitive control mechanisms. Neuron. 2004;41:473–487. doi: 10.1016/s0896-6273(03)00851-1. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Frontal lobe mechanisms that resolve proactive interference. Cereb Cortex. 2005;15:2003–2012. doi: 10.1093/cercor/bhi075. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J. Region of interest analysis using an SPM toolbox. Neuroimage. 2002;16(2):1140–1141. [Google Scholar]
- Bunge SA, Helskog EH, Wendelken C. Left, but not right, rostrolateral prefrontal cortex meets a stringent test of the relational integration hypothesis. Neuroimage. 2009;46:338–342. doi: 10.1016/j.neuroimage.2009.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Wendelken C, Badre D, Wagner AD. Analogical reasoning and prefrontal cortex: evidence for separable retrieval and integration mechanisms. Cereb Cortex. 2005;15:239–249. doi: 10.1093/cercor/bhh126. [DOI] [PubMed] [Google Scholar]
- Cho S, Moody T, Fernandino L, Mumford J, Poldrack R, Cannon T, Knowlton B, Holyoak K. Common and dissociable prefrontal Loci associated with component mechanisms of analogical reasoning. Cereb Cortex. 2010;20(3):524–533. doi: 10.1093/cercor/bhp121. [DOI] [PubMed] [Google Scholar]
- Christoff K, Gabrieli JDE. The frontopolar cortex and human cognition: evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology. 2000;28:168–186. [Google Scholar]
- Christoff K, Keramatian K, Gordon AM, Smith R, Madler B. Prefrontal organization of cognitive control according to levels of abstraction. Brain Res. 2009;1286:94–105. doi: 10.1016/j.brainres.2009.05.096. [DOI] [PubMed] [Google Scholar]
- Christoff K, Prabhakaran V, Dorfman J, Zhao Z, Kroger JK, Holyoak KJ, Gabrieli JD. Rostrolateral prefrontal cortex involvement in relational integration during reasoning. Neuroimage. 2001;14:1136–1149. doi: 10.1006/nimg.2001.0922. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Wagner AD. Domain-general and domain-sensitive prefrontal mechanisms for recollecting events and detecting novelty. Cereb Cortex. 2005;15:1768–1778. doi: 10.1093/cercor/bhi054. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. An adaptive coding model of neural function in prefrontal cortex. Nat Rev Neurosci. 2001;2:820–829. doi: 10.1038/35097575. [DOI] [PubMed] [Google Scholar]
- Duncan J. Prefrontal cortex and Spearman's g. In: Duncan J, Phillips LH, McLeod P, editors. Measuring the mind: speed, control, and age. Oxford: Oxford University Press; 2005. pp. 249–272. [Google Scholar]
- Duncan J. EPS Mid-Career Award 2004: brain mechanisms of attention. Q J Exp Psychol (Colchester) 2006;59:2–27. doi: 10.1080/17470210500260674. [DOI] [PubMed] [Google Scholar]
- Duncan J, Burgess P, Emslie H. Fluid intelligence after frontal lobe lesions. Neuropsychologia. 1995;33:261–268. doi: 10.1016/0028-3932(94)00124-8. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Duncan J, Seltz RJ, Kolodny J, Bor D, Herzog H, Ahmed A, Newell FN, Emslie H. A neural basis for general intelligence. Am J Ophthalmol. 2000;130:687. doi: 10.1016/s0002-9394(00)00752-2. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Henson RN. Frontal lobes and human memory: insights from functional neuroimaging. Brain. 2001;124:849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex. New York: Lippincott Williams and Wilkins; 1997. [Google Scholar]
- Gold BT, Balota DA, Jones SJ, Powell DK, Smith CD, Andersen AH. Dissociation of automatic and strategic lexical-semantics: functional magnetic resonance imaging evidence for differing roles of multiple frontotemporal regions. J Neurosci. 2006;26:6523–6532. doi: 10.1523/JNEUROSCI.0808-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A, Kraemer D, Fugelsang J, Gray J, Dunbar K. Connecting long distance: semantic distance in analogical reasoning modulates frontopolar cortex activity. Cereb Cortex. 2010;20(1):70–76. doi: 10.1093/cercor/bhp081. [DOI] [PubMed] [Google Scholar]
- Green AE, Fugelsang JA, Kraemer DJ, Shamosh NA, Dunbar KN. Frontopolar cortex mediates abstract integration in analogy. Brain Res. 2006;1096:125–137. doi: 10.1016/j.brainres.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Duncan J, Owen AM. Selective tuning of the blood oxygenation level-dependent response during simple target detection dissociates human frontoparietal subregions. J Neurosci. 2007;27:6219–6223. doi: 10.1523/JNEUROSCI.0851-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Gruszka A, Fallon SJ, Owen AM. Inefficiency in self-organized attentional switching in the normal aging population is associated with decreased activity in the ventrolateral prefrontal cortex. J Cogn Neurosci. 2008;20:1670–1686. doi: 10.1162/jocn.2008.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Owen AM. Fractionating attentional control using event-related fMRI. Cereb Cortex. 2006;16:1679–1689. doi: 10.1093/cercor/bhj116. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Thompson R, Duncan J, Owen AM. The target selective neural response–similarity, ambiguity, and learning effects. PLoS One. 2008;3(6):e2520. doi: 10.1371/journal.pone.0002520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Thompson R, Duncan J, Owen AM. Selective tuning of the right inferior frontal gyrus during target detection. Cogn Affect Behav Neurosci. 2009;9:103–112. doi: 10.3758/CABN.9.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature. 1999;399:148–151. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Corrado G, Pietrini P, Grafman J. Dissociating the role of the medial and lateral anterior prefrontal cortex in human planning. Proc Natl Acad Sci U S A. 2000;97:7651–7656. doi: 10.1073/pnas.130177397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, Corrado G, Pietrini P, Grafman J. Dissociating the functional properties of the medial and lateral anterior prefrontal cortex. Brain Cogn. 2001;47:93–97. doi: 10.1073/pnas.130177397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302:1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Kourtzi Z, DiCarlo JJ. Learning and neural plasticity in visual object recognition. Curr Opin Neurobiol. 2006;16:152–158. doi: 10.1016/j.conb.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Kroger JK, Sabb FW, Fales CL, Bookheimer SY, Cohen MS, Holyoak KJ. Recruitment of anterior dorsolateral prefrontal cortex in human reasoning: a parametric study of relational complexity. Cereb Cortex. 2002;12:477–485. doi: 10.1093/cercor/12.5.477. [DOI] [PubMed] [Google Scholar]
- Linden DEJ, Prvulovic D, Formisano E, Vollinger M, Zanella FE, Goebel R, Dierks T. The functional neuroanatomy of target detection: an fMRI study of visual and auditory oddball tasks. Cereb Cortex. 1999;9:815–823. doi: 10.1093/cercor/9.8.815. [DOI] [PubMed] [Google Scholar]
- Luo Q, Perry C, Peng D, Jin Z, Xu D, Ding G, Xu S. The neural substrate of analogical reasoning: an fMRI study. Brain Res Cogn Brain Res. 2003;17:527–534. doi: 10.1016/s0926-6410(03)00167-8. [DOI] [PubMed] [Google Scholar]
- Luria AR, Teuber HL, Pribram KH, Haigh B. Higher cortical functions in man. London: Tavistock; 1966. [Google Scholar]
- Mackintosh NJ. IQ and human intelligence. Oxford: Oxford University Press; 1998. [Google Scholar]
- Malach R, Levy I, Hasson U. The topography of high-order human object areas. Trends Cogn Sci. 2002;6:176–184. doi: 10.1016/s1364-6613(02)01870-3. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Norman DA, Shallice T. Attention to action: willed and automatic control of behaviour. In: Davidson R, Schwartz GE, Shapiro D, editors. Consciousness and self-regulation. New York: Plenun Times; 1980. [Google Scholar]
- Nyberg L, Marklund P, Persson J, Cabeza R, Forkstam C, Petersson KM, Ingvar M. Common prefrontal activations during working memory, episodic memory, and semantic memory. Neuropsychologia. 2003;41:371–377. doi: 10.1016/s0028-3932(02)00168-9. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Owen AM, Evans AC, Petrides M. Evidence for a two-stage model of spatial working memory processing within the lateral frontal cortex: a positron emission tomography study. Cereb Cortex. 1996;6:31–38. doi: 10.1093/cercor/6.1.31. [DOI] [PubMed] [Google Scholar]
- Owen AM, Lee ACH, Williams EJ. Dissociating aspects of verbal working memory within the human frontal lobe: further evidence for a “process-specific” model of lateral frontal organization. Psychobiology. 2000;28:146–155. [Google Scholar]
- Petrides M. Frontal lobes and working memory: evidence from investigations of the effects of cortical excisions in nonhuman primates. In: Boller F, Grafman J, editors. Handbook of neuropsychology. Amsterdam: Elsevier; 1994. pp. 59–82. [Google Scholar]
- Petrides M. Impairments on nonspatial self-ordered and externally ordered working-memory tasks after lesions of the mid-dorsal part of the lateral frontal-cortex in the monkey. J Neurosci. 1995;15:359–375. doi: 10.1523/JNEUROSCI.15-01-00359.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philos Trans R Soc Lond B Biol Sci. 2005;360:781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur J Neurosci. 2002;16:291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- Plomin R, Spinath FM. Intelligence: genetics, genes, and genomics. J Pers Soc Psychol. 2004;86:112–129. doi: 10.1037/0022-3514.86.1.112. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neurosci. 2004;5:184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Astafiev SV, Franke D, Pope DLW, Snyder AZ, McAvoy MP, Corbetta M. Interaction of stimulus-driven reorienting and expectation in ventral and dorsal frontoparietal and basal ganglia-cortical networks. J Neurosci. 2009;29:4392–4407. doi: 10.1523/JNEUROSCI.5609-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R, Keramatian K, Christoff K. Localizing the rostrolateral prefrontal cortex at the individual level. Neuroimage. 2007;36:1387–1396. doi: 10.1016/j.neuroimage.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Spearman C. “General intelligence” objectively determined and measured. Am J Psychol. 1904;15:201–293. [Google Scholar]
- Stuss DT, Benson DF. The frontal lobes. New York: Raven Press; 1986. [Google Scholar]
- Thompson-Schill SL. Neuroimaging studies of semantic memory: inferring “how” from “where”. Neuropsychologia. 2003;41:280–292. doi: 10.1016/s0028-3932(02)00161-6. [DOI] [PubMed] [Google Scholar]
- Thomson GH. The factor analysis of human ability. London: University of London Press; 1951. [Google Scholar]
- Wagner AD, Pare-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31:329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Wendelken C, Nakhabenko D, Donohue SE, Carter CS, Bunge SA. “Brain is to thought as stomach is to ??”: investigating the role of rostrolateral prefrontal cortex in relational reasoning. J Cogn Neurosci. 2008;20:682–693. doi: 10.1162/jocn.2008.20055. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Hampshire A, Barker RA, Owen AM. Attentional control in Parkinson's disease is dependent on COMT val 158 met genotype. Brain. 2008;131:397–408. doi: 10.1093/brain/awm313. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Hampshire A, Robbins TW, Owen AM, Barker RA. Catechol O-methyltransferase Val158Met genotype influences frontoparietal activity during planning in patients with Parkinson's disease. J Neurosci. 2007;27:4832–4838. doi: 10.1523/JNEUROSCI.0774-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]