Abstract
We have previously shown connexin mediated CO2-dependent ATP release from the surface of the medulla oblongata. Given the localization of connexin 26 (Cx26) to the chemosensing areas of the medulla, we have tested in a heterologous expression system (HeLa cells) whether Cx26 may be sensitive to changes in 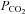 . Cx26 responded to an increase in
. Cx26 responded to an increase in 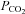 at constant extracellular pH by opening and to a decrease in
at constant extracellular pH by opening and to a decrease in 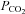 by closing. Furthermore, Cx26 was partially activated at a physiological
by closing. Furthermore, Cx26 was partially activated at a physiological 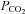 of around 40 mmHg. Cx26 in isolated patches responded to changes in
of around 40 mmHg. Cx26 in isolated patches responded to changes in 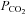 , suggesting direct CO2 sensitivity of the hemichannel to CO2. Heterologous expression of Cx26 in HeLa cells was sufficient to endow them with the capacity to release ATP in a CO2-sensitive manner. We have examined other heterologously expressed connexins for their ability to respond to changes in
, suggesting direct CO2 sensitivity of the hemichannel to CO2. Heterologous expression of Cx26 in HeLa cells was sufficient to endow them with the capacity to release ATP in a CO2-sensitive manner. We have examined other heterologously expressed connexins for their ability to respond to changes in 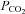 . The closely related β connexins Cx30 and Cx32 also displayed sensitivity to changes in
. The closely related β connexins Cx30 and Cx32 also displayed sensitivity to changes in 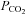 , but with slightly different characteristics from Cx26. The more distant Cx43 exhibited CO2-dependent closing (possibly mediated through intracellular acidification), while Cx36 displayed no CO2 sensitivity. These surprising findings suggest that connexins may play a hitherto unappreciated variety of signalling roles, and that Cx26 and related β connexins may impart direct sensitivity to CO2 throughout the brain.
, but with slightly different characteristics from Cx26. The more distant Cx43 exhibited CO2-dependent closing (possibly mediated through intracellular acidification), while Cx36 displayed no CO2 sensitivity. These surprising findings suggest that connexins may play a hitherto unappreciated variety of signalling roles, and that Cx26 and related β connexins may impart direct sensitivity to CO2 throughout the brain.
Introduction
Starting from the key demonstration of the causal link between ATP release from the chemosensitive regions of the medulla and the adaptive ventilatory response (Gourine et al. 2005), we have studied CO2 chemoreception in detail by utilizing CO2-dependent ATP release as a signal to assess the underlying transduction mechanisms (Huckstepp et al. 2010). Surprisingly we found that CO2-triggered ATP release in vitro in horizontal slices of the ventral surface of the medulla oblongata depended directly on changes in 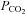 and did not require extracellular acidification. Furthermore ATP concentrations at the surface of the medulla could be altered in a bidirectional manner by increases and decreases in
and did not require extracellular acidification. Furthermore ATP concentrations at the surface of the medulla could be altered in a bidirectional manner by increases and decreases in 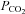 . Strikingly, CO2-dependent ATP release did not occur via neuronal exocytosis: it persisted in the absence of extracellular Ca2+. Gap junction hemichannel antagonists selective for pannexins had no effect on CO2-dependent ATP release. By contrast, CO2-dependent ATP release was greatly reduced by blockers that act on connexins.
. Strikingly, CO2-dependent ATP release did not occur via neuronal exocytosis: it persisted in the absence of extracellular Ca2+. Gap junction hemichannel antagonists selective for pannexins had no effect on CO2-dependent ATP release. By contrast, CO2-dependent ATP release was greatly reduced by blockers that act on connexins.
Several lines of evidence suggest that connexin 26 (Cx26) is located in the correct regions of the medulla oblongata to mediate the CO2-dependent ATP release that we have detected from the ventral surface of the medulla oblongata (Huckstepp et al. 2010). These locations of Cx26 correspond to proposed sites of CO2 chemosensors: the retrotrapezoid nucleus (RTN) (Mulkey et al. 2004); the raphé (Severson et al. 2003; Corcoran et al. 2009); the caudal area near the XIIth nerve identified by Loeschcke (1982); as well as our previously demonstrated sites of ATP release (Gourine et al. 2005; Huckstepp et al. 2010). Our evidence also suggests that hemichannel gating is involved in responses to changes in 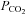 at the medullary surface as both the leptomeninges and sub-pial astrocytes load reversibly with dye in a CO2-dependent manner. Cx26 localizes to cells that exhibit CO2-dependent dye loading. Furthermore, blockers that exhibit some selectivity for this connexin reduce ATP release in vivo and the adaptive response to breathing. Our evidence therefore suggests that Cx26 is the most likely hemichannel to participate in CO2 sensing.
at the medullary surface as both the leptomeninges and sub-pial astrocytes load reversibly with dye in a CO2-dependent manner. Cx26 localizes to cells that exhibit CO2-dependent dye loading. Furthermore, blockers that exhibit some selectivity for this connexin reduce ATP release in vivo and the adaptive response to breathing. Our evidence therefore suggests that Cx26 is the most likely hemichannel to participate in CO2 sensing.
Gap junction hemichannels were first proposed to have a function of their own by Stout et al. (2002). Although their first signalling roles were observed in non-physiological situations, several studies have now suggested important roles during development (Weissman et al. 2004; Pearson et al. 2005; Dale, 2008). The idea that hemichannel gating could play significant physiological signalling roles has been supported by, for example, the recent observations that pannexin-1 hemichannels may play a role in sensory transduction in taste buds (Huang et al. 2007; Dando & Roper, 2009).
In this paper we investigate the CO2 sensitivity of Cx26 hemichannels in a heterologous expression system to test whether this molecule itself could act as a CO2 sensor and whether its properties are sufficient to underlie the previously observed CO2-dependent ATP release in the medulla oblongata. We find that Cx26 hemichannels can respond to changes in 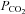 directly and that Cx26 is a sufficient molecular component to endow CO2 sensitivity in a heterologous expression system.
directly and that Cx26 is a sufficient molecular component to endow CO2 sensitivity in a heterologous expression system.
Methods
Cell culture
Wild-type (WT) and connexin-expressing HeLa cells were obtained from the laboratory of Dr Klaus Willecke, Bonn and maintained in Dulbecco's modified Eagle's medium (DMEM) with the following supplements: 1 mm glutamine (Melford Laboratories Ltd, Ipswich, UK), 10% fetal calf serum (Invitrogen) and penicillin–streptomycin (Sigma) at 10 U ml−1 and 10 mg, respectively. In addition, the connexin-expressing cells were under selective pressure with Puromycin (Sigma) at 1 μg ml−1. All cells were grown at 37°C in a humidified 95% O2–5% CO2 incubator. For patch clamp recordings the cells were plated out in 6-well plates at 2 × 106 cells per well for the connexin-expressing HeLa cells and 1 × 106 cells per well for the WT HeLa cells, and an additional 3 mm CaCl2 was added to the medium.
Recording solutions
Standard aCSF: 124 mm NaCl, 3 mm KCl, 1 mm CaCl2, 26 mm NaHCO3, 1.25 mm NaH2PO4, 1 mm MgSO4, 10 mm d-glucose saturated with 95% O2–5% CO2, pH 7.5, 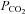 35 mmHg.
35 mmHg.
80 mmHCO3− aCSF: 70 mm NaCl, 3 mm KCl, 1 mm CaCl2, 80 mm NaHCO3, 1.25 mm NaH2PO4, 1 mm MgSO4, 10 mm d-glucose, saturated with 12% CO2 (with the balance being O2) to give a pH of 7.5 and a 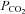 of 70 mmHg.
of 70 mmHg.
50 mmHCO3− aCSF: 100 mm NaCl, 3 mm KCl, 1 mm CaCl2, 50 mm NaHCO3, 1.25 mm NaH2PO4, 1 mm MgSO4, 10 mm d-glucose, saturated with 9% CO2 (with the balance being O2) to give a pH of 7.5 and a 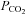 of 55 mmHg.
of 55 mmHg.
10 mmHCO3 aCSF: 140 mm NaCl, 3 mm KCl, 1 mm CaCl2, 10 mm NaHCO3, 1.25 mm NaH2PO4, 1 mm MgSO4, 10 mm d-glucose, saturated with 2% CO2 (with the balance being O2) to give a pH of 7.5 and a 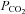 of 20 mmHg.
of 20 mmHg.
Low Cl− control aCSF: 10 mm NaCl, 114 mm sodium gluconate, 26 mm NaHCO3, 1.25 mm NaH2PO4, 3 mm KCl, 1 mm MgSO4, 1 mm CaCl2 and 10 mm glucose, equilibrated with 5% CO2–95% O2.
Low Cl− hypercapnic aCSF: 10 mm NaCl, 70 mm sodium gluconate, 80 mm NaHCO3, 1.25 mm NaH2PO4, 3 mm KCl, 1 mm MgSO4, 1 mm CaCl2 and 10 mm glucose, equilibrated with 12% CO2 (with the balance being O2).
Immunocytochemistry
The Cx26-expressing and wild-type HeLa cells were fixed in 4% paraformaldehyde in 0.1 m phosphate buffer pH 7.4 for 45 min at room temperature. Mouse anti-Cx26 (Invitrogen) was applied to the cells at a dilution of 1:200 and incubated overnight at 4°C. The cells were then washed in 0.1 m phosphate buffer and incubated for 2 h with a secondary antibody conjugated to Alexa Fluor-488. Cells were examined with an epifluorescence microscope.
PCR
The RNA of the HeLa cells was extracted using the RNeasy kit (Qiagen, Valencia, CA, USA). Reverse transcription (by standard methods) was performed and the resultant cDNA was used for subsequent PCR. Standard methods were used to run PCR reactions with Cx26 primers: forward, AGCATCCTCGGGGGTGTCAAC; backward, CCGGAAGAAGATGCTGGTGGTGTA.
Patch clamp recordings
Coverslips containing non-confluent cells were placed into a perfusion chamber at 28°C in sterile filtered standard aCSF. Standard patch clamp techniques were used to make whole-cell recordings and outside out or inside out membrane patch recordings. For whole-cell patch clamp the intracellular fluid in the patch pipette contained: 120 mm potassium gluconate, 10 mm CsCl, 10 mm TEA-Cl, 10 mm EGTA, 3 mm ATP, 1 mm MgCl2, 1 mm CaCl2, sterile filtered, pH adjusted to 7.2 with KOH. All whole-cell recordings were performed at a holding potential of −40 mV with regular steps of 5 s to −50 mV to assess whole-cell conductance.
For outside out patch recordings the pipette solution was: 120 mm potassium aspartate, 10 mm CsCl2, 10 mm TEACl2, 10 mm EGTA, 5 mm ATP, 6 mm MgCl2, 1 mm CaCl2, sterile filtered, pH adjusted to 7.2 with KOH. Inside-out patch pipettes contained standard aCSF. The isolated membrane patch recordings were analysed by measuring the integral of the channel currents in the control, hyper/hypocapnic solutions and wash relative to the zero-current baseline. Comparison of the change of this integral of current in the three conditions was performed with Friedman's two-way analysis of variance; exact P values are given in the text.
Dye loading
Subconfluent wild-type (WT) and connexin-expressing cells (on coverslips) were subjected to 200 μm carboxyfluorescein (CBF) for 5 min in standard aCSF (control) or 80 mm HCO3− aCSF saturated with 9% CO2 (hypercapnic conditions). This was then followed by a wash of 30 min in standard aCSF. The cell loading experiments were repeated at least 3 times. For analysis, 40 cells were chosen at random for each cell line and condition. Using ImageJ, a region of interest was drawn round each cell body and the median pixel intensity for the cell calculated. These medians were then ranked and the Mann–Whitney U test used to assess statistical significance; P values are reported in the text.
ATP release
ATP and null biosensors (Llaudet et al. 2005) were obtained from Sarissa Biomedical Ltd (Coventry, UK). They were used in conjunction with a Duostat ME200+ (Sycopel International Ltd, Jarrow, UK). The biosensors were bent so that the sensing portion (0.5 mm in length) could be laid flat against the cultured HeLa cells. Dual simultaneous recordings were made with the ATP and null biosensors. The null biosensors lack the ATP-sensing enzymes and act as a control for any non-specific signals (Llaudet et al. 2005; Gourine et al. 2005, 2008; Pearson et al. 2005).
Preloading of the HeLa cells with ATP was necessary to observe CO2-evoked ATP release. This is most probably because with the non-confluent monolayer of cells used in these experiments, relatively few cells (<20) were in contact with the sensor, and preloading was necessary to increase the signal size to a level detectable by the biosensors. This was achieved by exposing the cells (both Cx26-expressing and wild-type) to 10 mm ATP for 5 min in the presence of 80 mm HCO3− aCSF saturated with 9% CO2 (to open the Cx26 channels and load them with exogenous ATP). They were then washed for 30 min in standard aCSF, to remove any trace of the added ATP from the medium. The ATP and null biosensors were placed in close proximity to the cells and allowed to stabilize. The cells were then exposed to the hypercapnic stimulus to test for release.
Results
Cx26 responds to changes in 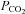
To examine whether Cx26 may be sensitive to changes in 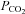 , we obtained HeLa cells expressing Cx26. We first verified that the wild-type HeLa cells were devoid of Cx26 and that the Cx26-expressing cells did indeed express Cx26 by both reverse transcriptase PCR and immunocytochemistry (Fig. 1).
, we obtained HeLa cells expressing Cx26. We first verified that the wild-type HeLa cells were devoid of Cx26 and that the Cx26-expressing cells did indeed express Cx26 by both reverse transcriptase PCR and immunocytochemistry (Fig. 1).
Figure 1. Cx26-expressing HeLa cells demonstrate Cx26 immunoreactive puncta and contain mRNA for Cx26.
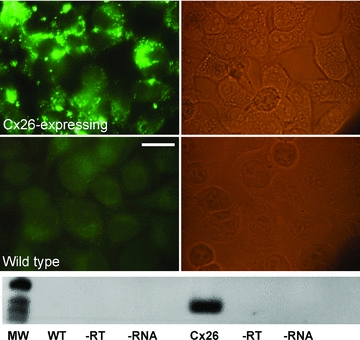
Wild-type cells do not express Cx26 mRNA and show no immunoreactivity. Epifluorescence images on the left, brightfield images on the right, scale bar 20 μm. The bottom section is a Northern blot demonstrating reverse transcription PCR for Cx26 expression in wild-type (WT) and Cx26-expressing cells. MW, molecular weight markers; −RT, no reverse transcriptase control; −RNA, no RNA control.
We performed whole-cell patch clamp recordings from the wild-type and connexin-expressing HeLa cells. We found that wild-type HeLa cells had a very low whole-cell conductance, whereas in Cx26-expressing HeLa cells the resting whole-cell conductance was much larger (Fig. 2A). When 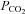 was changed from its control level of 35 mmHg to 70 mmHg, there was no response in the wild-type cells (Fig. 2A). However a rapid and large increase of conductance accompanied by an outward current and an increase in current noise was observed in the Cx26-expressing cells (Fig. 2A). This increase in whole-cell conductance would therefore appear to be due to the CO2-dependent opening of Cx26 hemichannels in the HeLa cells. The response of Cx26-expressing cells to an increase in
was changed from its control level of 35 mmHg to 70 mmHg, there was no response in the wild-type cells (Fig. 2A). However a rapid and large increase of conductance accompanied by an outward current and an increase in current noise was observed in the Cx26-expressing cells (Fig. 2A). This increase in whole-cell conductance would therefore appear to be due to the CO2-dependent opening of Cx26 hemichannels in the HeLa cells. The response of Cx26-expressing cells to an increase in 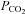 to 70 mmHg at a more physiological temperature of 33°C was no different (1.2 ± 0.2 nS, n = 8 versus 1.1 ± 0.3 nS, n = 9 at 28°C).
to 70 mmHg at a more physiological temperature of 33°C was no different (1.2 ± 0.2 nS, n = 8 versus 1.1 ± 0.3 nS, n = 9 at 28°C).
Figure 2. Cx26-expressing HeLa cells respond to changes in 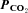 .
.
A, the Cx26-expressing cells show an increase in whole-cell conductance when  is increased to 70 mmHg and 55 mmHg and a decrease in whole-cell conductance when
is increased to 70 mmHg and 55 mmHg and a decrease in whole-cell conductance when  is reduced to 20 mmHg from the baseline level of 35 mmHg. The records for 55 mmHg and 20 mmHg
is reduced to 20 mmHg from the baseline level of 35 mmHg. The records for 55 mmHg and 20 mmHg 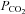 are from the same cell. Note the increase in current noise during elevated
are from the same cell. Note the increase in current noise during elevated 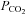 and the corresponding reduction in current noise during the reduction of
and the corresponding reduction in current noise during the reduction of 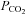 . The wild-type (WT) cells show a lower whole-cell conductance and no change in response to an increase of
. The wild-type (WT) cells show a lower whole-cell conductance and no change in response to an increase of 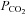 to 70 mmHg (bar, bottom). Holding potential −40 mV, with steps to −50 mV. B, summary graph showing the change in whole-cell conductance versus level of
to 70 mmHg (bar, bottom). Holding potential −40 mV, with steps to −50 mV. B, summary graph showing the change in whole-cell conductance versus level of 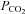 for Cx26-expressing and wild-type HeLa cells. Bars indicate s.e.m.; Cx26, n = 12 for 20 mmHg, 13 for 55 mmHg and 9 for 70 mmHg; ANOVA, F = 19.17, P = 3.9 × 10−7, all values significantly different from zero, P < 0.005; WT n = 4 for 20 mmHg, 4 for 55 mmHg and 7 for 70 mmHg.
for Cx26-expressing and wild-type HeLa cells. Bars indicate s.e.m.; Cx26, n = 12 for 20 mmHg, 13 for 55 mmHg and 9 for 70 mmHg; ANOVA, F = 19.17, P = 3.9 × 10−7, all values significantly different from zero, P < 0.005; WT n = 4 for 20 mmHg, 4 for 55 mmHg and 7 for 70 mmHg.
When the 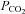 was reduced from 35 mmHg to 20 mmHg, there was a consequent whole-cell conductance decrease and a reduction of current noise (Fig. 2A). This implies that there must be some basal level of Cx26 hemichannel opening under control conditions of 35 mmHg
was reduced from 35 mmHg to 20 mmHg, there was a consequent whole-cell conductance decrease and a reduction of current noise (Fig. 2A). This implies that there must be some basal level of Cx26 hemichannel opening under control conditions of 35 mmHg 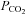 and reductions of
and reductions of 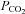 from this level must cause the closing of these Cx26 hemichannels. Plotting whole-cell conductance change against
from this level must cause the closing of these Cx26 hemichannels. Plotting whole-cell conductance change against 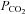 for wild-type and Cx26-expressing cells showed no sensitivity to changes in
for wild-type and Cx26-expressing cells showed no sensitivity to changes in 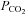 for wild-type cells, but a steep change centred around 40 mmHg for the Cx26-expressing cells (Fig. 2B). Thus the expression of Cx26 is sufficient to confer CO2 sensitive whole-cell conductance changes on the HeLa cells.
for wild-type cells, but a steep change centred around 40 mmHg for the Cx26-expressing cells (Fig. 2B). Thus the expression of Cx26 is sufficient to confer CO2 sensitive whole-cell conductance changes on the HeLa cells.
CO2 sensitivity of other connexins
To test whether CO2 sensitivity is specific to Cx26 or a more general property of connexins we examined four other connexin subtypes expressed in HeLa cells. These connexins were chosen on the basis that they are expressed in the medulla oblongata (Cx32, Cx36, Cx43) or resulted from a relatively recent gene duplication event relative to Cx26 (Cx30). The related β connexins Cx30 and Cx32 exhibited sensitivity to changes in 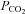 – they conferred CO2-dependent changes in whole-cell conductance (Fig. 3A). We examined the CO2 sensitivity of Cx32 in more detail and found that it was considerably less sensitive to
– they conferred CO2-dependent changes in whole-cell conductance (Fig. 3A). We examined the CO2 sensitivity of Cx32 in more detail and found that it was considerably less sensitive to 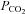 than Cx26. Cx32 exhibited statistically significant whole-cell conductance increases only when
than Cx26. Cx32 exhibited statistically significant whole-cell conductance increases only when 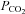 was increased to 70 mmHg (P < 0.001), and did not respond to decreases in
was increased to 70 mmHg (P < 0.001), and did not respond to decreases in 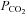 from the control level (Fig. 3B). The γ connexin, Cx36, was completely insensitive to changes in
from the control level (Fig. 3B). The γ connexin, Cx36, was completely insensitive to changes in 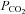 (Fig. 3A), whereas the α connexin, Cx43, responded with a conductance decrease and a loss of inward current (giving an apparent net outward current during the CO2 stimulus) (Fig. 3A). This reduction of the whole-cell conductance (and inward current) may have arisen because the resulting intracellular acidification reduced the gating of the Cx43 hemichannels. A summary of the changes in whole-cell conductance in response to an increase of
(Fig. 3A), whereas the α connexin, Cx43, responded with a conductance decrease and a loss of inward current (giving an apparent net outward current during the CO2 stimulus) (Fig. 3A). This reduction of the whole-cell conductance (and inward current) may have arisen because the resulting intracellular acidification reduced the gating of the Cx43 hemichannels. A summary of the changes in whole-cell conductance in response to an increase of 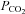 to 70 mmHg is shown in Fig. 3C. This confirms that the β connexin-expressing HeLa cells differ from those expressing Cx36 and Cx43 in exhibiting a conductance increase in response to an increase in
to 70 mmHg is shown in Fig. 3C. This confirms that the β connexin-expressing HeLa cells differ from those expressing Cx36 and Cx43 in exhibiting a conductance increase in response to an increase in 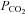 .
.
Figure 3. The CO2 sensitivity of other β, γ and α connexins.
Cx30- and -32-expressing cells (β connexins closely related to Cx26) exhibit sensitivity to an increase in 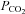 to 70 mmHg (bar). Cx36-expressing cells (a γ connexin) have no sensitivity to an increase in
to 70 mmHg (bar). Cx36-expressing cells (a γ connexin) have no sensitivity to an increase in 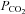 (70 mmHg, bar). Cx43-expressing cells (an α connexin) exhibit a CO2-dependent reduction in whole-cell conductance. B, detailed investigation of the sensitivity of Cx32 to increases in
(70 mmHg, bar). Cx43-expressing cells (an α connexin) exhibit a CO2-dependent reduction in whole-cell conductance. B, detailed investigation of the sensitivity of Cx32 to increases in 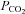 demonstrates that it requires elevations of
demonstrates that it requires elevations of 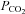 above 55 mmHg to give a significant change in whole-cell conductance. C, summary bar graph demonstrating the changes in whole-cell conductance to a
above 55 mmHg to give a significant change in whole-cell conductance. C, summary bar graph demonstrating the changes in whole-cell conductance to a 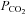 challenge of 70 mmHg for the 4 connexins in comparison to Cx26. The responses of the different connexins are highly significantly different, ANOVA F = 7.24, P = 0.000264; P values are given for where the mean conductance change is significantly different from zero.
challenge of 70 mmHg for the 4 connexins in comparison to Cx26. The responses of the different connexins are highly significantly different, ANOVA F = 7.24, P = 0.000264; P values are given for where the mean conductance change is significantly different from zero.
Cx26 responds to changes in 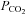 rather than Cl− or HCO3−
rather than Cl− or HCO3−
To accommodate the higher concentrations of HCO3−, concentrations of Cl− in aCSF were reduced accordingly. We therefore performed controls to check whether Cx26 remained responsive to changes in 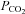 under conditions of constant extracellular [Cl−]. Under these conditions the Cx26-expressing cells exhibited a conductance change of 1.5 ± 0.4 nS in response to an increase in
under conditions of constant extracellular [Cl−]. Under these conditions the Cx26-expressing cells exhibited a conductance change of 1.5 ± 0.4 nS in response to an increase in 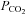 (n = 6, Fig. 4A). This was similar to a conductance change of 1.1 ± 0.1 nS (n = 9) under conditions where extracellular [Cl−] changed during application of the same CO2 stimulus (Figs 2A and B and 3C).
(n = 6, Fig. 4A). This was similar to a conductance change of 1.1 ± 0.1 nS (n = 9) under conditions where extracellular [Cl−] changed during application of the same CO2 stimulus (Figs 2A and B and 3C).
Figure 4. Cx26 is sensitive to changes in 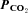 rather than Cl− or HCO3−.
rather than Cl− or HCO3−.
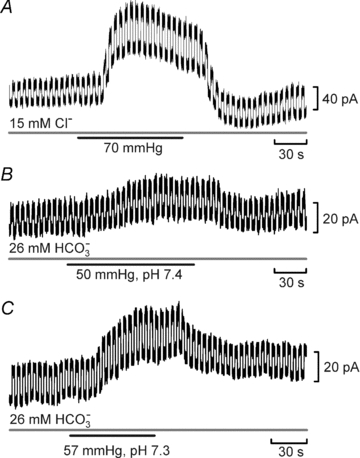
A, recording of the whole-cell response to 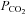 of 70 mmHg (bar) in the presence of constant extracellular Cl−. Note the large conductance change and increase in current noise. B, the whole-cell response of Cx26 to a 50 mmHg
of 70 mmHg (bar) in the presence of constant extracellular Cl−. Note the large conductance change and increase in current noise. B, the whole-cell response of Cx26 to a 50 mmHg 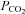 challenge delivered with constant extracellular HCO3−.
challenge delivered with constant extracellular HCO3−.
It is conceivable that the Cx26 channel responds to changes in [HCO3−] rather than 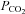 . We therefore performed experiments where the concentration of added HCO3− remained constant at 26 mm and we varied
. We therefore performed experiments where the concentration of added HCO3− remained constant at 26 mm and we varied 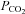 (with a small consequent change in pH). When
(with a small consequent change in pH). When 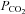 was increased to a drop in pH of 0.1 units (
was increased to a drop in pH of 0.1 units (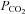 50 mmHg), a small increase in whole-cell conductance was seen (443 ± 88 pS, n = 8, Fig. 4B). A slightly bigger increase in
50 mmHg), a small increase in whole-cell conductance was seen (443 ± 88 pS, n = 8, Fig. 4B). A slightly bigger increase in 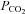 to cause a drop in pH of 0.2 units (
to cause a drop in pH of 0.2 units (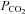 57 mmHg) caused a conductance change of 663 ± 128 pS, n = 6 (Fig. 4C). These changes are slightly lower than the values at a constant pH of 7.5 interpolated from the graph in Fig. 2B, probably as a result of the accompanying acidification – which reduces CO2-mediated release of ATP in the medulla (Huckstepp et al. 2010).
57 mmHg) caused a conductance change of 663 ± 128 pS, n = 6 (Fig. 4C). These changes are slightly lower than the values at a constant pH of 7.5 interpolated from the graph in Fig. 2B, probably as a result of the accompanying acidification – which reduces CO2-mediated release of ATP in the medulla (Huckstepp et al. 2010).
CO2-dependent dye loading of connexin-expressing HeLa cells
To provide methodologically independent confirmation of our patch clamp findings we examined CO2-dependent dye loading of the connexin-expressing HeLa cells. Baseline loading following exposure to 200 μm carboxyfluorescein (CBF) for 5 min under control conditions (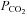 35 mmHg) was compared to loading of CBF for 5 min under hypercapnic conditions (
35 mmHg) was compared to loading of CBF for 5 min under hypercapnic conditions (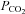 70 mmHg). Wild-type cells showed no loading under either condition (Fig. 5).
70 mmHg). Wild-type cells showed no loading under either condition (Fig. 5).
Figure 5. CO2-dependent dye loading into the connexin-expressing HeLa cells.
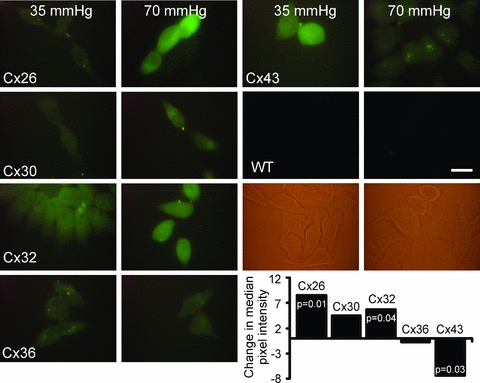
An increase of 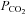 from 35 mmHg to 70 mmHg causes a large significant increase in dye loading for cells expressing Cx26 and Cx32. Cells expressing Cx30 show a smaller increase that failed to achieve statistical significance. Cx36 showed no change in dye loading, while Cx43 showed a decrease in loading. All connexin-expressing cells demonstrated increased loading of carboxyfluorescein under control conditions, whereas the wild-type cells showed no loading under the control or elevated CO2 conditions (yellow panels: bright field images of WT cells). Scale bar 20 μm. Statistical comparison of change in loading at 70 mmHg versus that at 35 mmHg by Mann–Witney U test, P values given where this difference was significant.
from 35 mmHg to 70 mmHg causes a large significant increase in dye loading for cells expressing Cx26 and Cx32. Cells expressing Cx30 show a smaller increase that failed to achieve statistical significance. Cx36 showed no change in dye loading, while Cx43 showed a decrease in loading. All connexin-expressing cells demonstrated increased loading of carboxyfluorescein under control conditions, whereas the wild-type cells showed no loading under the control or elevated CO2 conditions (yellow panels: bright field images of WT cells). Scale bar 20 μm. Statistical comparison of change in loading at 70 mmHg versus that at 35 mmHg by Mann–Witney U test, P values given where this difference was significant.
In contrast all of the connexin-expressing cells showed some CBF loading under control conditions (presumably due to spontaneous gating of the connexin hemichannels under these conditions). Cx26- and Cx32-expressing cells showed significant increases in loading following exposure to increased levels of 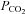 . Cx30 showed an increase that did not reach statistical significance. Cx36-expressing cells exhibited no change and Cx43-expressing cells a decrease in loading under hypercapnic conditions (Fig. 5). The change in median pixel intensity (quantification of dye loading) closely mirrored the changes in whole-cell conductance during hypercapnia for the cell lines expressing different connexins (compare Figs 3C and 5).
. Cx30 showed an increase that did not reach statistical significance. Cx36-expressing cells exhibited no change and Cx43-expressing cells a decrease in loading under hypercapnic conditions (Fig. 5). The change in median pixel intensity (quantification of dye loading) closely mirrored the changes in whole-cell conductance during hypercapnia for the cell lines expressing different connexins (compare Figs 3C and 5).
We therefore conclude that CO2 sensitivity is not a universal feature of all the connexins. Cx26 appears to be the most sensitive of the connexins tested here to changes in 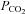 within the physiological range. It is capable of responding in a bidirectional manner to changes in
within the physiological range. It is capable of responding in a bidirectional manner to changes in 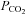 around its normal value of 40 mmHg. Cx30 and Cx32 are less sensitive.
around its normal value of 40 mmHg. Cx30 and Cx32 are less sensitive.
Cx26 channels release ATP
Cx26 is clearly capable of allowing the permeation of CBF – a molecule similar in size and charge to ATP. To verify that it can also allow release of ATP, we used biosensors to measure CO2-evoked ATP release from Cx26-expressing HeLa cells. Without preloading of the HeLa cells with ATP, we did not observe ATP release with biosensors placed next to the HeLa cells (see Methods). However following preloading with ATP, CO2 elicited ATP release from Cx26-expressing HeLa cells (Fig. 6). No ATP release in response to CO2 was seen from wild-type HeLa cells (Fig. 6). We therefore conclude that Cx26 is permeable to ATP and its expression alone is sufficient to endow cells with the capacity for CO2-dependent ATP release. The dynamics of this ATP release were remarkably similar to that seen for CO2-dependent release in the medulla oblongata (Huckstepp et al. 2010a).
Figure 6. Cx26 expression is sufficient for recapitulation of CO2-dependent ATP release.
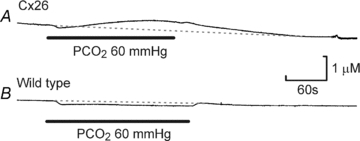
A, release of ATP in response to elevated 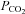 from Cx26-expressing cells. B, no ATP release was seen from wild-type cells.
from Cx26-expressing cells. B, no ATP release was seen from wild-type cells.
Gating of Cx26 in response to changes in 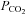 in isolated patches
in isolated patches
While expression of Cx26 alone is sufficient to make HeLa cells CO2 sensitive and suggests direct modulation of the channel by changes in 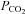 , this does not demonstrate a direct effect of CO2 on the channel. We therefore made patch clamp recordings from isolated membrane patches. The persistence of Cx26 gating in response to changes in
, this does not demonstrate a direct effect of CO2 on the channel. We therefore made patch clamp recordings from isolated membrane patches. The persistence of Cx26 gating in response to changes in 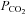 in isolated patches would favour a direct action of CO2 on the hemichannel, as this would remove all soluble components and leave only the possibility of indirect actions mediated through a membrane associated accessory protein in close spatial association with the hemichannel.
in isolated patches would favour a direct action of CO2 on the hemichannel, as this would remove all soluble components and leave only the possibility of indirect actions mediated through a membrane associated accessory protein in close spatial association with the hemichannel.
In the outside-out patches drawn from Cx26-expressing cells large channel openings could be observed. In two excised patches we were able to obtain a full current–voltage relation (Fig. 7A). Unlike many reports of hemichannel gating which demonstrate sustained gating, we did not observe long channel openings – instead the openings were very flickery in nature. Brief episodes of flickery hemichannel gating have been reported by others (Gonzalez et al. 2006). We measured the amplitudes of the most frequent channel openings at a range of transmembrane voltages, and fitted regression lines to subsets of points from these data. The currents had a reversal potential around 0 mV and our analysis suggested conductance states of 37, 69, 144 and 239 pS (Fig. 7A and B). It is very difficult to assign a main conductance state to these recordings, but 144 pS may represent the main conductance state of the channel and 37 and 69 pS may represent subconductance states (cf. Gassmann et al. 2009). These currents were absent from patches drawn from wild-type cells.
Figure 7. The gating of Cx26 in outside-out patches drawn from Cx26-expressing HeLa cells.
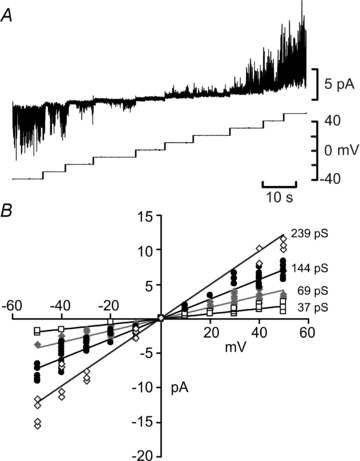
A, complete I–V relation demonstrating channel gating from −50 to +50 mV with a reversal potential around 0 mV. B, linear regression fits to the most prevalent different conductance levels detectable within the currents recorded.
When an isohydric hypercapnic stimulus was applied (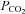 70 mmHg) no channel gating was seen in wild-type patches (10 patches, integral of membrane current in control and hypercapnia 0.61 × 105 and 0.43 × 105 pA s, respectively, not significantly different, Fig. 8A and D). However in patches drawn from the Cx26-expressing cells gating of a large conductance channel could be seen (Fig. 8B, eight membrane patches, integral of membrane current in control and hypercapnia 1.5 × 105 and 10.0 × 105 pA s, respectively, P = 0.00086, Fig. 8D). In membrane patches that exhibited a high degree of spontaneous channel gating at a
70 mmHg) no channel gating was seen in wild-type patches (10 patches, integral of membrane current in control and hypercapnia 0.61 × 105 and 0.43 × 105 pA s, respectively, not significantly different, Fig. 8A and D). However in patches drawn from the Cx26-expressing cells gating of a large conductance channel could be seen (Fig. 8B, eight membrane patches, integral of membrane current in control and hypercapnia 1.5 × 105 and 10.0 × 105 pA s, respectively, P = 0.00086, Fig. 8D). In membrane patches that exhibited a high degree of spontaneous channel gating at a 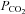 of 35 mmHg (presumably therefore containing a greater number of Cx26 hemichannels), a reduction of
of 35 mmHg (presumably therefore containing a greater number of Cx26 hemichannels), a reduction of 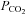 to 20 mmHg (at constant pH) resulted in a reversible decrease in channel openings (Fig. 8C, 7 membrane patches, integral of membrane current in control and hypercapnia 4.0 × 105 and 0.43 × 105 pA s respectively, P = 0.0012, Fig. 8D). We also observed CO2-dependent gating in patches in the inside-out configuration (3 patches, Fig. 8E). Given that we observed increased Cx26 openings in both inside-out and outside-out patches with an increase in
to 20 mmHg (at constant pH) resulted in a reversible decrease in channel openings (Fig. 8C, 7 membrane patches, integral of membrane current in control and hypercapnia 4.0 × 105 and 0.43 × 105 pA s respectively, P = 0.0012, Fig. 8D). We also observed CO2-dependent gating in patches in the inside-out configuration (3 patches, Fig. 8E). Given that we observed increased Cx26 openings in both inside-out and outside-out patches with an increase in 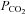 delivered to the bathing medium held at constant pH we can eliminate a local change in pH as the causative agent of gating. Instead our data strongly suggest a direct action of CO2 itself on the Cx26 hemichannel.
delivered to the bathing medium held at constant pH we can eliminate a local change in pH as the causative agent of gating. Instead our data strongly suggest a direct action of CO2 itself on the Cx26 hemichannel.
Figure 8. CO2-dependent gating of Cx26 in isolated patches.
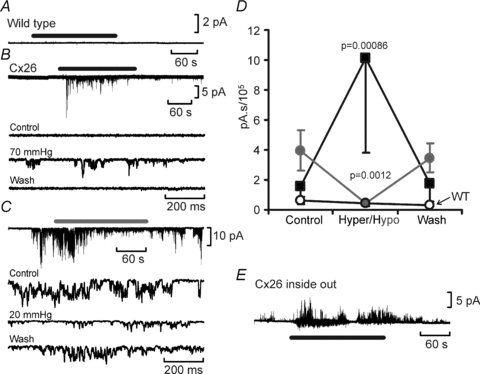
A, no changes in channel gating in response to an increase in 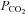 to 70 mmHg (bar) were seen in patches drawn from wild-type cells. B, patches drawn from Cx26-expressing cells demonstrated a reversible increase in channel openings in response to an increase in
to 70 mmHg (bar) were seen in patches drawn from wild-type cells. B, patches drawn from Cx26-expressing cells demonstrated a reversible increase in channel openings in response to an increase in 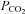 to 70 mmHg (bar). Expanded traces from before during and after the CO2 stimulus shown below. C, reversible reduction of channel gating in response to a decrease of
to 70 mmHg (bar). Expanded traces from before during and after the CO2 stimulus shown below. C, reversible reduction of channel gating in response to a decrease of 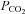 to 20 mmHg (grey bar). Expanded traces from before during and after the CO2 reduction shown below. D, summary graph showing the reversible change in the current integral recorded from the membrane patches drawn from Cx26-expressing cells in response to increases (black, hyper, n = 8) and decreases (grey, hypo, n = 7) of
to 20 mmHg (grey bar). Expanded traces from before during and after the CO2 reduction shown below. D, summary graph showing the reversible change in the current integral recorded from the membrane patches drawn from Cx26-expressing cells in response to increases (black, hyper, n = 8) and decreases (grey, hypo, n = 7) of 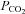 . The changes are highly significant (Friedman's two way ANOVA). The black trace shows the lack of any change in the current intregral for patches drawn from wild-type cells (increase of
. The changes are highly significant (Friedman's two way ANOVA). The black trace shows the lack of any change in the current intregral for patches drawn from wild-type cells (increase of 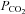 , n = 10). E, increase in channel gating of Cx26 in an inside out membrane patch in response to increasing
, n = 10). E, increase in channel gating of Cx26 in an inside out membrane patch in response to increasing 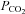 to 70 mmHg (bar). A small conductance outward current was present in this patch (downward deflections below baseline).
to 70 mmHg (bar). A small conductance outward current was present in this patch (downward deflections below baseline).
Discussion
Our results lead us to the surprising conclusion that Cx26 and closely related β connexins are directly sensitive to changes in 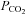 , which cause the hemichannels to open. We have demonstrated this in three ways – through observing CO2-dependent changes in the whole-cell conductance of cells heterologously expressing certain connexins, through CO2-dependent dye loading of these cells and via single channel recordings from isolated membrane patches drawn from Cx26-expressing cells. Furthermore we have shown that expression of Cx26 is sufficient by itself to reproduce the phenomenon of CO2-dependent ATP release. Nevertheless there are several aspects of our data that are at first sight apparently inconsistent with the current literature and thus deserve discussion.
, which cause the hemichannels to open. We have demonstrated this in three ways – through observing CO2-dependent changes in the whole-cell conductance of cells heterologously expressing certain connexins, through CO2-dependent dye loading of these cells and via single channel recordings from isolated membrane patches drawn from Cx26-expressing cells. Furthermore we have shown that expression of Cx26 is sufficient by itself to reproduce the phenomenon of CO2-dependent ATP release. Nevertheless there are several aspects of our data that are at first sight apparently inconsistent with the current literature and thus deserve discussion.
Connexin hemichannels and determinants of gating
Increases in 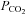 are often used to close rather than open hemichannels and gap junctions (e.g. Gonzalez et al. 2006). However in these cases very large amounts of CO2 are expressly given to cause intracellular acidification to around pH 6.0 and hence closure of the hemichannels and gap junctions. The stimuli that we have used are much more modest and designed to mimic physiological changes of
are often used to close rather than open hemichannels and gap junctions (e.g. Gonzalez et al. 2006). However in these cases very large amounts of CO2 are expressly given to cause intracellular acidification to around pH 6.0 and hence closure of the hemichannels and gap junctions. The stimuli that we have used are much more modest and designed to mimic physiological changes of 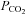 and pH and under these conditions hemichannel opening is readily evident. Interestingly in some reports (Young & Peracchia, 2004) a transient increase of gap junction conductance can be seen early on during the application of 100% CO2 presumably before
and pH and under these conditions hemichannel opening is readily evident. Interestingly in some reports (Young & Peracchia, 2004) a transient increase of gap junction conductance can be seen early on during the application of 100% CO2 presumably before 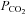 has equilibrated to its full level and intracellular acidification has occurred.
has equilibrated to its full level and intracellular acidification has occurred.
Native rat Cx26 unlike human Cx26 does not form hemichannels that are voltage sensitive (Gonzalez et al. 2006). However, this is unlikely to be due to the hemichannels not being physically formed – immunocytochemical staining shows puncta on the membrane surface. The rat and human versions of Cx26 share 90% amino acid identity, and introducing the point mutation N159D restores voltage sensitive gating to rat Cx26 hemichannels. Our observation of dye loading and gating of native rat hemichannels at negative resting potentials is on the surface inconsistent with these earlier findings. However, our experiments have examined a different facet of Cx26 gating which is likely to be mechanistically distinct from the voltage-dependent gating – opening mediated by CO2. Gonzalez et al. (like most in the field) examined hemichannel gating with Hepes buffered solutions that completely lacked HCO3− or CO2. Their conditions would therefore be unable to detect CO2-dependent gating of the hemichannels – our data show that lowering 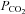 below 20 mmHg greatly reduces Cx26 gating (Fig. 8).
below 20 mmHg greatly reduces Cx26 gating (Fig. 8).
A further issue of difference with the earlier literature is that we saw only flickery channel openings (Figs 7 and 8) and never observed the classical sustained high conductance openings reported by others. While receiving less attention, some authors do report brief interludes of a flickery gating mode (Gonzalez et al. 2006) giving a precedent for our observations. A potential resolution of this issue is the presence of HCO3−/CO2 in our solutions, which is almost invariably absent from the solutions used for single channel recordings reported by others. The interaction with CO2 could conceivably bias the channels towards a flickery gating mode.
One difficulty with the concept of hemichannels performing a physiological signalling role is the idea that a large conductance channel could open for seconds at a time and potentially allow loss of many low molecular weight components from the cytosol, and equally ingress of similar sized molecules from the extracellular space. Under our more physiological recording conditions that utilize the native HCO3−/CO2 buffering system to buffer both intracellular and extracellular pH, we predominantly observed flickery modes of gating. This may mean that under physiological conditions hemichannel gating is far more transient and highly regulated and thus able to release suitable quantities of ATP for signalling purposes without radically depleting the intracellular contents. The issue remains, though, that extracellular components could enter the cells with potentially harmful consequences. We speculate that if hemichannels were localized in the plasma membrane of specific subcellular compartments then this potentially disruptive exchange of intra- and extracellular contents could be avoided or regulated.
Interestingly Cx26 is not unique in its sensitivity to changes in 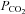 . The related β-connexins, Cx30 and Cx32, also exhibit sensitivity to CO2. Cx30 is highly homologous to Cx26 and arose from a relatively recent gene duplication (Cruciani & Mikalsen, 2006). Cx32 is also very close phylogenetically to Cx26. Both of these connexins can coassemble with Cx26 to form heteromeric and heterotypic hemichannels and gap junctions (Stauffer, 1995; Yum et al. 2007). Of the three connexins, Cx26 responded to smaller excursions (both up and down) of
. The related β-connexins, Cx30 and Cx32, also exhibit sensitivity to CO2. Cx30 is highly homologous to Cx26 and arose from a relatively recent gene duplication (Cruciani & Mikalsen, 2006). Cx32 is also very close phylogenetically to Cx26. Both of these connexins can coassemble with Cx26 to form heteromeric and heterotypic hemichannels and gap junctions (Stauffer, 1995; Yum et al. 2007). Of the three connexins, Cx26 responded to smaller excursions (both up and down) of 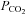 from its physiological level than Cx32. This suggests that heteromeric hemichannels (Cx26–Cx32 and possibly Cx26–Cx30) could differ in their sensitivity to changes in
from its physiological level than Cx32. This suggests that heteromeric hemichannels (Cx26–Cx32 and possibly Cx26–Cx30) could differ in their sensitivity to changes in 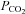 from homomeric Cx26 hemichannels. Cx26, -30 and -32 form a distinct branch of the β connexin family. Members of the other branch comprise Cx25, 30.3, 31 and 31.1. An interesting question is whether the entire β connexin family is CO2 sensitive. Cx25 is phylogenetically the oldest member of this latter branch – if it were to be CO2 sensitive, it would imply CO2 sensitivity for the entire β connexin family (or at least that any non-CO2 sensitive β connexins in this branch must have subsequently lost this property). Cx25 is thus an important connexin to study in determining the evolution of this property of the connexins.
from homomeric Cx26 hemichannels. Cx26, -30 and -32 form a distinct branch of the β connexin family. Members of the other branch comprise Cx25, 30.3, 31 and 31.1. An interesting question is whether the entire β connexin family is CO2 sensitive. Cx25 is phylogenetically the oldest member of this latter branch – if it were to be CO2 sensitive, it would imply CO2 sensitivity for the entire β connexin family (or at least that any non-CO2 sensitive β connexins in this branch must have subsequently lost this property). Cx25 is thus an important connexin to study in determining the evolution of this property of the connexins.
Physiological significance
Our results suggest a new way to think about hemichannel gating – that hemichannels can in effect act as receptors and play a role in physiological signalling. Interestingly another astrocytic connexin, Cx43, has been reported to respond directly to NO, probably via nitrosylation of cysteine residues (Retamal et al. 2006, 2009). By analogy we propose that CO2 interacts directly with Cx26 by a mechanism yet to be determined. One attractive possibility is the formation of a carbamate on one or more amino groups of lysine residues, or even the N-terminal amino group. The carbamate is formed though a labile covalent bond that would change the charge of these positively charged –NH3+ groups to negatively charged –NHCOO− groups. There is precedent for biological actions of carbamate formation – from the Bohr effect on haemoglobin (Kilmartin et al. 1973), to the activation of RuBisCO (Lundqvist & Schneider, 1991), the most abundant protein on the planet.
As Cx26 is widely distributed in the leptomeninges and subpial astrocytes of the brain (Mercier & Hatton, 2001; Solomon et al. 2001), it may give the capacity for the direct detection of changes in 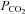 in many superficial brain areas. In the medulla oblongata it is well placed at the ventral surface and in astrocytes alongside penetrating blood vessels to detect early changes of arterial
in many superficial brain areas. In the medulla oblongata it is well placed at the ventral surface and in astrocytes alongside penetrating blood vessels to detect early changes of arterial 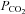 . Cerebrovascular blood flow is highly sensitive to arterial
. Cerebrovascular blood flow is highly sensitive to arterial 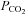 , with increases causing an increase in blood flow and decreases in
, with increases causing an increase in blood flow and decreases in 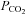 having the opposite effect (cerebrovascular CO2 reactivity, Ainslie & Duffin, 2009). The location of Cx26 next to penetrating blood vessels suggests that Cx26 could contribute to the CO2-dependent control of blood flow in the brain.
having the opposite effect (cerebrovascular CO2 reactivity, Ainslie & Duffin, 2009). The location of Cx26 next to penetrating blood vessels suggests that Cx26 could contribute to the CO2-dependent control of blood flow in the brain.
In our studies examining CO2-dependent ATP release at the ventral surface of the medulla, we recorded a large resting tone of ATP (Huckstepp et al. 2010). The properties of Cx26 help to explain the existence of this tone as Cx26 will open spontaneously at the resting levels of 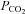 that we used (35 mmHg). This spontaneous gating at resting levels of
that we used (35 mmHg). This spontaneous gating at resting levels of 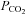 is potentially very significant as it gives the capacity to respond to both increases and decreases in
is potentially very significant as it gives the capacity to respond to both increases and decreases in 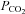 through Cx26-mediated chemosensory mechanisms.
through Cx26-mediated chemosensory mechanisms.
Winterstein (1949) and Loeschcke (1982) proposed that changes in 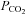 are detected as the consequent change in extracellular pH. There is ample evidence for a pH-sensitive component of respiratory chemosensitivity (Feldman et al. 2003; Nattie, 2001). Nevertheless, there are some observations that cannot be explained by this notion: respiratory responses induced by CO2 inhalation accompanied by acidification (‘respiratory acidosis’) are significantly greater than responses to identical changes in ventral medullary pH evoked without an increase in
are detected as the consequent change in extracellular pH. There is ample evidence for a pH-sensitive component of respiratory chemosensitivity (Feldman et al. 2003; Nattie, 2001). Nevertheless, there are some observations that cannot be explained by this notion: respiratory responses induced by CO2 inhalation accompanied by acidification (‘respiratory acidosis’) are significantly greater than responses to identical changes in ventral medullary pH evoked without an increase in 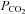 by infusion of acid into the blood (‘metabolic acidosis’; Eldridge et al. 1985; Shams, 1985; reviewed by Putnam et al. 2004). This has also been observed in vitro where responses of putative chemosensitive neurons in the medulla to respiratory acidosis are always greater compared to those induced by metabolic acidosis (Fukuda, 1983; Wang et al. 2002).
by infusion of acid into the blood (‘metabolic acidosis’; Eldridge et al. 1985; Shams, 1985; reviewed by Putnam et al. 2004). This has also been observed in vitro where responses of putative chemosensitive neurons in the medulla to respiratory acidosis are always greater compared to those induced by metabolic acidosis (Fukuda, 1983; Wang et al. 2002).
The possibility that CO2 can be detected both directly and via a change in extracellular pH would resolve this inconsistency in the literature. However in the absence of a molecular mechanism, the possibility of direct CO2 detection has received little attention. Our work showing that CO2 can be detected directly via Cx26, which opens to release ATP (which can then excite respiratory neurons), provides a potential mechanistic explanation as to why a stimulus that involves both an increase in 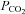 and a decrease in pH is more effective than changes in pH alone in inducing the cellular responses and adaptive changes of breathing.
and a decrease in pH is more effective than changes in pH alone in inducing the cellular responses and adaptive changes of breathing.
The CO2 sensitivity of Cx26 closely matches the CO2 sensitivity of ATP release in the medulla oblongata, giving further evidence that this connexin underlies CO2-dependent ATP release. While Cx32 is also present in the medulla, its sensitivity to changes in 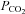 does not match that of the CO2-evoked ATP release, but is shifted towards significantly higher levels of
does not match that of the CO2-evoked ATP release, but is shifted towards significantly higher levels of 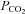 . Therefore, this channel is unlikely to play a major role in mediating the responses to physiological fluctuations in
. Therefore, this channel is unlikely to play a major role in mediating the responses to physiological fluctuations in 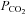 . Furthermore Cx32 is primarily expressed in ogliodendrocytes where its expression is inversely linked to glial fibrillary acidic protein (GFAP) expression (Nicholson et al. 2001). Since dye loading is associated with GFAP it is less likely that it occurs through this connexin.
. Furthermore Cx32 is primarily expressed in ogliodendrocytes where its expression is inversely linked to glial fibrillary acidic protein (GFAP) expression (Nicholson et al. 2001). Since dye loading is associated with GFAP it is less likely that it occurs through this connexin.
The vast majority of studies of CO2 chemosensitivity have concentrated on the medulla and its link to respiratory control. The widespread distribution of Cx26 throughout external surfaces of the brain suggests that many other areas of the brain (with little link to the control of breathing) may also be directly sensitive to CO2.
Acknowledgments
We thank the MRC and BBSRC for support. We thank Dr Klaus Willecke (University of Bonn) for the generous gift of the Cx-expressing and wild-type HeLa cells.
Glossary
Abbreviations
- CBF
carboxyfluorescein
- Cx
connexin
Author contributions
R.T.R.H. and N.D. performed the whole-cell experiments. R.T.R.H. performed all single channel recordings and dye loading experiments and analysed the data. R.E. performed the immunocytochemistry and RT-PCR and helped to culture the cells, R.T.R.H. and A.S. performed the ATP release experiments from HeLa and wild-type cells, and N.D. directed the work and wrote the manuscript. All authors read and commented on the manuscript. The work was performed at the University of Warwick.
References
- Ainslie PN, Duffin J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1473–1495. doi: 10.1152/ajpregu.91008.2008. [DOI] [PubMed] [Google Scholar]
- Corcoran AE, Hodges MR, Wu Y, Wang W, Wylie CJ, Deneris ES, Richerson GB. Medullary serotonin neurons and central CO2 chemoreception. Respir Physiol Neurobiol. 2009;168:49–58. doi: 10.1016/j.resp.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruciani V, Mikalsen SO. The vertebrate connexin family. Cell Mol Life Sci. 2006;63:1125–1140. doi: 10.1007/s00018-005-5571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N. Dynamic ATP signalling and neural development. J Physiol. 2008;586:2429–2436. doi: 10.1113/jphysiol.2008.152207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dando R, Roper SD. Cell-to-cell communication in intact taste buds through ATP signalling from pannexin 1 gap junction hemichannels. J Physiol. 2009;587:5899–5906. doi: 10.1113/jphysiol.2009.180083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge FL, Kiley JP, Millhorn DE. Respiratory responses to medullary hydrogen ion changes in cats: different effects of respiratory and metabolic acidoses. J Physiol. 1985;358:285–297. doi: 10.1113/jphysiol.1985.sp015551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda Y. Difference between actions of high PCO2 and low [HCO3−] on neurons in the rat medullary chemosensitive areas in vitro. Pflugers Arch. 1983;398:324–330. doi: 10.1007/BF00657242. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature. 2005;436:108–111. doi: 10.1038/nature03690. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Dale N, Korsak A, Llaudet E, Tian F, Huckstepp R, Spyer KM. Release of ATP and glutamate in the nucleus tractus solitarii mediate pulmonary stretch receptor (Breuer-Hering) reflex pathway. J Physiol. 2008;586:3963–3978. doi: 10.1113/jphysiol.2008.154567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann O, Kreir M, Ambrosi C, Pranskevich J, Oshima A, Roling C, Sosinsky G, Fertig N, Steinem C. The M34A mutant of Connexin26 reveals active conductance states in pore-suspending membranes. J Struct Biol. 2009;168:168–176. doi: 10.1016/j.jsb.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez D, Gomez-Hernandez JM, Barrio LC. Species specificity of mammalian connexin-26 to form open voltage-gated hemichannels. FASEB J. 2006;20:2329–2338. doi: 10.1096/fj.06-5828com. [DOI] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci U S A. 2007;104:6436–6441. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckstepp RTR, id Bihi R, Eason R, Spyer KM, Dicke N, Willecke K, Marina N, Gourine AV, Dale N. Connexin hemichannel-mediated CO2-dependent release of ATP in the medulla oblongata contributes to central respiratory chemosensitivity. J Physiol. 2010;588:3901–3920. doi: 10.1113/jphysiol.2010.192088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin JV, Fogg J, Luzzana M, Rossi-Bernardi L. Role of the α-amino groups of the α and β chains of human hemoglobin in oxygen-linked binding of carbon dioxide. J Biol Chem. 1973;248:7039–7043. [PubMed] [Google Scholar]
- Lundqvist T, Schneider G. Crystal structure of the ternary complex of ribulose-1,5-bisphosphate carboxylase, Mg(II), and activator CO2 at 2.3-Å resolution. Biochemistry. 1991;30:904–908. doi: 10.1021/bi00218a004. [DOI] [PubMed] [Google Scholar]
- Llaudet E, Hatz S, Droniou M, Dale N. Microelectrode biosensor for real-time measurement of ATP in biological tissue. Anal Chem. 2005;77:3267–3273. doi: 10.1021/ac048106q. [DOI] [PubMed] [Google Scholar]
- Loeschcke HH. Central chemosensitivity and the reaction theory. J Physiol. 1982;332:1–24. doi: 10.1113/jphysiol.1982.sp014397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier F, Hatton GI. Connexin 26 and basic fibroblast growth factor are expressed primarily in the subpial and subependymal layers in adult brain parenchyma: roles in stem cell proliferation and morphological plasticity? J Comp Neurol. 2001;431:88–104. doi: 10.1002/1096-9861(20010226)431:1<88::aid-cne1057>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Nattie EE. Central chemosensitivity, sleep, and wakefulness. Respir Physiol. 2001;129:257–268. doi: 10.1016/s0034-5687(01)00295-x. [DOI] [PubMed] [Google Scholar]
- Nicholson SM, Gomes D, de Nechaud B, Bruzzone R. Altered gene expression in Schwann cells of connexin32 knockout animals. J Neurosci Res. 2001;66:23–36. doi: 10.1002/jnr.1194. [DOI] [PubMed] [Google Scholar]
- Pearson RA, Dale N, Llaudet E, Mobbs P. ATP released via gap junction hemichannels from the pigment epithelium regulates neural retinal progenitor proliferation. Neuron. 2005;46:731–744. doi: 10.1016/j.neuron.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol. 2004;287:C1493–1526. doi: 10.1152/ajpcell.00282.2004. [DOI] [PubMed] [Google Scholar]
- Retamal MA, Yin S, Altenberg GA, Reuss L. Modulation of Cx46 hemichannels by nitric oxide. Am J Physiol Cell Physiol. 2009;296:C1356–1363. doi: 10.1152/ajpcell.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retamal MA, Cortes CJ, Reuss L, Bennett MV, Saez JC. S-nitrosylation and permeation through connexin 43 hemichannels in astrocytes: induction by oxidant stress and reversal by reducing agents. Proc Natl Acad Sci U S A. 2006;103:4475–4480. doi: 10.1073/pnas.0511118103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson CA, Wang W, Pieribone VA, Dohle CI, Richerson GB. Midbrain serotonergic neurons are central pH chemoreceptors. Nat Neurosci. 2003;6:1139–1140. doi: 10.1038/nn1130. [DOI] [PubMed] [Google Scholar]
- Shams H. Differential effects of CO2 and H+ as central stimuli of respiration in the cat. J Appl Physiol. 1985;58:357–364. doi: 10.1152/jappl.1985.58.2.357. [DOI] [PubMed] [Google Scholar]
- Stauffer KA. The gap junction proteins β1-connexin (connexin-32) and β2-connexin (connexin-26) can form heteromeric hemichannels. J Biol Chem. 1995;270:6768–6772. [PubMed] [Google Scholar]
- Solomon IC, Halat TJ, El-Maghrabi MR, O’Neal MH., 3rd Localization of connexin26 and connexin32 in putative CO2-chemosensitive brainstem regions in rat. Respir Physiol. 2001;129:101–121. doi: 10.1016/s0034-5687(01)00299-7. [DOI] [PubMed] [Google Scholar]
- Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- Wang W, Bradley SR, Richerson GB. Quantification of the response of rat medullary raphe neurones to independent changes in pHo and PCO2. J Physiol. 2002;540:951–970. doi: 10.1113/jphysiol.2001.013443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman TA, Riquelme PA, Ivic L, Flint AC, Kriegstein AR. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron. 2004;43:647–661. doi: 10.1016/j.neuron.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Winterstein H. The reaction theory of respiratory regulation. Experientia. 1949;5:221–226. doi: 10.1007/BF02166892. [DOI] [PubMed] [Google Scholar]
- Young KC, Peracchia C. Opposite Cx32 and Cx26 voltage-gating response to CO2 reflects opposite voltage-gating polarity. J Membr Biol. 2004;202:161–170. doi: 10.1007/s00232-004-0727-2. [DOI] [PubMed] [Google Scholar]
- Yum SW, Zhang J, Valiunas V, Kanaporis G, Brink PR, White TW, Scherer SS. Human connexin26 and connexin30 form functional heteromeric and heterotypic channels. Am J Physiol Cell Physiol. 2007;293:C1032–1048. doi: 10.1152/ajpcell.00011.2007. [DOI] [PubMed] [Google Scholar]


