Abstract
Phytochemical investigation of Hypericum empetrifolium Willd. (Clusiaceae), a species native to Greece and Turkey has led to the bioassay-guided identification of two acylphloroglucinol derivatives with potent in vitro anti-inflammatory activity. Using NMR spectroscopy and mass spectrometry, the acylphloroglucinol derivatives were characterized as 3-geranyl-1-(2′-methylpropanoyl)phloroglucinol (1) and 3-geranyl-1-(2′-methylbutanoyl)phloroglucinol (2). Hypotheses are proposed regarding the biosynthetic origin of these and similar acylphloroglucinols from related Hypericum species. Compounds 1 and 2 were evaluated for in vitro inhibitory activity against COX-1, COX-2 and 5-LOX catalyzed LTB4 formation. Compound 1 displayed good activity (IC50 values: 6.0, 29.9, and 2.2 μM, respectively) in all three assays. Compound 2 showed good activity (IC50 value: 5.8 μM) against LTB4 formation and moderate activity (IC50 value: 26.2 μM) against COX-1.
Keywords: Hypericum empetrifolium, Clusiaceae, Guttiferae, Section Coridium, Anti-inflammatory, Acylphloroglucinol derivative
1. Introduction
The region extending westward from northwestern Africa to the Caucausas is a rich zone of diversity for members of the flowering plant genus Hypericum L. (Clusiaceae), with more than 80 species occurring in Greece and Turkey alone (Robson, 1967). Endemism is also common in this region, and relatively few of the species have been phytochemically studied due to their restricted distributions and highly specific habitat requirements. However, the traditional medicinal use of many Hypericum species, as well as the contemporary usage of species such as Hypericum perforatum L. (common St. John's Wort), a valuable medicinal herb taken to treat symptoms of mild to moderate depression, supports the need for further phytochemical study of these morphologically and ecologically diverse plants (Avato, 2005).
Natural populations of Hypericum empetrifolium Willd. are found primarily throughout the southern part of the Grecian mainland and the coastal area of western Turkey (Robson, 1967). This species has been taxonomically placed in section Coridium, a section comprising six species, within which Hypericum amblycalyx Coust. & Gand. and Hypericum jovis Greuter (both endemic to Crete) represent those morphologically most closely allied with H. empetrifolium (Robson, 1981). Phytochemical studies have revealed the presence of acylphloroglucinol derivatives in H. amblycalyx (Winkelmann et al., 2003) and H. jovis (Athanasas et al., 2004), which possess cytotoxic, antibacterial or antioxidant bioactivities.
Previously, phytochemical investigations of H. empetrifolium have described the presence of naphthodianthrones and flavonoids in crude extracts of the flowering plant (Kitanov, 2001; Makovetska, 2000) and the composition of the essential oil (Petrakis, Couladis, & Roussis, 2005). The three other species in section Coridium are Hypericum asperulifolium (eastern Transcaucasia), Hypericum coris (central and western Alps) and Hypericum ericoides (southeastern Spain, Morocco and Tunisia). Flavonoids, xanthones and xantholignoids have been previously reported from H. ericoides (Cardona & Seoan, 1982a, 1982b, 1983), while flavonoids and naphthodianthrones have been reported from H. coris (Makovetska, 2000).
In Turkey, the local name for H. empetrifolium of sari piren (sari meaning yellow) alludes to the use of decoctions of the flowers to dye cloth in western Anatolia (Baytop, 1999). Medicinally, a decoction of the flowering tops of this species is taken against kidney stones and to treat gastric ulcers (Tuzlacy, 2006). In Greece, decoctions of H. empetrifolium, as well as other native Hypericum species, are taken internally as an anthelmintic and diuretic and used externally as a wash to speed wound-healing, heal scalds, and treat outbreaks of herpes (Vokou et al., 1993). The anti-inflammatory and analgesic effects of an uncharacterized crude methanol extract from the flowering aerial plant portion have been studied in vivo using male Wistar rats as test models (Trovato et al., 2001).
An initial in vitro assessment of the anti-inflammatory activity of a particular crude plant extract or purified compounds can be performed by measuring the inhibition of cyclooxygenase (COX) and lipoxygenase (LOX) enzymatic activity. COX-1, COX-2 and 5-LOX are the key enzymes of arachidonic acid metabolism that lead to the production of important mediators of inflammation. COX-1 and -2 catalyze the first two steps in prostaglandin synthesis and 5-LOX catalyzes the oxygenation of arachidonic acid in the first step of the leukotriene pathway.
COX-1 is constitutively expressed and COX-1 derived prostanoids maintain the integrity of gastrointestinal mucosa and, therefore, inhibition of COX-1-derived prostanoid production has been believed to cause side effects such as gastrointestinal damage and ulcers. Because COX-2 expression is primarily induced by various external stimuli, the inhibition of COX-2 derived prostanoids has been believed to be responsible for anti-inflammatory, analgesic and anti-pyretic effects (Calanni & Laufer, 2003; Smith, DeWitt, & Garavito, 2000). Thus, in recent years selective COX-2 inhibitors have been generally preferred over nonselective ones, although side effects such as cardiovascular problems have been reported for certain selective COX-2 inhibitors (Mukherjee, Nissen, & Topol, 2001), and it is now known that COX-2 is expressed constitutively in some tissues (Mattia & Coluzzi, 2005).
Leukotrienes such as LTB4, formed through the activity of 5-LOX, are potent mediators of inflammatory and allergic reactions. 5-LOX inhibitors are, therefore, considered to possess therapeutic potential against a variety of allergic and inflammatory conditions, with bronchial asthma figuring as the main 5-LOX related disease (Calanni & Laufer, 2003; Werz, 2007). An alternative approach to selective COX-2 inhibition might be the simultaneous blockage of COX and 5-LOX pathways by dual inhibitors. Drugs possessing these characteristics are believed to have a higher efficacy (i.e. stronger anti-inflammatory effect) and, because the preferential conversion of arachidonic acid to leukotrienes that is observed for medications inhibiting only the COX pathway is avoided, such drugs generally are believed to have lower gastric toxicity (Naveau, 2005).
As part of the current research, the bioactivity-guided fractionation of a crude extract from the fruits of H. empetrifolium was performed using cyclooxygenase-1 (COX-1), cyclooxygenase-2 (COX-2) assays and a 5-lipoxygenase (5-LOX) product formation assay, resulting in the isolation of two acylphloroglucinol derivatives with potent in vitro anti-inflammatory activities.
2. Results and discussion
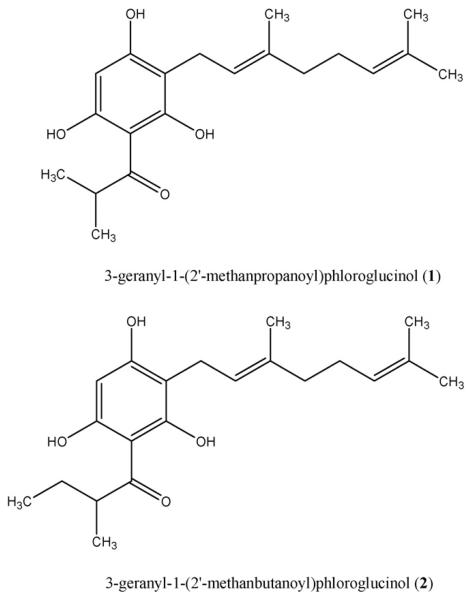
Plants of H. empetrifolium were grown from seed under common garden conditions. Fruits (collected while semi-ripe: green to yellowing) were collected, dried, and were rinsed exhaustively with dichloromethane. The goal was to capture compounds that could be expected in an oil-extract of the fruits, but also obtain some of the compounds of low to medium polarity that might reasonably be expected in a water-decoction. The methanol-soluble portion of the dichloromethane extract was then further fractionated by repeated normal- and reverse-phase chromatography, yielding two acylphloroglucinol derivatives, 3-geranyl-1-(2′-methylpropanoyl)phloroglucinol (1) and 3-geranyl-1-(2′-methylbutanoyl)phloroglucinol (2) (Fig. 1). The proton and carbon chemical shift values of these compounds were identical to those reported by Rios and Delgado (1992).
Fig. 1.
Acylphloroglucinols isolated from Hypericum empetrifolium.
Compounds 1 and 2 are acylphloroglucinol derivatives that have been previously described from several species of Helichrysum, Achyrocline (Asteraceae) and Esenbeckia (Rutaceae). Compound 1 has been most recently isolated from two additional species of Hypericum: Hypericum styphelioides (section Brathys) and, interestingly, H. jovis (section Coridium) (for references see Table 1). The current study represents the first report of the isolation of compound 2 from Hypericum.
Table 1.
Plants from which compounds 1 and 2 have been previously isolated
| Family | Species | Compound 1a | Compound 2a | Reference |
|---|---|---|---|---|
| Asteraceae | Achyrocline alata | 0.10 | 0.06 | Bohlmann, Zdero, Abraham, Suwita, and Grenz (1980) |
| Helichrysum indicum | 0.24 | 1.18 | Jakupovic et al. (1989) | |
| Helichrysum infaustum | 0.01 | ND | Bohlmann and Suwita (1979) | |
| Helichrysum krookii | 0.15–0.33 | ND | Bohlmann, Abraham, Robinson, and King (1980) | |
| Helichrysum moeserianum | 0.02 | 0.07 | Jakupovic et al. (1989) | |
| Helichrysum natalitium | 1.40 | 0.14 | Bohlmann and Zdero (1979) | |
| Helichrysum stenopterum | 0.01 | 0.01 | Jakupovic, Kuhnke, Schuster, Metwally, and Bohlmann (1986) | |
| Clusiaceae | Hypericum empetrifolium | 0.03 | 0.03 | Current study |
| Hypericum jovis | 0.40 | ND | Athanasas et al. (2004) | |
| Hypericum styphelioides | 0.001 | ND | Gamiotea-Turro et al. (2004) | |
| Rutaceae | Esenbeckia nesiotica | 0.01 | 0.04 | Rios and Delgado (1992) |
ND, not detected or reported.
Reported in % (w/w) extracted plant material.
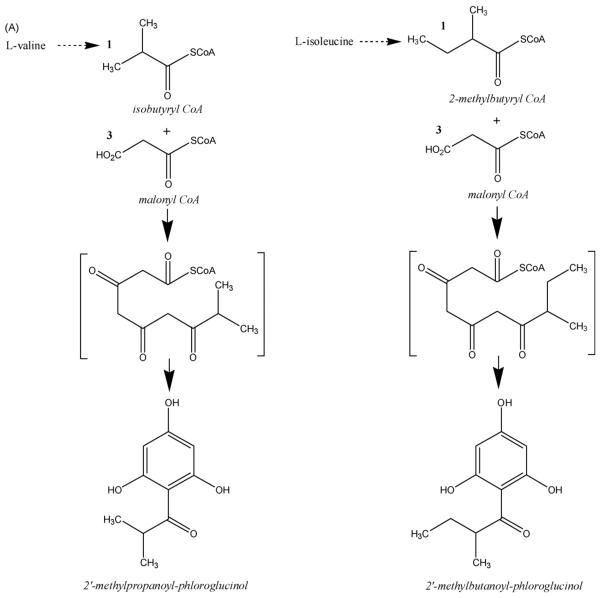
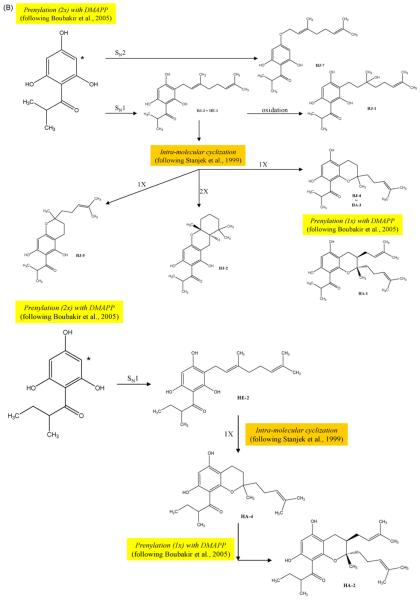
A working hypothesis that acylphloroglucinol compounds are chemotaxonomically valuable for assessing relationships within the genus of Hypericum has been proposed, but due to the fact that fewer than 40% of the species have been in any way phytochemically studied, this hypothesis has rarely been systematically tested. The isolation of these compounds from H. empetrifolium provided an interesting opportunity to compare the diversity of acylphloroglucinol structures present in a group of three Hypericum species, believed to be closely inter-related on the basis of morphological characteristics and geographic distribution patterns. Fig. 2 illustrates proposed biosynthetic origins for the various acylphloroglucinol structures that have been, to date, isolated from H. empetrifolium, H. amblycalyx and H. jovis.
Fig. 2.
(A) Proposed biosynthetic route to phloroglucinol moiety and acyl side chain in the skeleton (summarized from Adam, Arigoni, Bacher, & Eisenreich, 2002; Karppinen, Hokkanen, Tolonen, Mattila, & Hohtola, 2007; Klinglauf et al., 2005). (B) Proposed biosynthetic elaboration upon the skeleton, leading to acylphloroglucinols isolated from H. empetrifolium (HE), Hypericum jovis (HJ) (compound numbering from Athanasas et al., 2004) and Hypericum amblycalyx (HA) (compound numbering from Winkelmann et al., 2003) (Boubakir, Beuerle, Liu, & Beerhues, 2005; Stanjek, Miksch, Lueer, Matern, & Boland, 1999).
As an additional aspect of the current research, we wished to demonstrate that careful cultivation, selection of particular parts of the plant at the appropriate stage of growth, and use of a small-scale investigatory method, developed in our laboratory, could result in the isolation of structurally and medicinally interesting natural products from a relatively small amount of plant material in quantities sufficient for structural elucidation and in vitro bioactivity testing. A significant challenge to conducting phytochemical isolation and structural elucidation studies with endemic species and those with restricted distributions is overcoming the difficulty of access to appropriate amounts of plant material. Through this research, we demonstrate that cultivation of a desired plant species from cuttings or seeds represents a valuable alternative to the harvesting of plants from wild populations.
When compound 1 was previously isolated from H. styphelioides, it was tested for free-radical scavenging activity and displayed moderate activity as compared to the positive control substance, quercetin (Gamiotea-Turro et al., 2004). Its antioxidant activity, after being isolated from H. jovis, was also evaluated and found comparable to that of the positive control, Trolox (a water-soluble form of vitamin E) (Athanasas et al., 2004). The authors of the latter work suggested that the three free hydroxyl groups present in the molecule enhanced radical scavenging activity. A potential future research direction would be to examine the antioxidant activity of compound 2, as compared to both compound 1 and other compounds with three free hydroxyl groups isolated from H. jovis.
However, neither crude extracts nor purified compounds from these species, or other species believed to be closely related to H. empetrifolium, have been previously tested in vitro for their anti-inflammatory activity. In the in vivo study of anti-inflammatory and analgesic effects of a crude methanol extract (100 mg/kg i.p.) of H. empetrifolium by Trovato et al. (2001), an inhibition of edema (carrageenan-induced paw edema test) was observed, indicating anti-inflammatory activity. In addition a selective analgesic effect, in that an inhibition of writhing contractions (acetic-acid test) but not against pain induced by the thermal stimulus (hot-plate test), was observed. The authors hypothesized that the observed anti-inflammatory and analgesic effects of the studied H. empetrifolium extract may have been related to an inhibition of prostaglandin synthesis, but further work was not conducted to identify and isolate active substances from the uncharacterized methanolic extract.
In the current study, initial bioassay testing of the crude dichloromethane extract from fruits of H. empetrifolium against enzymes involved in inflammation response (COX-1, COX-2 and 5-LOX product formation) had indicated a very high (99.7 ± 0.1%) inhibition of LTB4 formation in the in vitro assay, tested at 20 μg/mL. COX-1 and COX-2 were also significantly inhibited (85.1 ± 4.0 and 49.1 ± 2.8% inhibition, respectively). The two acylphloroglucinol derivatives (compounds 1 and 2) present as significant components of the extracts of fruits of H. empetrifolium were subsequently isolated and their in vitro anti-inflammatory activity evaluated. Compound 1 demonstrated good activity against COX-1 (IC50 6.0 μM) and 5-LOX mediated LTB4 formation (IC50 2.2 μM), and moderate activity against COX-2 (IC50 29.9 μM).
Interestingly, despite the high degree in similarity between the structures, compound 2 demonstrated only moderate COX-1 (IC50 26.2 μM) activity, but maintained a significant inhibitory activity against 5-LOX product formation (IC50 5.8 μM). These results suggest that the lengthening of the side chain at position 1 (e.g. the moiety with proposed biosynthetic origin from l-isoleucine as opposed to l-valine), meta to the geranyl moiety, reduced bioactivity slightly in vitro against LT formation and significantly against COX-1 and COX-2.
The observed inhibitory activity against both prostaglandin and leukotriene formation indicated that the tested compounds might act as dual COX/LOX inhibitors. This hypothesis is corroborated by the results of Albert et al. (2002) who found that hyperforin, a structurally related acylphloroglucinol, acts as a dual COX-1/5-LOX inhibitor in cellular as well as in cell-free assays. These results could explain in part the observed activity of H. empetrifolium extracts against inflammatory pain in rats in vivo, and support their traditional usage to speed wound healing in humans. A desired next step for this research is to perform in vivo anti-inflammatory and analgesic tests with isolated compounds 1 and 2, as well as potentially with other acylphloroglucinols isolated from related species of Hypericum.
3. Experimental
3.1. General experimental procedures
VLC was performed using a C18 SepPak cartridge (AllTech Association, Deerfield, IL), eluting with a H2O/methanol gradient (10% steps, 80:20 to 0:100). Analytical TLC was performed on silica gel 60 F254 plates (Merck), eluting with hexane/EtOAc 75:25, visualization as reddish-purple bands by spraying with H2SO4 (10% solution, v/v in 95% aq. EtOH) and then vanillin (5% solution, w/v in 95% aq. EtOH) reagents, followed by heating at 150 °C for 45 s and detection under UV/VIS light at 254 and 365 nm. Preparative HPLC was performed on an Agilent 1100 Separations Module equipped with a photodiode array detector (Agilent Technologies, USA), using a LiChroCART RP-18 column (LiChrospher, 7 μm, 10 mm × 250 mm; Merck), flow rate 2 mL/min, UV detection at 254 nm, and an isocratic elution system: H2O/MeCN (20:80). HPLC-DAD/ESI-MS (neg.) was performed on a Thermo Finnigan Surveyor liquid chromatography instrument with Thermo Quest Surveyor photodiode array detector, autosampler, and MS pump, and a Thermo Finnigan LCQ-XP mass detector equipped with an electro-spray ionization (ESI) source run by Xcaliber software. Analytical HPLC was performed using a Zorbax SB RP-18 column (3 μm, 2.1 m × 150 m; Agilent Technologies), flow rate 250 μL/min, gradient elution H2O/MeOH, UV detection at 254 nm (25:75 to 0:100 over 20 min, 10 min held at 0.100, 10 min equilibration). Mass spectra were detected and recorded in a scan range of m/z 50–1000, using a transfer capillary temperature of 350 °C, a spray voltage of 5.00 kV and a sheath gas flow of 70 units. 1H-, 13C-, and 2D-NMR experiments (HSQC, HMBC, DQF-COSY) were performed with Varian Unity-Inova-400 and −600 MHz spectrometers. The compounds 1 and 2 were dissolved in CDCl3 and spectra were recorded at 25 °C. Experimental parameters were as published in (Seebacher, Simic, Weis, Saf, & Kunert, 2003).
3.2. Plant material
Seeds of H. empetrifolium were obtained as a personal gift from Dr. N.K.B. Robson and cultivated at the Graz Botanical Garden (Graz, Austria). The identity of the flowering plants was verified by S. Crockett using taxonomic keys published by Robson (1967). Mature fruits, collected while semi-ripe (dark green to slightly yellowing), were air-dried in a darkened, ventilated cabinet to a moisture content of less than 5%. A voucher specimen has been deposited at the Department of Pharmacognosy at Karl-Franzens-University (Graz, Austria).
3.3. Extraction and isolation
10 g of air-dried fruits of H. empetrifolium were exhaustively rinsed with DCM (5×, 100 mL). Extracts were combined and dried under vacuum in a rotary evaporator. The resulting ca. 250 mg of reddish-brown extract was dissolved in MeOH/H2O (8:2) and placed in the freezer overnight in order to facilitate the precipitation of waxes and triterpenes. Centrifugation of the dissolved extract (3000 rpm, 3 min) yielded 125 mg of precipitate. The supernatant was dried under vacuum and yielded 121 mg of extract, which was subsequently loaded on a RP-18 SepPak cartridge and subjected to VLC using an H2O/MeOH gradient (10% steps, 80:20 to 0:100). The resulting nine fractions were examined using analytical TLC and HPLC. Fraction 7 (26 mg) was further fractionated by semi-preparative HPLC (on a LiChrospher C18, 7 μm, 10 mm × 250 mm column) using an isocratic system of 20:80 H2O/MeCN, yielding compounds 1 (3 mg) and 2 (3 mg).
3.4. Anti-inflammatory testing
Sample preparation and evaluation of results: Samples were dissolved in absolute EtOH. Extracts were tested at a final concentration of 20 μg/mL in the assay mixture, and pure compounds at 50 μM. Each sample was tested in at least three independent experiments, and each experiment was performed in duplicate. Results are given as means of ±S.D. Compounds showing inhibitory activity at the screening concentration were subjected to IC50 determination by testing them in at least three different concentrations. Calculation of IC50 values was performed by semilogarithmic presentation of dose vs. inhibitory activity and logarithmic regression analysis. In vitro assay for COX-1 and COX-2 inhibitory activity: Assays for COX-1 and COX-2 inhibition were performed in a 96-well-plate format with purified prostaglandin H synthase (PGHS)-1 from ram seminal vesicles for COX-1 and purified PGHS-2 from sheep placental cotyledons for COX-2 (both Cayman Chemical Company, Ann Arbor, USA) as previously described (Fiebich et al., 2005; Reininger & Bauer, 2006). The concentration of PGE2, the main arachidonic acid metabolite in this reaction, was determined by a competitive PGE2 EIA kit (Assay Designs Inc., Ann Arbor, MI, USA). Indomethacin (ICN, Aurora, USA; IC50 COX-1 0.9 μM) and NS-398 (Cayman Chemical Company, Ann Arbor, USA; IC50 COX-2 2.6 μM) were used as positive controls. In vitro assay for leukotriene formation inhibitory activity: The bioassay for inhibition of leukotriene formation was carried out in 96-well-plate format as described by Adams, Kunert, Haslinger, and Bauer (2004) with slight modifications. Briefly, polymorpho-nuclear leukocytes with 5-LOX activity were isolated from venous human blood based on sedimentation rates and lysis tolerance. Cell viability was checked with 0.4% trypan blue solution. The cell suspension (4500 cells/mL) was incubated with the sample, CaCl2, calcimycin A23187 and arachidonic acid in a shaking water bath at 37 °C. After 10 min incubation was stopped by addition of 10% HCO2H. After centrifugation, the samples were diluted and the concentration of LTB4 formed during incubation was determined by means of a competitive LTB4 EIA kit (Assay Designs Inc., Ann Arbor, USA). Zileuton (Sequoia, Oxford, UK; IC50 5.0 μM) was used as positive control.
Acknowledgments
Sincere thanks to Dr. Norman K.B. Robson for the gift of H. empetrifolium seeds and to the Graz Botanical Garden for care and cultivation of the growing plants. Thanks to Andrea Fleck for assistance in collecting NMR spectra. Thanks to Dr. Betul Demirci for access to Turkish references on ethnobotanical usage of H. empetrifolium.
Footnotes
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
References
- Adam P, Arigoni D, Bacher A, Eisenreich W. Biosynthesis of hyperforin in Hypericum perforatum. Journal of Medicinal Chemistry. 2002;45:4786–4793. doi: 10.1021/jm0209782. [DOI] [PubMed] [Google Scholar]
- Adams M, Kunert O, Haslinger E, Bauer R. Inhibition of leukotriene biosynthesis by quinolone alkaloids from the fruits of Evodia rutaecarpa. Planta Medica. 2004;70:904–908. doi: 10.1055/s-2004-832614. [DOI] [PubMed] [Google Scholar]
- Albert D, Zündorf I, Dingermann T, Müller WE, Steinhilber D, Werz O. Hyperforin is a dual inhibitor of cyclooxygenase-1 and 5-lipoxygenase. Biochemical Pharmacology. 2002;64:1767–1775. doi: 10.1016/s0006-2952(02)01387-4. [DOI] [PubMed] [Google Scholar]
- Athanasas K, Magiatis P, Fokialakis N, Skaltsounis A-L, Pratsinis H, Kletsas D. Hyperjovinols A and B: Two new phloroglucinol derivatives from Hypericum jovis with antioxidant activity in cell cultures. Journal of Natural Products. 2004;67:973–977. doi: 10.1021/np034051w. [DOI] [PubMed] [Google Scholar]
- Avato P. A survey of the Hypericum genus: Secondary metabolites and bioactivity. Studies of Natural Products and Chemistry (Bioactive Natural Products, Part K) 2005;30:602–634. [Google Scholar]
- Baytop T. Therapy with plants in Turkey: Past and present. Nobel Typ Basymevi; Istanbul: 1999. [Google Scholar]
- Bohlmann F, Abraham W-R, Robinson H, King RM. A new labdane derivative and geranylphloroglucinols from Achyrocline alata. Phytochemistry. 1980b;19:2475–2477. [Google Scholar]
- Bohlmann F, Suwita A. Weitere phloroglucin-derivate aus Helichrysum arten. Phytochemistry. 1979;18:2046–2049. [Google Scholar]
- Bohlmann F, Zdero C. Neue phloroglucin-derivate aus Helichrysum natalitium und Helichrysum bellum. Phytochemistry. 1979;18:641–644. [Google Scholar]
- Bohlmann F, Zdero C, Abraham W-R, Suwita A, Grenz M. Neue diterpene und neue dihydrochalkon-derivate sowi weitere inhaltsstoffe aus Helichrysum arten. Phytochemistry. 1980a;19:873–879. [Google Scholar]
- Boubakir Z, Beuerle T, Liu B, Beerhues L. The first prenylation step in hyperforin biosynthesis. Phytochemistry. 2005;66:51–57. doi: 10.1016/j.phytochem.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Calanni F, Laufer S. Biochemistry and mediators of inflammation. In: Laufer S, Gay S, Brune K, editors. Inflammation and rheumatic diseases. The molecular basis of novel therapies. Georg Thieme Verlag; Stuttgart, Germany: 2003. pp. 15–57. [Google Scholar]
- Cardona ML, Seoan E. Flavonoids and xanthonolignoids of Hypericum ericoides. Phytochemistry. 1982a;21:2759–2760. [Google Scholar]
- Cardona ML, Seoan E. Xanthone constituents of Hypericum ericoides. Journal of Natural Products. 1982b;45:134–136. [Google Scholar]
- Cardona ML, Seoan E. Flavonoids and xanthones of Hypericum ericoides L. Anales de Quimica Serie C: Quimica Organica Bioquimica. 1983;79:144–148. [Google Scholar]
- Fiebich BL, Grozdeva M, Hess S, Hüll M, Danasch U, Bodensieck A, et al. Petasites hybridus extracts in vitro inhibit COX-2 and PGE2 release by direct interaction with the enzyme and by preventing p42/44 MAP kinase activation in rat primary microglial cells. Planta Medica. 2005;71:12–19. doi: 10.1055/s-2005-837744. [DOI] [PubMed] [Google Scholar]
- Gamiotea-Turro D, Cuesta-Rubio O, Prieto-González S, De Simone F, Passi S, Rastrelli L. Antioxidative constituents from the leaves of Hypericum styphelioides. Journal of Natural Products. 2004;67:869–871. doi: 10.1021/np030364f. [DOI] [PubMed] [Google Scholar]
- Jakupovic J, Kuhnke J, Schuster A, Metwally MA, Bohlmann F. Phloroglucinol derivatives and other constituents from South African Helichrysum species. Phytochemistry. 1986;25:1133–1142. [Google Scholar]
- Jakupovic J, Zdero C, Grenz M, Tsichritzis F, Lehmann L, Hashemi-Nejad S, et al. Twenty-one acylphloroglucinol derivatives and further constituents from South African Helichrysum species. Phytochemistry. 1989;28:1119–1131. [Google Scholar]
- Karppinen K, Hokkanen J, Tolonen A, Mattila S, Hohtola A. Biosynthesis of hyperforin and adhyperforin from amino acid precursors in shoot cultures of Hypericum perforatum. Phytochemistry. 2007;68:1038–1045. doi: 10.1016/j.phytochem.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Kitanov GM. Hypericin and pseudohypericin in some Hypericum species. Biochemical Systematics and Ecology. 2001;29:171–178. doi: 10.1016/s0305-1978(00)00032-6. [DOI] [PubMed] [Google Scholar]
- Klinglauf P, Beuerle T, Mellenthin A, El-Moghazy SAM, Boubakir Z, Beerhues L. Biosynthesis of the hyperforin skeleton in Hypericum calycinum cell cultures. Phytochemistry. 2005;66:139–145. doi: 10.1016/j.phytochem.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Makovetska OY. Research of biologically active substances of Hypericum L. species. Farmatsevticheskii Zhurnal. 2000;5:40–47. [Google Scholar]
- Mattia C, Coluzzi F. COX-2 inhibitors: Pharmacological data and adverse effects. Minerva Anestesiologica. 2005;71:461–470. [PubMed] [Google Scholar]
- Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. Journal of The American Medical Association. 2001;86:954–959. doi: 10.1001/jama.286.8.954. [DOI] [PubMed] [Google Scholar]
- Naveau B. Editorial: Dual inhibition of cyclo-oxygenases and 5-lipoxygenase: A novel therapeutic approach to inflammation? Joint Bone Spine. 2005;72:199–201. doi: 10.1016/j.jbspin.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Petrakis PV, Couladis M, Roussis V. A method for detecting the biosystematic significance of the essential oil composition: The case of five Hellenic Hypericum L. species. Biochemical Systematics and Ecology. 2005;33:873–898. [Google Scholar]
- Reininger EA, Bauer R. Prostaglandin-H-synthase (PGHS)-1 and 2 microtiter assays for the testing of herbal drugs and in vitro inhibition of PGHS-isoenzymes by polyunsaturated fatty acids from Platycodi radix. Phytomedicine. 2006;13:164–169. doi: 10.1016/j.phymed.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Rios M, Delgado G. Polyprenols and acylphloroglucinols from Esenbeckia nesiotica. Phytochemistry. 1992;31:3491–3494. [Google Scholar]
- Robson NKB. Hypericum. In: Davis PH, editor. Flora of Turkey and the East Aegean Islands. Vol. 2. Edinburgh University Press; Edinburgh: 1967. pp. 355–401. [Google Scholar]
- Robson NKB. Studies in the genus Hypericum L. (Guttiferae). 2. Characters of the genus. Bulletin of The British Museum of Natural History (Botany) 1981;8(2):55–226. [Google Scholar]
- Seebacher W, Simic N, Weis R, Saf R, Kunert O. Complete assignments of 1H and 13C NMR resonances of oleanolic acid, 18α-oleanlic acid, ursolic acid and their 11-oxo derivatives. Magnetic Resonance in Chemistry. 2003;41:636–638. [Google Scholar]
- Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: Structural, cellular and molecular biology. Annual Review of Biochemistry. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- Stanjek V, Miksch M, Lueer P, Matern U, Boland W. Biosynthesis of psoralen: Mechanism of a cytochrome P450 catalyzed oxidative bond cleavage. Angewandte Chemie—International Edition. 1999;38:400–402. doi: 10.1002/(SICI)1521-3773(19990201)38:3<400::AID-ANIE400>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Trovato A, Raneri E, Kouladis M, Tzakou O, Taviano MF, Galati EM. Anti-inflammatory and analgesic activity of Hypericum empetrifolium Willd. (Guttiferae) Il Farmaco. 2001;56:455–457. doi: 10.1016/s0014-827x(01)01061-8. [DOI] [PubMed] [Google Scholar]
- Tuzlacy E. Turkiye'nin Bitkisel Halk Ylaclary (Herbal folk medicines) Alfa Basymyaym Daoytym Pirketi; Istanbul: 2006. [Google Scholar]
- Vokou D, Katradi K, Kokkini S. Ethnobotanical survey of Zagori (Epirus, Greece), a renowed centre of folk medicine in the past. Journal of Ethnopharmacology. 1993;39:187–196. doi: 10.1016/0378-8741(93)90035-4. [DOI] [PubMed] [Google Scholar]
- Werz O. Inhibition of 5-lipoxygenase product synthesis by natural compounds of plant origin. Planta Medica. 2007;73:1331–1357. doi: 10.1055/s-2007-990242. [DOI] [PubMed] [Google Scholar]
- Winkelmann K, San M, Kypriotakis Z, Skaltsa H, Bosilij B, Heilmann J. Antibacterial and cytotoxic activity of prenylated bicyclic acylphloroglucinol derivatives from Hypericum amblycalyx. Zeitschrift fur Naturforschung C: A Journal of Biosciences. 2003;58:527–532. doi: 10.1515/znc-2003-7-814. [DOI] [PubMed] [Google Scholar]