Abstract
Arterial 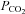 , a major determinant of breathing, is detected by chemosensors located in the brainstem. These are important for maintaining physiological levels of
, a major determinant of breathing, is detected by chemosensors located in the brainstem. These are important for maintaining physiological levels of 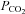 in the blood and brain, yet the mechanisms by which the brain senses CO2 remain controversial. As ATP release at the ventral surface of the brainstem has been causally linked to the adaptive changes in ventilation in response to hypercapnia, we have studied the mechanisms of CO2-dependent ATP release in slices containing the ventral surface of the medulla oblongata. We found that CO2-dependent ATP release occurs in the absence of extracellular acidification and correlates directly with the level of
in the blood and brain, yet the mechanisms by which the brain senses CO2 remain controversial. As ATP release at the ventral surface of the brainstem has been causally linked to the adaptive changes in ventilation in response to hypercapnia, we have studied the mechanisms of CO2-dependent ATP release in slices containing the ventral surface of the medulla oblongata. We found that CO2-dependent ATP release occurs in the absence of extracellular acidification and correlates directly with the level of 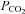 . ATP release is independent of extracellular Ca2+ and may occur via the opening of a gap junction hemichannel. As agents that act on connexin channels block this release, but compounds selective for pannexin-1 have no effect, we conclude that a connexin hemichannel is involved in CO2-dependent ATP release. We have used molecular, genetic and immunocytochemical techniques to demonstrate that in the medulla oblongata connexin 26 (Cx26) is preferentially expressed near the ventral surface. The leptomeninges, subpial astrocytes and astrocytes ensheathing penetrating blood vessels at the ventral surface of the medulla can be loaded with dye in a CO2-dependent manner, suggesting that gating of a hemichannel is involved in ATP release. This distribution of CO2-dependent dye loading closely mirrors that of Cx26 expression and colocalizes to glial fibrillary acidic protein (GFAP)-positive cells. In vivo, blockers with selectivity for Cx26 reduce hypercapnia-evoked ATP release and the consequent adaptive enhancement of breathing. We therefore propose that Cx26-mediated release of ATP in response to changes in
. ATP release is independent of extracellular Ca2+ and may occur via the opening of a gap junction hemichannel. As agents that act on connexin channels block this release, but compounds selective for pannexin-1 have no effect, we conclude that a connexin hemichannel is involved in CO2-dependent ATP release. We have used molecular, genetic and immunocytochemical techniques to demonstrate that in the medulla oblongata connexin 26 (Cx26) is preferentially expressed near the ventral surface. The leptomeninges, subpial astrocytes and astrocytes ensheathing penetrating blood vessels at the ventral surface of the medulla can be loaded with dye in a CO2-dependent manner, suggesting that gating of a hemichannel is involved in ATP release. This distribution of CO2-dependent dye loading closely mirrors that of Cx26 expression and colocalizes to glial fibrillary acidic protein (GFAP)-positive cells. In vivo, blockers with selectivity for Cx26 reduce hypercapnia-evoked ATP release and the consequent adaptive enhancement of breathing. We therefore propose that Cx26-mediated release of ATP in response to changes in 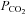 is an important mechanism contributing to central respiratory chemosensitivity.
is an important mechanism contributing to central respiratory chemosensitivity.
Introduction
Breathing is a vital function that maintains arterial partial pressures of O2 (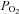 ) and CO2 (
) and CO2 (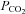 ) within physiological limits to provide O2 for metabolism, excrete CO2 and regulate acid–base balance. Chemosensory reflexes regulate breathing to ensure homoeostatic control of blood gases (Loeschcke, 1982; Feldman et al. 2003). Under some circumstances this control can be disturbed. For example at high altitude, enhanced ventilation, necessary to compensate for lower atmospheric O2 concentration, reduces the
) within physiological limits to provide O2 for metabolism, excrete CO2 and regulate acid–base balance. Chemosensory reflexes regulate breathing to ensure homoeostatic control of blood gases (Loeschcke, 1982; Feldman et al. 2003). Under some circumstances this control can be disturbed. For example at high altitude, enhanced ventilation, necessary to compensate for lower atmospheric O2 concentration, reduces the 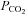 in the blood (hypocapnia) and hence the ventilatory drive – this can result in periodic breathing (Grocott et al. 2009; Wilson et al. 2009). During central sleep apnoea arterial
in the blood (hypocapnia) and hence the ventilatory drive – this can result in periodic breathing (Grocott et al. 2009; Wilson et al. 2009). During central sleep apnoea arterial 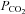 rises (hypercapnia) and
rises (hypercapnia) and 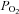 falls (hypoxia) causing arousal and resumption of breathing. Congenital central hypoventilation syndrome (CCHS) is a disorder of central chemoreception (Shea, 1997). Most patients with CCHS can adequately regulate their blood gases while awake but when asleep, these patients lose the drive to breathe and will die unless artificially ventilated (Chen & Keens, 2004).
falls (hypoxia) causing arousal and resumption of breathing. Congenital central hypoventilation syndrome (CCHS) is a disorder of central chemoreception (Shea, 1997). Most patients with CCHS can adequately regulate their blood gases while awake but when asleep, these patients lose the drive to breathe and will die unless artificially ventilated (Chen & Keens, 2004).
Despite the undoubted importance of central respiratory chemoreception for homoeostatic control of blood gases, the mechanisms by which this is achieved remain unclear. Several lines of evidence suggest that changes in 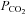 are detected as consequent changes in pH (Winterstein, 1949; Loeschcke, 1982; Feldman et al. 2003; Guyenet, 2008; Nattie & Li, 2008). There are several areas of the medulla oblongata that contain pH/CO2 sensitive neurons, especially near the highly vascularised ventral surface of the medulla oblongata (the ventral medullary surface, VMS). For example a population of neurons sensitive to changes in pH has been described in the retrotrapezoid nucleus (RTN) (Mulkey et al. 2004). These neurons send neurites to the VMS (Mulkey et al. 2004), are glutamatergic (Stornetta et al. 2006) and project to the ventral respiratory column. They are strong candidates for at least one class of central pH chemosensors. However the molecular mechanisms of central respiratory chemosensitivity have not been established and a possible molecular candidate – the pH sensitive TASK-1 channel – has recently been eliminated as the transducing molecule (Mulkey et al. 2007; Trapp et al. 2008). Nevertheless several other types of pH-sensitive K+ channels could play a role in chemosensing (Wu et al. 2004; Yamamoto et al. 2008). pH-sensitive neurons of the medullary raphé and locus coeruleus have also been proposed as candidate respiratory chemosensors (Filosa et al. 2002; Severson et al. 2003; Richerson, 2004; Corcoran et al. 2009). Note, however, that the existence of a pH-sensing chemosensory mechanism does not preclude the possibility of parallel chemosensing pathways (Ritucci et al. 2005; Putnam et al. 2005; cf. Summers et al. 2002).
are detected as consequent changes in pH (Winterstein, 1949; Loeschcke, 1982; Feldman et al. 2003; Guyenet, 2008; Nattie & Li, 2008). There are several areas of the medulla oblongata that contain pH/CO2 sensitive neurons, especially near the highly vascularised ventral surface of the medulla oblongata (the ventral medullary surface, VMS). For example a population of neurons sensitive to changes in pH has been described in the retrotrapezoid nucleus (RTN) (Mulkey et al. 2004). These neurons send neurites to the VMS (Mulkey et al. 2004), are glutamatergic (Stornetta et al. 2006) and project to the ventral respiratory column. They are strong candidates for at least one class of central pH chemosensors. However the molecular mechanisms of central respiratory chemosensitivity have not been established and a possible molecular candidate – the pH sensitive TASK-1 channel – has recently been eliminated as the transducing molecule (Mulkey et al. 2007; Trapp et al. 2008). Nevertheless several other types of pH-sensitive K+ channels could play a role in chemosensing (Wu et al. 2004; Yamamoto et al. 2008). pH-sensitive neurons of the medullary raphé and locus coeruleus have also been proposed as candidate respiratory chemosensors (Filosa et al. 2002; Severson et al. 2003; Richerson, 2004; Corcoran et al. 2009). Note, however, that the existence of a pH-sensing chemosensory mechanism does not preclude the possibility of parallel chemosensing pathways (Ritucci et al. 2005; Putnam et al. 2005; cf. Summers et al. 2002).
We have shown previously that release of ATP from the areas of the VMS corresponding to the classical chemosensory areas (Loeschcke, 1982) is an important signalling step in the central chemosensory transduction of the CO2 stimulus (Gourine et al. 2005a). The released ATP may excite respiratory neurons via both P2X and P2Y receptors on their ventrally directed dendrites that approach the VMS (Kawai et al. 1996; Gourine et al. 2003). We have now studied in detail the mechanisms of ATP release by using an in vitro slice of the VMS that reliably reproduces CO2-dependent ATP release. Our new data suggest that ATP is released in response to changes in 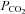 rather than extracellular pH and occurs via gap junction hemichannels, most likely connexin 26, which demonstrates appropriate expression patterns and properties (Huckstepp et al. 2010) to mediate this release.
rather than extracellular pH and occurs via gap junction hemichannels, most likely connexin 26, which demonstrates appropriate expression patterns and properties (Huckstepp et al. 2010) to mediate this release.
Methods
All methods required to obtain tissue for in vitro experiments and the in vivo recordings were performed in accordance with the ethical standards of The Journal of Physiology as set out by Drummond (2009).
In vitro experiments
Young adult Sprague–Dawley rats (4–6 weeks old) were humanely killed by an overdose of isoflurane in accordance with the UK Animals (Scientific Procedures) Act 1986. The medulla was rapidly dissected free from the skull in a bath of chilled aCSF with an additional 10 mm Mg2+ at a temperature between 4 and 6°C. To preserve the CO2-sensitive ATP release mechanism, it was essential to ensure that no blood found its way to the VMS, and that the pia mater remained intact and undisturbed throughout the whole procedure. The isolated medulla was glued onto a chilled metal block ventral surface upper most and, with the aid of a Microm HM650V (Walldorf, Germany) vibrating tissue slicer, a horizontal slice 400 μm thick was cut parallel to the VMS.
Following isolation, the tissue slice was left for 30 min to recover in standard artificial cerebrospinal fluid (aCSF) at 33°C under constant superfusion at a rate of approximately 6 ml min−1. The following solutions were used.
Standard aCSF: 124 mm NaCl, 3 mm KCl, 1 mm CaCl2, 26 mm NaHCO3, 1.25 mm NaH2PO4, 1 mm MgSO4, 10 mm d-glucose saturated with 95% O2–5% CO2, pH 7.5, 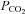 35 mmHg.
35 mmHg.
80 mmHCO3− aCSF: 70 mm NaCl, 3 mm KCl, 1 mm CaCl2, 80 mm NaHCO3, 1.25 mm NaH2PO4, 1 mm MgSO4, 10 mm d-glucose, saturated with 9%, 12% or 15% CO2 (with the balance being O2) to give a pH of 7.65, 7.5 or 7.35 and a 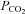 of 60, 70 or 90 mmHg, respectively.
of 60, 70 or 90 mmHg, respectively.
50 mmHCO3− aCSF: 100 mm NaCl, 3 mm KCl, 1 mm CaCl2, 50 mm NaHCO3, 1.25 mm NaH2PO4, 1 mm MgSO4, 10 mm d-glucose, saturated with 6%, 9% or 12% CO2 (with the balance being O2) to give a pH of 7.65, 7.5 or 7.35 and a 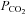 of 40, 55 or 70 mmHg, respectively.
of 40, 55 or 70 mmHg, respectively.
10 mmHCO3 aCSF: 140 mm NaCl, 3 mm KCl, 1 mm CaCl2, 10 mm NaHCO3, 1.25 mm NaH2PO4, 1 mm MgSO4, 10 mm d-glucose, saturated with 2% CO2 (with the balance being O2) to give a pH of 7.5 and a 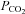 of 20 mmHg.
of 20 mmHg.
All chemicals and compounds were from Sigma, unless otherwise indicated. Glycerol (2 mm) was added to all solutions to enable operation of the ATP biosensor.
ATP biosensing
ATP and null biosensors (Llaudet et al. 2005) were obtained from Sarissa Biomedical Ltd (Coventry, UK). These biosensors have a permselectivity layer that greatly enhances their selectivity for ATP versus non-specific electroactive interferents (Fig. 1A). They were used in conjunction with a Duostat ME200+ (Sycopel International Ltd, Jarrow, UK). The biosensors were bent so that the sensing portion (0.5 mm in length) could be laid flat against the surface of the slice (inset Fig. 1B). Dual simultaneous recordings were made with the ATP and null biosensors. The null biosensors lack the ATP-sensing enzymes and act as a control for any non-specific signals (Gourine et al. 2005a,b; Llaudet et al. 2005; Pearson et al. 2005; Gourine et al. 2008). For every experiment the sensors were calibrated at the end of each recording, and tested against a potential interferent (5-HT) to assess selectivity and tested against aCSF with high 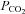 to test whether this treatment affected the null and ATP biosensors equally (Fig. 1A). All records are presented as the differential signal between the ATP and null biosensors (Fig. 1B). This removes any contamination of the specific ATP signal by non-specific signals (Fig. 1A). The blockers used in this study did not alter the biosensors’ responsiveness to ATP (Supplementary Fig. 1). Simultaneous pH measurements were made within the recording chamber by means of a miniature pH electrode (Harvard Apparatus, Edenbridge, Kent, UK).
to test whether this treatment affected the null and ATP biosensors equally (Fig. 1A). All records are presented as the differential signal between the ATP and null biosensors (Fig. 1B). This removes any contamination of the specific ATP signal by non-specific signals (Fig. 1A). The blockers used in this study did not alter the biosensors’ responsiveness to ATP (Supplementary Fig. 1). Simultaneous pH measurements were made within the recording chamber by means of a miniature pH electrode (Harvard Apparatus, Edenbridge, Kent, UK).
Figure 1. Selectivity, calibration and placement of biosensors.
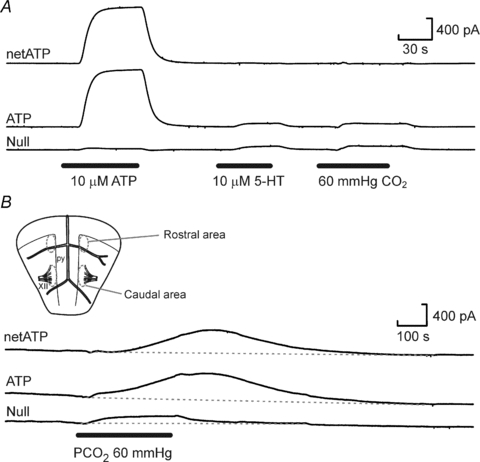
A, responses of ATP and null biosensors to ATP, 5-hydroxytryptamine (5-HT, a representative highly electroactive interferent) and the CO2 stimulus. The small responses to 5-HT and CO2 are very similar in the ATP and null biosensors and subtraction of the null sensor current from the ATP biosensor current gives the net ATP signal (netATP). B, inset shows the locations of the rostral and caudal recording areas relative to landmarks such as the pyramids (py) and XIIth nerve rootlets (XII). Responses of the ATP and null biosensors to the hypercapnic stimulus when placed at the caudal location on the ventral medullary surface.
Application of pharmacological agents
After the initial recovery period, a 5 min test stimulus of 80 mm HCO3− aCSF saturated with 9% CO2 (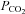 60 mmHg) was given followed by a 30 min wash in standard aCSF. The pharmacological agent (acetazolamide 500 μm, carbenoxolone 10 μm, carbenoxolone 100 μm, Co2+ 500 μm, 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB) 200 μm, proadifen 200 μm, probenecid 1 mm, or SQ22536 (Tocris) 100 μm) was then applied for 20 min in standard aCSF to allow it to take effect. A second hypercapnic stimulus in the presence of the appropriate drug was then given. After 30 min in standard aCSF containing the drug, the tissue was washed for a further 20 min in standard aCSF before the sensors were removed (to allow measurement of the basal levels of ATP release – the tone) and the sensors calibrated.
60 mmHg) was given followed by a 30 min wash in standard aCSF. The pharmacological agent (acetazolamide 500 μm, carbenoxolone 10 μm, carbenoxolone 100 μm, Co2+ 500 μm, 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB) 200 μm, proadifen 200 μm, probenecid 1 mm, or SQ22536 (Tocris) 100 μm) was then applied for 20 min in standard aCSF to allow it to take effect. A second hypercapnic stimulus in the presence of the appropriate drug was then given. After 30 min in standard aCSF containing the drug, the tissue was washed for a further 20 min in standard aCSF before the sensors were removed (to allow measurement of the basal levels of ATP release – the tone) and the sensors calibrated.
Removal of extracellular Ca2+
An initial test stimulus of 80 mm HCO3− aCSF saturated with 9% CO2 was given, followed by 30 min of wash. To test the dependence of ATP release on extracellular Ca2+, Ca2+-free aCSF (standard aCSF in which CaCl2 was replaced with MgCl2 with the addition of 1 mm EGTA) was then applied for 20 min before a second application of a Ca2+-free 80 mm HCO3− aCSF saturated with 9% CO2 (80 mm HCO3− aCSF in which CaCl2 was replaced with MgCl2 with addition of 1 mm EGTA). This was followed by 30 min in Ca2+-free aCSF. The tissue was then washed for a further 20 min in standard aCSF before the sensors were removed from the VMS (to allow measurement of the tone) and then calibrated.
Connexin blockers and zero Ca2+ evoked ATP release
Ca2+-free aCSF was applied for 10 min. The solution was switched to Ca2+-free aCSF containing a connexin hemichannel blocker (carbenoxolone 100 μm, Co2+ 500 μm, NPPB 200 μm or Proadifen 200 μm), followed by a 30 min wash in Ca2+-free aCSF. The tissue was then washed for a further 20 min in standard aCSF before the sensors were removed (to allow measurement of the tone) and calibrated.
The effects of all drug applications on the magnitude of CO2-evoked ATP release were compared to the second episode of ATP release in time-matched controls.
In vitro superfusion and timings of drug applications
Although the slice chamber (volume 1 ml) was superfused at a rapid rate, there was sufficient dead-space (volume 5 ml) in the tubing that it took around 40–50 s for the contents of the chamber to exchange following the switch of solutions. For practical reasons of accuracy, solution changes were marked on the digital recordings (and in the figures of the paper) at the time they were made rather than when the new solutions were expected to enter the chamber. This gives rise to the misleading impression of a relatively long delay in the response to changes in 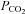 , which can be readily appreciated in the figures from comparing the bars marking the time at which solution changes were made with the recordings of pH, which reflects the solutions in the tissue chamber.
, which can be readily appreciated in the figures from comparing the bars marking the time at which solution changes were made with the recordings of pH, which reflects the solutions in the tissue chamber.
In vivo experiments
In vivo experiments were conducted in accordance with the UK Animals (Scientific Procedures) Act 1986. Adult male rats (260–300 g) were anaesthetised with urethane (1.6 g kg−1i.p.). Adequate anaesthesia was ensured by maintaining stable levels of arterial blood pressure, heart rate and central respiratory rate and showing lack of responses to paw pinch. The femoral artery and vein were cannulated for measurement of arterial blood pressure and administration of anaesthetic, respectively. The trachea was cannulated and the animal was ventilated with a mixture of 50% oxygen and 50% nitrogen (unless otherwise required by the protocol) using a positive pressure ventilator (Harvard rodent ventilator, model 683) with a tidal volume of ∼2 ml and a pump frequency similar to the resting respiratory rate (∼60 strokes min−1). The animal was then injected with gallamine triethiodide (Flaxedil™, 30 mg kg−1i.v.; then 10 mg kg−1 h−1i.v.) and was placed in a stereotaxic frame. The VMS was then exposed as described previously (Gourine et al. 2005a,b;).
Activity of the phrenic nerve was recorded as an indicator of central respiratory drive. The signal was amplified (×20,000), filtered (500–1500 Hz) and rectified and smoothed (τ = 50 ms). Partial pressures of O2 and CO2 as well as pH of the arterial blood were measured every 1–2 h. End-tidal levels of CO2 were monitored on-line using a fast-response CO2 analyser (model Capstar-100, CWE Inc., Ardmore, PA, USA) and kept at a designated level by altering tidal volume and respiratory frequency. In all the experiments 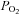 in the arterial blood was kept at >100 mmHg to ensure minimal drive from the peripheral chemoreceptors. The body temperature was maintained with a servo-controlled heating pad at 37.0 ± 0.2°C.
in the arterial blood was kept at >100 mmHg to ensure minimal drive from the peripheral chemoreceptors. The body temperature was maintained with a servo-controlled heating pad at 37.0 ± 0.2°C.
ATP and null sensors were each connected to a separate MicroC potentiostat (WPI, Sarasota, FL, USA) and held on a stereotaxic micromanipulator. The sensors were aligned parallel to the pyramidal tracts and then were placed in a direct contact with the VMS 0.1–0.5 mm lateral from the tracts (adjacent to, but not on top of, the major blood vessels, see Fig. 1c of Gourine et al. 2005a). The sensitive part of the sensor was ∼0.5 mm in length placed over a significant portion of the VMS chemosensitive areas just rostral from the XII nerve roots (corresponding to the caudal position used with the in vitro slices). The identical placement of the ATP sensors and null sensors was achieved by aligning them to the pyramidal tracts, landmark blood vessels and by means of the vernier scale of the manipulator. Once the sensors were placed, the exposed area of the brain and both sensors were covered with an excessive volume of modified Bulmer's buffer (100 mm NaCl, 10 mm phosphate buffer, 1 mm MgCl2 and 2 mm glycerol) and a period of 20–30 min was allowed until a steady baseline was obtained.
Quantitative PCR
A sequence of 300 μm horizontal slices (cf. Fig. 1) were cut along the ventral–dorsal axis of medullas isolated from 5- to 6-week-old rats. The RNA of each slice was extracted using the RNeasy kit (Qiagen, Valencia, CA, USA). Reverse transcription (by standard methods) was performed and the resultant cDNA was used in subsequent quantitative PCR (QPCR) analysis using an Applied Biosystems (Foster City, CA, USA) Real Time 7000. Rat β-actin, connexin 26, 32, 36 and 43, and pannexin 2 primers were designed using the primer design package (Primer Express) supplied with the Applied Biosystems Real Time 7000 and were synthesised by Invitrogen. Each QPCR reaction was performed in triplicate and the experiment was performed three independent times (3 rats).
Primer sequences were: β-actin forward AGGCCAACCGTGAAAAGATG and reverse GCCTGGATGGCTACGTACATG; Cx26 forward GGGAGAGGATGAGGCAACCT and reverse AATGTTTGCCCGGGAGATG; Cx32 forward CCAACACGGTGGACTGCTT and reverse AGGCGGCGAGCATAAAGA; Cx36 forward CCTACGGAGAAGACGGTCTTTC and reverse AGGCGGCGAGCATAAAGA; Cx43 forward TTTCCCCGACGACAACCA and reverse TGGCTAATGGCTGGAGTTCAT; and pannexin 2 forward GCTGGTCACCCTGGTCTTCA and reverse CAGTAGCCACGGGCGTACA.
Cx26+/LacZ knock-in mouse
This mouse strain was generated in the Institute of Genetics, University of Bonn (Germany). The mice were kept under standard housing conditions with a 12 h–12 h dark–light cycle with food and water provided ad libitum. Experiments were carried out in accordance with local and state regulations for research with animals in Gemany. The knock-in mouse strain was generated to study Cx26 expression by analyses of LacZ reporter gene expression or, alternatively, substitution of Cx26 by Cx32. In Cx26+/LacZ mice the coding region of Cx26 was heterozygously replaced by the lox-P-sites-flanked (floxed) LacZ coding region preceded by a nuclear localization signal and followed by the coding region of Cx32. Endogenous Cx26 promoter activity causes expression of Cx26 from the wild-type allele and LacZ reporter gene expression from the transgenic allele. Cx32 expression can only be driven under control of the endogenous Cx26 promoter (Cx26+/Cx32 mice) after Cre mediated excision of the floxed LacZ-coding DNA. In this report only the floxed Cx26+/LacZ mice were used to study Cx26 expression in the brain by analyses of β-galactosidase activity. Enquiries about these mice should be directed to K. Willecke (Bonn).
Immunocytochemistry
Horizontal slices 400 μm thick containing the VMS from 5- to 6-week-old rats were fixed in 4% paraformaldehyde (PFA) in 0.1 m phosphate buffer pH 7.4 for 45 min at room temperature. Brains from the Cx26+/lacZ mice were fixed with 4% PFA in phosphate-buffered saline (PBS) via cardiac perfusion and then post-fixed in 4% PFA by the Bonn lab in accordance with local and state regulations for research with animals in Germany. Fixed tissue was placed in 30% sucrose in 0.1 m phosphate buffer, pH 7.4 and left overnight at 4°C. The fixed tissue was then embedded in Cryo-M-Bed (Bright Instruments, Huntingdon, UK) and re-sectioned into 20 or 60 μm transverse sections. All primary and secondary antibodies were made up in immunosolution (0.1 m phosphate buffer, 0.1% BSA, 0.1% sodium azide, 0.4% Triton X-100, pH 7.4). Slices were incubated overnight at 4°C in 10% normal horse serum. Chick anti-glial fibrillary acidic protein (GFAP) (Chemicon, Temecula, CA, USA) and mouse anti-Cx26 (Invitrogen) were applied at 1:500 and 1:200 respectively and incubated overnight at 4°C. The slices were then washed in 0.1 m phosphate buffer and were incubated with appropriate secondary antibodies for 2 h (Alexa Fluor 488 goat anti-mouse for Cx26 and Alexa Fluor 594 goat anti-chick for GFAP (both obtained from Invitrogen). In rats, astrocytes residing in close proximity to the VMS were also identified by cell-specific green fluorescent protein (GFP) expression driven by the GFAP promoter followed by immunohistochemical detection of Cx26. In accordance with the UK Animals (Scientific Procedures) Act 1986, adult male Sprague–Dawley rats were injected intramuscularly with a mixture of ketamine (60 mg kg−1) and medetomidine (250 μg kg−1) and were placed in a stereotaxic frame. Animals received two bilateral microinjections (1 μl each, stereotaxic coordinates from Bregma, AP: −10.5 mm, L −2.0 mm, V −8.5 mm) into the rostral ventrolateral medulla of an adenoviral vector to express GFP under the control of the enhanced astrocyte-specific GFAP promoter (generously provided by Prof. S. Kasparov, University of Bristol). The rats were intracardially perfused 10 days after the injections, the brainstem was isolated, and sliced and coronal sections (30 μm) were immunostained for Cx26 as described above.
Some sections (in this case 60 μm thick) were processed as free-floating sections. In these instances sections were also stained for microtubule associated protein 2 (MAP2; 1:500, Sigma) and β-galactosidase (1:1000, Sigma) with appropriate secondary antibodies. Slices were finally mounted in Vectashield DAPI (Vector Laboratories, Inc., Burlingame, CA, USA) and examined with an SP2 Leica confocal microscope.
Dye loading
Medullary slice
After preparing the slice as above and an initial 20 min recovery period, 80 mm HCO3− aCSF saturated with 9% CO2 containing either carboxyfluorescein (CBF, 100 μm) or fluorescein isothiocyanate (FITC, 100 μm) was applied for 5 min, followed by a wash of 30 min in standard aCSF. Controls underwent a 5 min application of the fluorescent compound in standard aCSF followed by a 30 min wash. To wash out the dye previously loaded into the tissue, a 5 min application of 80 mm HCO3− aCSF saturated with 9% CO2 was given in the absence of the dye, followed by a 10 min wash in standard aCSF. These experiments were performed on the ventral medullary slice from three rats – each replicate gave the same result.
Statistical analysis
Statistical comparisons were made by one-way ANOVA, the Kruskal–Wallis test (for data with higher variance) or Student's paired sample t test where appropriate. Values quoted in the text are means ± s.e.m.
Results
In vitro recapitulation of CO2-evoked ATP release from the VMS
We first used in vitro preparations (horizontal slices of the medulla oblongata) to examine in detail the mechanisms of CO2-evoked ATP release from the VMS chemosensitive areas.
Hypercapnic acidosis (an increase in 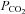 at constant [HCO3−] resulting in a decrease of extracellular pH to 7.1) can evoke ATP release but this was rarely repeatable more than once in a single preparation (Supplementary Fig. 2, cf. Gourine et al. 2005a). During moderate hypercapnia in vivo, the extracellular pH at the ventral surface of the medulla oblongata usually decreases by 0.1–0.2 units of pH (Loeschcke, 1982; Eldridge et al. 1985). We have consequently explored the dependence of ATP release on changes in
at constant [HCO3−] resulting in a decrease of extracellular pH to 7.1) can evoke ATP release but this was rarely repeatable more than once in a single preparation (Supplementary Fig. 2, cf. Gourine et al. 2005a). During moderate hypercapnia in vivo, the extracellular pH at the ventral surface of the medulla oblongata usually decreases by 0.1–0.2 units of pH (Loeschcke, 1982; Eldridge et al. 1985). We have consequently explored the dependence of ATP release on changes in 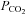 and pH by also altering bicarbonate levels during the CO2 stimulus.
and pH by also altering bicarbonate levels during the CO2 stimulus.
An increase in 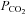 in aCSF containing 80 mm HCO3− saturated with 9% CO2–91% O2 (
in aCSF containing 80 mm HCO3− saturated with 9% CO2–91% O2 (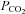 60 mmHg, pH 7.65) reliably evoked ATP release in the caudal chemosensitive area of the VMS (just medial to the emergence of the rootlets of the XIIth nerve Fig. 2A, B and E). ATP was also released in response to CO2 from the dorsal side of the 400 μm thick ventral slice (Fig. 2C and E); but not from the ventral side of the second slice of the medulla cut in the ventral to dorsal progression (Fig. 2C and E). We also observed CO2-dependent ATP release from the rostral chemosensitive area (immediately ventral to the RTN, Fig. 2D and E). Outside of the rostral and caudal VMS areas, e.g. at more lateral locations, very little CO2-evoked ATP release was detected (Fig. 2F). The rostral and caudal release areas for ATP release correspond to those described in our earlier localization of sites of CO2-evoked ATP release at the VMS in vivo (Gourine et al. 2005a) and respectively lie ventral to the RTN and the serotonergic neurons of the medullary raphé (Bradley et al. 2002).
60 mmHg, pH 7.65) reliably evoked ATP release in the caudal chemosensitive area of the VMS (just medial to the emergence of the rootlets of the XIIth nerve Fig. 2A, B and E). ATP was also released in response to CO2 from the dorsal side of the 400 μm thick ventral slice (Fig. 2C and E); but not from the ventral side of the second slice of the medulla cut in the ventral to dorsal progression (Fig. 2C and E). We also observed CO2-dependent ATP release from the rostral chemosensitive area (immediately ventral to the RTN, Fig. 2D and E). Outside of the rostral and caudal VMS areas, e.g. at more lateral locations, very little CO2-evoked ATP release was detected (Fig. 2F). The rostral and caudal release areas for ATP release correspond to those described in our earlier localization of sites of CO2-evoked ATP release at the VMS in vivo (Gourine et al. 2005a) and respectively lie ventral to the RTN and the serotonergic neurons of the medullary raphé (Bradley et al. 2002).
Figure 2. CO2-evoked ATP release in vitro.
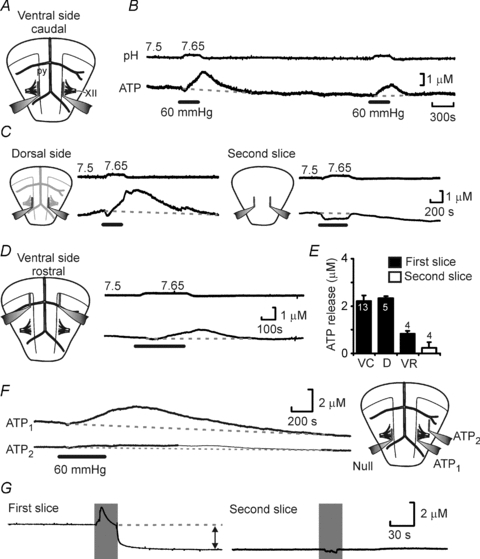
A, diagram of the ventral surface of the ventral medullary slice showing location of the pyramids (py) and XIIth nerve rootlets (XII) and the major blood vessels along with ATP and Null biosensor placements. B, simultaneous recording of bath pH and net ATP release (difference in current between ATP and Null sensors) in response to a CO2 stimulus of 60 mmHg. C, ATP release can be observed from the dorsal surface of the first slice (left) but not from the ventral surface of the second slice in the sequence (right). The negative shift in the sensor trace is due to pH sensitivity of the biosensor. D, CO2-evoked ATP release could also be observed in a rostral position close to the RTN. E, summary comparing the magnitude of CO2 evoked ATP release in the ventral slice caudal position (VC), ventral slice dorsal surface (D) and ventral slice rostral position (VR). The groups are significantly different (Kruskal–Wallis test P = 0.001); the release from the second slice is not significantly different from zero; all other groups are significantly different from zero. F, ATP release is localized on the ventral surface. Recordings from two ATP biosensors (positions shown in diagram on right) showing excellent release from the caudal position but little release from a lateral position. G, there is a substantial ATP tone present at the surface of the ventral slice (left) but not on surface of the second slice (right). The sensors were removed from the slice (indicated by grey shading). With the first slice there was a substantial fall of sensor signal indicative of a tone but no such change was seen with the second slice.
Interestingly, we detected a high basal concentration (tone) of ATP of 3.2 ± 0.9 μm (n = 13) at the VMS (Fig. 2G). It was absent from the ventral side of the second more dorsal slice (0.1 ± 0.3 μm, n = 4, Fig. 2G), suggesting that this tone is a hallmark of the ventral medullary chemosensitive areas. To our knowledge, this is an unprecedented high basal level of extracellular ATP, as in most areas of the brain ATP is rapidly broken down to adenosine and other downstream purines. That this tone exists, suggests both a significant continuous rate of basal release and a slower rate of hydrolysis of extracellular ATP in the medullary areas near the VMS compared to other brain regions.
ATP release depends on 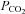
We next examined the dependence of ATP release from the VMS on extracellular pH and 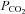 by systematically changing
by systematically changing 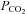 , pH and [HCO3−] in the milieu (Fig. 3). We found that at a given extracellular pH, ATP release depended on the level of
, pH and [HCO3−] in the milieu (Fig. 3). We found that at a given extracellular pH, ATP release depended on the level of 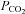 . Increasing
. Increasing 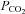 to 55 mmHg and further to 70 mmHg (at constant extracellular pH) evoked release of progressively larger concentrations of ATP (Fig. 4A). Thus, CO2-dependent ATP release does not require extracellular acidification, but instead depends upon prevailing levels of
to 55 mmHg and further to 70 mmHg (at constant extracellular pH) evoked release of progressively larger concentrations of ATP (Fig. 4A). Thus, CO2-dependent ATP release does not require extracellular acidification, but instead depends upon prevailing levels of 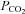 (Fig. 4C). At a constant pH of 7.5, when
(Fig. 4C). At a constant pH of 7.5, when 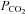 was reduced to 20 mmHg (from the control level of 35 mmHg) a loss of the ATP tone was seen (Fig. 4A). This is significant as it suggests that bidirectional changes in ATP can signal both decreases and increases in
was reduced to 20 mmHg (from the control level of 35 mmHg) a loss of the ATP tone was seen (Fig. 4A). This is significant as it suggests that bidirectional changes in ATP can signal both decreases and increases in 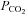 .
.
Figure 3. The dependence of ATP release on 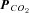 , HCO3− and pH.
, HCO3− and pH.
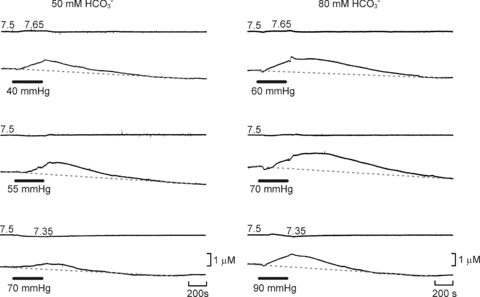
Examples of ATP release evoked by elevations in 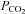 at different levels of HCO3−. At any given pH, higher levels of
at different levels of HCO3−. At any given pH, higher levels of 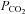 (and HCO3−) evoke more ATP release. Reducing extracellular pH gives less ATP release. Each recording is from a separate slice.
(and HCO3−) evoke more ATP release. Reducing extracellular pH gives less ATP release. Each recording is from a separate slice.
Figure 4. ATP release correlates with 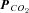 .
.
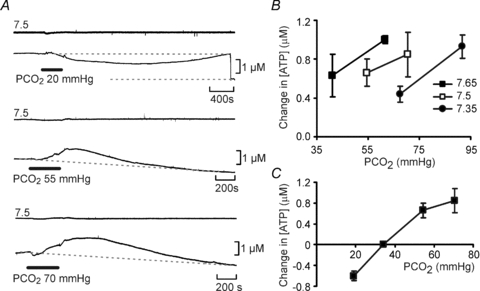
A, at a constant pH (7.5), 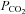 was altered from its control value (35 mmHg) to 20, 55 and 70 mmHg (bar). When
was altered from its control value (35 mmHg) to 20, 55 and 70 mmHg (bar). When 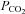 was reduced there was a loss of tone (indicated by grey dashed lines – sensor removed from slice at the end of the record. Increasing
was reduced there was a loss of tone (indicated by grey dashed lines – sensor removed from slice at the end of the record. Increasing 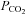 resulted in progressively greater ATP release. Each recording from a different slice. B, summary of ATP release versus
resulted in progressively greater ATP release. Each recording from a different slice. B, summary of ATP release versus 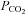 at three different levels of pH. Acidification decreases the sensitivity of ATP release to changes in
at three different levels of pH. Acidification decreases the sensitivity of ATP release to changes in 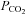 – rightward shift of the points (n = 4 slices for each point). C, summary of evoked ATP release versus
– rightward shift of the points (n = 4 slices for each point). C, summary of evoked ATP release versus 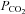 at pH 7.5.
at pH 7.5.
The systematic experimental variation of pH and  also revealed that pH alters the set point of the relationship between CO2 and ATP release from the VMS (Fig. 4B). At alkaline pH smaller absolute levels of
also revealed that pH alters the set point of the relationship between CO2 and ATP release from the VMS (Fig. 4B). At alkaline pH smaller absolute levels of 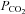 were required to achieve the same concentrations of ATP release in comparison to that at lower levels of pH.
were required to achieve the same concentrations of ATP release in comparison to that at lower levels of pH.
We next studied the possible mechanisms by which CO2 could evoke release of ATP. In principle this release could be triggered by changes in extracellular pH, intracellular pH, [HCO3−] or 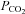 itself. Our data (Figs 3 and 4) exclude a decrease in extracellular pH as a stimulus for ATP release. Changes in intracellular pH, which follow changes in
itself. Our data (Figs 3 and 4) exclude a decrease in extracellular pH as a stimulus for ATP release. Changes in intracellular pH, which follow changes in 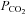 , could potentially induce ATP release. Applications of NH4Cl (20 mm, n = 5) to alter intracellular pH did not cause a statistically significant change in the extracellular level of ATP at the VMS (mean change −0.3 ± 0.3 μm); however in 3 of these 5 cases this treatment caused a loss of extracellular ATP (Fig. 5A). Addition of 5 mm propionate did not cause any release of ATP (n = 3, data not shown). CO2 could conceivably trigger release via an increase in intracellular [HCO3−]. We therefore used acetazolamide to inhibit the enzyme carbonic anhydrase (cf. Nattie & Li, 1996), which catalyses the reaction of CO2 with water. Acetazolamide by itself did not induce ATP release. However, by reducing the rate of formation of HCO3−, it should delay and reduce CO2-evoked ATP release, if this were to depend on either HCO3− or intracellular pH. However it had no effect (Fig. 5B and D), suggesting that changes in intracellular [HCO3−] or pH are not the proximal stimulus for ATP release. Although there is evidence for a CO2/HCO3−-sensitive adenylate cyclase (Mittag et al. 1993; Raven, 2006), SQ22536, a blocker of adenylate cyclase, had no effect on the amplitude of CO2-evoked ATP release (Fig. 5C and D). Furthermore we analysed the integral of CO2-triggered ATP release in the presence of SQ22536 (area under the curve) and found that it was 764 ± 238 mm s (n = 7) compared to a control value of 551 ± 98 mm s (n = 16). This eliminates the potential contribution of a cAMP-dependent signalling pathway to CO2-dependent ATP release.
, could potentially induce ATP release. Applications of NH4Cl (20 mm, n = 5) to alter intracellular pH did not cause a statistically significant change in the extracellular level of ATP at the VMS (mean change −0.3 ± 0.3 μm); however in 3 of these 5 cases this treatment caused a loss of extracellular ATP (Fig. 5A). Addition of 5 mm propionate did not cause any release of ATP (n = 3, data not shown). CO2 could conceivably trigger release via an increase in intracellular [HCO3−]. We therefore used acetazolamide to inhibit the enzyme carbonic anhydrase (cf. Nattie & Li, 1996), which catalyses the reaction of CO2 with water. Acetazolamide by itself did not induce ATP release. However, by reducing the rate of formation of HCO3−, it should delay and reduce CO2-evoked ATP release, if this were to depend on either HCO3− or intracellular pH. However it had no effect (Fig. 5B and D), suggesting that changes in intracellular [HCO3−] or pH are not the proximal stimulus for ATP release. Although there is evidence for a CO2/HCO3−-sensitive adenylate cyclase (Mittag et al. 1993; Raven, 2006), SQ22536, a blocker of adenylate cyclase, had no effect on the amplitude of CO2-evoked ATP release (Fig. 5C and D). Furthermore we analysed the integral of CO2-triggered ATP release in the presence of SQ22536 (area under the curve) and found that it was 764 ± 238 mm s (n = 7) compared to a control value of 551 ± 98 mm s (n = 16). This eliminates the potential contribution of a cAMP-dependent signalling pathway to CO2-dependent ATP release.
Figure 5. CO2-evoked ATP release cannot be mimicked by changes in intracellular pH or altered by inhibition of carbonic anhydrase or adenylate cyclase.
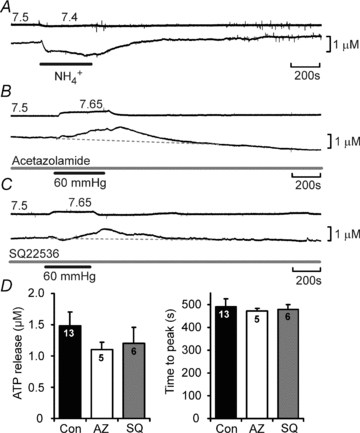
A, application of 20 mm NH4Cl to alter intracellular pH did not cause evoked ATP release. B, acetazolamide, a blocker of carbonic anhydrase, at 500 μm had no effect on the magnitude or time course of CO2-evoked ATP release. C, SQ22536, a blocker of adenylate cyclase, at 100 μm had no effect on the magnitude or time course of CO2-evoked ATP release. D, summary bar graphs demonstrating that neither acetazolamide nor SQ22536 had an effect on the magnitude or timing of ATP release.
CO2-evoked ATP release does not require extracellular Ca2+ and is gap junction hemichannel dependent
We next tested the Ca2+ dependence of CO2-evoked ATP release. Application of aCSF lacking Ca2+ (with 2 mm Mg2+ to maintain the total divalent ion concentration and 1 mm EGTA to chelate any residual Ca2+) by itself induced a profound release of ATP (4.0 ± 0.6 μm, n = 4, Fig. 6A). However, an increase in  still evoked release of ATP on top of this elevated baseline (2.0 ± 0.5 μm, n = 4, not significantly different from the concentration of ATP released under control conditions, Fig. 2A and E). CO2-evoked ATP release is therefore independent of extracellular Ca2+.
still evoked release of ATP on top of this elevated baseline (2.0 ± 0.5 μm, n = 4, not significantly different from the concentration of ATP released under control conditions, Fig. 2A and E). CO2-evoked ATP release is therefore independent of extracellular Ca2+.
Figure 6. CO2-dependent ATP release does not require extracellular Ca2+.
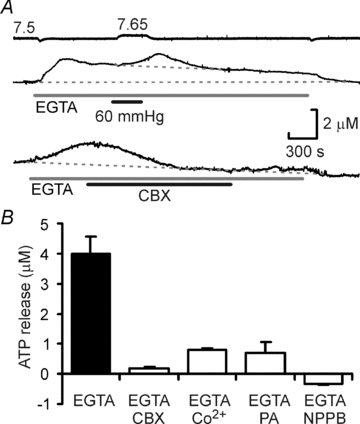
A, removal of extracellular Ca2+ (and addition of 1 mm EGTA) evokes ATP release. Top, however an increase in  can still triggers further ATP release on top of this elevated baseline. Bottom, the EGTA/zero Ca2+ evoked ATP release can be blocked by 100 μm carbenoxolone (CBX). B, summary of the effect of several gap junction channel blockers on the EGTA-evoked ATP release. The blockers significantly reduce the magnitude of ATP release (P < 0.001, Kruskal–Wallis test).
can still triggers further ATP release on top of this elevated baseline. Bottom, the EGTA/zero Ca2+ evoked ATP release can be blocked by 100 μm carbenoxolone (CBX). B, summary of the effect of several gap junction channel blockers on the EGTA-evoked ATP release. The blockers significantly reduce the magnitude of ATP release (P < 0.001, Kruskal–Wallis test).
That lowered extracellular Ca2+ could evoke ATP release suggested gap junction hemichannels as conduits for ATP release, as all of these channels will gate open in the absence of extracellular Ca2+ (Muller et al. 2002). We found that several gap junction hemichannel blockers including carbenoxolone, Co2+, proadifen and NPPB all blocked the zero Ca2+/EGTA induced release of ATP (Fig. 6A and B). These agents also greatly reduced the CO2-evoked ATP release and to a lesser extent the basal tone of ATP found at the caudal area of the VMS (Figs 7A and B and 8).
Figure 7. Gap junction hemichannel antagonists greatly reduce CO2-evoked ATP release.
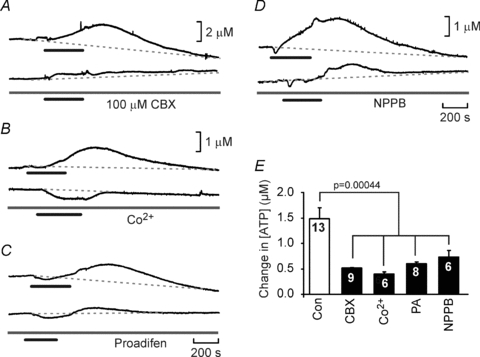
A–D, recordings of CO2-evoked ATP release before and after the application of 100 μm CBX (A), 500 μm Co2+ (B), 200 μm proadifen (C) and 200 μm NPPB (D). All these agents greatly reduced the release of ATP. E, summary statistics demonstrating the reduction of ATP release by these agents. For the comparison the second episode of ATP release was analysed in both the control (no drug) and drug treated slices to allow for the rundown of ATP release apparent in the slice preparation (see Methods). The blockers significantly reduced ATP release (ANOVA, P = 0.00044).
Figure 8. The basal levels of ATP at the ventral surface of the medulla can be blocked by gap junction hemichannel antagonists.
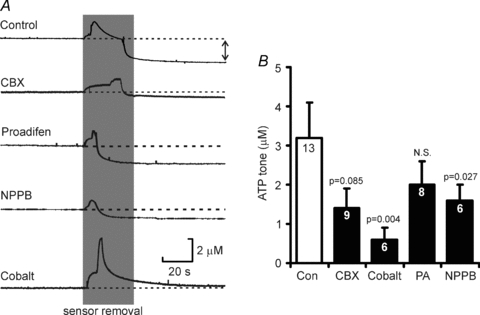
A, the effect of several agents on the ATP tone recorded following removal of ATP biosensors from the surface of the slice (indicated by double headed arrow for control case). B, summary statistics showing the effect of these antagonists on the magnitude of the resting ATP tone. Analysis suggests that the samples are not drawn from the same population (P = 0.029, Kruskal–Wallis test). The probabilities next to the bars indicate pairwise comparisons to the control.
Pannexin 1 forms hemichannels and has been reported to release ATP. Its action can be discriminated by application of carbenoloxolone at lower concentrations (which do not block connexin hemichannels) and by the use of probenecid (Bruzzone et al. 2005; Silverman et al. 2008). While carbenoxolone, blocked ATP release at 100 μm (see above and Fig. 7), it had no effect on CO2-evoked ATP release at the lower dose of 10 μm (Fig. 9A and B). Similarly, CO2-evoked ATP release was not affected by 1 mm probenecid (Fig. 9A and B). This pharmacological profile eliminates pannexin-1 as the conduit for ATP release but is consistent with a connexin hemichannel being involved.
Figure 9. Pannexin-1 hemichannels are not involved in CO2-evoked ATP release.
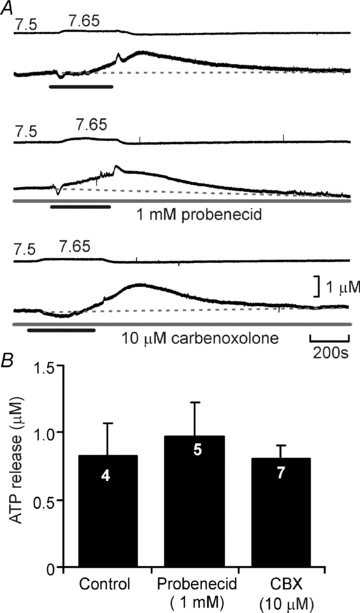
A, neither probenecid at 1mM, nor carbenoxolone at 10 μm, both agents selective for pannexin-1 hemichannels, affected CO2-evoked ATP release. B, summary bar graph demonstrating the lack of effect of probenecid and carbenoxolone on ATP release compared to interleaved controls.
Expression of gap junction proteins in the medulla oblongata
There are many different types of gap junction proteins that can form functional hemichannels including connexins and pannexins. After first using conventional RT-PCR to probe the presence of connexin and pannexin mRNA in the whole medulla oblongata, we used quantitative PCR (QPCR) to determine how the expression of connexin and pannexin mRNA varied at different levels along its ventro-dorsal axis. Whilst connexins 32, 36 and 43, and pannexin 2 were roughly uniformly distributed throughout the medulla, connexin 26 (Cx26) was expressed predominantly in the first 300 μm of tissue (relative to the ventral surface, Fig. 10A). Whilst Cx26 demonstrated a preferential localization in the first 300 μm of tissue relative to the other tissue sections, Cx43 and Cx32 were more highly expressed in this location (6.5 ± 2.5- and 2.6 ± 0.96-fold more prevalent, respectively). By contrast Cx36 and pannexin 2 were much less abundant (0.27 ± 0.12- and 0.11 ± 0.10-fold less prevalent, respectively).
Figure 10. Localization of Cx26 in the ventral medulla oblongata.
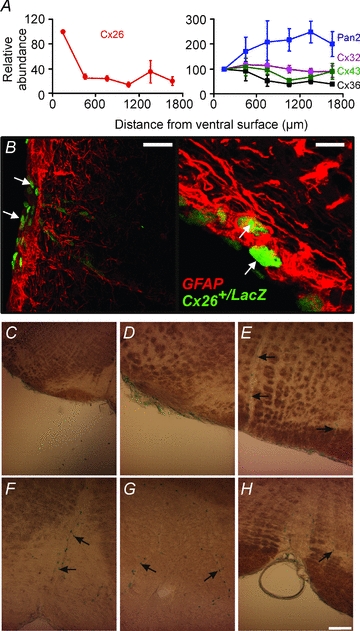
A, quantitative PCR demonstrates that mRNA for Cx26 is enriched within the first 300 μm of tissue from the ventral surface of the medulla in rat, while the mRNA of other connexins is more uniformly distributed. B, localization of LacZ immunoreactivity at the ventral surface of the medulla oblongata (white arrows) in mouse Cx26+/LacZ tissue. It is found in the leptomeninges, marginal glial cells and cells close to blood vessels. Tissue was co-stained with GFAP to label glial cells. Scale bar 40 μm (left) and 10 μm (right). C–E, localization of LacZ activity in the leptomeninges and along penetrating blood vessels in the ventrolateral medulla, at the same rostrocaudal level of the sites of ATP release. F–H, localization near the midline of the ventral medulla in the vicinity of the raphé nucleus. Black arrows indicate lacZ-positive nuclei adjacent to blood vessels (C–H). Scale bar 100 μm.
The QPCR data demonstrate the relative abundance of mRNA but do not demonstrate cellular localization. We therefore examined tissue in which a LacZ reporter had been knocked into the Cx26 locus (see Methods; cf. Filippov et al. 2003). The tissue was derived from heterozygotes as deletion of the Cx26 gene is embryonic lethal. We found that in these transgenic mice LacZ was expressed in the leptomeninges, the glia limitans, and in nuclei near the penetrating blood vessels (Fig. 10B–H). This pattern of Cx26 expression was also confirmed by immunocytochemical localization of Cx26 protein in the rat medulla oblongata. As reported by others (Nagy et al. 2001; Mercier & Hatton, 2001; Solomon et al. 2001), Cx26 immunoreactivity in the ventral medulla oblongata was confined to the very ventral margin of the parenchyma, the surface of astrocytes surrounding penetrating vessels at the VMS and the pia mater (Fig. 11). Interestingly Cx26-positive puncta were also observed on many processes of astrocytes which reside at and near the VMS (Fig. 11). We note that the distribution of Cx26 that we found both by knock-in reporter expression and immunocytochemical staining was much more restricted than that reported in earlier studies (Solomon et al. 2001).
Figure 11. Immunocytochemical localization of Cx26 on astrocytes.
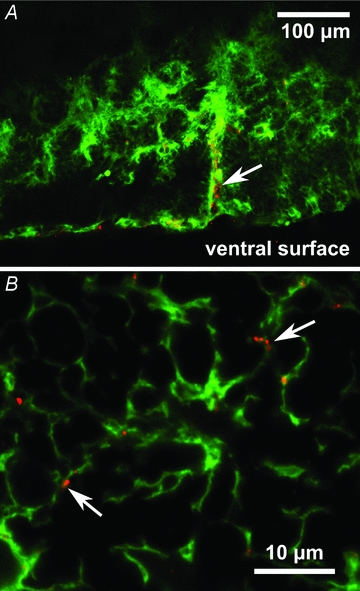
A, Cx26-positive puncta (red) on astrocytes (green) located near the ventral surface (glia limitans) and ensheathing penetrating blood vessels (white arrow). B, at higher resolution Cx26 positive puncta can be seen (in a different tissue section) to be associated with astrocytic processes (white arrows). Tissue from rat.
Identity of VMS cells responding to changes in 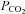
Our data suggest that CO2-dependent ATP release occurs via a hemichannel opening. Furthermore we have demonstrated that within the medulla oblongata Cx26 has a specific pattern of expression predominantly localized to near the VMS. This suggests that Cx26 may mediate the ATP release in response to CO2. If this hemichannel opens when 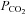 increases to allow egress of ATP it should also allow entry of a dye (of appropriate size and charge) that would enable identification of the cells that express the hemichannel and respond to changes in
increases to allow egress of ATP it should also allow entry of a dye (of appropriate size and charge) that would enable identification of the cells that express the hemichannel and respond to changes in 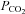 .
.
We therefore attempted to load ventral medullary slices in a CO2-dependent manner either with CBF for live tissue studies or FITC for subsequent fixation and counter-staining. The presence of CBF under control conditions caused some background loading (presumably through the spontaneous gating of hemichannels). This loading was greatly increased following exposure of the slice to increased levels of  (n = 3, Fig. 12A). Subsequent exposure of the slice to hypercapnia in the absence of CBF reduced the loading as expected (Fig. 12A).
(n = 3, Fig. 12A). Subsequent exposure of the slice to hypercapnia in the absence of CBF reduced the loading as expected (Fig. 12A).
Figure 12. CO2-dependent dye loading of the leptomeninges and astrocytes at the ventral medullary surface.
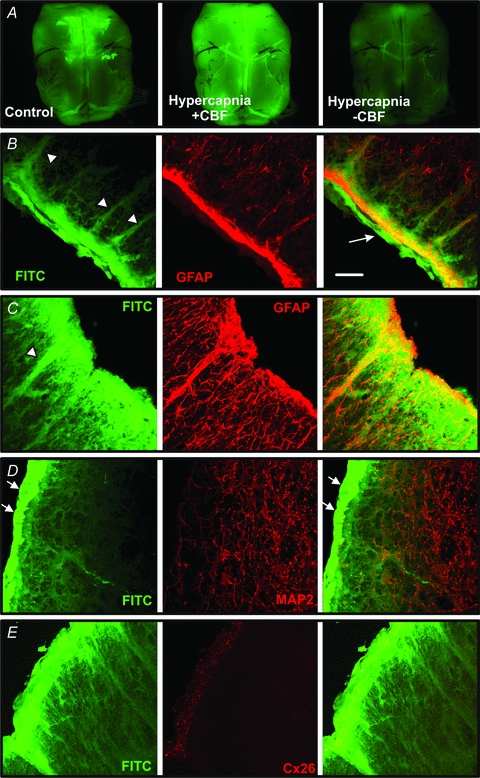
A, whole mount images of a ventral medullary slice showing baseline loading following exposure to carboxyfluorescein (CBF) in control aCSF, greatly enhanced loading when exposed to CBF during hypercapnia ( 60 mmHg) and diminished loading when
60 mmHg) and diminished loading when  was increased in the absence of CBF to flush out the previously loaded CBF. B–E, transverse sections showing FITC loading into the pia mater (arrow), the glia limitans and along the tracks of penetrating blood vessels (arrowheads). Note how the FITC colocalizes with GFAP staining suggesting that astrocytes are CO2 sensitive (B and C). However, neurons stained with MAP2 do not dye load with FITC. The bright strip of FITC loaded tissue (white arrows) is the leptomeningeal layer. D and E, Cx26 immunostaining is located in the glia limitans where a high degree of CO2-dependent dye loading occurs. Scale bar, 40 μm. All tissue from rat.
was increased in the absence of CBF to flush out the previously loaded CBF. B–E, transverse sections showing FITC loading into the pia mater (arrow), the glia limitans and along the tracks of penetrating blood vessels (arrowheads). Note how the FITC colocalizes with GFAP staining suggesting that astrocytes are CO2 sensitive (B and C). However, neurons stained with MAP2 do not dye load with FITC. The bright strip of FITC loaded tissue (white arrows) is the leptomeningeal layer. D and E, Cx26 immunostaining is located in the glia limitans where a high degree of CO2-dependent dye loading occurs. Scale bar, 40 μm. All tissue from rat.
To determine which cells loaded with the dye in a CO2-dependent manner, the slices containing the VMS were loaded with FITC, and the cellular localization of this dye was compared to that of conventional glial (GFAP) and neuronal (MAP2) markers. We found that increased 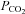 allowed FITC entry into both the leptomeninges and the marginal glial layer (Fig. 12B–E). Interestingly the FITC loading often followed the tracks of penetrating blood vessels, reminiscent of the localization of Cx26 (Fig. 12B–E compare with Figs 10 and 11). FITC loading was colocalized with GFAP (Fig. 12B and C), but not with MAP2 (Fig. 12D). FITC labelling also colocalized with Cx26 immunoreactivity at the surface (glia limitans) and around penetrating blood vessels (Fig. 12E).
allowed FITC entry into both the leptomeninges and the marginal glial layer (Fig. 12B–E). Interestingly the FITC loading often followed the tracks of penetrating blood vessels, reminiscent of the localization of Cx26 (Fig. 12B–E compare with Figs 10 and 11). FITC loading was colocalized with GFAP (Fig. 12B and C), but not with MAP2 (Fig. 12D). FITC labelling also colocalized with Cx26 immunoreactivity at the surface (glia limitans) and around penetrating blood vessels (Fig. 12E).
Connexin hemichannel-mediated ATP release at the VMS contributes to the adaptive respiratory response to hypercapnia
The agents that we found to reduce CO2-dependent ATP release in vitro (Co2+, NPPB and proadifen, cf. Ripps et al. 2004; Zhao, 2005) are capable of partial but substantial block of Cx26 hemichannels. To explore whether there is a causal link between Cx26 hemichannel gating and the adaptive changes in breathing we used these compounds in vivo, and made recordings of the central respiratory drive (phrenic nerve discharge) and ATP release from the VMS in anaesthetized and artificially ventilated rats. Application of either NPPB or proadifen to the VMS had no effect on resting phrenic nerve discharge (P > 0.1) but greatly diminished CO2-evoked ATP release and significantly reduced the resultant increases in respiratory activity (Fig. 13A–C). The degree of reduction in the CO2-induced respiratory response following inhibition of hemichannel activity at the VMS was quantitatively similar to that previously reported for the action of ATP receptor antagonists applied to the VMS (Gourine et al. 2005a) and reflects the contribution of the VMS chemosensitive sites to the overall ventilatory response to CO2 (Nattie, 2001).
Figure 13. Hemichannel blockers reduce CO2-evoked ATP release in vivo and diminish the adaptive respiratory response to CO2.
A and B, the effects of proadifen (PA, 200 μm) and NPPB (200 μm) on ATP release and the adaptive respiratory response to CO2 in anaesthetized and artificially ventilated rats (PNG – integrated phrenic neurogram). Both ATP release and the respiratory responses were reduced. C, summary data showing the concentrations of CO2-evoked ATP release and reduction of the respiratory response in the absence and presence of proadifen and NPPB on the ventral surface of the medulla oblongata (comparisons paired sample t tests).
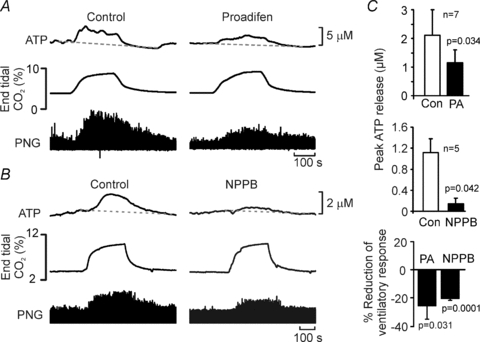
Pharmacological studies of the roles of gap junction hemichannels have been hampered by a lack of highly selective agents to block these channels. It is possible therefore that the effects of NPPB and proadifen on the CO2-evoked respiratory response, which have multiple targets, could have resulted from non-specific depression of respiratory network activity. As a control, we examined the effect of these agents on the respiratory responses to hypoxia in a similar rat preparation. Although responses to hypoxia depend on peripheral inputs from the carotid body, we have previously shown that there is a central component mediated by ATP release in the medulla oblongata (Gourine et al. 2005b). A brief period of hypoxia greatly enhances inspiratory activity (Fig. 14). Neither proadifen nor NPPB when applied to the VMS either separately or in combination had any effect on the resultant respiratory response (Fig. 14). Thus, these agents, at the concentrations used, neither induce a non-specific depression of respiratory network nor interfere with the operation of the reflex pathway responsible for increases in breathing following activation of the peripheral chemoreceptors. Together, our in vivo data strongly suggest that ATP released via Cx26 hemichannels contribute to the adaptive increases in ventilation in response to an increase in the levels of inspired CO2.
Figure 14. Hemichannel blockers do not affect the respiratory response to systemic hypoxia in vivo.
A, neither proadifen (PA, 200 μm) nor NPPB (200 μm) altered the increases in phrenic nerve activity (PNG – integrated phrenic neurogram) evoked by 5 min of 10% hypoxia (thick bars) in an anaesthetized and ventilated rat. The combination of both agents had no effect either. (n = 4 rats.) B, changes in phrenic nerve amplitude in response to hypoxia under control conditions and in the presence of the hemichannel blockers.
Discussion
In this study we have replicated in an in vitro slice preparation of the ventral medulla containing the VMS of young adult rats an important central chemosensory transduction event – CO2-dependent ATP release (Gourine et al. 2005a). This has allowed the detailed investigation of the facets of the chemosensory stimulus necessary for triggering ATP release, and the mechanisms by which ATP is released during hypercapnia.
Nature of the chemosensory stimulus which evokes ATP release from the VMS
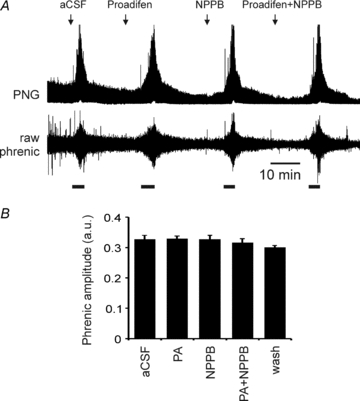
As CO2 and H2O on are in equilibrium with H+, HCO3−, fluctuations in CO2 levels could in principle be detected either via a change in 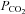 directly, extracellular or intracellular acidification, or via a change in [HCO3−]. There is evidence that small changes in extracellular pH trigger ATP release from the VMS (Gourine et al. 2010). The data presented here indicate that an increase in
directly, extracellular or intracellular acidification, or via a change in [HCO3−]. There is evidence that small changes in extracellular pH trigger ATP release from the VMS (Gourine et al. 2010). The data presented here indicate that an increase in 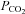 alone is also a sufficient stimulus to evoke release of ATP – the latter was observed in response to rising
alone is also a sufficient stimulus to evoke release of ATP – the latter was observed in response to rising 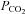 delivered at different constant levels of extracellular pH.
delivered at different constant levels of extracellular pH.
The case for intracellular HCO3− as the effective stimulus for triggering ATP release is weakened by the fact that acetazolamide, a membrane permeant inhibitor of carbonic anhydrase (Buckler et al. 1991), which catalyses the formation of H2CO3 and ultimately HCO3− and H+, had no effect on either the amplitude or speed of CO2 evoked ATP release. Furthermore acetazolamide had no effect on the resting level of ATP. Nevertheless all of the chemosensory stimulation protocols that we used did involve parallel changes in extracellular HCO3− (to control extracellular pH) and we cannot completely exclude the possibility that ATP release was triggered in part by changes in extracellular HCO3−. However, this is unlikely as in the accompanying paper we demonstrate that an increase in 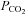 at constant HCO3− still gates open Cx26 hemichannels (Huckstepp et al. 2010).
at constant HCO3− still gates open Cx26 hemichannels (Huckstepp et al. 2010).
To what extent could changes in intracellular pH represent the effective stimulus? Two agents that can directly alter intracellular pH, NH4Cl and propionate, had no effect on the extracellular concentration of ATP at the VMS. NH4Cl will initially cause intracellular alkalinization, but this is followed by acidification in many cells (Ritucci et al. 1998). The concentration of propionate that we used was rather low to avoid confounding signals on the biosensor, but these levels have been shown to reduce intracellular pH by as much as 0.3 units (Shono et al. 2010). That acetazolamide had no effect on the dynamics of ATP release also weakens the case for a change in intracellular pH, as carbonic anhydrase is needed to mediate the rapid intracellular acidification that accompanies an increase in 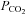 – thus we would have expected ATP release to be slowed in the presence of acetazolamide if this were the case. Although we cannot absolutely eliminate intracellular pH as the effective signal, as we did not measure this variable directly, in the accompanying paper we demonstrate under conditions of constant pH that CO2 modulates Cx26 hemichannel opening in inside-out and outside-out excised membrane patches (Huckstepp et al. 2010). This further weakens the case for intracellular pH as the chemosensory stimulus for inducing the release of ATP.
– thus we would have expected ATP release to be slowed in the presence of acetazolamide if this were the case. Although we cannot absolutely eliminate intracellular pH as the effective signal, as we did not measure this variable directly, in the accompanying paper we demonstrate under conditions of constant pH that CO2 modulates Cx26 hemichannel opening in inside-out and outside-out excised membrane patches (Huckstepp et al. 2010). This further weakens the case for intracellular pH as the chemosensory stimulus for inducing the release of ATP.
A further issue that we addressed here is whether a CO2/HCO3-sensitive adenylate cyclase might be involved and cause ATP release through cAMP-dependent signalling. However blockade of adenylate cyclases with SQ22536 had no effect on CO2-evoked ATP release. We therefore propose that CO2 itself is one of the main stimuli, which evokes the ATP release from the VMS during hypercapnia.
Gap junction hemichannels mediate ATP release in response to CO2
CO2-dependent ATP release appears to occur via the opening of a gap junction hemichannel. Several lines of evidence support this. ATP release is independent of extracellular Ca2+, and removal of extracellular Ca2+ itself, a condition that triggers opening of gap junction hemichannels, causes ATP release from the VMS. This manipulation presumably increases the opening of all connexin hemichannel types present in the ventral medulla, giving rise to the large level of ATP release observed. That a CO2 stimulus under these conditions can still cause enhanced ATP release is presumably due to its acting specifically on Cx26 and via a mechanism independent of the divalent modulation of the hemichannel gating, such that it could cause even greater channel opening and hence additional ATP release.
Both the CO2-evoked ATP release and the zero Ca2+ evoked release are substantially inhibited by the application of several compounds that can block hemichannels. None of these compounds are specific for hemichannels; nevertheless they all have different off target effects – that they all block both forms of ATP release strongly suggests a role for gap junction hemichannels.
The role of hemichannels in mediating CO2-evoked ATP release is further strengthened by the results of the dye-loading experiments, which demonstrate opening of a sufficiently large channel in response to an increase in 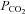 to allow the influx of carboxyfluorescein or FITC (molecular weights of ∼380). The dye loads into the leptomeninges and subpial astrocytes as well as astrocytes that ensheath the penetrating blood vessels. These two lines of evidence strongly suggest that hemichannels gate open to allow the efflux of ATP during elevated
to allow the influx of carboxyfluorescein or FITC (molecular weights of ∼380). The dye loads into the leptomeninges and subpial astrocytes as well as astrocytes that ensheath the penetrating blood vessels. These two lines of evidence strongly suggest that hemichannels gate open to allow the efflux of ATP during elevated 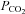 .
.
Interestingly there is a high basal tone of ATP at the VMS. This basal tone also appears to arise from release via hemichannels – it was greatly reduced by agents that act on these channels. Furthermore we observed a background level of dye loading in the slices at normal physiological levels of 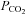 . This is again consistent with the idea of a resting level of hemichannel gating that is sufficient to allow a low level of tonic efflux of ATP (and hence dye loading). That such a large resting tone is measurable suggests a continual source of ATP release.
. This is again consistent with the idea of a resting level of hemichannel gating that is sufficient to allow a low level of tonic efflux of ATP (and hence dye loading). That such a large resting tone is measurable suggests a continual source of ATP release.
The identity of the gap junction hemichannel mediating CO2-evoked ATP release from the VMS
There are two major gene families of gap junction proteins capable of forming hemichannels – the pannexins and connexins. Our pharmacological data eliminate pannexin-1 as a mediator of ATP release. CO2-dependent ATP release is unaffected by the presence of blockers selective for this channel (low doses of carbenoxolone and probenecid). All of the other agents that we used to block CO2-dependent ATP release act on Cx26 hemichannels and indeed Cx26 is our favoured candidate to mediate CO2-dependent ATP release.
Although Cx26 is not the most abundant connexin in the ventral medulla, of the connexins present in the medulla oblongata, only Cx26 has a selective distribution near the VMS as determined by quantitative PCR, immunocytochemistry and a knock-in reporter. It is present in the leptomeninges, the glia limitans and cells close to penetrating blood vessels. In addition there was great similarity between the distribution of Cx26-expressing cells and the cell types and pattern of CO2-dependent dye loading. Indeed we find that Cx26 is expressed on the surface of the cells that dye load in response to increased 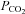 . Our finding, reported in the accompanying paper (Huckstepp et al. 2010), that Cx26 is directly sensitive to changes in
. Our finding, reported in the accompanying paper (Huckstepp et al. 2010), that Cx26 is directly sensitive to changes in 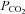 in the physiological range, leaves little doubt that this connexin hemichannel contributes to CO2-dependent ATP release.
in the physiological range, leaves little doubt that this connexin hemichannel contributes to CO2-dependent ATP release.
Physiological significance
Our pharmacological data obtained with the use of a range of hemichannel blockers support a causal link between gating of Cx26 hemichannel at the VMS and the adaptive respiratory responses to changes in inspired CO2. We utilized two different blockers (NPPB and proadifen), both known to inhibit Cx26. As a control for non-specific actions of these agents on the respiratory network or reflex pathways from the peripheral chemoreceptors, we demonstrated that neither NPPB nor proadifen altered hypoxia-evoked increases in central respiratory drive. However, since deletion of Cx26 is embryonic lethal (Gabriel et al. 1998), and Cx26 is involved in the early development of brain (Elias et al. 2007) and other tissues, genetic dissection of the role of Cx26 in central chemosensory transduction will await the development of tissue specific inducible knock-out animal models.
We have demonstrated here that hypercapnia-evoked ATP release at the VMS depends in part directly on changes in 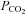 . The CO2-dependent release of ATP is modulated by extracellular pH. Acidification causes a rightward shift in the relationship between ATP release and CO2, while alkalinization shifts the relation leftwards and allows smaller absolute levels of
. The CO2-dependent release of ATP is modulated by extracellular pH. Acidification causes a rightward shift in the relationship between ATP release and CO2, while alkalinization shifts the relation leftwards and allows smaller absolute levels of 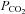 to evoke ATP release. This inhibitory effect of acidification on the CO2-evoked release of ATP could potentially reduce its contribution to chemosensing depending on the extent of the accompanying pH change. However the critical factor is the pH at the vicinity of Cx26 and local buffering could conceivably limit the pH change around the glial cells and Cx26 compared to that of the bulk extracellular fluid.
to evoke ATP release. This inhibitory effect of acidification on the CO2-evoked release of ATP could potentially reduce its contribution to chemosensing depending on the extent of the accompanying pH change. However the critical factor is the pH at the vicinity of Cx26 and local buffering could conceivably limit the pH change around the glial cells and Cx26 compared to that of the bulk extracellular fluid.
Interestingly, at resting levels of 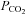 , we found a high basal tone of ATP in the chemosensitive areas, supporting the hypothesis that ATP provides a continual excitatory drive to the respiratory network (Thomas et al. 1999). Fluctuations in
, we found a high basal tone of ATP in the chemosensitive areas, supporting the hypothesis that ATP provides a continual excitatory drive to the respiratory network (Thomas et al. 1999). Fluctuations in 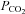 above and below the normal physiological level respectively increase and decrease release of ATP from the VMS. Thus, this is a bidirectional control system, which has all the features required to contribute to modulation of the activity of the respiratory network and, therefore, ventilation in accord with prevailing CO2 tension in the brain parenchyma.
above and below the normal physiological level respectively increase and decrease release of ATP from the VMS. Thus, this is a bidirectional control system, which has all the features required to contribute to modulation of the activity of the respiratory network and, therefore, ventilation in accord with prevailing CO2 tension in the brain parenchyma.
In man mutations of Cx26 (also known as GJB2) are some of the commonest causes of non-syndromal sensorineural hearing loss (Rabionet et al. 2000; Petersen & Willems 2006). Yet altered ventilatory drive has not been reported in these patient cohorts. There are several possible reasons for this. Firstly, the CO2-sensitive feature of Cx26 and its role in the supporting cells of the cochlea may involve different and independent aspects of the molecule. Thus a mutation that affects one role may not affect the other. Secondly, for those mutations that prevent expression of functional protein (e.g. premature stop codons, Kelley et al. 1998), compensatory changes in the expression of other CO2-sensitive connexins such as Cx30 and Cx32 (Huckstepp et al. 2010) may diminish any breathing phenotype. Thirdly, if the nature of the respiratory deficit were to be highly variable (taking a known defect of central chemoreception – congenital central hypoventilation syndrome – as a precedent), it may simply have gone unremarked in the potentially small proportion of profoundly deaf patients where this could arise. Our work suggests that it is worth investigating whether altered ventilatory drive can be observed in a cohort of genotyped Cx26-deficient patients.
Cx26 is expressed in the leptomeninges and glia limitans but not in neurons, and thus both cell types are potentially capable of releasing ATP in a CO2-dependent manner. This suggests that the leptomeninges and glia limitans may play a much more important role in the central respiratory chemosensory reflex than has been hitherto appreciated. As Cx26 is expressed in cells that line penetrating blood vessels (arterioles and capillaries), it might conceivably play a role in CO2-dependent vasodilatation and thus contribute to the regulation of cerebral blood flow during the chemosensory reflex, which will alter the relationship between arterial 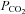 , and pH and those of the brain parenchyma.
, and pH and those of the brain parenchyma.
Parallel pathways of central respiratory chemosensory transduction
The possibility that CO2 levels are detected via changes in extracellular pH is well documented (Winterstein, 1949; Loeschcke, 1982). However several studies have revealed that changes in extracellular pH unaccompanied by an increase in 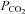 evoke much weaker adaptive changes in respiration compared to similar levels of acidification driven by an elevation of
evoke much weaker adaptive changes in respiration compared to similar levels of acidification driven by an elevation of 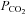 (‘metabolic’ and ‘respiratory’ acidosis, respectively; Eldridge et al. 1985; Shams, 1985; Putnam et al. 2004). Some have suggested that intracellular acidification is an important aspect of central chemosensitivity (Putnam et al. 2004). While our data do not support intracellular pH as a factor for triggering ATP release during hypercapnia, they do strongly suggest that ATP release depends on the prevailing
(‘metabolic’ and ‘respiratory’ acidosis, respectively; Eldridge et al. 1985; Shams, 1985; Putnam et al. 2004). Some have suggested that intracellular acidification is an important aspect of central chemosensitivity (Putnam et al. 2004). While our data do not support intracellular pH as a factor for triggering ATP release during hypercapnia, they do strongly suggest that ATP release depends on the prevailing 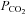 directly. This identifies a parallel CO2-sensitive pathway that could act additively with the pH sensing pathway and thus account for the different magnitudes of cellular and systemic respiratory responses evoked by metabolic versus respiratory acidosis.
directly. This identifies a parallel CO2-sensitive pathway that could act additively with the pH sensing pathway and thus account for the different magnitudes of cellular and systemic respiratory responses evoked by metabolic versus respiratory acidosis.
CO2 chemoreception seems to involve several brainstem nuclei including the medullary raphé, the RTN and the locus coeruleus (Nattie & Li, 2009). Our observation that only about 20% of the response to hypercapnia is blocked by inhibitors of hemichannel mediated ATP release when applied to the VMS is consistent with this hypothesis of multiple sites of chemosensitivity. The Cx26-mediated mechanism of CO2-evoked ATP release may also contribute to chemosensing in several of these other nuclei. We note that populations of neurons proposed as central respiratory pH chemosensors, including the RTN, medullary raphé and locus coeruleus, are all sensitive to ATP (Nieber et al. 1997; Yao et al. 2000; Mulkey et al. 2006; Cao & Song, 2007). These neural populations could therefore represent the distributed sites of convergence for the neuronally mediated pH sensing and the ATP-mediated CO2 sensing components of central respiratory chemosensitivity. We have demonstrated here that the ATP released during hypercapnia can diffuse at least 400 μm deep into the tissue, suggesting that it should be able to activate the chemosensitive neurons of the RTN and medullary raphé. Indeed, in contrast to earlier observations by Mulkey et al. (2006), recent evidence in organotypic slices demonstrates that both pH-evoked depolarizations and Ca2+ responses of Phox2b-expressing RTN neurones are almost entirely mediated by prior release of ATP (Gourine et al. 2010). Equally it remains to be determined whether this mechanism also operates at the dorsal surface of the brainstem where glial-derived ATP released via Cx26 could excite locus coeruleus neurons.
The RTN neurons express the transcription factor Phox2b (Stornetta et al. 2006). Mutations of this gene are associated with central congenital hypoventilation syndrome (CCHS) in humans. Introduction of these mutations into mice or selective depletion of Phox2b-expressing RTN neurons can severely disrupt breathing and results in a complete loss of CO2 sensitivity in the neonate (Dubreuil et al. 2008, 2009). The effect of these mutations on adult chemosensitivity has not been reported. In humans with CCHS, the symptoms often lessen with age (Shea, 1997; Chen & Keens, 2004). It is entirely possible that the role of Phox2b-expressing neurons is particularly important for chemoreception in the neonate but lessens with development as other chemosensory transduction mechanisms develop and other areas of the brainstem become important. Indeed in Phox2b+/− heterozygotes, impaired responses to hypercapnia evident at age P2 are largely normalized by P10 (Dauger et al. 2003). We propose that the CO2-sensitive Cx26 hemichannel-mediated mechanism of ATP release may play an important role in parallel to the chemosensitivity mediated by specific populations of pH-sensitive neurons in central respiratory chemosensitivity in the adult.
Acknowledgments
We thank the MRC (N.D.), BBSRC (N.D.) and Wellcome Trust (A.V.G.) for generous support. A.V.G. is a Wellcome Trust Senior Research Fellow. The generation and characterization of Cx26+/LacZ mice in the Bonn laboratory has been supported by the German Research Association (SFB 645, B2) and the Juergen Manchot Foundation.
Glossary
Abbreviations
- Cx
connexin
- CBX
carbenoxolone
- CBF
carboxyfluorescein
- FITC
fluorescein isothiocyanate
- GFAP
glial fibrillary acidic protein
- MAP2
microtubule associated protein 2
- NPPB
5-nitro-2-(3-phenylpropylamino)benzoic acid
- RTN
retrotrapezoid nucleus
- VMS
ventral medullary surface
Author contributions
R.T.R.H. and R.idB. performed the in vitro slice experiments and analysed the data. R.E. assisted by R.T.R.H. performed and analysed the immunocytochemistry and QPCR, K.M.S. advised on in vivo experiments, A.V.G. helped to design the study, and performed the in vivo work assisted by R.T.R.H., and N.M. performed Cx26 immunolabelling. N.D. (Bonn) and K.W. generated the Cx26+/lacZ mice and provided the tissue. N.D. (Warwick) conceived the study, directed the work and wrote the manuscript. All authors read and commented on the manuscript. All in vitro experiments were performed at Warwick, the in vivo experiments were performed at University College London.
Supplemental material
References
- Bradley SR, Pieribone VA, Wang W, Severson CA, Jacobs RA, Richerson GB. Chemosensitive serotonergic neurons are closely associated with large medullary arteries. Nat Neurosci. 2002;5:401–402. doi: 10.1038/nn848. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem. 2005;92:1033–1043. doi: 10.1111/j.1471-4159.2004.02947.x. [DOI] [PubMed] [Google Scholar]
- Buckler KJ, Vaughan-Jones RD, Peers C, Nye PC. Intracellular pH and its regulation in isolated type I carotid body cells of the neonatal rat. J Physiol. 1991;436:107–129. doi: 10.1113/jphysiol.1991.sp018542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Song G. Purinergic modulation of respiration via medullary raphe nuclei in rats. Respir Physiol Neurobiol. 2007;155:114–120. doi: 10.1016/j.resp.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Chen ML, Keens TG. Congenital central hypoventilation syndrome: not just another rare disorder. Paediatr Respir Rev. 2004;5:182–189. doi: 10.1016/j.prrv.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Corcoran AE, Hodges MR, Wu Y, Wang W, Wylie CJ, Deneris ES, Richerson GB. Medullary serotonin neurons and central CO2 chemoreception. Respir Physiol Neurobiol. 2009;168:49–58. doi: 10.1016/j.resp.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauger S, Pattyn A, Lofaso F, Gaultier C, Goridis C, Gallego J, Brunet JF. Phox2b controls the development of peripheral chemoreceptors and afferent visceral pathways. Development. 2003;130:6635–6642. doi: 10.1242/dev.00866. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil V, Ramanantsoa N, Trochet D, Vaubourg V, Amiel J, Gallego J, Brunet JF, Goridis C. A human mutation in Phox2b causes lack of CO2 chemosensitivity, fatal central apnea, and specific loss of parafacial neurons. Proc Natl Acad Sci U S A. 2008;105:1067–1072. doi: 10.1073/pnas.0709115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil V, Thoby-Brisson M, Rallu M, Persson K, Pattyn A, Birchmeier C, Brunet JF, Fortin G, Goridis C. Defective respiratory rhythmogenesis and loss of central chemosensitivity in Phox2b mutants targeting retrotrapezoid nucleus neurons. J Neurosci. 2009;29:14836–14846. doi: 10.1523/JNEUROSCI.2623-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge FL, Kiley JP, Millhorn DE. Respiratory responses to medullary hydrogen ion changes in cats: different effects of respiratory and metabolic acidoses. J Physiol. 1985;358:285–297. doi: 10.1113/jphysiol.1985.sp015551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias LA, Wang DD, Kriegstein AR. Gap junction adhesion is necessary for radial migration in the neocortex. Nature. 2007;448:901–907. doi: 10.1038/nature06063. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippov MA, Hormuzdi SG, Fuchs EC, Monyer H. A reporter allele for investigating connexin 26 gene expression in the mouse brain. Eur J Neurosci. 2003;18:3183–3192. doi: 10.1111/j.1460-9568.2003.03042.x. [DOI] [PubMed] [Google Scholar]
- Filosa JA, Dean JB, Putnam RW. Role of intracellular and extracellular pH in the chemosensitive response of rat locus coeruleus neurones. J Physiol. 2002;541:493–509. doi: 10.1113/jphysiol.2001.014142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel HD, Jung D, Butzler C, Temme A, Traub O, Winterhager E, Willecke K. Transplacental uptake of glucose is decreased in embryonic lethal connexin26-deficient mice. J Cell Biol. 1998;140:1453–1461. doi: 10.1083/jcb.140.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Atkinson L, Deuchars J, Spyer KM. Purinergic signalling in the medullary mechanisms of respiratory control in the rat: respiratory neurones express the P2X2 receptor subunit. J Physiol. 2003;552:197–211. doi: 10.1113/jphysiol.2003.045294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature. 2005a;436:108–111. doi: 10.1038/nature03690. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Dale N, Spyer KM. Release of ATP in the ventral medulla during hypoxia in rats: Role in hypoxic ventilatory response. J Neurosci. 2005b;25:1211–1218. doi: 10.1523/JNEUROSCI.3763-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Dale N, Korsak A, Llaudet E, Tian F, Huckstepp R, Spyer KM. Release of ATP and glutamate in the nucleus tractus solitarii mediate pulmonary stretch receptor (Breuer-Hering) reflex pathway. J Physiol. 2008;586:3963–3978. doi: 10.1113/jphysiol.2008.154567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grocott MP, Martin DS, Levett DZ, McMorrow R, Windsor J, Montgomery HE. Arterial blood gases and oxygen content in climbers on Mount Everest. N Engl J Med. 2009;360:140–149. doi: 10.1056/NEJMoa0801581. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. The 2008 Carl Ludwig Lecture: retrotrapezoid nucleus, CO2 homeostasis, and breathing automaticity. J Appl Physiol. 2008;105:404–416. doi: 10.1152/japplphysiol.90452.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckstepp RTR, Eason R, Sachdev A, Dale N. CO2-dependent opening of connexin 26 and related β connexins. J Physiol. 2010;588:3921–3931. doi: 10.1113/jphysiol.2010.192096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai A, Ballantyne D, Muckenhoff K, Scheid P. Chemosensitive medullary neurones in the brainstem–spinal cord preparation of the neonatal rat. J Physiol. 1996;492:277–292. doi: 10.1113/jphysiol.1996.sp021308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley PM, Harris DJ, Comer BC, Askew JW, Fowler T, Smith SD, Kimberling WJ. Novel mutations in the connexin 26 gene (GJB2) that cause autosomal recessive (DFNB1) hearing loss. Am J Hum Genet. 1998;62:792–799. doi: 10.1086/301807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llaudet E, Hatz S, Droniou M, Dale N. Microelectrode biosensor for real-time measurement of ATP in biological tissue. Anal Chem. 2005;77:3267–3273. doi: 10.1021/ac048106q. [DOI] [PubMed] [Google Scholar]
- Loeschcke HH. Central chemosensitivity and the reaction theory. J Physiol. 1982;332:1–24. doi: 10.1113/jphysiol.1982.sp014397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier F, Hatton GI. Connexin 26 and basic fibroblast growth factor are expressed primarily in the subpial and subependymal layers in adult brain parenchyma: roles in stem cell proliferation and morphological plasticity? J Comp Neurol. 2001;431:88–104. doi: 10.1002/1096-9861(20010226)431:1<88::aid-cne1057>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Mittag TW, Guo WB, Kobayashi K. Bicarbonate-activated adenylyl cyclase in fluid-transporting tissues. Am J Physiol Renal Physiol. 1993;264:F1060–1064. doi: 10.1152/ajprenal.1993.264.6.F1060. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Mistry AM, Guyenet PG, Bayliss DA. Purinergic P2 receptors modulate excitability but do not mediate pH sensitivity of RTN respiratory chemoreceptors. J Neurosci. 2006;26:7230–7233. doi: 10.1523/JNEUROSCI.1696-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, Chen X, Sen N, Mistry AM, Guyenet PG, Bayliss DA. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J Neurosci. 2007;27:14049–14058. doi: 10.1523/JNEUROSCI.4254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller DJ, Hand GM, Engel A, Sosinsky GE. Conformational changes in surface structures of isolated connexin 26 gap junctions. EMBO J. 2002;21:3598–3607. doi: 10.1093/emboj/cdf365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy JI, Li X, Rempel J, Stelmack G, Patel D, Staines WA, Yasumura T, Rash JE. Connexin26 in adult rodent central nervous system: demonstration at astrocytic gap junctions and colocalization with connexin30 and connexin43. J Comp Neurol. 2001;441:302–323. doi: 10.1002/cne.1414. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. Central chemoreception in the region of the ventral respiratory group in the rat. J Appl Physiol. 1996;81:1987–1995. doi: 10.1152/jappl.1996.81.5.1987. [DOI] [PubMed] [Google Scholar]
- Nattie EE. Central chemosensitivity, sleep, and wakefulness. Respir Physiol. 2001;129:257–268. doi: 10.1016/s0034-5687(01)00295-x. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. Multiple central chemoreceptor sites: cell types and function in vivo. Adv Exp Med Biol. 2008;605:343–347. doi: 10.1007/978-0-387-73693-8_60. [DOI] [PubMed] [Google Scholar]
- Nattie E, Li A. Central chemoreception is a complex system function that involves multiple brain stem sites. J Appl Physiol. 2009;106:1464–1466. doi: 10.1152/japplphysiol.00112.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieber K, Poelchen W, Illes P. Role of ATP in fast excitatory synaptic potentials in locus coeruleus neurones of the rat. Br J Pharmacol. 1997;122:423–430. doi: 10.1038/sj.bjp.0701386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RA, Dale N, Llaudet E, Mobbs P. ATP released via gap junction hemichannels from the pigment epithelium regulates neural retinal progenitor proliferation. Neuron. 2005;46:731–744. doi: 10.1016/j.neuron.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Petersen MB, Willems PJ. Non-syndromic, autosomal-recessive deafness. Clin Genet. 2006;69:371–392. doi: 10.1111/j.1399-0004.2006.00613.x. [DOI] [PubMed] [Google Scholar]
- Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol. 2004;287:C1493–1526. doi: 10.1152/ajpcell.00282.2004. [DOI] [PubMed] [Google Scholar]
- Putnam RW, Conrad SC, Gdovin MJ, Erlichman JS, Leiter JC. Neonatal maturation of the hypercapnic ventilatory response and central neural CO2 chemosensitivity. Respir Physiol Neurobiol. 2005;149:165–179. doi: 10.1016/j.resp.2005.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabionet R, Gasparini P, Estivill X. Molecular genetics of hearing impairment due to mutations in gap junction genes encoding beta connexins. Hum Mutat. 2000;16:190–202. doi: 10.1002/1098-1004(200009)16:3<190::AID-HUMU2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Raven JA. Sensing inorganic carbon: CO2 and HCO3. Biochem J. 2006;396:e5–7. doi: 10.1042/BJ20060574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- Ritucci NA, Chambers-Kersh L, Dean JB, Putnam RW. Intracellular pH regulation in neurons from chemosensitive and nonchemosensitive areas of the medulla. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1152–1163. doi: 10.1152/ajpregu.1998.275.4.R1152. [DOI] [PubMed] [Google Scholar]
- Ritucci NA, Erlichman JS, Leiter JC, Putnam RW. Response of membrane potential and intracellular pH to hypercapnia in neurons and astrocytes from rat retrotrapezoid nucleus. Am J Physiol Regul Integr Comp Physiol. 2005;289:R851–861. doi: 10.1152/ajpregu.00132.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripps H, Qian HH, Zakevicius J. Properties of connexin26 hemichannels expressed in Xenopus oocytes. Cell Mol Neurobiol. 2004;24:647–665. doi: 10.1023/B:CEMN.0000036403.43484.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson CA, Wang W, Pieribone VA, Dohle CI, Richerson GB. Midbrain serotonergic neurons are central pH chemoreceptors. Nat Neurosci. 2003;6:1139–1140. doi: 10.1038/nn1130. [DOI] [PubMed] [Google Scholar]
- Shams H. Differential effects of CO2 and H+ as central stimuli of respiration in the cat. J Appl Physiol. 1985;58:357–364. doi: 10.1152/jappl.1985.58.2.357. [DOI] [PubMed] [Google Scholar]
- Shea SA. Life without ventilatory chemosensitivity. Respir Physiol. 1997;110:199–210. doi: 10.1016/s0034-5687(97)00084-4. [DOI] [PubMed] [Google Scholar]
- Shono Y, Kamouchi M, Kitazono T, Kuroda J, Nakamura K, Hagiwara N, Ooboshi H, Ibayashi S, Iida M. Change in intracellular pH causes the toxic Ca2+ entry via NCX1 in neuron- and glia-derived cells. Cell Mol Neurobiol. 2010;30:453–460. doi: 10.1007/s10571-009-9470-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman W, Locovei S, Dahl GP. Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Cell Physiol. 2008;295:C761–767. doi: 10.1152/ajpcell.00227.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon IC, Halat TJ, El-Maghrabi MR, O’Neal MH., 3rd Localization of connexin26 and connexin32 in putative CO2-chemosensitive brainstem regions in rat. Respir Physiol. 2001;129:101–121. doi: 10.1016/s0034-5687(01)00299-7. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci. 2006;26:10305–10314. doi: 10.1523/JNEUROSCI.2917-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers BA, Overholt JL, Prabhakar NR. CO2 and pH independently modulate L-type Ca2+ current in rabbit carotid body glomus cells. J Neurophysiol. 2002;88:604–612. doi: 10.1152/jn.2002.88.2.604. [DOI] [PubMed] [Google Scholar]
- Thomas T, Ralevic V, Gadd CA, Spyer KM. Central CO2 chemoreception: a mechanism involving P2 purinoceptors localized in the ventrolateral medulla of the anaesthetized rat. J Physiol. 1999;517:899–905. doi: 10.1111/j.1469-7793.1999.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp S, Aller MI, Wisden W, Gourine AV. A role for TASK-1 (KCNK3) channels in the chemosensory control of breathing. J Neurosci. 2008;28:8844–8850. doi: 10.1523/JNEUROSCI.1810-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MH, Newman S, Imray CH. The cerebral effects of ascent to high altitudes. Lancet Neurol. 2009;8:175–191. doi: 10.1016/S1474-4422(09)70014-6. [DOI] [PubMed] [Google Scholar]
- Winterstein H. The reaction theory of respiratory regulation. Experientia. 1949;5:221–226. doi: 10.1007/BF02166892. [DOI] [PubMed] [Google Scholar]
- Wu J, Xu H, Shen W, Jiang C. Expression and coexpression of CO2-sensitive Kir channels in brainstem neurons of rats. J Membr Biol. 2004;197:179–191. doi: 10.1007/s00232-004-0652-4. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Ishikawa R, Omoe K, Yoshikawa N, Yamaguchi-Yamada M, Taniguchi K. Immunohistochemical distribution of inwardly rectifying K+ channels in the medulla oblongata of the rat. J Vet Med Sci. 2008;70:265–271. doi: 10.1292/jvms.70.265. [DOI] [PubMed] [Google Scholar]
- Yao ST, Barden JA, Finkelstein DI, Bennett MR, Lawrence AJ. Comparative study on the distribution patterns of P2X1-P2X6 receptor immunoreactivity in the brainstem of the rat and the common marmoset (Callithrix jacchus): association with catecholamine cell groups. J Comp Neurol. 2000;427:485–507. [PubMed] [Google Scholar]
- Zhao HB. Connexin26 is responsible for anionic molecule permeability in the cochlea for intercellular signalling and metabolic communications. Eur J Neurosci. 2005;21:1859–1868. doi: 10.1111/j.1460-9568.2005.04031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


