Abstract
Endothelium-dependent vasodilatation is reduced with advancing age in humans, as evidenced by blunted vasodilator responsiveness to acetylcholine (ACh). Circulating adenosine triphosphate (ATP) has been implicated in the control of skeletal muscle vascular tone during mismatches in oxygen delivery and demand (e.g. exercise) via binding to purinergic receptors (P2Y) on the endothelium evoking subsequent vasodilatation, and ageing is typically associated with reductions in muscle blood flow under such conditions. Therefore, we tested the hypothesis that ATP-mediated vasodilatation is impaired with age in healthy humans. We measured forearm blood flow (venous occlusion plethysmography) and calculated vascular conductance (FVC) responses to local intra-arterial infusions of ACh, ATP, and sodium nitroprusside (SNP) before and during ascorbic acid (AA) infusion in 13 young and 13 older adults. The peak increase in FVC to ACh was significantly impaired in older compared with young adults (262 ± 71%vs. 618 ± 97%; P < 0.05), and this difference was abolished during AA infusion (510 ± 82%vs. 556 ± 71%; not significant, NS). In contrast, peak FVC responses were not different between older and young adults to either ATP (675 ± 105%vs. 734 ± 126%) or SNP (1116 ± 111%vs. 1138 ± 148%) and AA infusion did not alter these responses in either age group (both NS). In another group of six young and six older adults, we determined whether vasodilator responses to adenosine and ATP were influenced by P1-receptor blockade via aminophylline. The peak FVC responses to adenosine were not different in young (350 ± 65%) versus older adults (360 ± 80%), and aminophylline blunted these responses by ∼50% in both groups. The peak FVC responses to ATP were again not different in young and older adults, and aminophylline did not impact the vasodilatation in either group. Thus, in contrast to the observed impairments in ACh responses, the vasodilatory response to exogenous ATP is not reduced with age in healthy humans. Further, our data also indicate that adenosine mediated vasodilatation is not reduced with age, and that ATP-mediated vasodilatation is independent of P1-receptor stimulation in both young and older adults.
Introduction
Human ageing is associated with a number of changes in cardiovascular structure and function that lead to declines in overall cardiovascular health, and therefore is the predominant uncontrollable risk factor for the development of cardiovascular disease (Lloyd-Jones et al. 2009). One such change that has received much attention relates to the progressive decline in endothelial function that occurs with human ageing (Celermajer et al. 1994; Taddei et al. 2001). A normal, healthy endothelium functions to regulate cardiovascular homeostasis by inhibiting platelet and monocyte adhesion, and thus is considered vasoprotective and thromboresistant. Further, the endothelium is an important regulator of local vascular tone by producing vasodilator and vasoconstrictor substances (Wu & Thiagarajan, 1996). In humans, endothelium-dependent vasodilatation (as determined via local vascular responses to shear stress or acetylcholine (ACh) infusions) is significantly impaired in older healthy compared with young adults, highlighting the independent effect of age per se on vasomotor control (Yasue et al. 1990; Vanhoutte, 1997; Shimokawa, 1999; DeSouza et al. 2000; Taddei et al. 2001). Thus, given the important functions of the endothelium in maintaining cardiovascular homeostasis, understanding how endothelial dysfunction with advancing age impacts vascular control is of both physiological and clinical interest.
With respect to metabolic stress, older adults typically have an attenuated hyperaemic response to exercise, which may be mediated via reductions in endothelium-dependent vasodilatation (Poole et al. 2003; Proctor & Parker, 2006). As such, we recently demonstrated that acute improvements in endothelial function is associated with significant increases in muscle blood flow during dynamic exercise in ageing humans (Kirby et al. 2009). Further, ageing is associated with an impaired ability to blunt sympathetic vasoconstriction within the active muscle (Koch et al. 2003; Dinenno et al. 2005), which may involve endothelial derived vasodilating factors (Dinenno & Joyner, 2004; Schrage et al. 2007). Collectively, the control of vascular tone and thus oxygen delivery during exercise is impaired in older adults, and may be related to decrements in overall endothelial health and function.
Recently, circulating adenosine triphosphate (ATP) has been implicated in the control of skeletal muscle vascular tone during exercise via binding to purinergic receptors (P2Y) on the endothelium facilitating blood flow and oxygen delivery to contracting muscles (Gonzalez-Alonso et al. 2002; Kirby et al. 2008). The potential source(s) and mechanisms of the increase in circulating ATP during exercise include the deoxygenated red blood cell (which could be augmented during concomitant hypercapnia and acidosis) (Bergfeld & Forrester, 1992; Ellsworth et al. 1995; Gonzalez-Alonso et al. 2002), endothelial cells subjected to elevations in shear stress (a known endothelium-dependent stimulus) (Bodin & Burnstock, 1995), and mechanical influences on the red blood cell or vasculature (Sprague et al. 1998; Crecelius et al. 2010). Once bound to the P2Y receptors, ATP evokes ‘spreading’ or ‘ascending’ vasodilatation that is primarily endothelium dependent (Winter & Dora, 2007), and such a conducted dilatory response is believed to be requisite for the full expression of exercise hyperaemia (Segal, 1994; Segal & Kurjiaka, 1995). Further, circulating ATP is capable of attenuating sympathetic vasoconstriction in a graded fashion, an observation consistent with what is observed in contracting muscle (Tschakovsky et al. 2002; Kirby et al. 2008). Finally, exogenous ATP infusions can increase muscle blood flow to a similar extent as observed in maximally exercising muscle (Rosenmeier et al. 2004). These are some unique vasomotor properties of ATP that highlight the potential importance for understanding its role in vascular control during physiological stressors in humans such as exercise. To date, the effects of human ageing on endothelium-dependent ATP-mediated vasodilatation are unclear.
Once in circulation, ATP can be rapidly hydrolysed and degraded to ADP, AMP and eventually adenosine by ecto-enzymes (Yegutkin, 2008). Despite this, data obtained from young healthy adults indicate that exogenous ATP infusions are not influenced by adenosine receptor (P1-receptor) blockade, and this is consistent with the differential control of vascular tone in humans whereby ATP clearly blunts sympathetic vasoconstriction and adenosine does not (Kirby et al. 2008; Rosenmeier et al. 2008). However, circulating ectonucleotidases (which hydrolyse and break down ATP toward adenosine) have been suggested to be elevated in disease states that are increased with age and these patients exhibit endothelial dysfunction (Duarte et al. 2007; Schetinger et al. 2007; Lunkes et al. 2008). Thus, it is possible that in older adults ATP is degraded to adenosine and is evoking vasodilatation via P1-receptor stimulation, as opposed to P2Y receptors in young adults. This could have significant implications for the regulation of blood flow and oxygen delivery during physiological stressors that result in competing influences of local vasodilatation and sympathetic vasoconstriction.
In the present study, we tested the hypothesis that ATP-mediated vasodilatation is impaired in older compared with young healthy humans. Given that ATP-mediated vasodilatation is endothelium dependent, we also determined whether infusion of ascorbic acid would improve or restore ATP-mediated vasodilatation as has been shown for the classic endothelium-dependent vasodilator ACh (Taddei et al. 2001; Kirby et al. 2009). Finally, since ATP can be rapidly degraded to its downstream by-products via interaction with endothelial ectonucleotidases and that these enzymes have been suggested to be elevated in disease states that are increased with advancing age, in a subgroup of subjects we determined ATP-mediated vasodilatation during P1-receptor blockade via aminophylline.
Methods
Subjects
With Institutional Review Board approval and after written informed consent, a total of 19 young and 19 older healthy adult men and women participated in the present study. All subjects were normotensive and free from overt cardiovascular disease as assessed from casual blood pressure measurements and a medical history. Older subjects were further evaluated for clinical evidence of cardiopulmonary disease with a physical examination and resting and maximal exercise electrocardiograms. All subjects were sedentary to moderately active, non-smokers and not taking any medications including antioxidants, and studies were performed after a minimum of a 4 h fast. Subjects provided written, informed consent after all potential risks and procedures were explained. The study was approved by the Human Research Committee of Colorado State University and was performed according to the Declaration of Helsinki.
Arterial catheterization
A 20-gauge, 7.6 cm catheter was placed in the brachial artery of the non-dominant arm under aseptic conditions after local anaesthesia (2% lidocaine) for local administration of study drugs. The catheter was connected to a three-port connector as well as a pressure transducer for mean arterial pressure (MAP) measurement and continuously flushed at 3 ml h−1 with heparinized saline. The two side ports were used for infusions of vasoactive drugs (Kirby et al. 2008, 2009).
Blood samples
Measures of total cholesterol, low- and high-density lipoproteins (LDL and HDL), and triglycerides were performed via conventional methods by the clinical laboratory of the Poudre Valley Hospital (Fort Collins, CO, USA). Oxidized LDL was measured via standard enzyme-linked immunosorbent assay (ELISA) (Mercodia, Inc., Uppsala, Sweden) as a marker of circulating oxidative stress by the General Clinical Research Center of the Milton S. Hershey Medical Center (Hershey, PA, USA).
Body composition and forearm volume
Body composition was determined by dual-energy X-ray absorptiometry (DEXA; Hologic, Inc.; Bedford, MA, USA). Total forearm volume was calculated from regional analysis of the experimental forearm (from the proximal to distal radioulnar joint) from whole-body DEXA scans with QDR series software for normalization of individual drug doses. Body mass index was calculated as bodyweight (kg) divided by height (meters) squared.
Forearm blood flow and vascular conductance
Forearm blood flow (FBF) was measured via venous occlusion plethysmography using mercury-in-Salistic strain gauges (Greenfield et al. 1963; Dinenno et al. 2002). A paediatric blood pressure cuff was placed around the wrist of the experimental arm and inflated to suprasystolic pressure (∼200 mmHg) to arrest the hand circulation. Additionally, a venous occlusion cuff was placed around the upper portion of the experimental arm and cycled between rapid inflation at ∼50 mmHg (7 s) and deflation (8 s) yielding one blood flow measurement every 15 s. FBF was expressed as millilitres per 100 ml of tissue per minute (ml (100 ml)−1 min−1). As an index of forearm vasodilatation and to account for individual differences in baseline vascular tone, forearm vascular conductance (FVC) was calculated as (FBF/MAP) × 100 expressed as ml min−1 (100 mmHg)−1. In an effort to minimize the contribution of cutaneous blood flow to FBF measurements, a fan was directed at the experimental arm throughout the experimental protocol.
Vasoactive drug administration
As a standard test of endothelium-dependent vasodilatation, the muscarinic receptor agonist acetylcholine (ACh; Miochol-E, Novartis Inc.) was infused via a brachial artery catheter at 1, 4, 8 and 16 μg (100 ml forearm volume)−1 min−1 for 4 min each. Additionally, endothelium-independent vasodilatation was assessed via intra-arterial infusion of sodium nitroprusside (SNP; Nitropress, Hospira Inc., Lake Forest, IL, USA) at 0.25, 0.5, 1 and 2 μg (100 ml forearm volume)−1 min−1 for 4 min each (Taddei et al. 2001; DeSouza et al. 2002). To test our primary hypothesis, the P2Y receptor agonist ATP (Sigma A7699) was infused at 1.25, 2.5, 5 and 10 μg (100 ml forearm volume)−1 min−1 for 4 min each (Rongen et al. 1994; Kirby et al. 2008). ATP was confirmed sterile and free of fungus/endotoxin with a standard microbiology report (JCB-Analytical Research Labs, Witchita, KS, USA) prior to use (Kirby et al. 2008). As a method of acutely improving endothelium-dependent vasodilatation, the potent antioxidant ascorbic acid (AA; vitamin C; American Regent Inc., Shirley, NY, USA) was infused at 8 mg (100 ml forearm volume)−1 min−1 for 10 min as a loading dose (see Experimental protocol below), and at 40% of this loading dose for maintenance infusion throughout the remainder of the experiment (Taddei et al. 2001; Kirby et al. 2009).
In a second group of subjects, adenosine (Sicor, Irvine, CA, USA) was infused at 3.125, 6.25 and 12.5 μg (100 ml forearm volume)−1 min−1 for 4 min as described by Martin et al. (2006). In an effort to produce vasodilatation of a similar magnitude to adenosine, the doses of ATP were estimated to be 1.25, 2.5 and 5 μg (100 ml forearm volume)−1 min−1 for 4 min based on data from the first group of subjects. The P1-receptor blocker, aminophylline (American Reagent, Shirley, NY, USA), was infused for 20 min at 100 μg (100 ml forearm volume)−1 min−1 prior to experimental trials and continued through the remainder of the study (Leuenberger et al. 1999; Martin et al. 2006). Pilot data in our laboratory indicated this dose of aminophylline markedly decreases adenosine-mediated vasodilatation without affecting basal forearm vascular tone.
Experimental protocol
The primary purpose of the main experimental protocol was to determine whether ATP-mediated vasodilatation is impaired in older compared with young adults. A secondary purpose of this protocol was to determine whether ATP-mediated vasodilatation would be improved in older adults by acute antioxidant administration of AA similar to that observed previously to ACh (Taddei et al. 2001; Kirby et al. 2009). Thirteen young (eight men; five women) and 13 older (eight men; five women) people were studied in the supine position with the experimental (non-dominant) arm abducted 90 deg laterally at heart level. The most distal portion of the arm was slightly elevated in order to facilitate venous return during blood flow measurements with venous occlusion plethysmography. After a minimum of 30 min following catheterization and experimental set-up, the first of three vasoactive drugs (ACh, SNP, or ATP) was infused for 4 min at each dose totalling 16 min of infusion time. Four minutes of quiet rest preceded all vasodilator trials where baseline measurements were performed and saline was infused. Twenty minutes of quiet rest was allotted between all trials and the order of vasodilator drug infusion was randomized and counterbalanced across subjects. After completion of these initial trials, ascorbic acid was infused at the loading dose for 10 min, then reduced to 40% of this dose for the remainder of the study. The infusions of ACh, SNP, and ACh were then repeated in the same order as before AA for each subject.
In a second experimental protocol in an additional six young (4 men; 2 women) and six older (3 men; 3 women) subjects, we determined whether adenosine-mediated vasodilatation was impaired with age and whether any age-associated differences in ATP-mediated vasodilatation could be attributed to greater breakdown to adenosine and subsequent P1-receptor stimulation. Therefore, in randomized and counterbalanced fashion, adenosine and ATP were infused before and after local P1-receptor inhibition via aminophylline. The timeline for this protocol was similar to that described above for the primary experimental protocol.
Data acquisition and analysis
Data were collected and stored on computer at 250 Hz and analysed off-line with signal-processing software (WinDaq, DATAQ Instruments, Akron, OH, USA). FBF was determined from the derivative of the forearm plethysmogram. Mean arterial pressure (MAP) was determined from the arterial pressure waveform and heart rate (HR) was determined via standard three-lead ECG. Baseline FBF, FVC, HR and MAP represent an average of the last minute of the resting time period prior to all pharmacological vasodilatory tests. In addition, all haemodynamic responses from drug infusions represent an average of the last minute of data from that specific dose of infusion. The percentage change in FBF and FVC during drug infusions was calculated as: ((FBF drug − FBF baseline)/(FBF baseline)) × 100. Changes in FVC were calculated in a similar fashion.
Statistics
All values are reported as means ± s.e.m. Comparison of subject characteristics and the haemodynamic values at specific time points between groups were made with Student's unpaired t test, and the within group values for each hyperaemic condition with a paired t test. Specific hypothesis testing within trials was performed to assess mean group differences between young and older adults using two-way repeated measures analysis of variance. Post hoc analysis was performed using Tukey's test when significance was observed. Significance was set at P < 0.05.
Results
Subject characteristics
The mean age difference between young and older subjects was ∼45 years (Table 1). There were no significant differences between young and older adults in forearm volume, HDL-cholesterol, or triglycerides. Older adults had a greater BMI, body fat percentage, total cholesterol and LDL-cholesterol (all P < 0.05), although these values were within normal levels. Baseline FBF, FVC, and HR for all trials were not different between young and older adults. Although normotensive, MAP was elevated in older compared with young adults (P < 0.05).
Table 1.
Subject characteristics and baseline haemodynamics
| Variable | Young (18–31 yrs) | Older (57–86 yrs) |
|---|---|---|
| Male:female | 8:5 | 8:5 |
| Age (years) | 21 ± 1 | 66 ± 3* |
| Body mass index (kg m−2) | 22.0 ± 0.5 | 24.4 ± 0.9* |
| Body fat (%) | 17.4 ± 2.1 | 28.1 ± 2.2* |
| Forearm volume (ml) | 931 ± 77 | 876 ± 59 |
| Total cholesterol (mmol l−1) | 3.4 ± 0.2 | 4.4 ± 0.2* |
| LDL cholesterol (mmol l−1) | 2.0 ± 0.1 | 2.9 ± 0.2* |
| HDL cholesterol (mmol l−1) | 1.2 ± 0.1 | 1.1 ± 0.1 |
| Triglycerides (mmol l−1) | 0.7 ± 0.1 | 0.9 ± 0.1 |
| Mean arterial pressure (mmHg) | 85 ± 2 | 97 ± 2* |
| Heart rate (beats min−1) | 56 ± 2 | 57 ± 1 |
| Forearm blood flow (ml (dl FAV)−1 min−1) | 2.2 ± 0.3 | 2.0 ± 0.2 |
| Forearm vascular conductance (ml (dl FAV) min−1 (100 mmHg)−1) | 2.7 ± 0.4 | 2.0 ± 0.2 |
Data presented as mean ± s.e.m. LDL, low density lipoprotein; HDL, high density lipoprotein; FAV, forearm volume.
P < 0.05 vs. younger.
Vasodilator drug administration: effect of age
The vasodilatory responses expressed as the percentage increase in FVC from baseline during all doses of ACh, ATP and SNP are shown in Fig. 1A–C. As expected, older adults demonstrated an impaired vasodilatory response to ACh compared to that observed in young adults (Fig. 1A; P < 0.05). The vasodilatory response to the endothelium-independent vasodilator, SNP, was not affected by age (Fig. 1C). In contrast to our hypothesis, older adults had a preserved vasodilator response to ATP infusion (Fig. 1B). FBF responses followed a similar pattern to that of FVC for all conditions (Table 2). Within both age groups, MAP was unchanged during ACh and ATP; however at the highest two doses of SNP, MAP was significantly lower than baseline (P < 0.05; Table 2). Similarly, HR was not different between or within ACh and ATP infusion, yet the two highest doses of SNP resulted in elevated HR compared to baseline for both age groups (P < 0.05; Table 2).
Figure 1. Forearm vasodilatory responses to acetylcholine, ATP and sodium nitroprusside infusion.
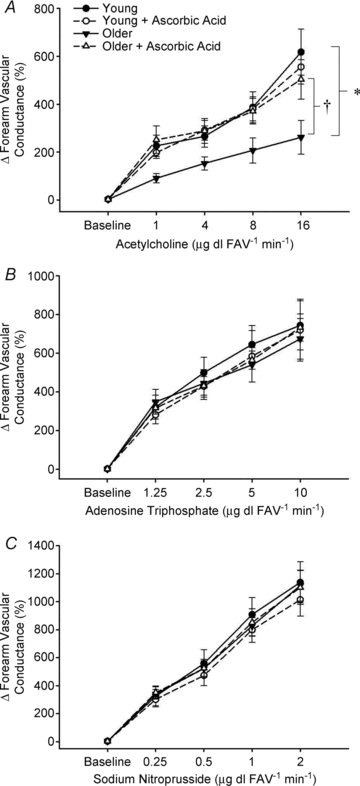
Vasodilatation was significantly impaired in older adults during muscarinic receptor agonist infusion (A; ACh) and this was abolished during simultaneous AA administration. There was no significant age-related impairment in vasodilatation to the purinergic receptor agonist ATP (B) or to the NO donor SNP (C), and these responses were unaffected by AA. *P < 0.05 vs. young within condition; †P < 0.05 vs. without AA in older adults.
Table 2.
Forearm and systemic haemodynamics for subjects in primary experimental protocol
| Baseline | Dose 1 | Dose 2 | Dose 3 | Dose 4 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FBF | MAP | HR | FBF | MAP | HR | FBF | MAP | HR | FBF | MAP | HR | FBF | MAP | HR | ||
| ACh | Young | 2.1 ± 0.3 | 86 ± 2 | 55 ± 1 | 6.7 ± 1.0 | 86 ± 2 | 57 ± 3 | 7.4 ± 1.0 | 86 ± 2 | 55 ± 2 | 9.6 ± 1.0 | 87 ± 2 | 55 ± 2 | 14.4 ± 1.9 | 86 ± 2 | 55 ± 2 |
| Young + AA | 2.1 ± 0.2 | 89 ± 2 | 54 ± 2 | 6.3 ± 0.7 | 89 ± 2 | 54 ± 1 | 8.2 ± 1.0 | 89 ± 2 | 55 ± 2 | 10.1 ± 1.1 | 89 ± 2 | 54 ± 2 | 13.6 ± 1.5 | 89 ± 2 | 57 ± 2 | |
| Older | 1.9 ± 0.1 | 98 ± 2 | 56 ± 1 | 3.5 ± 0.4*† | 99 ± 2 | 57 ± 1 | 4.6 ± 0.3*† | 100 ± 2 | 58 ± 2 | 5.3 ± 0.6*† | 99 ± 2 | 58 ± 2 | 6.3 ± 0.9*† | 100 ± 2 | 58 ± 2 | |
| Older + AA | 1.9 ± 0.1 | 101 ± 2 | 56 ± 2 | 6.8 ± 1.1 | 102 ± 3 | 55 ± 2 | 7.7 ± 1.1 | 103 ± 2 | 57 ± 2 | 9.4 ± 1.2 | 103 ± 2 | 57 ± 2 | 12.2 ± 1.9 | 103 ± 2 | 57 ± 1 | |
| ATP | Young | 2.1 ± 0.2 | 86 ± 2 | 53 ± 3 | 8.2 ± 1.0 | 87 ± 2 | 55 ± 2 | 11.6 ± 1.5 | 88 ± 2 | 56 ± 2 | 14.2 ± 1.7 | 87 ± 2 | 56 ± 2 | 15.7 ± 2.1 | 87 ± 2 | 55 ± 2 |
| Young + AA | 2.0 ± 0.2 | 90 ± 2 | 54 ± 2 | 7.5 ± 1.0 | 91 ± 2 | 54 ± 2 | 10.1 ± 1.2 | 91 ± 2 | 55 ± 2 | 12.5 ± 1.9 | 90 ± 2 | 56 ± 1 | 15.1 ± 2.5 | 91 ± 2 | 57 ± 2 | |
| Older | 2.0 ± 0.2 | 98 ± 2 | 56 ± 1 | 7.9 ± 0.9 | 99 ± 2 | 57 ± 2 | 9.8 ± 1.0 | 98 ± 2 | 58 ± 2 | 11.6 ± 1.6 | 98 ± 2 | 58 ± 2 | 13.9 ± 1.7 | 98 ± 2 | 58 ± 2 | |
| Older + AA | 1.9 ± 0.1 | 100 ± 3 | 56 ± 2 | 8.1 ± 0.1 | 101 ± 3 | 56 ± 1 | 10.7 ± 1.4 | 102 ± 2 | 57 ± 2 | 12.9 ± 1.3 | 101 ± 2 | 58 ± 2 | 16.4 ± 1.7 | 102 ± 3 | 58 ± 2 | |
| SNP | Young | 2.1 ± 0.3 | 86 ± 2 | 55 ± 2 | 8.2 ± 1.2 | 87 ± 2 | 56 ± 2 | 11.7 ± 1.4 | 85 ± 2 | 56 ± 2 | 17.7 ± 1.9 | 83 ± 2 | 61 ± 2 | 21.3 ± 2.1 | 82 ± 2 | 62 ± 2 |
| Young + AA | 2.1 ± 0.2 | 89 ± 2 | 55 ± 2 | 8.4 ± 1.0 | 92 ± 2 | 56 ± 2 | 11.8 ± 1.3 | 90 ± 2 | 55 ± 2 | 17.9 ± 1.8 | 87 ± 2 | 57 ± 2 | 21.5 ± 2.0 | 85 ± 2 | 60 ± 2 | |
| Older | 1.8 ± 0.1 | 98 ± 3 | 57 ± 1 | 7.8 ± 0.5 | 99 ± 3 | 57 ± 1 | 11.1 ± 1.2 | 96 ± 3 | 58 ± 1 | 15.6 ± 1.2 | 94 ± 2 | 59 ± 2 | 19.4 ± 1.8 | 89 ± 2 | 61 ± 2 | |
| Older + AA | 2.0 ± 0.2 | 101 ± 3 | 56 ± 2 | 8.8 ± 1.1 | 103 ± 3 | 56 ± 2 | 12.0 ± 1.6 | 101 ± 2 | 57 ± 2 | 17.0 ± 1.6 | 96 ± 2 | 58 ± 2 | 20.6 ± 1.9 | 93 ± 2 | 61 ± 2 | |
FBF, forearm blood flow (ml (100 ml)−1 min−1); MAP, mean arterial pressure (mmHg); HR, heart rate (beats min−1)
P < 0.05 vs. young
P < 0.05 vs. with AA; note, all absolute MAP values for older adults are significantly greater than young.
Vasodilator drug administration: effect of ascorbic acid
Acute infusion of AA restored endothelium-dependent vasodilatation to ACh in older adults but had no effect on the dilatory response in young adults (Fig. 1A). In contrast, concurrent AA administration during ATP and SNP infusion did not impact the vasodilatory responses in either young or older adults (Fig. 1B–C). FBF responses followed a similar pattern to that of FVC for all conditions (Table 2). Neither MAP nor HR was significantly affected by administration of AA within any drug condition (not significant, NS; Table 2).
Effect of P1-receptor blockade on adenosine-and ATP-mediated vasodilatation
The vasodilatory responses expressed as the percentage increase in FVC from baseline during all doses of adenosine and ATP are shown in Fig. 2A and B. Adenosine-mediated vasodilatation was not different between young and older adults at any dose (Fig. 2A). Similar to subjects from the primary experiment, vasodilatory responsiveness to ATP infusion was again not different between young and older adults (Fig. 2B). P1-receptor blockade via aminophylline blunted peak adenosine-mediated vasodilatation in both young (∼47%) and older (∼44%) adults (both P < 0.05 vs. zero); however it had little to no impact on ATP-mediated vasodilatation (∼10%vs.∼10%; both NS vs. zero). Importantly, the effect of aminophylline during either adenosine or ATP infusion was not different between age groups (adenosine: P = 0.8; ATP: P = 0.9, Fig. 2A–B). FBF responses followed a similar pattern to that of FVC in both age groups and within all drug conditions (Table 3). Neither MAP nor HR was significantly affected during ATP or adenosine infusions before or during aminophylline administration (NS; Table 3).
Figure 2. Forearm vasodilatory responses to ATP and adenosine infusion.
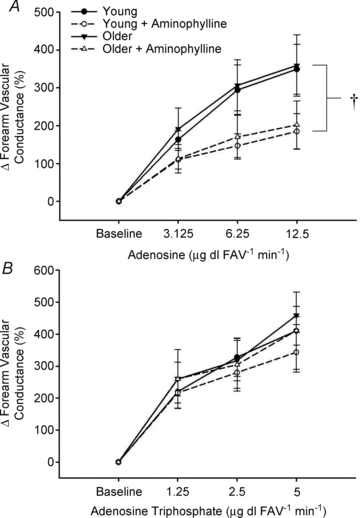
Vasodilatation to P1-receptor agonist adenosine infusion was not significantly impaired in older adults (A). Aminophylline significantly reduced vasodilatation to adenosine at all drug doses in both young and older adults. No significant age-related impairment in vasodilatation to the purinergic receptor agonist ATP was observed and this vasodilatation was unaffected during aminophylline infusion regardless of age (B). †P < 0.05 vs. without aminophylline.
Table 3.
Forearm and systemic haemodynamics for subjects in aminophylline protocol
| Baseline | Dose 1 | Dose 2 | Dose 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FBF | MAP | HR | FBF | MAP | HR | FBF | MAP | HR | FBF | MAP | HR | ||
| ADO | Young | 1.7 ± 0.3 | 91 ± 3 | 60 ± 5 | 4.5 ± 0.9 | 94 ± 4 | 58 ± 4 | 6.2 ± 0.9 | 95 ± 4 | 59 ± 4 | 7.0 ± 0.8 | 95 ± 5 | 58 ± 5 |
| Young + APH | 2.0 ± 0.5 | 93 ± 4 | 57 ± 5 | 4.1 ± 0.9 | 98 ± 5 | 58 ± 4 | 4.8 ± 1.0 | 97 ± 5 | 57 ± 4 | 5.6 ± 1.2 | 98 ± 6 | 58 ± 4 | |
| Older | 1.2 ± 0.2 | 98 ± 5 | 57 ± 2 | 3.6 ± 0.8 | 99 ± 5 | 58 ± 2 | 4.8 ± 0.9 | 99 ± 5 | 57 ± 2 | 5.6 ± 1.1 | 100 ± 4 | 58 ± 2 | |
| Older + APH | 1.2 ± 0.3 | 102 ± 6 | 56 ± 2 | 2.9 ± 1.1 | 102 ± 6 | 56 ± 2 | 3.5 ± 1.4† | 101 ± 6 | 56 ± 2 | 3.9 ± 1.5 | 102 ± 7 | 58 ± 2 | |
| ATP | Young | 1.8 ± 0.3 | 92 ± 5 | 59 ± 5 | 5.8 ± 1.2 | 93 ± 4 | 59 ± 5 | 7.5 ± 1.5 | 93 ± 5 | 58 ± 5 | 9.1 ± 1.7 | 93 ± 5 | 59 ± 5 |
| Young + APH | 2.0 ± 0.3 | 92 ± 5 | 58 ± 5 | 6.2 ± 1.0 | 98 ± 5 | 57 ± 4 | 7.3 ± 1.1 | 96 ± 5 | 59 ± 4 | 8.6 ± 1.2 | 97 ± 5 | 58 ± 4 | |
| Older | 1.3 ± 0.2 | 98 ± 3 | 58 ± 2 | 4.7 ± 0.9 | 98 ± 4 | 58 ± 1 | 5.8 ± 1.4 | 102 ± 5 | 57 ± 2 | 7.4 ± 1.1 | 99 ± 4 | 57 ± 2 | |
| Older + APH | 1.3 ± 0.2 | 98 ± 5 | 56 ± 1 | 4.6 ± 1.0 | 102 ± 5 | 56 ± 2 | 5.4 ± 1.1 | 102 ± 5 | 56 ± 2 | 6.5 ± 1.0 | 101 ± 6 | 57 ± 2 | |
APH, aminophylline; ADO, adenosine; FBF, forearm blood flow (ml (100 ml)−1 min−1); MAP, mean arterial pressure (mmHg); HR, heart rate (beats min−1).
Plasma markers of oxidative stress
At baseline, plasma oxidized-LDL was greater in the older compared with young subjects (53 ± 3 vs. 39 ± 3 U l−1; P < 0.05). Infusion of ascorbic acid did not affect these plasma levels in either young (38 ± 3 U l−1) or older adults (54 ± 3 U l−1), which most likely reflects that the ascorbic acid was administered locally via brachial artery catheter and was dose-adjusted to forearm volume. Importantly, the improvement in acetylcholine-mediated vasodilatation (see above) in older subjects is consistent with prior studies and provides evidence that ascorbic acid was effective at the level of the forearm vasculature (Taddei et al. 2001; Kirby et al. 2009).
Discussion
Circulating ATP has been implicated in the control of skeletal muscle vascular tone during mismatches in oxygen delivery and demand (e.g. exercise) via binding to purinergic receptors on the endothelium, and ageing is typically associated with reductions in muscle blood flow under such conditions. Further, diminished endothelial function is observed in aged humans and has been closely related to oxidative stress (Taddei et al. 2001; Forstermann & Munzel, 2006; Ashfaq et al. 2008). The key finding of the present investigation is that vasodilator responsiveness to exogenous ATP in ageing humans is preserved despite the presence of local endothelial dysfunction as evidenced by impaired vasodilatation to ACh infusion. Additionally, the presence of the antioxidant AA reversed the observed impairments to ACh, but had no impact on the vasodilatory response to exogenous ATP. Importantly, the vasodilatory response to exogenous ATP was unaffected under conditions of P1-receptor blockade (with aminophylline), negating the possibility that adenosine-mediated vasodilatation is, in part, masking a genuine diminished vasodilatory response to exogenous ATP. Collectively, our findings demonstrate that older adults with endothelial dysfunction respond with a similar vasodilatory response to ATP and adenosine to that observed in young adults.
Ageing and endothelium-dependent vasodilatation
In older adults, endothelium-dependent vasodilatation in response to ACh infusion is attenuated compared to young adults, and this impaired vasodilatory response is classically referred to as ‘endothelial dysfunction’ (Yasue et al. 1990). Despite the preponderance of evidence supporting blunted ACh-mediated vasodilatation in older adults, data suggest that not all endothelium-dependent agonists demonstrate reduced vasodilator responsiveness in aged humans (DeSouza et al. 2002). Recently, ATP has become more recognized as a significant endogenous circulating modulator of vascular tone that affects oxygen delivery to meet the metabolic demand of active tissue (Ellsworth, 2004; Gonzalez-Alonso, 2008). Further, isolated vessels studies clearly indicate that vasodilatation to ATP is largely dependent on the presence of a healthy endothelium (Busse et al. 1988; Winter & Dora, 2007). Therefore, the primary purpose of the present experiment was to test the hypothesis that the vasodilatory response to exogenous infusion of ATP is impaired in ageing humans. As expected, healthy older adults demonstrated obvious blunted endothelium-dependent vasodilatation in response to the muscarinic agonist, ACh, and unaltered vasodilatation to the endothelium-independent dilator, SNP, compared with young adults. However, in contrast to our hypothesis, no age-associated decrement in ATP-mediated vasodilatation (in the same subjects that demonstrated blunted ACh responses) was observed.
To the best of our knowledge, only one other group has attempted to determine ATP-mediated vasodilatory responsiveness in healthy older adults (Imaizumi et al. 1990). Similar to the present study, ATP-mediated vasodilatation was not attenuated in older subjects (age 57 ± 1 years). However, the findings from this previous study were difficult to interpret given that vasodilatation to ACh was not impaired with age (i.e. endothelial dysfunction was not present). Although there is limited direct information in healthy ageing humans, there appears to be a fairly robust vasodilator response to intra-coronary artery infusion of ATP in coronary artery diseased patients, a population which typically exhibits endothelial dysfunction (Takase et al. 1998). Interestingly, ACh infusion in this population often results in vasoconstriction or profoundly weak vasodilatation further supporting different vasodilator responses between the endothelium-dependent agonists ACh and ATP (Vita et al. 1990; Yasue et al. 1990). Nonetheless, the present findings clearly indicate that vasodilatory responsiveness to ATP is not impaired in older adults demonstrating typical ‘endothelial dysfunction’ as evidenced by substantially blunted responses to ACh.
Our laboratory and others have demonstrated that acute intra-arterial administration of AA can abolish age-associated impairments in endothelium-dependent vasodilatation to ACh (Taddei et al. 2001; Kirby et al. 2009). Thus, in the present study we used intra-arterial AA as a research tool to acutely improve endothelial function in older adults, and we hypothesized that ATP-mediated vasodilatation would be impaired in older adults and that AA would acutely restore ATP responsiveness as has been observed with ACh. In contrast to our hypothesis, ATP-mediated vasodilatation was not impaired with age, and thus it follows that AA had no effect on ATP vasodilator responsiveness. Although not directly determined in this study, the majority of studies examining the mechanism by which AA can modulate vascular function indicate an ability of AA to scavenge superoxide or stabilize tetrahydrobiopterin (cofactor for nitric oxide (NO) synthesis), both of which would preserve the bioavailability of NO (Forstermann & Munzel, 2006). Consequently, the contribution of NO to ACh-mediated vasodilatation in aged humans has been shown to be greatly enhanced following AA administration (Taddei et al. 1998, 2001). Further, in vitro experiments indicate that antioxidant treatment to endothelial cells can acutely increase vasodilatory prostaglandin (PG) production (Wu et al. 2004). With respect to ATP-mediated vasodilatation, the underlying signalling mechanisms are at present unclear as some in vitro studies and one human study suggest a role for both NO and PGs (Burnstock, 1990; Hammer et al. 2001; Mortensen et al. 2009), whereas a number of studies in humans (including unpublished observations from our laboratory) indicate that ATP evokes vasodilatation independent of NO and PGs (Rongen et al. 1994; Shiramoto et al. 1997; Hrafnkelsdottir et al. 2001; van Ginneken et al. 2004). Collectively, our observations of preserved vasodilatation to exogenous ATP which was unaffected by AA administration in older adults with endothelial dysfunction indirectly supports these previous studies, indicating a minor contribution of NO and PGs to ATP-mediated vasodilatation in humans.
Ageing and adenosine-mediated vasodilatation
The finding that vasodilatation to ATP was not different between groups was unexpected given the endothelium dependence of ATP and current knowledge of ACh responses in this population. In this context, we questioned whether ectonucleotidases that assist in the breakdown of ATP towards adenosine are elevated in older individuals thereby allowing adenosine-mediated vasodilatation to mask any true impairment to ATP. There is evidence for increases in ATP-degrading ectonucleotidases in a variety of diseased populations that demonstrate endothelial dysfunction, presumably as a means to offset inflammatory mediated platelet aggregation by ADP (Schetinger et al. 2007; Lunkes et al. 2008). In particular, ATP hydrolysis is closely related to ox-LDL, and older adults typically have elevated levels of ox-LDL compared to younger adults as was observed in the present study (Duarte et al. 2007). Therefore, in a subgroup of subjects we determined adenosine-mediated vasodilatation in older adults before and after P1-receptor blockade via aminophylline. We observed that vasodilatation to adenosine infusion was not different in older adults and that the contribution of adenosine-mediated vasodilatation during ATP infusion was not significant in both young and older adults. Our findings in older adults are similar to other observations in young adults using adenosine receptor blocking agents (Rongen et al. 1994; Mortensen et al. 2009). Although ectonucleotidase activity in older adults under these conditions is not directly known, our observations clearly indicate that adenosine-mediated vasodilatation does not appear to mask a true decrement in vasodilatory responsiveness to ATP administration in ageing humans.
Experimental considerations
Since we observed that the vasodilatation to ATP was not different between age groups and evidence suggests that this population may have increased ectonucleotidase activity (Duarte et al. 2007), we aimed to test in a subgroup of adults whether vasodilatation to adenosine was masking a true vasodilatory responsiveness to ATP in older adults. Therefore, we infused a P1-receptor antagonist during both adenosine and ATP infusions and observed ∼50% reduction to adenosine-mediated vasodilatation in both age groups but a non-significant reduction in vasodilatation during ATP infusions. Despite our attempts to uncover extraneous vasodilatation via the ATP-breakdown product adenosine, ADP as well as AMP also results in vasodilatation (Rosenmeier et al. 2008). Whether the balance of nucleotide-mediated vasodilatation is shifted in older adults is difficult to address since ATP, ADP and AMP all have the potential to bind to P2 receptors on the endothelium and specific pharmacological antagonists are currently unavailable for human use (Ralevic & Burnstock, 1998). Hence, we were unable to determine the contribution of ADP and AMP to exogenous ATP infusion. Regardless, our data are the first clear evidence that adenosine-mediated forearm vasodilatation is not impaired in older adults and that vasodilatation via adenosine binding to P1 receptors during ATP infusion is minimal in both young and older adults.
Physiological perspectives
The data from the present investigation are consistent with previous findings in humans indicating that the responsiveness to endothelium-dependent vasodilators are not always impaired, and that the most common impairment observed is in response to the muscarinic receptor agonist ACh (DeSouza et al. 2002). Why this is the case is not clearly known but it most likely indicates that downstream signalling from muscarinic receptor and the dependence on NO in mediating the vasodilatation is the most prominent age-associated impairment (Taddei et al. 2001; DeSouza et al. 2002). Our interest in the vasoactive purine ATP has stemmed from accumulating evidence that it is released during a variety of physiological stressors and can clearly contribute to the regulation of tissue blood flow and oxygen delivery (Gonzalez-Alonso et al. 2002; Farias et al. 2005; Ellsworth et al. 2009). The potential insight gained from our observations of preserved ATP responsiveness with age and the lack of effect of AA on this response in older adults points towards a NO-independent vasodilator property of ATP. This is consistent with the majority of studies in humans (Rongen et al. 1994; Shiramoto et al. 1997; Hrafnkelsdottir et al. 2001; van Ginneken et al. 2004), and we believe highlights the need for future studies designed to further understand the mechanisms by which ATP evokes vasodilatation in humans.
Exogenous ATP administration can modulate vascular tone in a multifaceted manner as a result of evoking both profound dilatation as well as having the ability to blunt sympathetic vasoconstriction (Rosenmeier et al. 2004; Gonzalez-Alonso, 2008; Kirby et al. 2008). Thus, from a physiological standpoint, the extent to which ATP is present and acts in circulation is of considerable interest. For many years nucleotides have been identified in circulation (Forrester, 1969); however only more recently have investigators begun to determine the stimuli that increase [ATP] in blood. In view of this, plasma [ATP] elevate during exercise (Gonzalez-Alonso et al. 2002), and specific red blood cell release of ATP is enhanced under conditions of hypoxia, hypercapnia, acidosis and mechanical deformation (Sprague et al. 1996; Ellsworth et al. 2009). Despite this understanding in young adults, whether an age-associated impairment in circulating [ATP] exists at rest or during various stimuli is uncertain. Corroborating this perspective is the fact that older adults typically demonstrate reduced exercise and hypoxic vasodilatation compared to younger individuals in conditions where ATP would be presumed to be high in concentration and result in active vasodilatation (Kravec et al. 1972; Proctor & Parker, 2006; Kirby et al. 2009). Building upon the present observations that vasodilatory responsiveness to exogenous ATP infusion appears intact in ageing humans, future work should be aimed at determining whether circulating ATP is hampered with age and how this may contribute to altered haemodynamic regulation.
Conclusions
The findings from the present investigation indicate that vasodilatory responsiveness to exogenous ATP is not impaired in ageing humans despite the presence of endothelial dysfunction as evidenced by attenuated ACh-mediated vasodilatation. Further, adenosine-mediated vasodilatation is intact in older adults and the degradation of ATP to adenosine does not appear to ‘mask’ a genuine impairment in endothelium-dependent vasodilatation to ATP. Given the potential significance of circulating ATP in regulating vascular tone during physiological stressors which are often impaired in older individuals, it is plausible that advancing age has little impact on P2Y receptor sensitivity but results in diminished endogenous release of ATP in circulation.
Acknowledgments
We would like to thank the subjects who volunteered to participate in this study as well as Julia Davis for her administrative assistance. This research was supported by National Institutes of Health awards AG022337, AG027150, and HL087952 (F.A.D.).
Glossary
Abbreviations
- AA
ascorbic acid
- ACh
acetylcholine
- ADO
adenosine
- APH
aminophylline
- ATP
adenosine triphosphate
- DEXA
dual-energy X-ray absorptiometry
- ECG
electrocardiogram
- FAV
forearm volume
- FBF
forearm blood flow
- FVC
forearm vascular conductance
- HDL
high-density lipoprotein
- HR
heart rate
- LDL
low-density lipoprotein
- MAP
mean arterial pressure
- MBV
mean blood velocity
- MVC
maximal voluntary contraction
- NOS
nitric oxide synthase
- NS
not significant
- P-receptor
purinergic-receptor
- SNP
sodium nitroprusside
Author contributions
B.S.K. contributed to the experimental design, data acquisition, data analysis, data interpretation, and drafting of the manuscript. A.R.C. contributed to data acquisition and interpretation, and critical review of the manuscript. W.F.V. provided clinical support and invasive methodology, and contributed to data acquisition and interpretation, as well as critical review of the manuscript. F.A.D. contributed to the conception and experimental design, data acquisition and interpretation, and critical review of the manuscript. All authors approved the final version of the manuscript. All work performed in the Human Cardiovascular Physiology Laboratory at Colorado State University.
References
- Ashfaq S, Abramson JL, Jones DP, Rhodes SD, Weintraub WS, Hooper WC, Vaccarino V, Alexander RW, Harrison DG, Quyyumi AA. Endothelial function and aminothiol biomarkers of oxidative stress in healthy adults. Hypertension. 2008;52:80–85. doi: 10.1161/HYPERTENSIONAHA.107.097386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res. 1992;26:40–47. doi: 10.1093/cvr/26.1.40. [DOI] [PubMed] [Google Scholar]
- Bodin P, Burnstock G. Synergistic effect of acute hypoxia on flow-induced release of ATP from cultured endothelial cells. Experientia. 1995;51:256–259. doi: 10.1007/BF01931108. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Dual control of local blood flow by purines. Ann N Y Acad Sci. 1990;603:31–44. doi: 10.1111/j.1749-6632.1990.tb37659.x. discussion 44–35. [DOI] [PubMed] [Google Scholar]
- Busse R, Ogilvie A, Pohl U. Vasomotor activity of diadenosine triphosphate and diadenosine tetraphosphate in isolated arteries. Am J Physiol Heart Circ Physiol. 1988;254:H828–832. doi: 10.1152/ajpheart.1988.254.5.H828. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- Crecelius AR, Kirby BS, Richards JC, Voyles WF, Dinenno FA. Rhythmic mechanical deformation of the human forearm increases venous plasma [ATP] and ATP release. FASEB J. 2010;24:1039.10. (abstract) [Google Scholar]
- DeSouza CA, Clevenger CM, Greiner JJ, Smith DT, Hoetzer GL, Shapiro LF, Stauffer BL. Evidence for agonist-specific endothelial vasodilator dysfunction with ageing in healthy humans. J Physiol. 2002;542:255–262. doi: 10.1113/jphysiol.2002.019166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Dietz NM, Joyner MJ. Aging and forearm postjunctional α-adrenergic vasoconstriction in healthy men. Circulation. 2002;106:1349–1354. doi: 10.1161/01.cir.0000028819.64790.be. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Joyner MJ. Combined NO and PG inhibition augments α-adrenergic vasoconstriction in contracting human skeletal muscle. Am J Physiol Heart Circ Physiol. 2004;287:H2576–2584. doi: 10.1152/ajpheart.00621.2004. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Masuki S, Joyner MJ. Impaired modulation of sympathetic α-adrenergic vasoconstriction in contracting forearm muscle of ageing men. J Physiol. 2005;567:311–321. doi: 10.1113/jphysiol.2005.087668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte MM, Loro VL, Rocha JB, Leal DB, Bem AF, Dorneles A, Morsch VM, Schetinger MR. Enzymes that hydrolyze adenine nucleotides of patients with hypercholesterolemia and inflammatory processes. FEBS J. 2007;274:2707–2714. doi: 10.1111/j.1742-4658.2007.05805.x. [DOI] [PubMed] [Google Scholar]
- Ellsworth ML. Red blood cell-derived ATP as a regulator of skeletal muscle perfusion. Med Sci Sports Exerc. 2004;36:35–41. doi: 10.1249/01.MSS.0000106284.80300.B2. [DOI] [PubMed] [Google Scholar]
- Ellsworth ML, Ellis CG, Goldman D, Stephenson AH, Dietrich HH, Sprague RS. Erythrocytes: oxygen sensors and modulators of vascular tone. Physiology (Bethesda) 2009;24:107–116. doi: 10.1152/physiol.00038.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol Heart Circ Physiol. 1995;269:H2155–2161. doi: 10.1152/ajpheart.1995.269.6.H2155. [DOI] [PubMed] [Google Scholar]
- Farias M, 3rd, Gorman MW, Savage MV, Feigl EO. Plasma ATP during exercise: possible role in regulation of coronary blood flow. Am J Physiol Heart Circ Physiol. 2005;288:H1586–1590. doi: 10.1152/ajpheart.00983.2004. [DOI] [PubMed] [Google Scholar]
- Forrester T. The identification of adenosine triphosphate in fresh human plasma. J Physiol. 1969;200:53–54P. (abstract) [PubMed] [Google Scholar]
- Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alonso J. ATP: a double-edged signalling molecule regulating the flow of oxygen. J Physiol. 2008;586:4033–4034. doi: 10.1113/jphysiol.2008.160358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res. 2002;91:1046–1055. doi: 10.1161/01.res.0000044939.73286.e2. [DOI] [PubMed] [Google Scholar]
- Greenfield ADM, Whitney RJ, Mowbray JF. Methods for the investigation of peripheral blood flow. Brit Med Bull. 1963;19:101–109. doi: 10.1093/oxfordjournals.bmb.a070026. [DOI] [PubMed] [Google Scholar]
- Hammer LW, Ligon AL, Hester RL. ATP-mediated release of arachidonic acid metabolites from venular endothelium causes arteriolar dilation. Am J Physiol Heart Circ Physiol. 2001;280:H2616–H2622. doi: 10.1152/ajpheart.2001.280.6.H2616. [DOI] [PubMed] [Google Scholar]
- Hrafnkelsdottir T, Erlinge D, Jern S. Extracellular nucleotides ATP and UTP induce a marked acute release of tissue-type plasminogen activator in vivo in man. Thromb Haemost. 2001;85:875–881. [PubMed] [Google Scholar]
- Imaizumi T, Takeshita A, Suzuki S, Yoshida M, Ando S, Nakamura M. Age-independent forearm vasodilatation by acetylcholine and adenosine 5′-triphosphate in humans. Clin Sci (Lond) 1990;78:89–93. doi: 10.1042/cs0780089. [DOI] [PubMed] [Google Scholar]
- Kirby BS, Voyles WF, Carlson RE, Dinenno FA. Graded sympatholytic effect of exogenous ATP on postjunctional α-adrenergic vasoconstriction in the human forearm: implications for vascular control in contracting muscle. J Physiol. 2008;586:4305–4316. doi: 10.1113/jphysiol.2008.154252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BS, Voyles WF, Simpson CB, Carlson RE, Schrage WG, Dinenno FA. Endothelium-dependent vasodilatation and exercise hyperaemia in ageing humans: impact of acute ascorbic acid administration. J Physiol. 2009;587:1989–2003. doi: 10.1113/jphysiol.2008.167320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch DW, Leuenberger U, Proctor DN. Augmented leg vasoconstricion in dynamically exercising older men during acute sympathetic stimulation. J Physiol. 2003;551:337–344. doi: 10.1113/jphysiol.2003.042747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravec TF, Eggers GW, Jr, Kettel LJ. Influence of patient age on forearm and systemic vascular response to hypoxaemia. Clin Sci. 1972;42:555–565. doi: 10.1042/cs0420555. [DOI] [PubMed] [Google Scholar]
- Leuenberger UA, Gray K, Herr MD. Adenosine contributes to hypoxia-induced forearm vasodilation in humans. J Appl Physiol. 1999;87:2218–2224. doi: 10.1152/jappl.1999.87.6.2218. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O’Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics – 2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- Lunkes GI, Lunkes DS, Leal D, Araujo Mdo C, Correa M, Becker L, Rosa CS, Morsch VM, Schetinger MR. Effect of high glucose levels in human platelet NTPDase and 5′-nucleotidase activities. Diabetes Res Clin Pract. 2008;81:351–357. doi: 10.1016/j.diabres.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Martin EA, Nicholson WT, Eisenach JH, Charkoudian N, Joyner MJ. Influences of adenosine receptor antagonism on vasodilator responses to adenosine and exercise in adenosine responders and nonresponders. J Appl Physiol. 2006;101:1678–1684. doi: 10.1152/japplphysiol.00546.2006. [DOI] [PubMed] [Google Scholar]
- Mortensen SP, Gonzalez-Alonso J, Bune LT, Saltin B, Pilegaard H, Hellsten Y. ATP-induced vasodilation and purinergic receptors in the human leg: roles of nitric oxide, prostaglandins, and adenosine. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1140–1148. doi: 10.1152/ajpregu.90822.2008. [DOI] [PubMed] [Google Scholar]
- Poole JG, Lawrenson L, Kim J, Brown C, Richardson RS. Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am J Physiol Heart Circ Physiol. 2003;284:H1251–1259. doi: 10.1152/ajpheart.00790.2002. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Parker BA. Vasodilation and vascular control in contracting muscle of the aging human. Microcirculation. 2006;13:315–327. doi: 10.1080/10739680600618967. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Rongen GA, Smits P, Thien T. Characterization of ATP-induced vasodilation in the human forearm vascular bed. Circulation. 1994;90:1891–1898. doi: 10.1161/01.cir.90.4.1891. [DOI] [PubMed] [Google Scholar]
- Rosenmeier JB, Hansen J, Gonzalez-Alonso J. Circulating ATP-induced vasodilatation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J Physiol. 2004;558:351–365. doi: 10.1113/jphysiol.2004.063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmeier JB, Yegutkin GG, Gonzalez-Alonso J. Activation of ATP/UTP-selective receptors increases blood flow and blunts sympathetic vasoconstriction in human skeletal muscle. J Physiol. 2008;586:4993–5002. doi: 10.1113/jphysiol.2008.155432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schetinger MR, Morsch VM, Bonan CD, Wyse AT. NTPDase and 5′-nucleotidase activities in physiological and disease conditions: new perspectives for human health. Biofactors. 2007;31:77–98. doi: 10.1002/biof.5520310205. [DOI] [PubMed] [Google Scholar]
- Schrage WG, Eisenach JH, Joyner MJ. Ageing reduces nitric-oxide- and prostaglandin-mediated vasodilatation in exercising humans. J Physiol. 2007;579:227–236. doi: 10.1113/jphysiol.2006.124313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal SS. Cell-to-cell communication coordinates blood flow control. Hypertension. 1994;23:1113–1120. doi: 10.1161/01.hyp.23.6.1113. [DOI] [PubMed] [Google Scholar]
- Segal SS, Kurjiaka DT. Coordination of blood flow control in the resistance vasculature of skeletal muscle. Med Sci Sports Exerc. 1995;27:1158–1164. [PubMed] [Google Scholar]
- Shimokawa H. Primary endothelial dysfunction: atherosclerosis. J Mol Cell Cardiol. 1999;31:23–37. doi: 10.1006/jmcc.1998.0841. [DOI] [PubMed] [Google Scholar]
- Shiramoto M, Imaizumi T, Hirooka Y, Endo T, Namba T, Oyama J, Hironaga K, Takeshita A. Role of nitric oxide towards vasodilator effects of substance P and ATP in human forearm vessels. Clin Sci (Lond) 1997;92:123–131. doi: 10.1042/cs0920123. [DOI] [PubMed] [Google Scholar]
- Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz ME, Lonigro AJ. Deformation-induced ATP release from red blood cells requires CFTR activity. Am J Physiol Heart Circ Physiol. 1998;275:H1726–1732. doi: 10.1152/ajpheart.1998.275.5.H1726. [DOI] [PubMed] [Google Scholar]
- Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. ATP: the red blood cell link to NO and local control of the pulmonary circulation. Am J Physiol Heart Circ Physiol. 1996;271:H2717–2722. doi: 10.1152/ajpheart.1996.271.6.H2717. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Magagna A, Salvetti A. Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation. 1998;97:2222–2229. doi: 10.1161/01.cir.97.22.2222. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- Takase B, Uehata A, Akima T, Nagai T, Nishioka T, Hamabe A, Satomura K, Ohsuzu F, Kurita A. Endothelium-dependent flow-mediated vasodilation in coronary and brachial arteries in suspected coronary artery disease. Am J Cardiol. 1998;82:1535–1539. A1537–1538. doi: 10.1016/s0002-9149(98)00702-4. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Sujirattanawimol K, Ruble SB, Valic Z, Joyner MJ. Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle? J Physiol. 2002;541:623–635. doi: 10.1113/jphysiol.2001.014431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ginneken EE, Meijer P, Verkaik N, Smits P, Rongen GA. ATP-induced vasodilation in human skeletal muscle. Br J Pharmacol. 2004;141:842–850. doi: 10.1038/sj.bjp.0705589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte PM. Endothelial dysfunction and atherosclerosis. Eur Heart J. 1997;18(Suppl E):E19–29. doi: 10.1016/s0195-668x(97)90005-1. [DOI] [PubMed] [Google Scholar]
- Vita JA, Treasure CB, Nabel EG, McLenachan JM, Fish RD, Yeung AC, Vekshtein VI, Selwyn AP, Ganz P. Coronary vasomotor response to acetylcholine relates to risk factors for coronary artery disease. Circulation. 1990;81:491–497. doi: 10.1161/01.cir.81.2.491. [DOI] [PubMed] [Google Scholar]
- Winter P, Dora KA. Spreading dilatation to luminal perfusion of ATP and UTP in rat isolated small mesenteric arteries. J Physiol. 2007;582:335–347. doi: 10.1113/jphysiol.2007.135202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Liu L, Meydani M, Meydani SN. Effect of vitamin E on prostacyclin (PGI2) and prostaglandin (PG) E2 production by human aorta endothelial cells: mechanism of action. Ann N Y Acad Sci. 2004;1031:425–427. doi: 10.1196/annals.1331.063. [DOI] [PubMed] [Google Scholar]
- Wu KK, Thiagarajan P. Role of endothelium in thrombosis and hemostasis. Annu Rev Med. 1996;47:315–331. doi: 10.1146/annurev.med.47.1.315. [DOI] [PubMed] [Google Scholar]
- Yasue H, Matsuyama K, Matsuyama K, Okumura K, Morikami Y, Ogawa H. Responses of angiographically normal human coronary arteries to intracoronary injection of acetylcholine by age and segment. Possible role of early coronary atherosclerosis. Circulation. 1990;81:482–490. doi: 10.1161/01.cir.81.2.482. [DOI] [PubMed] [Google Scholar]
- Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]


