Abstract
We tested the hypothesis that carotid artery stiffening with ageing is associated with transforming growth factor-β1 (TGF-β1)-related increases in adventitial collagen and reductions in medial elastin, which would be reversed by voluntary aerobic exercise. Ex vivo carotid artery incremental stiffness was greater in old (29–32 months, n = 11) vs. young (4–7 months, n = 8) cage control B6D2F1 mice (8.84 ± 1.80 vs. 4.54 ± 1.18 AU, P < 0.05), and was associated with selective increases in collagen I and III and TGF-β1 protein expression in the adventitia (P < 0.05), related to an increase in smooth muscle α-actin (SMαA) (myofibroblast phenotype) (P < 0.05). In cultured adventitial fibroblasts, TGF-β1 induced increases in superoxide and collagen I protein (P < 0.05), which were inhibited by Tempol, a superoxide dismutase. Medial elastin was reduced with ageing, accompanied by decreases in the pro-synthetic elastin enzyme, lysyl oxidase, and increases in the elastin-degrading enzyme, matrix metalloproteinase 2. Fibronectin was unchanged with ageing, but there was a small increase in calcification (P < 0.05). Increased incremental stiffness in old mice was completely reversed (3.98 ± 0.34 AU, n = 5) by 10–14 weeks of modest voluntary wheel running (1.13 ± 0.29 km day−1), whereas greater voluntary wheel running (10.62 ± 0.49 km day−1) had no effect on young mice. The amelioration of carotid artery stiffness by wheel running in old mice was associated with reductions in collagen I and III and TGF-β1, partial reversal of the myofibroblast phenotype (reduced SMαA) and reduced calcification (all P < 0.05 vs. old controls), whereas elastin and its modulating enzymes were unaffected. Adventitial TGF-β1-related oxidative stress may play a key role in collagen deposition and large elastic artery stiffening with ageing and the efficacious effects of voluntary aerobic exercise.
Cardiovascular diseases (CVDs) remain the leading cause of death in modern societies and much of this mortality is caused by dysfunction of arteries (Lloyd-Jones et al. 2010). Advancing age is the major risk factor for CVD and this is attributable in part to the development of large elastic artery stiffening, which can lead to numerous CV pathologies including systolic hypertension, stroke and heart failure (Lakatta & Levy, 2003). Thus, understanding the mechanisms by which large elastic arteries stiffen with age and interventions that reverse this stiffening are of major physiological and biomedical importance.
Increases in the deposition of the major load-bearing isoforms of collagen (I and III) and reductions in elastin are believed to be important mechanisms mediating large elastic artery stiffening with ageing (Zieman et al. 2005; Diez, 2007). Increases in the extracellular matrix glycoprotein fibronectin and calcification also may contribute to arterial stiffness with ageing (Boumaza et al. 2001; Atkinson, 2008). However, several aspects of these processes are poorly understood. For example, it is unknown if the changes in these collagens and elastin with ageing occur in the medial layer of arteries, the adventitial layer or both; nor do we understand the mechanisms by which such region-specific changes could be mediated.
Habitual aerobic exercise is a first-line therapeutic strategy for reducing the risk of CVD with ageing (Blair et al. 1989). Middle-aged and older adults who regularly perform aerobic exercise demonstrate less age-associated stiffening of large elastic arteries compared with their sedentary peers (Vaitkevicius et al. 1993; Tanaka et al. 1998; Tanaka et al. 2000; Seals et al. 2008, 2009). However, the mechanisms by which regular aerobic exercise exerts its favourable effects on large elastic artery stiffening with ageing have not been established, partly because of lack of access to these tissues in humans. The limited available data in experimental animals (forced swimming in rats) do not support an influence of voluntary exercise on whole artery collagen or elastin (Matsuda et al. 1993; Nosaka et al. 2003).
In the present study we hypothesized that stiffening of the carotid artery with ageing would be associated with increased deposition of collagen primarily in the adventitia because cultured fibroblasts synthesize more collagen than vascular smooth muscle cells (Patel et al. 2000), and that this would be related to increased expression of the profibrotic cytokine transforming growth factor-β1 (TGF-β1) and a shift to a myofibroblast (i.e. ‘secretory’ or collagen synthesizing) phenotype. We further hypothesized that because cultured vascular smooth muscle cells produce more elastin than fibroblasts (Ruckman et al. 1994), age-associated reductions in elastin would occur primarily in the medial layer of the carotid artery and be related to changes in the elastin-modulating enzymes lysyl oxidase and matrix metalloproteinase 2 (MMP-2). We also hypothesized that increases in fibronectin and/or calcification may be associated with arterial stiffening with ageing. Finally, we hypothesized that regular aerobic exercise would reverse some or all of the age-associated stiffening of large elastic arteries by reducing adventitial collagen, increasing medial elastin, or both. We postulated that exercise would produce these respective effects in old mice by inhibiting expression of TGF-β1 and reversing the shift to a myofibroblast phenotype, and by ameliorating the age-related changes in lysyl oxidase and MMP. We also assessed the possibility that exercise could normalize the increase in fibronectin and calcification of large elastic arteries observed in old mice.
To test these hypotheses, we performed ex vivo analyses of carotid arteries using a recently established B6D2F1 mouse model of arterial ageing (Lesniewski et al. 2009), combined with selective in vitro experiments on adventitial fibroblasts. As is the case in humans (Kawasaki et al. 1987; Tanaka et al. 1998), the B6D2F1 mouse demonstrates stiffening of the carotid artery with ageing in the absence of increases in peripheral artery stiffness (Lesniewski et al. 2009). Voluntary wheel running was used as a model of voluntary aerobic exercise in humans (Bradley et al. 2008; Durrant et al. 2009).
Methods
Animals
B6D2F1 mice were obtained from the National Institute on Ageing rodent colony. All mice were housed in an animal care facility at the University of Colorado at Boulder on a 12:12 light:dark cycle. Twenty young (4–7 months) and 20 old (29–32 months) male B6D2F1 mice were fed normal rodent chow ad libitum and housed in standard mouse cages or in cages with running wheels 10–14 weeks prior to killing. The 10–14 weeks of voluntary running was based on prior work from our laboratory demonstrating that exercise interventions of this length were sufficient to induce improvements in vascular function in both older mice (Durrant et al. 2009) and middle-aged and older adult humans (DeSouza et al. 2000; Tanaka et al. 2000). Running distance was measured as reported recently (Durrant et al. 2009). All procedures were approved by University of Colorado at Boulder Animal Care and Use Committee and conformed to the US National Institutes of Health guidelines set out in the Guide to the Care and Use of Laboratory Animals (NIH publication no. 85-23, revised 1996).
Carotid artery stiffness measurements
Mice were killed in random order by exsanguinations via cardiac puncture while under isoflurane anaesthesia. Carotid arteries were excised and incubated in a myograph chamber (DMT, Inc., Atlanta, GA, USA) containing Ca2+-free PSS for 1 h at 37°C. Intraluminal pressure was increased (5 and 20–200 mmHg, in 20 mmHg increments) and lumen diameter and medial wall thickness were measured. To account for potential individual or group differences in baseline diameter, passive-pressure relations were expressed relative to lumen diameter measured at 100 mmHg. Circumferential stress, stretch and incremental stiffness were calculated as previously described (Muller-Delp et al. 2002; Lesniewski et al. 2009). Wall thickness was measured at each pressure increment and used in the calculation of circumferential stress.
Immunohistochemistry
Carotid arteries were excised and frozen in optimal cutting temperature compound (Fisher Scientific, Pittsburgh, PA, USA) in liquid nitrogen cooled isopentane. Sections (7 μm) were fixed with acetone and washed in Tris buffer. All slides were stained with the Dako EnVision+ System-HRP-DAB kit according to the manufacturer's protocol (Dako North America Inc., Carpinteria, CA, USA). Briefly, primary antibodies for collagen type I (1:4000, Millipore), collagen type III (1:2000, Millipore), α-elastin (1:25, Abcam Inc., Cambridge, MA, USA), lysyl oxidase (1:1000, Imgenex Corp., San Diego, CA, USA), MMP-2 (1:500, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), TGF-β1 (1:1000, Santa Cruz), fibronectin (1:4000, Sigma) and smooth muscle α-actin (1:1000, Epitomics Inc., Burlingame, CA, USA) were incubated for 1 h at 4°C. Labelled polymer secondary was applied for 30 min and staining was visualized after a 2-min exposure to diaminobenzidine. Slides were counterstained with haematoxylin to visualize the nuclei. Slides were dehydrated and coverslipped.
Picro Sirius Red was used to stain for collagen fibres. Slides were stained with direct red (0.5 g, Sigma) in a saturated aqueous solution of picric acid (500 ml, Ricca Chemical Co., Stamford, CT, USA) for 30 min. Slides were washed twice in acidified water (5 ml acetic acid, glacial in 1 litre distilled water), dehydrated in three changes of 100% ethanol and coverslipped.
The Von Kossa stain (Polysciencs, Inc., Warrington, PA, USA) was used to stain calcification. Three per cent silver nitrate was applied to samples and exposed to UV light for 60 min. The slides were rinsed in three changes of distilled water, place in 5% sodium thiosulfate for 2 min and again rinsed in three changes of distilled water. Slides were counterstained with nuclear fast red for 5 min, dehydrated and coverslipped.
Quantification
Digital photomicrographs were obtained using a Nikon Eclipse TS100 photomicroscope, and quantification was performed with Image-Pro Plus software (Media Cybernetics, Bethesda, MD, USA). To determine density, images were converted to a grey scale in the green channel. The entire region of interest, either media or adventitia, was quantified. A pixel-by-pixel analysis was performed with a predetermined and optimized model (Image-Pro Plus software). The data are presented as relative density (sample density/positive control density stained on the same day). To determine the percentage of smooth muscle α-actin cells in the adventitia, positive stained cells (brown) were divided by total cells (blue nuclei) and multiplied by 100. Percentage staining was determined by taking the area stained divided by the total area for the same region of interest.
Primary adventitial fibroblast cell culture
Two male Sprague–Dawley rats (6 months of age) were obtained from the University of Colorado at Boulder breeding colony. Rats were killed with an overdose of carbon dioxide. Adventitial fibroblasts were isolated as previously described (Pagano, 1997). Briefly, thoracic aortas were excised and cleared of perivascular adipose tissue. The vessel was cut longitudinally, endothelial cells scraped off and the medial smooth muscle cells were peeled from the adventitia. The adventitia was placed in Dulbecco's modified Eagle's medium (DMEM)–F12 containing 1 mg ml−1 collagenase for 4 h at 37°C. The digested tissue was centrifuged for 5 min at 675 g and the re-suspended pellet was plated in a T-75 flask and cells allowed to grow for 4–5 days. Cells were passaged using 1:1 phosphate-buffered saline (PBS)–trypsin–EDTA, plated at a density of 30% and 90–95% confluent cells were studied between passages 3 and 6.
To determine the effects of TGF-β1 (R&D Systems, Minneapolis, MN, USA) on collagen production, cells were grown to 90–95% confluence and serum starved for 24 h prior to experimentation. Cells were treated with TGF-β1 (10 ng ml−1) and/or Tempol (100 μm) for 24 h and subsequently lysed in ice-cold RIPA buffer (0.5 m Tris-HCl, pH 7.4, 1.5 m NaCl, 2.5% deoxycholic acid, 10% NP-40, 10 mm EDTA, 1 mm NaF, 1 mm sodium orthovanadate, 1 mm phenylmethylsulfonylfluoride) containing protease (Protease Inhibitor Cocktail Tablet, Roche) and phosphatase (0.01% phoshatase inhibitor cocktail, Sigma) inhibitors. Protein concentrations were determined using a BCA colorimetric protein assay (Pierce (Thermo Scientific), Rockford, IL, USA). Five micrograms of protein containing 1 m DTT was loaded into a polyacrylamide gel, separated by electrophoresis and transferred onto a nitrocellulose membrane. The membrane was blocked in Tris-buffered saline (TBS)–0.05% Tween (TBST) containing 5% non-fat milk for 1 h. The membrane was washed for 30–60 min with frequent changes of TBST. Membranes were incubated overnight at 4°C on a rocker with anti-type I collagen (1:2000, Millipore, Billerica, MA, USA). The membrane was washed for 30–60 min in TBST and a secondary HRP-conjugated antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) was applied and incubated on a rocker at room temperature for 1 h. Blots were developed using ECL (Pierce/Thermo Scientific) and bands were visualized using a digital acquisition system (ChemiDoc-It, UVP Inc., Upland, CA, USA). The blot was stripped and re-probed for glyceraldehyde phosphate dehydrogenase (GAPDH) (1:2000, Cell Signaling Technology, Inc., Danvers, MA, USA). Bands were analysed with ImageJ software (NIH). Collagen type I bands were normalized to GAPDH protein expression and the control group.
Superoxide production was assessed in control and TGF-β1 (10 ng ml−1) treated cells via electron paramagnetic resonance spectroscopy using modified procedures that have been described previously (Rippe et al. 2010). Cells were grown to 90–95% confluence in a six-well plate and serum starved for 24 h before treatment. After the 24 h treatment period, the medium was removed and cells were washed once with oxygenated Krebs–Henseleit bicarbonate (KHB) buffer. Cells were lysed in 60 μl per well deoxygenated KHB buffer, the lysate was centrifuged at 500 g for 10 min and supernatant was removed. The pellet was resuspended in 90 μl of deoxygenated KHB buffer and 10 μl of 10 mm 1-hydroxy-3-carboxy-pyrrolidine (CPH) spin trap and incubated at 37°C for 1 h. Electron paramagnetic resonance (EPR) was then performed on each sample using a capillary tube.
Statistical analysis
Data are presented as means ± s.e.m. Statistical analysis was performed with SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). A two-way ANOVA was used to analyse stiffness and animal characterization data. All histology and Western blot data were analysed with a one-way ANOVA and EPR data were analysed with Student's t test. Least squared difference post hoc tests were used where appropriate. Significance was set at P < 0.05.
Results
Animal characteristics are shown in Table 1. Body mass was lower in the old compared with the young cage control mice, and in the wheel running compared with the cage control groups (both P < 0.05). Heart weight was greater in the old compared with the young groups (P < 0.05), and the heart to body weight ratio was greater in all groups compared with the young cage controls (P < 0.05). Gastrocnemius muscle weight (absolute and the ratio to body weight) was lower in the old compared with the young groups (P < 0.05), and increased (young, P < 0.05) or tended to increase (old, P = 0.06) with wheel running.
Table 1.
Animal characteristics
| YC | OC | YVR | OVR | |
|---|---|---|---|---|
| Body weight (g) | 39.2 ± 1.4 | 34.5 ± 0.7* | 31.1 ± 0.5* | 31.2 ± 1.3*† |
| Heart weight (mg) | 188 ± 5 | 232 ± 12** | 186 ± 4 | 205 ± 7** |
| Heart:body ratio (g/g × 100) | 0.50 ± 0.03 | 0.65 ± 0.03* | 0.60 ± 0.02* | 0.65 ± 0.02* |
| Gastrocnemius (mg) | 183 ± 9 | 138 ± 5** | 204 ± 13 | 153 ± 11** |
| Gastrocnemius:body ratio (g/g × 100) | 0.47 ± 0.03 | 0.41 ± 0.02** | 0.65 ± 0.04†‡ | 0.48 ± 0.03**†‡ |
| Carotid artery diameter (μm) | 395 ± 5 | 430 ± 6** | 398 ± 5 | 416 ± 6** |
| Average running distance (km) | 10.6 ± 0.49 | 1.13 ± 0.29* |
Values and means ± s.e.m.
P < 0.05 vs. YC
P < 0.05 vs. OC
P < 0.05 main effect of age
P < 0.05 main effect of voluntary running.
YC, young control; OC, old control; YVR, young voluntary running; OVR, old voluntary running.
Maximal carotid artery diameter was greater in the old compared with the young groups (P < 0.05). Both young and old mice ran voluntarily when placed in cages equipped with running wheels; however the daily running distance of the old mice was only ∼10% of that observed in the young mice (P < 0.05), as described previously (Durrant et al. 2009).
Carotid artery stiffness
Incremental stiffness (Fig. 1A) was greater (P < 0.05) in old compared with young cage control mice due to a combination of reduced passive distention to high intraluminal pressures (Fig. 1B) and increased circumferential stretch to low intraluminal pressures (Fig. 1C) (both P < 0.05). The resultant reductions in passive distention and increased circumferential stress in aged arteries demonstrate an inability of the vessels to adequately dilate at high pressures and relax at low pressures, respectively, both of which contribute to arterial stiffening. Wheel running had no effect on incremental stiffness in young mice, but completely reversed carotid artery stiffening with ageing (P < 0.05). The normalization of carotid artery stiffness by wheel running in old mice was mediated by the combination of greater distention and circumferential stretch compared with old cage control animals (both P < 0.05). Because wheel running affected carotid artery stiffness only in the old mice, histochemical analyses are presented below for young and old cage control and old wheel running groups, with one noted exception.
Figure 1. Carotid artery stiffness.
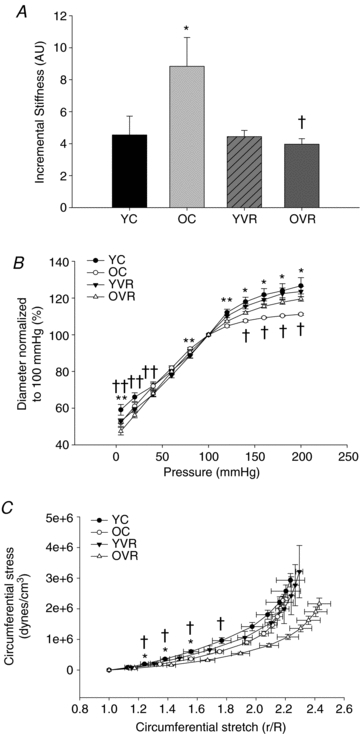
Carotid artery incremental stiffness (A), passive pressure–diameter relations (B) and circumferential stress and stretch relations (C) from young control (YC), old control (OC), young voluntary running (YVR) and old voluntary running (OVR) mice (n = 8–11/group); values are means ± s.e.m. *P < 0.05 vs. YC; †P < 0.05 vs. OC; **P < 0.05 main effect of age; ††P < 0.05 main effect of training.
Carotid artery collagen and related proteins
As assessed by Picro Sirius Red staining, total collagen did not differ with age in the whole artery, media or adventitia in the cage control mice, nor were there differences between the old cage control and wheel running groups (Fig. 2A). Total collagen in the whole artery was slightly (∼5%) lower (P < 0.05) in the old wheel running mice than in the young cage controls, but mean values in the media and adventitia were not significantly different among the three groups (Fig. 2A).
Figure 2. Carotid artery collagen protein expression.
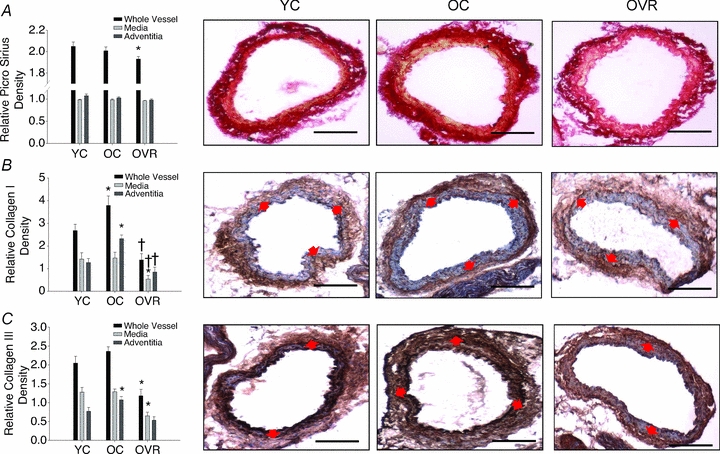
Carotid artery staining for total collagen (Picro Sirius Red) (A), collagen type I (B) and collagen type III (C) from young control (YC), old control (OC) and old voluntary running (OVR) mice (n = 5–7/group); values are means ± s.e.m. *P < 0.05 vs. YC, †P < 0.05 vs. OC. Representative images are presented and red arrows demarcate the medial–adventitial border; bar = 100 μm.
In contrast to total collagen, collagen I (Fig. 2B) was greater (P < 0.05) and collagen III (Fig. 2C) tended to be greater (P = 0.19) in the whole artery of old compared with young cage control mice, solely as a result of greater expression in the adventitia (both P < 0.05). Wheel running reversed the age-associated increases in collagen I and III in the adventitia, and also reduced the expression of these proteins in the media and whole artery (all P < 0.05). Because expression of collagen I and III in old wheel running mice was below that of young cage control mice, we also assessed these proteins in the carotid arteries of the young wheel running mice (Table 2). Although collagen I and III were lower in medial layer of young running vs. cage control mice (P < 0.05), there were no differences in the adventitia of the two groups, coinciding with the stiffness measurements.
Table 2.
Collagens I and III in carotid arteries
| Collagen I density (AU) | Collagen III density (AU) | |||||
|---|---|---|---|---|---|---|
| Whole vessel | Media | Adventitia | Whole vessel | Media | Adventitia | |
| YC | 2.45 ± 0.23 | 1.42 ± 0.29 | 1.27 ± 0.18 | 2.06 ± 0.18 | 1.28 ± 0.12 | 0.77 ± 0.10 |
| OC | 3.79 ± 0.41* | 1.48 ± 0.26 | 2.32 ± 0.17* | 2.36 ± 0.12 | 1.29 ± 0.07 | 1.07 ± 0.09* |
| YVR | 1.90 ± 0.42 | 0.98 ± 0.35†† | 0.91 ± 0.14 | 1.71 ± 0.32 | 0.97 ± 0.24†† | 0.74 ± 0.10 |
| OVR | 1.39 ± 0.29† | 0.54 ± 0.16†† | 0.84 ± 0.22† | 1.18 ± 0.17† | 0.65 ± 0.10†† | 0.53 ± 0.10† |
Values and means ± s.e.m.
P < 0.05 vs. YC
P < 0.05 vs. OC
P < 0.05 main effect of voluntary running.
YC, young control; OC, old control; YVR, young voluntary running; OVR, old voluntary running.
Given that the increases in collagen I and III with ageing were confined to the adventitia, we first determined if TGF-β1, a profibrotic cytokine implicated in stimulating collagen deposition, was altered in the adventitial layer of the carotid artery. Similar to collagen I and III, expression of TGF-β1 protein was greater (P < 0.05) in the adventitia of carotid arteries from old compared with young cage control mice, and this was reversed by wheel running (Fig. 3A). The greater collagen I and III and TGF-β1 in adventitia of old compared with young cage control animals was associated with a substantial increase in smooth muscle α-actin, a marker of adventitial fibroblast transformation to a secretory (i.e. myofibroblast or collagen-producing) phenotype (Fig. 3B). Wheel running reduced the age-associated increase in smooth muscle α-actin by ∼50% (P < 0.05 vs. old cage control).
Figure 3. Carotid artery transforming growth factor-β1 and smooth muscle α-actin protein expression.
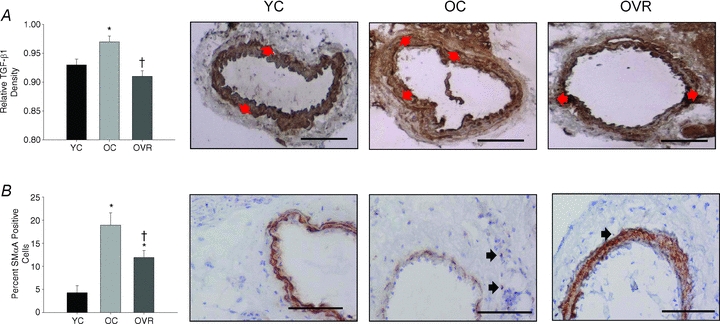
Adventitial transforming growth factor-β1 (TGF-β1) density (A) and the percentage smooth muscle α-actin (SMαA) positive cells (B) in the adventitia from young control (YC), old control (OC) and old voluntary running (OVR) mice (n = 4–11/group); values are means ± s.e.m. *P < 0.05 vs. YC, †P < 0.05 vs. OC. Representative images are presented and black arrows denote smooth muscle alpha actin positive cells; bar = 100 μm.
TGF-β1 stimulation of collagen I expression in vitro: role of oxidative stress
To obtain further evidence that the increases in TGF-β1 may have contributed to increased collagen deposition in carotid arteries with ageing (and normalization of collagen with wheel running) and that superoxide signalling may be involved, we performed additional experiments in cultured fibroblasts. TGF-β1 increased superoxide production (Fig. 4A) and collagen I protein expression (Fig. 4B) in fibroblasts (both P < 0.05). The TGF-β1 induced increase in collagen I was abolished by Tempol, a superoxide dismutase mimetic (Fig. 4B), suggesting that the increase was mediated by superoxide-associated oxidative stress.
Figure 4. Effects of transforming growth factor-β1 on superoxide and collagen I protein production.
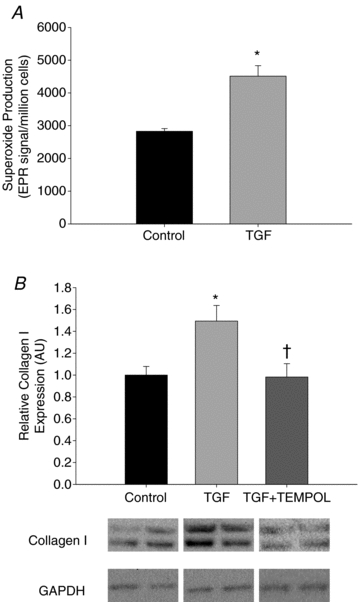
Superoxide production (A) and collagen I expression (B) in cultured adventitial fibroblasts treated with transforming growth factor-β1 (TGF-β1, 10 ng ml−1) or TGF-β1 + Tempol for 24 h (n = 4–8/group); values are means ± s.e.m. *P < 0.05 vs. Control, †P < 0.05 vs. TGF-β1 treatment.
Carotid artery elastin and related proteins
Elastin was lower (P < 0.05) in the whole carotid artery in the old compared with young cage control mice, and this was solely the result of lower expression in the media (P < 0.05), as adventitial elastin was not different in the two groups (Fig. 5A). The lower elastin in the whole carotid artery and media in the old cage control mice was associated with lower expression of the pro-synthetic elastin enzyme lysyl oxidase (Fig. 5B), and greater expression of the elastin degrading enzyme MMP-2 (Fig. 5C). In general, wheel running did not affect the age-associated changes in elastin, lysyl oxidase and MMP-2 (Fig. 5A–C).
Figure 5. Carotid artery elastin, lysyl oxidase and matrix metalloproteinase 2 protein expression.
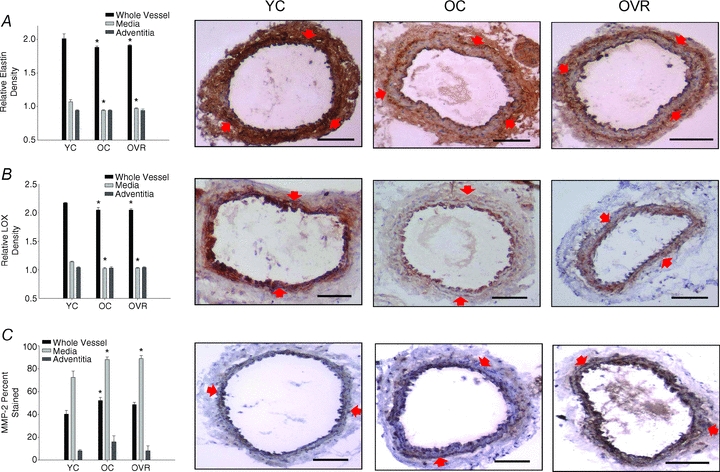
Elastin (A), lysyl oxidase (LOX) (B) and matrix metalloproteinase 2 (MMP-2) (C) densities from young control (YC), old control (OC) and old voluntary running (OVR) mice (n = 4–8/group; values are means ± s.e.m. *P < 0.05 vs. YC. Representative images are presented and red arrows demarcate the medial–adventitial border; bar = 100 μm.
Carotid artery fibronectin and calcification
Fibronectin did not differ with age in the cage control mice and was unaffected by wheel running (Table 3). There was only modest staining for calcification in the carotid arteries in all groups. However, calcification was greater in the old compared with the young cage control animals, and this effect of ageing was abolished in the old exercising mice (Table 3, all P < 0.05).
Table 3.
Fibronectin and calcification in carotid arteries
| Fibronectin density (AU) | Calcification (% stained) | |||||
|---|---|---|---|---|---|---|
| Whole vessel | Media | Adventitia | Whole vessel | Media | Adventitia | |
| YC | 3.78 ± 0.30 | 2.50 ± 0.07 | 1.29 ± 0.05 | 0.22 ± 0.06 | 0.70 ± 0.36 | 0.07 ± 0.05 |
| OC | 3.86 ± 0.33 | 2.48 ± 0.08 | 1.38 ± 0.05 | 2.39 ± 0.87* | 3.54 ± 1.70 | 1.24 ± 0.45* |
| OVR | 3.57 ± 0.30 | 2.35 ± 0.11 | 1.22 ± 0.05 | 0.64 ± 0.30† | 1.24 ± 0.55 | 0.22 ± 0.06† |
Values and means ± s.e.m.
P < 0.05 vs. YC
P < 0.05 vs. OC.
YC, young control; OC, old control; OVR, old voluntary running.
Discussion
The present study is the first to assess the possible cellular and molecular mechanisms by which voluntary aerobic exercise reduces large elastic artery stiffening with ageing. To our knowledge, these also are the first data on mechanisms of age-associated arterial stiffening using a mouse model.
We found that the increase in carotid artery stiffness with ageing in B6D2F1 mice was associated with increases in collagen I and III in the adventitia, which were, in turn, related to increases in adventitial TGF-β1 and evidence for a shift to a myofibroblast (secretory) phenotype. In vitro experiments in fibroblasts confirmed the collagen-stimulating effects of TGF-β1 in these cells by a mechanism dependent on superoxide. Carotid stiffening with age also was associated with reductions in elastin in the media, which were related to decreases in lysyl oxidase and increases in MMP-2. Fibronectin was not altered with ageing, but a small degree of calcification was observed. Importantly, voluntary aerobic exercise ameliorated carotid artery stiffening in old mice. This was associated with reductions in collagen I and III, decreased TGF-β1 and a partial reversal of the myofibroblast phenotype. Voluntary exercise also reduced calcification in old mice, but did not affect elastin or its modulating enzymes.
Carotid artery stiffness
In the present study, ex vivo carotid artery stiffness was increased with ageing in cage control B6D2F1 mice as reported previously (Cox, 1983; Simon & Danneman, 2005; Lesniewski et al. 2009), and observed in vivo in older healthy adult humans (Tanaka et al. 2000; Moreau et al. 2003). The present investigation extends earlier findings on ageing by demonstrating that 10–14 weeks of voluntary wheel running completely reverses carotid artery stiffening in old mice, restoring levels to those observed in young adult animals. Remarkably, this occurred despite the fact that old mice ran only ∼10% as much as the young mice. Thus, even small amounts of voluntary wheel running exert a potent physiological stimulus on carotid artery stiffness. Wheel running had no effect on the already normal levels of stiffness in young mice, as noted previously in humans (Tanaka et al. 2000). The present results are in partial agreement with earlier observations in rats in which forced swimming reduced the incremental elastic modulus in aorta by ∼40% in old animals (Nosaka et al. 2003). It is not clear if the different degrees of adaptation in the two studies are due to differences in species, type or voluntariness of the exercise (Niederhoffer et al. 2000), or other factors.
Collagen, myofibroblast phenotype and TGF-β1
Collagen I and III are the primary load-bearing proteins that comprise ∼90% of all collagens in the arterial wall (Diez, 2007). Increases in collagen expression and cross-linking are believed to contribute importantly to increases in large-elastic artery stiffness with ageing (Lakatta & Levy, 2003). Although results of previous studies vary (Cox, 1983; Maurel et al. 1987; Brüel & Oxlund, 1996; Wang & Lakatta, 2002; Qiu et al. 2007a,b; Wang et al. 2007), increased expression of both collagen I and collagen III is found in aorta of older compared with young adult human donors in the absence of clinical CVD (Wang et al. 2007). In agreement with this, in the present study we found increased abundance of collagens I and III in whole carotid arteries of old compared with young cage control mice as a result of greater expression in the adventitia, whereas total and medial collagen were not increased. Carotid artery stiffness also was selectively associated with adventitial collagen in the young cage control and wheel running groups. These observations are consistent with results from a recent ex vivo analysis of human carotid arteries suggesting that the adventitia is the key load-bearing component of the wall under physiological conditions (Sommer et al. 2010).
The mechanisms by which ageing causes greater accumulation of these collagens in the adventitia of carotid arteries is unknown. We hypothesized that increases in TGF-β1, a profibrotic cytokine implicated in both general and vascular-specific fibrosis (Blobe et al. 2000; Ruiz-Ortega et al. 2007), could be involved, perhaps in part by stimulating a change in adventitial fibroblasts to a myofibroblast (collagen secreting) phenotype. In agreement with this, we found that carotid adventitia of old mice demonstrate greater expression of TGF-β1 and smooth muscle α-actin, a myofibroblast marker (Siow & Churchman, 2007). Additional experiments in cultured fibroblasts demonstrated that TGF-β1 stimulates superoxide production and collagen I deposition. The latter was dependent on superoxide, as it was blocked by administration of Tempol, a superoxide dismutase mimetic. It also is possible that increases in nitric oxide bioavailability with voluntary running in old mice (Durrant et al. 2009) was involved in the exercise-related inhibition of adventitial TGF-β1 expression observed, as demonstrated in other models (Koyanagi et al. 2000). Taken together, our results provide new evidence that TGF-β1 may stimulate collagen deposition in the adventitia, which, in turn, contributes to the stiffening of carotid arteries with ageing, perhaps in part by an oxidative stress-related transformation of adventitial fibroblasts into collagen synthesizing/secreting myofibroblasts.
A previous study reported no effects of forced swimming on total collagen in aorta of old rats, despite improvements in stiffness (Nosaka et al. 2003). In the present study, we observed that wheel running had only a small influence on total collagen in the carotid artery. However, we found that normalization of carotid artery stiffness by wheel running in old mice was associated with reductions in collagen I and III to, or even below, levels observed in young control mice. Unlike ageing, the effects of exercise were observed in both the adventitia and media. Wheel running also completely reversed the increase in carotid adventitial TGF-β1 observed with ageing and reduced smooth muscle α-actin. Collectively, these findings support the possibility that voluntary aerobic exercise may ‘de-stiffen’ the carotid artery of old B6D2F1 mice by reversing the age-associated increases in collagen I and III. The latter may be mediated in part by reversing the age-related transformation of fibroblasts into myofibroblasts in the adventitia, a layer implicated in arterial stiffening with ageing (Schulze-Bauer et al. 2002; Sommer et al. 2010). However, the fact that wheel running reduced collagen I and III even in the media of carotid arteries suggests that other, yet to be identified, mechanisms may have been acting on vascular smooth muscle cells.
Elastin and related enzymes
Consistent with data from several previous investigations (Cox, 1983; Sauvage et al. 1998; Wang et al. 2002; Qiu et al. 2007a,b;), we found that elastin was lower, albeit only modestly, in whole carotid arteries of old compared with young cage control mice. In agreement with an earlier report in aorta of rats (Wang & Lakatta, 2002), this was the result of a small reduction in expression of elastin in the media. The lower whole artery and medial elastin in old cage control mice was associated with reduced expression of lysyl oxidase, an enzyme involved in elastin maturation (Bedell-Hogan et al. 1993; Kothapalli & Ramamurthi, 2009), and increased expression of MMP-2, an enzyme involved in elastin fibre degradation and remodelling (Emonard & Hornebeck, 1997; Sternlicht & Werb, 2001). Although MMP-2 has been shown to increase with age in the whole carotid (Wang et al. 2005), to our knowledge, this is the first report of reduced lysyl oxidase expression with large elastic artery ageing and the first evidence that the changes in expression of both enzymes in the whole artery are primarily the result of changes in the media.
Importantly, here we show that neither elastin nor these two elastin-modulating enzymes appear to be key mechanisms involved in the de-stiffening effects of wheel running in carotid arteries of B6D2F1 mice, as they were not different in old exercising compared with cage control animals. The observation of unaltered whole artery elastin in old mice with wheel running in the present study is in agreement with previous observations in aorta of old rats in response to swimming (Nosaka et al. 2003). Collectively, our results and those from the previous study (Nosaka et al. 2003) suggest that regular aerobic exercise does not reduce large elastic artery stiffness via changes in elastin.
Fibronectin and calcification
Expression of fibronectin, a glycoprotein that influences stiffness by binding to extracellular matrix proteins including integrins, collagen and proteoglycans (Hynes & Yamada, 1982), has been reported to increase in whole aorta with ageing in rats (Li et al. 1999; Wang et al. 2006). However, in the present study we found no influence of either age or wheel running on fibronectin in the whole carotid artery or in the media or adventitia. As such, age-related increases in carotid artery stiffness are not obviously associated with increased fibronectin, nor does it appear that wheel running de-stiffens the carotid arteries of old mice by reducing this protein.
Calcification of aorta in rats is reported to either increase (Kieffer et al. 2000) or not change (Brüel & Oxlund, 1996) with ageing in the absence of disease. Here we found modest, but greater calcification of the carotid artery with ageing in B6D2F1 mice. We also found that wheel running reversed this calcification in old mice to levels not significantly different from that observed in young mice. It is uncertain if such small changes in calcification could contribute to increases in carotid artery stiffness with ageing or play a role in the stiffness-reducing influence of wheel running. Our findings are in contrast to a previous report in rats that observed no consistent associations between age or forced swimming and aortic calcification (Nosaka et al. 2003), but in agreement with an earlier study in young rats (Matsuda et al. 1993).
Calcification is mediated by several cellular events that include oxidant stress, inflammation and elastolysis. In our aged B6D2F1 mice, elastin content was reduced in the medial layer of the carotid artery. Such elastolysis can produce elastin-derived peptides that, in turn, promote an osteogenic phenotype in smooth muscle cells (Simionescu et al. 2005). However, elastin content was not altered with voluntary running. On the other hand, adventitial TGF-β1 was increased with ageing and reduced with voluntary wheel running. TGF-β1 can induce an osteogenic phenotype in fibroblasts (Simionescu et al. 2007), suggesting this molecule could have contributed to altered calcification in the adventitia. Changes in other proteins also could be involved. For example, bone morphogenetic proteins 2 and 7 are members of the TGF-β superfamily that are implicated in promoting (Hruska et al. 2005) and reversing (Kang et al. 2010) calcification, respectively, and are additional potential mechanisms of action for modulation of vascular calcification with ageing and voluntary wheel running in the present study.
Limitations
The present study has several limitations that should be noted. Cross-linking of extracellular proteins contributes to large elastic artery stiffening with ageing (Semba et al. 2009), and reductions in cross-linking reduce stiffness (Kass et al. 2001). However, protein cross-linking was not assessed in the present study. Moreover, only male mice were studied. Although in humans, large elastic arteries stiffen with ageing and this is reduced with regular aerobic exercise in both men and women (Tanaka et al. 1998; Tanaka et al. 2000; Moreau et al. 2003), it is possible that the effects of ageing and exercise differ in female mice. Finally, our results regarding the mechanisms by which ageing and regular exercise modulate carotid artery stiffness in mice are association-based. Direct cause and effect evidence linking these events is needed to more definitively establish the mechanisms involved. In this context, the ageing and exercise training of conditional knockout mice may be required to demonstrate the role of TGF-β1 in arterial stiffening.
Conclusions
The results of this study provide evidence that increases in carotid artery stiffness with ageing are associated with TGF-β1-stimulated increases in adventitial collagen I and III related to changes in fibroblasts to the more secretory myofibroblast phenotype. Our findings also indicate that reductions in elastin in the medial layer of the carotid may contribute to stiffening, in part as a result of alterations in the elastin-modulating proteins lysyl oxidase and MMP-2. A small degree of calcification also occurs with ageing and could contribute to stiffening.
Importantly, even modest levels of regular voluntary aerobic exercise, a first-line therapy for preventing CVD in humans, completely reverse carotid artery stiffening with ageing in B6D2F1 mice. The de-stiffening effects of aerobic exercise on carotid arteries of old mice are associated with reductions in collagen I and III in both the adventitia and the media, as well as adventitial TGF-β1, and a partial reversal of the age-related shift to a myofibroblast phenotype. Voluntary aerobic exercise also may contribute to reductions in stiffness by reversing age-associated calcification of arteries.
Acknowledgments
This work was supported by NIH AG013038, AG033196, AG000279, HL007822.
Glossary
Abbreviations
- CVD
cardiovascular disease
- MMP-2
matrix metalloproteinase 2
- SMαA
smooth muscle α-actin
- TGF-β1
transforming growth factor-β1
Author contributions
B.S.F., D.R.S. and L.A.L. contributed to the conception and design of the studies. All authors contributed to analysis and interpretation of data and the drafting and revision of the article, and provided final approval of the version to be published. All experiments were carried out at the University of Colorado at Boulder.
References
- Atkinson J. Age-related elastocalcinosis in arteries: mechanisms, animal models, and physiological consequences. J Appl Physiol. 2008;105:1643–1651. doi: 10.1152/japplphysiol.90476.2008. [DOI] [PubMed] [Google Scholar]
- Bedell-Hogan D, Trackman P, Abrams W, Rosenbloom J, Kagan H. Oxidation, cross-linking, and insolubilization of recombinant tropoelastin by purified lysyl oxidase. J Biol Chem. 1993;268:10345–10350. [PubMed] [Google Scholar]
- Blair S, Kohl H, 3rd, Paffenbarger RS, Jr, Clark D, Cooper K, Gibbons L. Physical fitness and all-cause mortality. A prospective study of healthy men and women. J Am Med Assoc. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor β in human disease. N Engl J Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- Boumaza S, Arribas SM, Osborne-Pellegrin M, McGrath JC, Laurent S, Lacolley P, Challande P. Fenestrations of the carotid internal elastic lamina and structural adaptation in stroke-prone spontaneously hypertensive rats. Hypertension. 2001;37:1101–1107. doi: 10.1161/01.hyp.37.4.1101. [DOI] [PubMed] [Google Scholar]
- Bradley RL, Jeon JY, Liu F-F, Maratos-Flier E. Voluntary exercise improves insulin sensitivity and adipose tissue inflammation in diet-induced obese mice. Am J Physiol Endocrinol Metab. 2008;295:E586–594. doi: 10.1152/ajpendo.00309.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüel A, Oxlund H. Changes in biomechanical properties, composition of collagen and elastin, and advanced glycation endproducts of the rat aorta in relation to age. Atherosclerosis. 1996;127:155–165. doi: 10.1016/s0021-9150(96)05947-3. [DOI] [PubMed] [Google Scholar]
- Cox RH. Age-related changes in arterial wall mechanics and composition of NIA Fischer rats. Mech Ageing Dev. 1983;23:21–36. doi: 10.1016/0047-6374(83)90096-9. [DOI] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- Diez J. Arterial stiffness and extracellular matrix. Adv Cardiol. 2007;44:76–95. doi: 10.1159/000096722. [DOI] [PubMed] [Google Scholar]
- Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol. 2009;587:3271–3285. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emonard H, Hornebeck W. Binding of 92 kDa and 72 kDa progelatineases to insoluble elastin modulates their proteolytic activation. Biol Chem. 1997;378:265–271. doi: 10.1515/bchm.1997.378.3-4.265. [DOI] [PubMed] [Google Scholar]
- Hruska KA, Mathew S, Saab G. Bone morphogenetic proteins in vascular calcification. Circ Res. 2005;97:105–114. doi: 10.1161/01.RES.00000175571.53833.6c. [DOI] [PubMed] [Google Scholar]
- Hynes RO, Yamada KM. Fibronectins: multifunctional modular glycoproteins. J Cell Biol. 1982;95:369–377. doi: 10.1083/jcb.95.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YH, Jin JS, Yi DW, Son SM. Bone morphogenetic protein-7 inhibits vascular calcification induced by high vitamin D in mice. Tohoku J Exp Med. 2010;221:299–307. doi: 10.1620/tjem.221.299. [DOI] [PubMed] [Google Scholar]
- Kass DA, Shapiro EP, Kawaguchi M, Capriotti AR, Scuteri A, deGroof RC, Lakatta EG. Improved arterial compliance by a novel advanced glycation end-product crosslink breaker. Circulation. 2001;104:1464–1470. doi: 10.1161/hc3801.097806. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Sasayama S, Yagi S-I, Asakawa T, Hirai T. Non-invasive assessment of the age related changes in stiffness of major branches of the human arteries. Cardiovasc Res. 1987;21:678–687. doi: 10.1093/cvr/21.9.678. [DOI] [PubMed] [Google Scholar]
- Kieffer P, Robert A, Capdeville-Atkinson C, Atkinson J, Lartaud-Idjouadiene I. Age-related arterial calcification in rats. Life Sci. 2000;66:2371–2381. doi: 10.1016/s0024-3205(00)00567-1. [DOI] [PubMed] [Google Scholar]
- Kothapalli CR, Ramamurthi A. Lysyl oxidase enhances elastin synthesis and matrix formation by vascular smooth muscle cells. J Tissue Eng Regenerative Med. 2009;3:655–661. doi: 10.1002/term.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi M, Egashira K, Kubo-Inoue M, Usui M, Kitamoto S, Tomita H, Shimokawa H, Takeshita A. Role of transforming growth factor β1 in cardiovascular inflammatory changes induced by chronic inhibition of nitric oxide synthesis. Hypertension. 2000;35:86–90. doi: 10.1161/01.hyp.35.1.86. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- Lesniewski LA, Connell ML, Durrant JR, Folian BJ, Anderson MC, Donato AJ, Seals DR. B6D2F1 mice are a suitable model of oxidative stress-mediated impaired endothelium-dependent dilation with aging. J Gerontol A Biol Sci Med Sci. 2009;64A:9–20. doi: 10.1093/gerona/gln049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Froehlich J, Galis ZS, Lakatta EG. Increased expression of matrix metalloproteinase-2 in the thickened intima of aged rats. Hypertension. 1999;33:116–123. doi: 10.1161/01.hyp.33.1.116. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J, on behalf of the American Heart Association Statistics Committee and Stroke Statistics S Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Nosaka T, Sato M, Ohshima N. Effects of physical exercise on the elasticity and elastic components of the rat aorta. Eur J Appl Physiol Occup Physiol. 1993;66:122–126. doi: 10.1007/BF01427052. [DOI] [PubMed] [Google Scholar]
- Maurel E, Shuttleworth CA, Bouissou H. Interstitial collagens and ageing in human aorta. Virchows Archiv. 1987;410:383–390. doi: 10.1007/BF00712757. [DOI] [PubMed] [Google Scholar]
- Moreau KL, Donato AJ, Seals DR, DeSouza CA, Tanaka H. Regular exercise, hormone replacement therapy and the age-related decline in carotid arterial compliance in healthy women. Cardiovasc Res. 2003;57:861–868. doi: 10.1016/s0008-6363(02)00777-0. [DOI] [PubMed] [Google Scholar]
- Muller-Delp J, Spier SA, Ramsey MW, Lesniewski LA, Papadopoulos A, Humphrey JD, Delp MD. Effects of aging on vasoconstrictor and mechanical properties of rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol. 2002;282:H1843–1854. doi: 10.1152/ajpheart.00666.2001. [DOI] [PubMed] [Google Scholar]
- Niederhoffer N, Kieffer P, Desplanches D, Lartaud-Idjouadiene I, Sornay M-H, Atkinson J. Physical exercise, aortic blood pressure, and aortic wall elasticity and composition in rats. Hypertension. 2000;35:919–924. doi: 10.1161/01.hyp.35.4.919. [DOI] [PubMed] [Google Scholar]
- Nosaka H, Tanaka H, Watanabe I, Sato M, Matsuda M. Influence of regular exercise on age-related changes in arterial elasticity: mechanistic insights from wall compositions in rat aorta. Can J Appl Physiol. 2003;28:204–212. doi: 10.1139/h03-016. [DOI] [PubMed] [Google Scholar]
- Pagano P, Clark JK, Cifuentes-Pagano ME, Clark SM, Callis GM, Quinn MT. Localization of a constitutively active, phagocyte-like NADPH oxidase in rabbit aortic adventitia: Enhancement by angiotensin II. Proc Natl Acad Sci U S A. 1997;94:14483–14488. doi: 10.1073/pnas.94.26.14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Shi Y, Niculescu R, Chung EH, Martin JL, Zalewski A. Characteristics of coronary smooth muscle cells and adventitial fibroblasts. Circulation. 2000;101:524–532. doi: 10.1161/01.cir.101.5.524. [DOI] [PubMed] [Google Scholar]
- Qiu H, Depre C, Ghosh K, Resuello RG, Natividad FF, Rossi F, Peppas A, Shen Y-T, Vatner DE, Vatner SF. Mechanism of gender-specific differences in aortic stiffness with aging in nonhuman primates. Circulation. 2007a;116:669–676. doi: 10.1161/CIRCULATIONAHA.107.689208. [DOI] [PubMed] [Google Scholar]
- Qiu H, Tian B, Resuello RG, Natividad FF, Peppas A, Shen Y-T, Vatner DE, Vatner SF, Depre C. Sex-specific regulation of gene expression in the aging monkey aorta. Physiol Genomics. 2007b;29:169–180. doi: 10.1152/physiolgenomics.00229.2006. [DOI] [PubMed] [Google Scholar]
- Rippe C, Lesniewski L, Connell M, LaRocca T, Donato A, Seals D. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell. 2010;9:304–312. doi: 10.1111/j.1474-9726.2010.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckman JL, Luvalle PA, Hill KE, Giro MG, Davidson JM. Phenotypic stability and variation in cells of the porcine aorta: collagen and elastin production. Matrix Biol. 1994;14:135–145. doi: 10.1016/0945-053x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ortega M, Rodriguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J. TGF-β signaling in vascular fibrosis. Cardiovasc Res. 2007;74:196–206. doi: 10.1016/j.cardiores.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Sauvage M, Hinglais N, Mandet C, Badier C, Deslandes F, Michel JB, Jacob MP. Localization of elastin mRNA and TGF-β1 in rat aorta and caudal artery as a function of age. Cell Tissue Res. 1998;291:305–314. doi: 10.1007/s004410051000. [DOI] [PubMed] [Google Scholar]
- Schulze-Bauer CAJ, Regitnig P, Holzapfel GA. Mechanics of the human femoral adventitia including the high-pressure response. Am J Physiol Heart Circ Physiol. 2002;282:H2427–2440. doi: 10.1152/ajpheart.00397.2001. [DOI] [PubMed] [Google Scholar]
- Seals DR, DeSouza CA, Donato AJ, Tanaka H. Habitual exercise and arterial aging. J Appl Physiol. 2008;105:1323–1332. doi: 10.1152/japplphysiol.90553.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Walker AE, Pierce GL, Lesniewski LA. Habitual exercise and vascular ageing. J Physiol. 2009;587:5541–5549. doi: 10.1113/jphysiol.2009.178822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba RD, Najjar SS, Sun K, Lakatta EG, Ferrucci L. Serum carboxymethyl-lysine, an advanced glycation end product, is associated with increased aortic pulse wave velocity in adults. Am J Hypertens. 2009;22:74–79. doi: 10.1038/ajh.2008.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu A, Philips K, Vyavahare N. Elastin-derived peptides and TGF β1 induce osteogenic responses in smooth muscle cells. Biochem Biophys Res Commun. 2005;334:524–532. doi: 10.1016/j.bbrc.2005.06.119. [DOI] [PubMed] [Google Scholar]
- Simionescu A, Simionescu D, Vyavahare N. Osteogenic responses in fibroblasts activated by elastin degradation products and transforming growth factor β1. Am J Pathol. 2007;171:116–123. doi: 10.2353/ajpath.2007.060930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon G, Danneman KJ. Dilation and reduced distensibility of rat carotid artery with aging. Clin Exp Hypertens. 2005;6:459–466. doi: 10.1081/CEH-200067652. [DOI] [PubMed] [Google Scholar]
- Siow RCM, Churchman AT. Adventitial growth factor signalling and vascular remodelling: Potential of perivascular gene transfer from the outside-in. Cardiovasc Res. 2007;75:659–668. doi: 10.1016/j.cardiores.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Sommer G, Regitnig P, Koltringer L, Holzapfel GA. Biaxial mechanical properties of intact and layer-dissected human carotid arteries at physiological and supraphysiological loadings. Am J Physiol Heart Circ Physiol. 2010;298:H898–912. doi: 10.1152/ajpheart.00378.2009. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, DeSouza CA, Seals DR. Absence of age-related increase in central arterial stiffness in physically active women. Arterioscler Thromb Vasc Biol. 1998;18:127–132. doi: 10.1161/01.atv.18.1.127. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102:1270–1275. doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]
- Vaitkevicius PV, Fleg JL, Engel JH, O'Connor FC, Wright JG, Lakatta LE, Yin FC, Lakatta EG. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88:1456–1462. doi: 10.1161/01.cir.88.4.1456. [DOI] [PubMed] [Google Scholar]
- Wang M, Lakatta EG. Altered regulation of matrix metalloproteinase-2 in arotic remodeling during aging. Hypertension. 2002;39:865–873. doi: 10.1161/01.hyp.0000014506.13322.66. [DOI] [PubMed] [Google Scholar]
- Wang M, Lakatta EG. Altered regulation of matrix metalloproteinase-2 in aortic remodeling during aging. Hypertension. 2002;39:865–873. doi: 10.1161/01.hyp.0000014506.13322.66. [DOI] [PubMed] [Google Scholar]
- Wang M, Zhang J, Jiang L-Q, Spinetti G, Pintus G, Monticone R, Kolodgie FD, Virmani R, Lakatta EG. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension. 2007;50:219–227. doi: 10.1161/HYPERTENSIONAHA.107.089409. [DOI] [PubMed] [Google Scholar]
- Wang M, Zhang J, Spinetti G, Jiang L-Q, Monticone R, Zhao D, Cheng L, Krawczyk M, Talan M, Pintus G, Lakatta EG. Angiotensin II activates matrix metalloproteinase type II and mimics age-associated carotid arterial remodeling in young rats. Am J Pathol. 2005;167:1429–1442. doi: 10.1016/S0002-9440(10)61229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Zhao D, Spinetti G, Zhang J, Jiang L-Q, Pintus G, Monticone R, Lakatta EG. Matrix metalloproteinase 2 activation of transforming growth factor-β1 (TGF-β1) and TGF-β1-type II receptor signaling within the aged arterial wall. Arterioscler Thromb Vasc Biol. 2006;26:1503–1509. doi: 10.1161/01.ATV.0000225777.58488.f2. [DOI] [PubMed] [Google Scholar]
- Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]


