Abstract
Few data are available on soil ciliates from Asia. Thus, seven samples were collected in Singapore in February 1987 and investigated between December 1987 and May 1989, using the non-flooded Petri dish method, live observation, and silver impregnation. One hundred and three ciliate taxa, all new for the fauna of Singapore and Malaysia, were found. This applies also to Hemimastix amphikineta, a highly characteristic, euglenid flagellate with Gondwanan distribution. At least three undescribed ciliate species were discovered, viz., Ottowphrya magna, which has been published by Foissner (1993), Dileptus microstoma Vd’ačný & Foissner (2008), and Suturothrix monoarmata, which is described in the present paper. The new genus Suturothrix belongs to the order Haptorida and is unique in having a heteromorphic dorsal brush consisting of three staggered rows, thus forming a suture with the last right side ciliary row. Suturothrix monoarmata is a slender, middle-sized (~ 100 × 15 μm) ciliate easily recognisable by the single or two thick extrusomes in the centre of the minute oral bulge. The species is not restricted to Asia but has been found also in soil from the Amazon floodplain, Brazil.
Keywords: Asian soil ciliates, Brazil soil ciliates, Dileptus microstoma, Hemimastix amphikineta, Ottowphrya magna
1. Introduction
About 1000 ciliate species have been recorded from soil (Foissner et al. 2002), but statistical analyses suggest a much higher global diversity, viz., about 2000 species (Chao et al. 2006) or more, if semiterrestrial habitats are included, floodplain soils for instance (Foissner et al. 2008). Most described soil ciliate diversity is from Central Europe, Africa, and Antarctica (for reviews, see Foissner 1987a, b, 1996, 2000, Foissner et al. 2002), while only scattered information is available from America, Australia, and Asia (Shibuya 1930, Foissner 1986, 1988, 1993, Blatterer & Foissner 1988, Berger & Foissner 1989, Foissner et al. 2002, Foissner & Xu 2007).
The present study contributes to the Asian soil ciliate diversity by investigating seven samples from Singapore collected during a short stay on the way to Australia. Singapore and Malaysia are interesting from the biogeographical perspective because they are located in the transition zone of Gondwana and Laurasia, i.e. the two super-continents formed after the break up of Pangaea (Müller 1981). There is strong evidence that this deeply influenced not only the distribution of vascular plants and larger animals but also of protists (Foissner 2006).
2. Materials and methods
The material is from Singapore Island, i.e., from the south end of Malaysia, E103° N1°. Two additional sites from other countries are mentioned in the section on occurrence and ecology of Suturothrix monoarmata nov. spec. Seven samples were taken in February 1987, air-dried for one month, sealed in plastic bags, and investigated in December 1987 (samples 1 – 3, 7), June 1988 (sample 4), and May 1989 (samples, 5, 6). Sampling, taxonomic methods, and identification follow Foissner (1991) and Foissner et al. (2002). The samples were processed with the non-flooded Petri dish method (NFPM), and species were identified in vivo; identification of difficult taxa was checked in protargol preparations. Briefly, the NFPM involves placing 50 – 500 g litter and soil in a Petri dish (13 – 18 cm wide, 2 – 3 cm high) and saturating, but not flooding it, with distilled water. Such culture is analysed for ciliates by inspecting about 2 ml of the run-off on days 2, 7, 14, 21, and 28; for a detailed description of the NFPM, see Foissner et al. (2002).
Site 1: East coast, about 200 m inshore. Grassland with shrubs. Soil loamy and very hard, but not saline, pH 6 in water. Thus, mainly the litter layer (0 – 2 cm) was sampled.
Site 2: East coast, small sand dune with sparse vegetation on sea shore. Litter, roots, and sand were mixed to a composite sample with pH 7.1 in water.
Site 3: East coast, sea shore. Litter and the upper, sandy soil layer under a large tree were mixed to a composite sample with pH 5.7 in water.
Site 4: Primary rain forest in the Bukit Timah Nature Reserve about 12 km NW of the city. Mosses and adhering soil on granitic rocks were collected; pH 3.9 in water.
Site 5: As site 4; the upper 0 – 5 cm litter and soil layer, including the root carpet were mixed to a composite sample with pH 3.3 in water.
Site 6: As sample 5, but from another site of the Nature Reserve; pH 3.6 in water.
Site 7: East coast, sea shore. Dry leaves, sandy soil, and grass roots under a large tree were mixed to a composite sample with pH 7 in water; not saline.
3. Results and discussion
Faunistics
One hundred and three species were found in the seven samples investigated (Tab. 1). Of these, 93 were determined to species and subspecies level, while ten taxa could not be identified, either because they were too rare or were found only in the protargol slides. Three of the ten unidentified species are possibly undescribed, especially the species from sample 4, which is a Pseudokeronopsis-like, hypotrichous ciliate possibly representing even a new genus. Although there were sufficient specimens in the protargol slides, I could not describe it due to the lack of live observations.
Tab. 1.
Ciliate species diversity in six soil samples from Singapore
| Species a | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Amphisiella australis | − | − | − | + | − | − | − |
| Anteholosticha australis | − | − | − | − | − | − | + |
|
Arcuospathidium namibiense tristicha |
+ | − | − | − | − | − | + |
| Birojimia muscorum | − | − | − | − | − | − | + |
| Blepharisma hyalinum | + | − | − | − | − | − | − |
| Bresslaua vorax | + | + | − | − | − | − | − |
| Bresslauides terricola b | − | − | + | − | − | − | − |
| Bursaria truncatella | + | − | − | − | − | − | − |
|
Byrometopus pseudochilodon |
+ | − | − | + | − | − | − |
|
Caudiholosticha sylvatica |
+ | − | + | − | − | − | + |
|
Chilodontopsis muscorum c |
− | − | + | − | − | − | − |
|
Cinetochilum margaritaceum |
− | − | + | + | − | − | + |
| Colpoda cucullus | + | + | + | + | − | − | + |
| Colpoda edaphoni | − | − | + | − | − | − | − |
| Colpoda henneguyi | − | + | + | − | − | − | + |
| Colpoda inflata | + | + | + | + | − | − | + |
| Colpoda lucida | + | − | + | − | − | − | − |
| Colpoda magna | − | − | − | − | − | − | + |
| Colpoda maupasi | + | + | + | + | − | − | + |
| Colpoda steinii | − | + | + | + | + | + | + |
| Colpoda tripartita | − | + | − | − | − | − | − |
| Cultellothrix atypicum | + | − | − | − | − | − | − |
| Cyrtohymena candens | + | − | + | − | − | − | + |
| Cyrtohymena citrina | + | + | − | − | − | − | − |
|
Cyrtohymena quadrinucleata |
− | + | − | − | − | − | − |
| Cyrtolophosis mucicola | + | + | + | + | − | + | − |
|
Decliviostoma namibiensis d |
+ | − | − | − | − | − | − |
| Dileptus anguillula | − | − | − | − | + | − | − |
| Dileptus microstoma (n. sp; see Vd’ačný & Foissner, 2008) | − | − | − | − | − | − | + |
|
Dimacrocaryon amphileptoides |
− | − | − | − | − | − | + |
|
Drepanomonas musicola |
+ | − | + | − | − | − | − |
|
Drepanomonas pauciciliata |
+ | − | + | + | − | − | − |
| Drepanomonas revoluta | − | − | − | − | − | − | + |
| Drepanomonas sphagni | − | − | − | + | − | − | − |
| Enchelys multinucleata | − | + | − | − | − | − | − |
|
Epispathidium amphoriforme |
+ | − | − | − | − | − | − |
| Epispathidium terricola | + | − | + | − | − | − | + |
| Euplotes muscicola | − | − | − | − | − | − | + |
| Fuscheria terricola e | + | − | − | − | − | − | − |
| Gastronauta derouxi | − | − | − | − | − | − | + |
| Gonostomum affine | + | − | + | − | − | + | + |
| Grossglockneria acuta | + | − | − | − | − | − | − |
|
Grossglockneria hyalina |
− | − | + | − | − | − | − |
| Halteria grandinella | + | − | − | − | − | − | − |
|
Hausmanniella discoidea |
− | − | + | − | − | − | + |
| Hausmanniella patella | + | + | − | + | − | + | − |
|
Hemiamphisiella terricola |
− | + | − | − | − | − | + |
| Hemisincirra inquieta | + | + | − | − | − | − | + |
| Hemisincirra wenzeli | − | − | − | + | − | − | − |
| Holostichides terricola | − | − | + | − | − | − | − |
| Homalogastra setosa | − | + | + | − | − | − | + |
|
Leptopharynx costatus (microstomes and macrostomes) |
+ | + | + | + | − | + | + |
|
Microdiaphanosoma arcuatum |
− | − | + | + | + | + | − |
|
Mycophagophrys terricola |
+ | − | + | − | − | − | − |
| Nivaliella plana | − | + | + | − | − | − | − |
|
Odontochlamys gouraudi |
− | − | + | − | − | − | − |
| Ottowphrya dragescoi | − | − | + | − | − | − | − |
| Ottowphrya magna (n . sp.; see Foissner 1993) | − | + | − | − | − | − | − |
| Oxytricha granulifera (MA with crystals in sample 1) | + | − | + | − | − | − | + |
| Oxytricha longa | + | − | − | − | − | − | − |
|
Oxytricha longigranulosa |
+ | − | − | − | − | − | − |
| Oxytricha setigera | + | − | − | − | − | − | + |
| Paracineta lauterborni | + | − | − | − | − | − | − |
| Paraenchelys wenzeli | − | − | + | − | − | − | − |
| Parentocirrus hortualis | − | − | − | + | − | − | − |
|
Periholosticha paucicirrata |
+ | − | − | − | − | − | − |
|
Phacodinium metchnikoffi |
+ | − | − | − | − | − | − |
|
Platyophrya macrostoma |
− | − | − | − | + | − | − |
| Platyophrya spumacola | − | + | − | + | − | − | + |
| Platyophrya vorax | + | + | − | + | − | − | + |
| Plesiocaryon elongatum | − | − | + | − | − | − | − |
| Pleuroplites australis | + | − | − | − | − | − | − |
|
Protocyclidium muscicola |
+ | − | + | − | − | − | + |
|
Pseudocyrtolophosis alpestris |
+ | + | + | + | − | − | + |
|
Pseudoholophrya terricola |
+ | − | − | − | − | − | − |
|
Pseudoplatyophrya nana |
+ | + | − | + | + | + | − |
|
Pseudoplatyophrya saltans |
+ | − | + | − | − | + | + |
| Pseudourostyla franzi | + | − | − | − | − | − | − |
| Sathrophilus muscorum | + | + | + | − | − | − | + |
| Sikorops woronowiczae | − | − | − | + | − | − | − |
| Stammeridium kahli | − | − | − | − | − | − | + |
| Sterkiella cavicola | + | − | − | − | − | − | − |
|
Sterkiella histriomuscorum |
+ | − | + | − | − | − | − |
| Suturothrix monoarmata n. g., n. sp. | + | − | − | − | − | − | − |
|
Tachysoma humicola humicola |
+ | − | − | − | − | − | − |
|
Tachysoma humicola longisetum |
− | − | − | − | − | − | + |
| Terricirra livida | − | − | − | − | − | − | + |
| Tetrahymena rostrata | + | + | + | − | − | − | + |
|
Trachelophyllum apiculatum f |
+ | − | − | − | − | − | + |
| Urosomoida agiliformis | − | − | − | − | − | − | + |
| Urosomoida agilis | − | − | − | − | − | − | + |
| Vorticella astyliformis | + | − | − | − | − | − | − |
|
Woodruffiides metabolicus |
+ | − | − | − | − | − | − |
| Number of species identified | 52 | 24 | 36 | 20 | 5 | 9 | 35 |
| Number of species not identified | 4 | 1 | 0 | 2 | 0 | 0 | 3 |
| Unidentified species possibly not described | 1 | 0 | 0 | 1 | 0 | 0 | 1 |
For names of authors of species see Foissner (1998), Foissner et al. (2002), Berger (2006), and Foissner & Xu (2007).
Differs from other populations in having only a single micronucleus. Thus, it possibly represents a distinct subspecies; see Colpoda cavicola amicronucleata in Foissner et al. (2002).
Very slender, i.e., almost tail-like narrowed posteriorly. Kahl (1931) supposes that such forms might represent a new species. Unfortunately, the silver preparations are too poor to permit a decision. The number of basket rods and the location of the contractile vacuole match the European population of C. muscorum (Kahl 1931, Foissner 1984).
Replaces the homonym Obliquostoma.
Macronucleus ellipsoidal, i.e., dissimilar to that usually found in F. terricola. Body shape, however, more or less obclavate, and thus similar to F. terricola.
Identification not checked by SEM analysis of cortical scales.
A new species each was found in samples 1, 2, and 7, viz., Suturothrix monoarmata, described below, Platyophryides magnus (now Ottowphrya magna Foissner et al., 2002) described by Foissner (1993), and Dileptus microstoma Vd’ačný & Foissner, 2008. Ottowphrya magna might be a local or regional endemic because I did not find it in over 1000 soil samples taken globally. Several species are of doubtful identity (see footnotes in Tab. 1), while others are remarkable for their distribution, especially because the samples are from the Gondwanan-Laurasian transition zone. Bresslauides terricola is likely a Gondwanan species, previously only known from Kenya (Africa) and Australia (Foissner 1993). The same possibly applies to Sikorops woronowiczae, which has been known only from Kenya (Africa) and two sites in South America (Foissner 1999). Decliviostoma namibiensis has been known only from Namibia (Foissner et al. 2002), while Parentocirrus hortualis, a limnetic species, has been known only from Central Europe (Voss 1997, Blatterer & Foissner 2003). Periholosticha paucicirrata has been described recently from three sites in Europe (Foissner et al. 2005). The Singapore record supports the notion of Foissner et al. (2005) that this tiny species has a wide distribution not recognised due to problems in identification. Last but not least, Hemimastix amphikineta, which occurred in sample 4, should be mentioned. Although being only about 20 μm long, H. amphikineta is a flagship species because it has a unique organisation, including two flagella rows and a highly characteristic shape, both easily recognisable in the light microscope (Foissner et al. 1988). As yet, this curious protist has been found only in Gondwanan localities, such as Australia and South America.
Do these new records indicate a cosmopolitan distribution of soil ciliates? Very likely, they do not. In our detailed study on Namibian soil ciliates, we found 128 new species in 73 samples (Foissner et al. 2002). Only two of them occurred in the Singapore samples, viz., Decliviostoma namibiensis and Arcuospathidium namibiense tristicha, a tiny, ‘difficult’ species recently found also in Austria (Foissner et al. 2005). On the other hand, the three new species from Singapore, Ottowphrya magna, Dileptus microstoma, and Suturothrix monoarmata did not occur in the Namibian samples. This shows that such differences are not only caused by different sampling effort but possibly by restricted geographic distribution of certain species. This is emphasized by ‘flagship’ species, such as Bresslauides terricola, a large colpodid ciliate restricted to Gondwanan areas (Foissner 1993) and, as the Singapore record shows, the transition zone to Laurasia. See Foissner (2006, 2008) and Foissner et al. (2008) for a detailed discussion of the distribution problem.
As concerns the number of species, the seven samples split into two groups, viz., the ‘rich’ coastal sites (samples 1 – 3, 7) and the ‘poor’ rainforest sites (samples 4 – 6). However, this is a methodological artifact, that is, the rainforest samples were stored too long. When I collected and stored these samples, I did not know that resting cysts of rainforest ciliates do not withstand prolonged desiccation, i.e., most die within one year (Foissner 1997a, 2006).
Suturothrix nov. gen.
Diagnosis
Haptorida with heteromorphic dorsal brush consisting of three staggered rows, forming a suture with the last right side ciliary row.
Type species
Suturothrix monoarmata nov. spec.
Etymology
Composite of the Latin noun sutura (suture) and the Greek noun thrix (hair = ciliate s. l.), meaning a ‘ciliate with a suture’. Feminine gender.
Comparison with related genera
The new genus and species has all characteristics of the litostomate haptorids, as defined by Corliss (1979), Foissner & Foissner (1988), and Lynn & Small (2002): a simple cylindroidal to bursiform body with the oral opening occupying the anterior end; a holotrichous ciliature with several ciliary rows specialised anteriorly to form the so-called dorsal brush, which might be important for prey recognition; and extrusomes of the toxicyst type, used to paralyse or kill the prey (Figs 1 – 13). As concerns the extrusomes of Suturothrix, they look quite similar to those found in other haptorids (Foissner & Xu 2007). Thus, there is no doubt that they are toxicysts. The only uncommon feature is the low number (one or two) attached to the oral bulge.
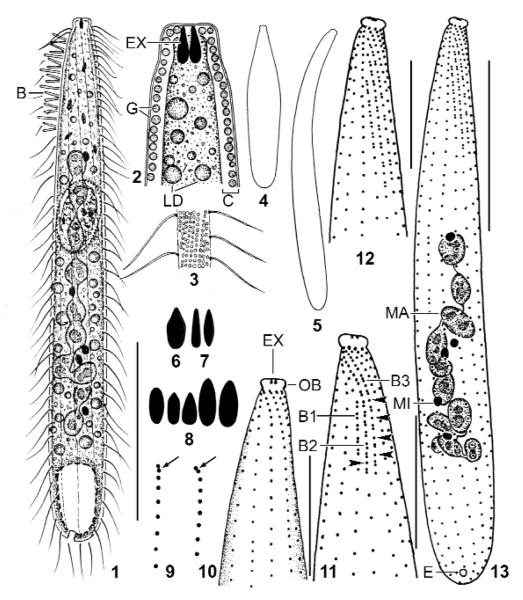
Figs 1 – 13.
Suturothrix monoarmata, Singapore (1, 3, 6, 12, 13), Thailand (2, 4, 5, 7, 9 – 11), and Brazil (8) specimens from life (1 – 8) and after protargol impregnation (9 – 13). 1: Right side overview. 2: Semischematic view of oral region. 3: Cortical granulation. 4, 5: Contracted (80 μm) and extended (120 μm) specimen. 6 – 8: Oral bulge extrusomes. 9: Anterior region of ciliary rows with supposed dikinetids marked by arrows. 10, 11: Ventral and dorsal view of same specimen. Arrowheads mark monokinetids between dikinetids. 12, 13: Dorsal ciliary pattern and nuclear apparatus of holotype specimen. B, B1–3: dorsal brush (rows), C: cortex, E: excretory pore; EX: extrusomes; G: cortical granules; LD: lipid droplets; MA: macronucleus; MI: micronucleus; OB: oral bulge. Bars 10 μm (Figs 10 – 12), 20 μm (13), 30 μm (1).
The most distinct feature of the new genus is the dorsal brush whose staggered rows cause a suture in the right side somatic ciliature (Figs 11 – 14, 24, 28). The staggered pattern is distinct also in the morphometric data (Tab. 2, last three characteristics). Very likely, this specific arrangement is not caused by spatial constraints because, for instance, the very slender Chaenea species have an ordinary, isomorphic brush (Foissner 1984, Petz et al. 1995). Except for the dileptids, where staggering brush rows are common (Foissner et al. 2002), we found only one other species with slightly staggered brush rows and a rather distinct suture, viz., Enchelys geleii, as described by Foissner (2000). However, this species has an isomorphic brush, and thus possibly needs a distinct genus because other Enchelys species have an ordinary brush (Foissner 1984, 1987a). The heteromorphic brush of Suturothrix is not unique but a rare feature found only in six other haptorid genera (for literature, see Foissner 1988, 2003, Foissner et al. 2002): Pleuroplites (Fam. Pleuroplitidae); Pseudoholophrya and Paraenchelys (Fam. Pseudoholophryidae); Kahlophrya and Songophrya (Fam. Myriokaryonidae); and Apobryophyllum (Fam. Bryophyllidae). Obviously, such brush pattern evolved convergently in several haptorid families. As concerns the bristles, Suturothrix is unique in that the brush monokinetids do not bear ordinary somatic cilia as in the genera mentioned above, but bristles which are, however, slightly longer than those of the dikinetids.
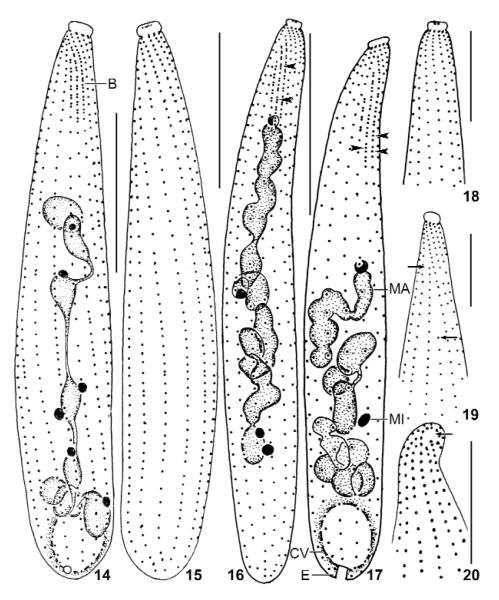
Figs 14 – 20.
Suturothrix monoarmata, Thailand (14, 15, 20), and Brazil (16 – 19) specimens after protargol impregnation. 14, 15: Ciliary pattern of dorsal and ventral side of same specimen. 16, 18: Ciliary pattern of dorsal and ventral side of same specimen. Arrowheads mark triads of basal bodies in brush row 2. 17: Dorsal view showing triads of basal bodies in brush rows 2 and 3 (arrowheads). 19: Ventral view showing two shortened ciliary rows (arrows). 20: Frontal view with minute oral area marked by arrow. B: dorsal brush; CV: contractile vacuole; E: excretory pore; MA: macronucleus; MI: micronucleus. Bars 10 μm (Figs 18 – 20), 20 μm (14 – 17).
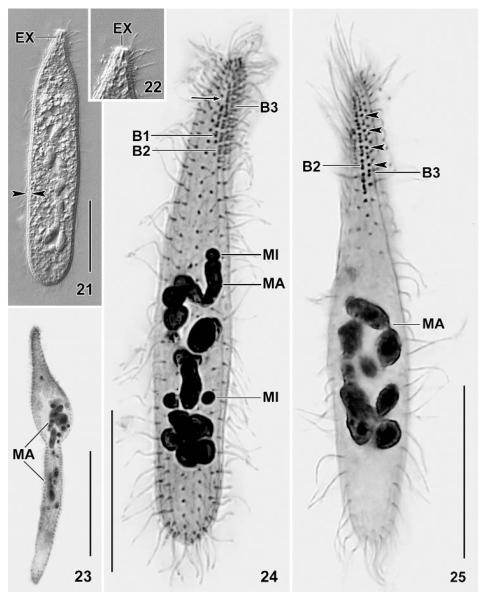
Figs 21 – 25.
Suturothrix monoarmata, Singapore (21, 22), Thailand (23) and Brazil (24, 25) specimens from life (21, 22) and after protargol impregnation (23 – 25). 21, 22: A contracted slightly pressed specimen showing the distinct cortex (opposed arrowheads) and the single extrusome. 23: A malformed specimen. 24, 25: Dorsal views showing the right side suture (arrow) and monokinetids between dikinetids (arrowheads). Note triads in brush row 2. B, B1 – 3: dorsal brush rows; EX: extrusome; MA: macronucleus; MI: micronuclei. Bars 20 μm (Figs 21, 24, 25), 40 μm (23).
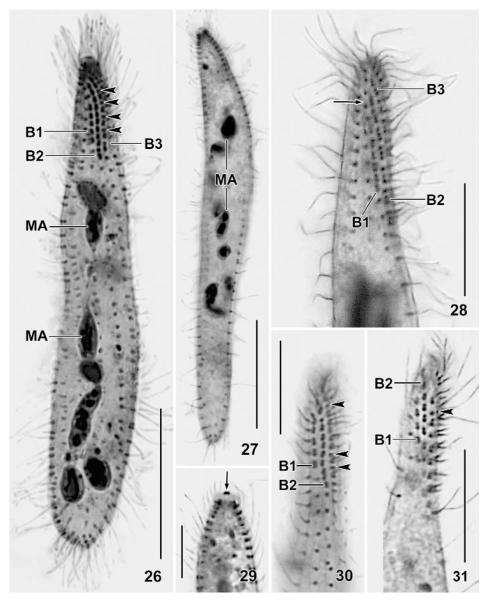
Figs 26 – 31.
Suturothrix monoarmata, ciliary pattern and macronucleus of Thailand (26, 27, 29 – 31) and Brazil (28) specimens after protargol impregnation. Arrowheads mark monokinetids between dikinetids of brush row 3. 26, 27: Overviews. 29: The centre of the oral bulge contains one or two argyrophilic granules (arrow). 28, 30, 31: Dorsal views showing the dorsal brush and the suture (28, arrow) formed by the staggering brush rows. B1 – 3: dorsal brush rows; MA: macronucleus. Bars 20 μm (Figs 26, 27), 10 μm (28 – 31).
Tab. 2.
Morphometric data from Suturothrix monoarmata. Upper line: Singapore population; middle line: Thailand population; lower line: Brazil population
| Characteristica | M | SD | SE | CV | Min | Max | n | |
|---|---|---|---|---|---|---|---|---|
| Body. length | 76.4 | 79 | 10.7 | 2.7 | 13.9 | 55 | 89 | 16 |
| 64.7 | 65 | 8.7 | 2 | 13.5 | 45 | 78 | 19 | |
| 69.7 | 65 | 15.9 | 3.7 | 22.8 | 43 | 110 | 19 | |
| Body, width | 8.1 | 8 | 1.3 | 0.3 | 16.1 | 7 | 11 | 16 |
| 10.4 | 10 | 2.1 | 0.5 | 19.9 | 7 | 14 | 19 | |
| 11.5 | 11 | 2.8 | 0.7 | 24.7 | 8 | 20 | 19 | |
| Body length : width, ratio | 9.6 | 9.6 | 1.8 | 0.5 | 18.8 | 6.3 | 12.6 | 16 |
| 6.4 | 5.9 | 1.5 | 0.4 | 23.9 | 4.6 | 9.1 | 19 | |
| 6.5 | 6.1 | 2.4 | 0.6 | 37.5 | 3.2 | 12.2 | 19 | |
| Oral bulge, width | 2.5 | 2.5 | - | - | - | 2 | 3 | 16 |
| 2.4 | 2.5 | - | - | - | 1.5 | 3 | 19 | |
| 2.6 | 2.5 | - | - | - | 2 | 3 | 19 | |
| Oral bulge, height | 0.9 | 1 | - | - | - | 0.5 | 1 | 16 |
| 1.1 | 1 | - | - | - | 0.7 | 2 | 19 | |
| 1.1 | 1 | - | - | - | 1 | 1.5 | 19 | |
| Anterior body end to macronucleus, distance |
23.4 | 21 | 5.8 | 1.5 | 24.7 | 12 | 36 | 16 |
| 19.2 | 17 | 6.8 | 1.6 | 35.5 | 13 | 40 | 19 | |
| 20.6 | 23 | 4.9 | 1.1 | 23.7 | 11 | 26 | 19 | |
| Macronucleus figure, length |
38.3 | 37.5 | 7.3 | 1.8 | 19.2 | 30 | 51 | 16 |
| 31.7 | 29 | 8.8 | 2 | 27.7 | 22 | 49 | 19 | |
| 38.2 | 35 | 9.9 | 2.3 | 26 | 23 | 60 | 19 | |
| Macronucleus nodules, length |
6.3 | 6 | 2.4 | 0.6 | 38.3 | 3 | 13 | 16 |
| 6.2 | 6 | 2.8 | 0.7 | 45.7 | 3 | 13 | 19 | |
| 5.8 | 5 | 2 | 0.5 | 35 | 3 | 10 | 19 | |
| Macronucleus nodules, width |
3.2 | 3 | 0.6 | 0.1 | 18 | 2 | 4 | 16 |
| 3.2 | 3 | 0.8 | 0.2 | 23.8 | 2 | 5 | 19 | |
| 3 | 3 | 0.6 | 0.1 | 19 | 2 | 4 | 19 | |
| Macronucleus nodules, number |
10.4 | 10.5 | 2.6 | 0.7 | 25.4 | 5 | 14 | 16 |
| 8.1 | 9 | 2.8 | 0.7 | 35.2 | 2 | 12 | 19 | |
| 13.9 | 14 | 2.3 | 0.5 | 16.3 | 10 | 18 | 19 | |
| Micronucleus, length | 1.5 | 1.5 | - | - | - | 1 | 2 | 16 |
| 1.6 | 1.5 | - | - | - | 1.2 | 2 | 19 | |
| 2 | 2 | - | - | - | 1.5 | 2.2 | 19 | |
| Micronucleus, width | 1.4 | 1.5 | - | - | - | 1 | 1.5 | 16 |
| 1.4 | 1.3 | - | - | - | 1 | 2 | 19 | |
| 1.6 | 1.5 | - | - | - | 1 | 2 | 19 | |
| Micronucleus, number |
5.4 | 5.5 | 1.4 | 0.3 | 25.1 | 4 | 9 | 16 |
| 5.3 | 5 | 1.2 | 0.3 | 23.6 | 2 | 7 | 19 | |
| 4.8 | 5 | 1.2 | 0.3 | 25.1 | 2 | 7 | 19 | |
| Ciliary rows, number | 11.6 | 12 | 0.7 | 0.2 | 6.3 | 10 | 12 | 16 |
| 11.2 | 11 | 1 | 0.2 | 8.6 | 10 | 13 | 19 | |
| 11 | 11 | 0.8 | 0.2 | 6.8 | 10 | 13 | 19 | |
| Ciliary rows, number of kinetids in a ventral row b |
53.9 | 53 | 10.3 | 2.6 | 19.1 | 36 | 73 | 16 |
| 48.3 | 50 | 8.9 | 2.1 | 18.5 | 31 | 62 | 19 | |
| 47.4 | 46 | 9.7 | 2.2 | 20.5 | 27 | 64 | 19 | |
| Dorsal brush rows, number | 3 | 3 | 0 | 0 | 0 | 3 | 3 | 16 |
| 3.5 | 3 | 0.7 | 0.2 | 19 | 2 | 5 | 14 | |
| 3 | 3 | 0 | 0 | 0 | 3 | 3 | 19 | |
| Dorsal brush row 1, length c | 10.6 | 10 | 1.5 | 0.4 | 13.8 | 8 | 13 | 16 |
| 8.9 | 9 | 2.3 | 0.6 | 26.2 | 4 | 13 | 14 | |
| 10.1 | 10 | 1.2 | 0.3 | 11.9 | 8 | 13 | 19 | |
| Dorsal brush row 1, number of dikinetids |
5.6 | 5.5 | 0.8 | 0.2 | 14.6 | 4 | 7 | 16 |
| 4.8 | 5 | 1.4 | 0.4 | 28.6 | 2 | 7 | 14 | |
| 5.2 | 5 | 1.1 | 0.3 | 20.8 | 3 | 7 | 19 | |
| Dorsal brush row 1, number of monokinetids d |
0.5 | 0.5 | - | - | - | 0 | 4 | 16 |
| 0.1 | 0 | - | - | - | 0 | 1 | 14 | |
| 0.1 | 0 | - | - | - | 0 | 1 | 19 | |
| Dorsal brush row 2, length c | 14.8 | 15 | 2.2 | 0.6 | 15 | 10 | 19 | 16 |
| 12 | 11.5 | 2.1 | 0.6 | 17.3 | 8 | 15 | 14 | |
| 13.3 | 13 | 1.5 | 0.3 | 10.9 | 11 | 17 | 19 | |
| Dorsal brush row 2, number of dikinetids |
9 | 9 | 1.7 | 0.4 | 19 | 5 | 12 | 16 |
| 10.1 | 10 | 2.4 | 0.7 | 24.1 | 6 | 14 | 14 | |
| 8.2 | 8 | 1.3 | 0.3 | 16 | 5 | 11 | 19 | |
| Dorsal brush row 2, number of monokinetids d |
5.4 | 6 | 1.7 | 0.4 | 30.8 | 2 | 8 | 16 |
| 0.8 | 0 | - | - | - | 0 | 4 | 14 | |
| 5.2 | 5 | 1.7 | 0.4 | 32.4 | 2 | 9 | 19 | |
| Dorsal brush row 2, number of triads | 0.8 | 0 | - | - | - | 0 | 5 | 16 |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 14 | |
| 4.3 | 5 | 1.3 | 0.3 | 30.1 | 2 | 6 | 19 | |
| Dorsal brush row 3, length c | 13.2 | 13 | 2.3 | 0.6 | 17.1 | 8 | 18 | 16 |
| 10.3 | 10.5 | 2.4 | 0.7 | 23.6 | 6 | 14 | 14 | |
| 12.4 | 12 | 1.7 | 0.4 | 13.8 | 10 | 17 | 19 | |
| Dorsal brush row 3, number of dikinetids |
7.5 | 7.5 | 1.6 | 0.4 | 21.2 | 5 | 12 | 16 |
| 7.6 | 8 | 1.7 | 0.4 | 21.5 | 5 | 11 | 14 | |
| 7.3 | 7 | 1.2 | 0.3 | 16.5 | 5 | 9 | 19 | |
| Dorsal brush row 3, number of monokinetids d |
4.1 | 4 | 1.4 | 0.4 | 35.4 | 0 | 6 | 16 |
| 2.9 | 3 | 1.8 | 0.5 | 64.2 | 0 | 5 | 14 | |
| 4.6 | 4 | 1.3 | 0.3 | 28.1 | 2 | 8 | 19 | |
| Dorsal brush, distance from anterior end of kineties to first dikinetid of row 1 |
5 | 5 | 0.6 | 0.1 | 11.6 | 3.5 | 6 | 19 |
| 4.2 | 4 | 1.7 | 0.4 | 39.2 | 2.5 | 10 | 19 | |
| 5.3 | 5.5 | 0.9 | 0.2 | 17.5 | 3 | 6.5 | 19 | |
| Dorsal brush, distance from anterior end of kineties to first dikinetid of row 2 |
2.6 | 2.5 | 0.4 | 0.1 | 14.8 | 2 | 3.5 | 19 |
| 2.2 | 2 | 0.6 | 0.1 | 25.6 | 1.5 | 3 | 19 | |
| 3.1 | 3 | 0.6 | 0.1 | 18.8 | 1.5 | 4 | 19 | |
| Dorsal brush, distance from anterior end of kineties to first dikinetid of row 3 |
1.8 | 2 | 0.4 | 0.1 | 21 | 1 | 2.5 | 19 |
| 1.6 | 1.5 | 0.6 | 0.1 | 34.5 | 1 | 3 | 19 | |
| 2 | 2 | 0.6 | 0.1 | 27.9 | 1 | 3.5 | 19 |
Data based on mounted, protargol-impregnated (Foissner’s specimens from non-flooded Petri dish cultures. Measurements in μm. CV: coefficient of variation in %, M: median, Max: maximum, Min: minimum, n: number of specimens investigated, SD: standard deviation, SE: standard error of mean, – arithmetic mean.
Very closely spaced (di)kinetids counted as 1 kinetid if only one basal body was ciliated.
In specimens with more that 3 rows, the supposed extra rows (often recognisable by some malformations) were omitted. Length measured from begin of ciliary rows to last dikinetid.
Only those which are within the dikinetidal part were counted, i.e., monokinetids at anterior and posterior end of brush rows were excluded.
Unfortunately, we could not clarify the oral structures of S. monoarmata, although the preparations were excellent. Thus, transmission electron microscopy is needed to find the family home of Suturothrix. However, there is strong indication that each kinety commences with a dikinetid (Fig. 9), quite similar as in Chaenea which has, additionally, oralised somatic monokinetids (Foissner 1984, Lipscomb & Riordan 1990). Oralised monokinetids are possibly present also in S. monoarmata because we could not find an oral basket. Accordingly, Suturothrix might belong to the families Acropisthiidae Foissner & Foissner 1988 or Fuscheriidae Foissner et al. 2002.
Suturothrix monoarmata nov. spec.
Diagnosis
(based on three populations): Size about 100 × 15 μm in vivo. Clavate to very narrowly clavate with minute oral bulge (~ 3 × 2 μm). On average 8 – 14 ellipsoidal macronucleus nodules and about 5 globular micronuclei. Extrusomes highly variable in shape, basically oblong with a size of 2.5 × 1 μm, usually only 1 or 2 attached to centre of oral bulge. On average 11 ciliary rows, 3 anteriorly differentiated to a distinctly heterostichad dorsal brush occupying an average of 19 % of body length. Brush bristles up to 6 μm long: row 1 with about 5 dikinetids and 1 monokinetid, row 2 with about 9 dikinetids and 4 monokinetids, row 3 with about 7 dikinetids and 4 monokinetids.
Type locality
Upper soil layer (0 – 5 cm) from a coastal bushland in Singapore, Asia, E 103° N 1°.
Type material
One holotype and two paratype slides with protargol-impregnated specimens from the type locality have been deposited in the Oberösterreichische Landesmuseum in Linz (LI), Biologiezentrum. Further, we deposited two voucher slides each with cells from Thailand and Brazil. Relevant specimens are marked by black ink circles on the cover slip.
Etymology
Composite of the Greek numeral mono (one) and the Latin adjective armatus (armed), referring to the single, thick extrusome usually found in the centre of the oral bulge.
Description
The three populations studied are very likely conspecific because they have a very similar morphology and morphometry (Figs 13, 14, 16; Tab. 2). Thus, we separate the observations only when features deviate more than usual.
Size 70 – 120 × 10 – 15 μm in vivo, usually about 100 × 15 μm, as calculated from some in vivo measurements and the morphometric data; considerably smaller in protargol preparations (~ 70 × 10 μm; Tab. 2) due to some shrinkage and a slight contractility difficult to recognise because contraction occurs slowly and hardly exceeds 30 %, except under slight cover-slip pressure where specimens may contract up to 50 % and become flask-shaped (Figs 4, 21). Length : width ratio highly variable, that is, 3 : 1 – 13 : 1, on average about 7 : 1, very likely caused by unequal contraction and a life cycle with theront and trophont, as typical for predaceous ciliates and indicated by the high (~ 20 %) variability coefficients of body width (Tab. 2); macrostomes do not develop. Shape also highly variable, difficult to classify due to a rather abrupt narrowing in anterior fifth, basically clavate/oblong to very narrowly clavate/oblong, rarely roughly bursiform or cylindroidal; widest in or underneath mid-body, oral bulge five times narrower than widest body region; flattened up to 1.5 : 1; slender specimens sometimes slightly curved (Figs 1, 5, 13, 14, 16, 17, 24, 25 – 27). Nuclear apparatus usually slightly underneath central quarters of cell (Figs 1, 13, 14, 16, 17, 24-27). Macronucleus a slightly irregular, moniliform strand consisting of about 8 – 14 nodules, depending on population; rarely almost rod-shaped. Individual macronucleus nodules more or less separated from each other, frequently connected by a fine argyrophilic strand; shape highly variable, that is, globular to very narrowly ellipsoidal (6 : 1), on average ellipsoidal in all populations; one or several rather large nucleoli, depending on nodule size. About five globular to broadly ellipsoidal micronuclei attached to macronucleus strand, rarely some scattered throughout cytoplasm. Contractile vacuole in posterior body end, usually a single, rarely two excretory pores in pole centre (Figs 1, 13, 17). Egestion vacuoles with granular contents migrate through the contractile vacuole to leave cell at posterior end. Extrusomes 2 – 3 × 1 – 1.5 μm in size, usually only one or two attached to centre of oral bulge, an outstanding feature making this species easily recognisable; some or many scattered throughout cytoplasm, especially in anterior half; anterior end often with an argyrophilic granule (Figs 10 – 13, 18, 29), while extrusome body impregnates very rarely with the method used. Extrusome shape rather variable within and between populations, that is, lanceolate, elliptical, bluntly fusiform, or oblong with conical anterior end (Figs 1, 2, 6 – 8, 21, 22); some variability possibly caused by partial explosion and/or observation problems due to the minute size. Cortex about 1 μm thick, distinctly separate from cytoplasm and thus conspicuous (Figs 1, 2, 21). Cortical granules colourless and less than 0.5 μm in size, very narrowly spaced in oral region, while rather loosely arranged in main part of body, especially in the Singapore population (Figs 2, 3). Cytoplasm colourless, contains few to many lipid droplets 1 – 3 μm across and some food vacuoles with naked amoebae about 20 μm in size and, possibly, euglenid flagellates (Peranema?). Swims and glides moderately rapid; serpentine when creeping between soil particles.
Cilia about 9 μm long in vivo, densely spaced (on average < 2 μm), especially in the oral region, arranged in an average of 11 very narrowly spaced rows extending more or less spirally, possibly due to varying cell contraction; rarely, some rows shortened anteriorly and/or posteriorly (Figs 1, 13 – 19, 24, 26; Tab. 2). Three rows anteriorly modified to dorsal brush, occupying an average of only 19 % of body length in all populations. Number of brush rows highly variable (2 – 5) in Thailand specimens for unknown reasons. Dorsal brush rows in parallel and very narrowly spaced, composed of monokinetids and dikinetids, with distinct differences between populations (Figs 1, 11 – 14, 16, 17, 24 – 26, 28, 30, 31; Tab. 2): row 1 less than half as long as rows 2 and 3, i.e., about 5 μm long, usually without or with few monokinetids; rows 2 and 3 of almost same length, i.e., about 13 μm long, composed of an average of 8 dikinetids and 4 monokinetids in Singapore and Brazil specimens, both frequently forming distinct triads in the latter; Thailand specimens with monokinetids only in row 3. Brush bristles 3 – 6 μm long in vivo, 2 – 4 μm in protargol preparations, those of monokinetids slightly longer than those of dikinetids. Anterior brush tails lacking or with few cilia, posterior tails lacking in all rows.
Oral bulge indistinctly to moderate distinctly set off from body proper, occupies anterior end of cell and thus minute, i.e., 2.5 – 4 μm wide and 1.5 – 2.5 μm high in vivo. Bulge centre slightly concave, usually occupied by only one or two extrusomes with argyrophilic anterior end appearing as a dark dot in protargol preparations (Figs 1, 2, 10 – 19, 22, 29; Tab. 2). Circumoral kinety and oral basket not recognisable (for details, see genus discussion).
Several malformed specimens occur in the Thailand population. They are longer than ordinary cells ( 94 × 14, n 6 vs. 65 × 10, n 19) and more ore less bipartited by a furrow, resembling that found in late dividers. Further, the ciliary and nuclear pattern is more or less disturbed (Fig. 23). Interestingly, the number of dorsal brush rows is also highly variable in this population (Tab. 2), indicating some toxic (?) influence.
Occurrence and ecology
To date found in two soil samples from Asia and in a floodplain soil from Brazil, South America. The Singapore type population was discovered in the upper 0 – 2 cm soil layer of a bushland ~ 200 m inshore. The loamy, non-saline soil was very compact and had pH 6 in water. In Thailand, S. monoarmata occurred in a very similar habitat, viz., in coastal soil from the small Ju Pa Island near to the town of Kata Karon, Phuket Peninsula. The soil, which was collected by Mag. Margit Palzenberger, was brownish, contained some rotten leaves, and had pH 7.4 in water. The third population was found in floodplain soil from a small island in the Rio Amazonas near to the town of Manaus. The very loamy, brownish soil was covered by a thin litter layer, contained only few roots, and had pH 5.1 in water.
These data indicate that S. monoarmata prefers poor coastal soils. It is well adapted to the soil habitat by the slender, flexible body. In spite of its narrowness (mouth area ~ 3 μm wide), it can feed on about 20 μm-sized amoebae ingested whole. Possibly, S. monoarmata is a cosmopolite, although we have not found it in Austria yet.
Comparison with related species
At low magnification (≤ × 100), S. monoarmata is an inconspicuous organism because of its moderate size, the slender shape, and the minute oral bulge. However, at higher magnification it is highly distinct due to the extrusomes of which only one or two are attached to the centre of the oral bulge, a curious pattern found only in one other genus, viz., Chaenea. Indeed, S. monoarmata is easily confused with Chaenea spp. in vivo, especially with C. torrenticola (re-described in Foissner 1984) which has a similar size, shape, and extrusome pattern. In protargol preparations, these genera are easily distinguished by the nuclear pattern (macronucleus moniliform vs. scattered nodules) and the dorsal brush (three-rowed and heteromorphic vs. four-rowed and isomorphic).
Kahl (1935) very briefly mentioned an Enchelys tokkuri Shibuya, 1930, which looks rather similar to S. monoarmata. However, Kahl (1935) confused the narrow side view of Spathidium furcatum Shibuya, 1930 with E. tokkuri.
4. Acknowledgements
This study was supported by the Austrian Science Foundation, FWF project P19699-B17. The technical assistance of Mag. Gudrun Fuss and Robert Schörghofer is greatly acknowledged.
5. References
- Berger H. Monograph of the Urostyloidea (Ciliophora, Hypotricha) Monographiae Biologicae. 2006;85:i–xvi. 1–1303. [Google Scholar]
- Berger H, Foissner W. Morphology and biometry of some soil hypotrichs (Protozoa, Ciliophora) from Europe and Japan. Bulletin of the British Museum of Natural History. 1989;55:19–46. [Google Scholar]
- Blatterer H, Foissner W. Beitrag zur terricolen Ciliatenfauna (Protozoa: Ciliophora) Australiens. Stapfia, Linz. 1988;17:1–84. [Google Scholar]
- Blatterer H, Foissner W. Morphological and ontogenetic comparison of two populations of Parentocirrus hortualis Voss 1997 (Ciliophora, Hypotrichida) Linzer biologische Beiträge. 2003;35:831–854. [Google Scholar]
- Chao A, Li PC, Agatha S, Foissner W. A statistical approach to estimate soil ciliate diversity and distribution based on data from five continents. Oikos. 2006;114:479–493. [Google Scholar]
- Corliss JO. Characterization, classification and guide to the literature. Pergamon Press; Oxford, New York, Toronto, Sydney, Paris, Frankfurt: 1979. The ciliated protozoa; pp. i–xvi.pp. 455 [Google Scholar]
- Foissner W. Infraciliatur, Silberliniensystem und Biometrie einiger neuer und wenig bekannter terrestrischer, limnischer und mariner Ciliaten (Protozoa: Ciliophora) aus den Klassen Kinetofragminophora, Colpodea und Polyhymenophora. Stapfia, Linz. 1984;12:1–165. [Google Scholar]
- Foissner W. Beitrag zur Kenntnis der Bodenciliaten (Protozoa: Ciliophora) des Himalaja. Zoologisches Jahrbuch, Systematik. 1986;113:45–53. [Google Scholar]
- Foissner W. Neue terrestrische und limnische Ciliaten (Protozoa, Ciliophora) aus Österreich und Deutschland. Sitzungsberichte der Österreichischen Akademie der Wissenschaften, Wien. 1987a;195:217–268. [Google Scholar]
- Foissner W. Soil protozoa: fundamental problems, ecological significance, adaptations in ciliates and testaceans, bioindicators, and guide to the literature. Progress in Protistology. 1987b;2:69–212. [Google Scholar]
- Foissner W. Gemeinsame Arten in der terricolen Ciliatenfauna (Protozoa: Ciliophora) von Australien und Afrika. Stapfia, Linz. 1988;17:85–133. [Google Scholar]
- Foissner W. Basic light and scanning electron microscopic methods for taxonomic studies of ciliated protozoa. European Journal of Protistology. 1991;27:313–330. doi: 10.1016/S0932-4739(11)80248-8. [DOI] [PubMed] [Google Scholar]
- Foissner W. Colpodea (Ciliophora) Protozoenfauna. 1993;4:i–x. 798. [Google Scholar]
- Foissner W. Faunistics, taxonomy and ecology of moss and soil ciliates (Protozoa, Ciliophora) from Antarctica, with description of new species, including Pleuroplitoides smithi gen. n., sp. n. Acta Protozoologica. 1996;35:95–123. [Google Scholar]
- Foissner W. Soil ciliates (Protozoa: Ciliophora) from evergreen rain forests of Australia, South America and Costa Rica: diversity and description of new species. Biology and Fertility of Soils. 1997a;25:317–339. [Google Scholar]
- Foissner W. Faunistic and taxonomic studies on ciliates (Protozoa, Ciliophora) from clean rivers in Bavaria (Germany), with descriptions of new species and ecological notes. Limnologica. 1997b;27:179–238. [Google Scholar]
- Foissner W. An updated compilation of world soil ciliates (Protozoa, Ciliophora), with ecological notes, new records, and descriptions of new species. European Journal of Protistology. 1998;34:195–235. [Google Scholar]
- Foissner W. Notes on the soil ciliate biota (Protozoa, Ciliophora) from the Shimba Hills in Kenya (Africa): diversity and description of three new genera and ten new species. Biodiversity and Conservation. 1999;8:319–389. [Google Scholar]
- Foissner W. A compilation of soil and moss ciliates (Protozoa, Ciliophora) from Germany, with new records and descriptions of new and insufficiently known species. European Journal of Protistology. 2000;36:253–283. [Google Scholar]
- Foissner W. The Myriokaryonidae fam. n., a new family of spathidiid ciliates (Ciliophora: Gymnostomatea) Acta Protozoologica. 2003;42:113–143. [Google Scholar]
- Foissner W. Biogeography and dispersal of micro-organisms: a review emphasizing protists. Acta Protozoologica. 2006;45:111–136. [Google Scholar]
- Foissner W. Protist diversity and distribution: some basic considerations. Biodiversity and Conservation. 2008;17:235–242. [Google Scholar]
- Foissner W, Foissner I. The fine structure of Fuscheria terricola Berger et al., 1983 and a proposed new classification of the subclass Haptoria Corliss, 1974 (Ciliophora, Litostomatea) Archiv für Protistenkunde. 1988;135:213–235. [Google Scholar]
- Foissner W, Xu K. Monograph of the Spathidiida (Ciliophora, Haptoria). Volume I: Protospathidiidae, Arcuospathidiidae, Apertospathulidae. Monographiae Biologicae. 2007;81:1–485. [Google Scholar]
- Foissner W, Blatterer H, Foissner I. The Hemimastigophora (Hemimastix amphikineta nov. gen., nov. spec.), a new protistan phylum from Gondwanian soils. European Journal of Protistology. 1988;23:361–383. doi: 10.1016/S0932-4739(88)80027-0. [DOI] [PubMed] [Google Scholar]
- Foissner W, Agatha S, Berger H. Soil ciliates (Protozoa, Ciliophora) from Namibia (Southwest Africa), with emphasis on two contrasting environments, the Etosha region and the Namib Desert. Denisia. 2002;5:1–1459. [Google Scholar]
- Foissner W, Berger H, Xu K, Zechmeister-Boltenstern S. A huge, undescribed soil ciliate (Protozoa: Ciliophora) diversity in natural forest stands of Central Europe. Biodiversity and Conservation. 2005;14:617–701. [Google Scholar]
- Foissner W, Chao A, Katz LA. Diversity and geographic distribution of ciliates (Protista: Ciliophora) Biodiversity and Conservation. 2008;17:345–363. [Google Scholar]
- Kahl A. Urtiere oder Protozoa I: Wimpertiere oder Ciliata (Infusoria) 4. Peritricha und Chonotricha. Tierwelt Deutschlands. 1931;30:651–886. [Google Scholar]
- Kahl A. Urtiere oder Protozoa I: Wimpertiere oder Ciliata (Infusoria) 2. Holotricha außer den im 1. Teil behandelten Prostomata. Tierwelt Deutschlands. 1935;21:181–398. [Google Scholar]
- Lipscomb DL, Riordan GP. The ultrastructure of Chaenea teres and an analysis of the phylogeny of the haptorid ciliates. Journal of Protozoology. 1990;37:287–300. [Google Scholar]
- Lynn DH, Small EB. Phylum Ciliophora. In: Lee JJ, Leedale GF, Bradbury P, editors. An illustrated guide to the Protozoa. Second edition. Volume I. Society of Protozoologists; Lawrence: 2002. pp. 371–656. [This edition of the illustrated guide is dated with 2000, however, it was first available (first published) in spring 2002.] [Google Scholar]
- Müller P. Arealsysteme und Biogeographie. Ulmer; Stuttgart: 1981. p. 704. [Google Scholar]
- Petz W, Song W, Wilbert N. Taxonomy and ecology of the ciliate fauna (Protozoa, Ciliophora) in the endopagial and pelagial of the Weddell Sea, Antarctica. Stapfia, Linz. 1995;40:1–223. [Google Scholar]
- Shibuya M. Ciliates found in soils from some parts of Japan. Journal of the Imperial Agricultural Experiment Station. 1930;1:199–214. [Google Scholar]
- Vd’ačný P, Foissner W. Description of four new soil dileptids (Ciliophora, Haptoria), with notes on adaptations to the soil environment. Acta Protozoologica. 2008 (in press) [PMC free article] [PubMed] [Google Scholar]
- Voss H-J. Morphology and morphogenesis of Parentocirrus hortualis nov. gen., nov. spec.: a new genus within the redefined family Kahliellidae sensu Eigner 1995 (Ciliophora, Hypotrichida) European Journal of Protistology. 1997;33:30–47. [Google Scholar]