Abstract
Purpose
Obese women and women who gain weight after a breast cancer diagnosis are at a greater risk for breast cancer recurrence and death compared with lean women and women who do not gain weight. In this population-based study, we assessed weight and body fat changes from within the first year of diagnosis to within the third year after diagnosis, and whether any changes in weight and body fat varied by demographic, prognostic, and lifestyle factors in 514 women with incident Stage 0–IIIA breast cancer.
Methods
Women were participants in the Health, Eating, Activity, and Lifestyle (HEAL) Study. Weight and body fat (via DEXA) were measured during the baseline visit and two years later at a follow-up visit. Analysis of covariance methods were used to obtain mean weight and body fat changes adjusted for potential cofounders.
Results
Women increased their weight and percent body fat by 1.7 ± 4.7 kg and 2.1 ± 3.9 %, respectively, from within their first year of diagnosis to within their third year of diagnosis. A total of 68% and 74% gained weight and body fat. Greater increases in weight were observed among women diagnosed with a higher disease stage, younger age, being postmenopausal, and women who decreased their physical activity from diagnosis to within 3 years after diagnosis (p for trend < 0.05).
Conclusions
Weight and body fat increased in the post-diagnosis period. Future research should focus on the effect of physical activity on weight and fat loss and breast cancer prognosis.
Keywords: obesity, physical activity, diet, prognosis, treatment
INTRODUCTION
Weight gain and obesity are common occurrences in women diagnosed with breast cancer (1–6). Gains in weight usually range from 2.0 to 6.0 kg during the first year of diagnosis; however, greater gains are not uncommon. Evidence exists that post-diagnosis weight gain may adversely affect survival among breast cancer survivors (7–9), and obesity at the time of diagnosis is a negative prognostic factor that is associated with recurrent disease and decreased survival (10–14). Both weight gain and obesity adversely affect risk for cardiovascular disease, hypertension, and diabetes (15–17), conditions for which women who have been diagnosed with breast cancer are at increased risk (18,19).
Multiple reasons for post-diagnosis weight gain in breast cancer survivors have been suggested, including receiving chemotherapy, being or becoming postmenopausal after diagnosis, decreased physical activity, and increased total caloric intake, although these explanations have not been extensively studied (1–6). Most studies that have examined changes in body weight after a diagnosis of breast cancer and factors associated with weight gain have been of short study duration (e.g., only during treatment or within the first year) and with small sample sizes (2,3,5, 20,21). Few studies have measured body weight (most rely on self-report) and even fewer studies have measured changes in body fat. Studies with a larger number of patients, with a longer follow-up, using valid measures of body composition are necessary so as to better define the real prognostic impact of gains in body fat and weight in this setting.
To further investigate changes in body composition in the post diagnosis period, we examined changes in body fat and weight from diagnosis to within three years after diagnosis in 514 breast cancer survivors enrolled in the Health, Eating, Activity, and Lifestyle (HEAL) Study, a population-based prospective cohort study. We also examined the associations of demographic, prognostic, and lifestyle factors with changes in body fat and weight.
METHODS
Study Setting, Subjects, and Recruitment
The HEAL study is a population-based, multi-center, multi-ethnic prospective cohort study that has enrolled 1,223 breast cancer survivors who are being followed to determine whether weight, physical activity, diet, sex hormones, and other exposures affect breast cancer prognosis (22–24). Women were recruited into the HEAL study through Surveillance, Epidemiology, End Results (SEER) registries in New Mexico, Los Angeles County (CA), and Western Washington. Details of the aims, study design, and recruitment procedures have been published previously (22–24). Comparable data on body weight collected at both visits were available only for New Mexico and Washington. Therefore these analyses were limited to participants from those two sites.
Briefly, in New Mexico, 654 women, aged 18 years or older, diagnosed with in situ to Stage IIIA breast cancer between July 1996 and March 1999, and living in Bernalillo, Sante Fe, Sandoval, Valencia, or Taos Counties were recruited for the HEAL Study. In Western Washington, 202 women, between the ages of 40 and 64 years, diagnosed with in situ to Stage IIIA breast cancer between September 1997 and September 1998, and living in King, Pierce, or Snohomish Counties were recruited. In both New Mexico and Washington, stage of disease was based on the American Joint Committee on Cancer (AJCC) stage groupings: 0 (‘in situ’), I, IIA, IIB, IIIA, which are based on the tumor-node-metastasis system (25). During the years of 1996 through 1999 when these women were diagnosed, there were no major changes in the AJCC codes within SEER.
Participants completed in-person interviews at baseline (within their first year after diagnosis, 6 ± 2 months from diagnosis) and two-years after the baseline visit (within their third year after diagnosis, 31 ± 4 months from diagnosis). Among the 856 women enrolled at baseline, a total of 39 women had a previous breast cancer diagnosis, a total of 47 women did not have a baseline body weight measure; 220 women did not have a follow-up body weight measure, 6 women did not complete the physical activity questionnaire at baseline, and a total of 30 women had a recurrence of breast cancer between the two visits. Our analyses are based on the remaining 514 women. Analyses involving body fat include a reduced sample size of 132 women because of limited funds available to perform DEXA scans. Women who had a DEXA scan were similar to the cohort of women included in the full analyses in terms of baseline characteristics. Written informed consent was obtained from each subject. The study was performed with the approval of the Institutional Review Boards of participating centers, in accord with an assurance filed with and approved by the U.S. Department of Health and Human Services.
Data Collection
Anthropometrics
Trained staff measured weight and height in a standard manner at the clinic visit. With the women wearing light indoor clothing and no shoes, weight was measured to the nearest 0.1 kg using a balance-beam laboratory scale. Height was measured, also without shoes, to the nearest 0.1 cm using a stadiometer. All measurements were performed and recorded twice in succession, averaged for a final value for analyses. Body mass index (BMI) was computed as weight in kg divided by height in m2. Three mutually exclusive BMI groups were created: lean weight (BMI < 25 kg/m2), overweight (25 kg/m2 ≤ BMI < 30.0 kg/m2), and obese (BMI ≥ 30.0 kg/m2) (26).
Percent body fat was measured from whole-body scans using a dual-energy x-ray absorptiometry (DEXA) scanner (Lunar model DPX [GE Medical Systems, Milwaukee, WI] in New Mexico; Hologic model QDR 1500 [Hologic Inc, Waltham, MA] in Washington).
Physical Activity Assessment
We collected information on physical activity using an interview-administered questionnaire at a visit scheduled within the first and third years after diagnosis. The questionnaire was based on the Modifiable Activity Questionnaire developed by Kriska and colleagues, which was designed to be easily modified for use with different populations, and which has been shown to be reliable and valid (27). The type, duration, and frequency of activities performed in the past year were assessed. The sports/recreation and household activity section of the questionnaire addressed 29 popular activities.
We then estimated hours per week for each activity by multiplying frequency and duration together. Two mutually exclusive groups were created based on type of activity, sports/recreation including walking or household/gardening. Each activity was also categorized as light (< 3 METs)-, moderate (3–6 METs)-, or vigorous (> 6 METs)-intensity based on Ainsworth et al’s ‘Compendium of Physical Activities’ (28, 29).
Stage of Disease and Cancer Treatment
We obtained data on disease stage from the local SEER registries prior to recruitment of women into the HEAL Study (30). Adjuvant treatment was categorized into three mutually exclusive groups: surgery only (including those taking or not taking tamoxifen), surgery + radiation (including those taking or not taking tamoxifen), or any chemotherapy (including surgery, those taking or not taking tamoxifen, as well as those having radiation or not). We also were interested in looking at tamoxifen use (adjusted for treatment group), thus two mutually exclusive groups were created: not taking tamoxifen and taking tamoxifen.
Other Variables
Standardized questionnaire information was collected on medical history, reproductive histyor, family history of cancer, physician-diagnosed type 2 diabetes, smoking status, and selected demographic data. Information on diet was collected via a food frequency questionnaire (31).
Statistical Analyses
Means and standard deviations of physiological and demographic characteristics of the study sample were calculated by study site, and differences in means were compared using t-tests for continuous variables and chi-square analyses for categorical variables. We also examined the proportion of breast cancer survivors gaining or losing body weight or body fat over the two-year follow-up period.
We used analysis of covariance methods to estimate least squares means and test for differences in body weight and body fat across categories of disease stage, adjuvant treatment, age, menopausal status, tamoxifen use, BMI, study site, ethnicity, smoking status, changes in total caloric intake, and changes in physical activity. Each analysis was adjusted for the other factors except the variable of interest; for example, in comparing body weight across disease stage, we adjusted for the other factors, but not disease stage. We used Tukey's Honestly Significant Difference (HSD) test to identify statistically significant differences between groups with the overall level of statistical significance constrained to 5%.
RESULTS
The mean age and BMI of participants were 56.3 ± 10.5 years and 26.3 ± 5.3 kg/m2, respectively (Table I). Sixty-nine percent of women were postmenopausal. Seventy-eight percent of the women had completed 12 years of high school, and 85% and 15% of the women were non-Hispanic White and Hispanic White, respectively. Twenty-five percent, 57%, and 18% of the patients were diagnosed with in situ, Stage I, and Stage II to III-A breast cancer, respectively.
Table I.
Baseline characteristics of breast cancer survivors in the HEAL Study by study site (N = 514).
| All | Washington | New Mexico | |
|---|---|---|---|
| (N = 514) | (N = 161) | (N = 353) | |
| Mean ± SD | Mean ± SD | Mean ± SD | |
| Age, years, mean ± SD | 56.3 ± 10.5 | 52.4 ± 6.3 | 58.1 ± 11.5* |
| Postmenopausal at baseline | 69% | 63% | 71% |
| Education, % High School graduates | 96% | 99% | 95% |
| Months from diagnosis to initial interview, mean ± SD | 6 ± 2 | 7 ± 2 | 6 ± 2 |
| Ethnicity | |||
| Non-Hispanic White | 85% | 98% | 79%* |
| Hispanic White | 15% | 2% | 21% |
| Stage of Disease | |||
| 0, in situ | 25% | 32% | 21%* |
| I | 57% | 49% | 61% |
| II – IIIA | 18% | 19% | 18% |
| Treatment | |||
| Surgery only | 30% | 25% | 33%* |
| Surgery ± Radiation only | 42% | 49% | 43% |
| Any chemotherapy | 27% | 19% | 24% |
| Tamoxifen users | 48% | 53% | 46% |
| Weight, kg, mean ± SD | 69.8 ± 15.0 | 73.8 ± 16.3 | 68.0 ± 14.0* |
| Height, cm, mean ± SD | 162.9 ± 6.9 | 164.2 ± 6.6 | 162.4 ± 6.9 |
| Body mass index, kg/m2, mean ± SD | 26.3 ± 5.3 | 27.3 ± 5.7 | 25.8 ± 5.1 |
| Baseline sports/recreational activity levels, hrs/wk, mean ± SD | 2.4 ± 3.5 | 2.2 ± 3.3 | 2.5 ± 3.6 |
| Baseline total daily caloric intake, mean ± SD | 1545 ± 562 | 1597 ± 576 | 1503 ± 591 |
| Current smokers | 9% | 8% | 10% |
| Physician-diagnosed type 2 diabetes | 6% | 2% | 7%* |
| Family history of breast cancer | 25% | 21% | 27%* |
significantly different from Washington (p < .05).
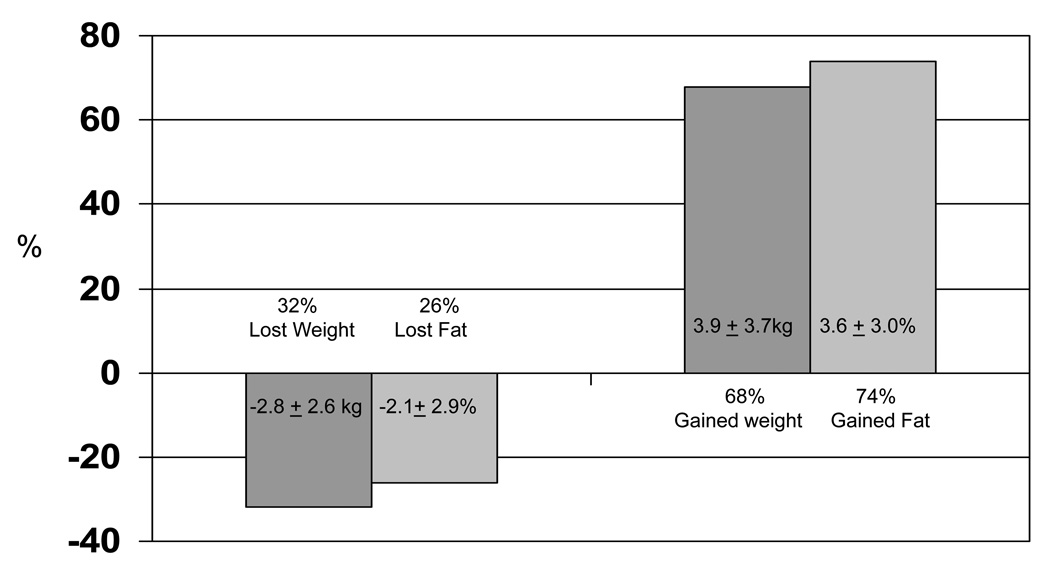
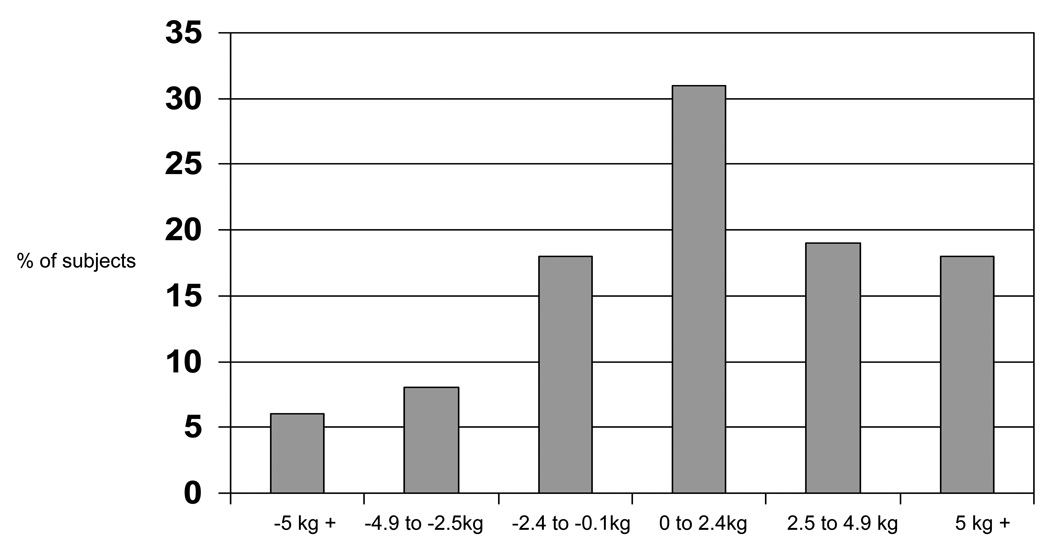
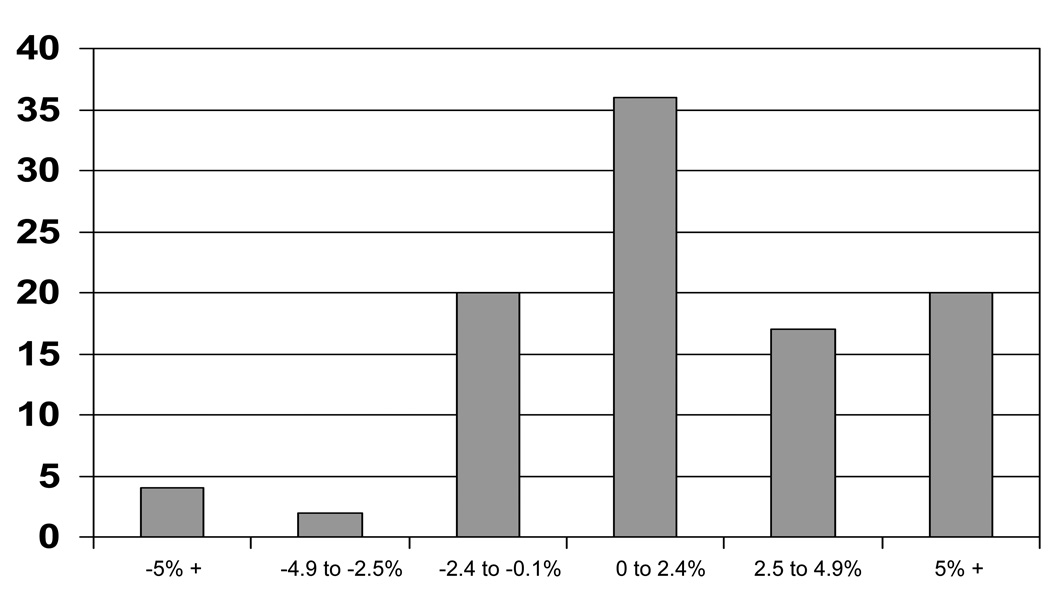
The mean weight and percent body fat change of participants were 1.7 ± 4.7 kg and 2.1 ± 3.9%, respectively with 68% of the sample gaining weight (mean weight gain = 3.9 ± 3.7 kg) and 74% gaining percent body fat (mean percent body fat gain = 3.6 ± 3.0%) (Figure I). Among the 68% of women who gained weight, 31% gained less than 2.5 kg, 19% gained between 2.5 and 4.9 kg, and 18% gained 5 kg or more (Figure II). Among the 74% of women who gained body fat, 36% gained less than 2.5%, 17% gained between 2.5 and 4.9%, and 21% gained 5% or more (Figure III).
Figure I.
Body weight (N = 514) and fat changes (N = 132) from baseline to within 3 years. Overall mean weight change = 1.7 ± 4.7kg (−13.2 to 27.0 kg); Overall mean fat change = 2.1 ± 3.9% (−14.5 to 15.0%)
Figure II.
Distribution of weight changes from baseline to within 3 years (n = 514).
Figure III.
Distribution of body fat changes from baseline to within 3 years (n = 132).
We observed a statistically significant trend of increasing gains in weight with increasing category of disease stage (p for trend = .0039), younger age groups (p for trend = .0001), postmenopausal women (p < .05), and decreasing sports/recreational physical activity from baseline to within 3 years after diagnosis (p for trend = .032) (Table II). Because of the observation of increasing weight gain among younger women, yet also with postmenopausal women, we further examined changes in body weight stratified by both age groups and menopausal status, and by age groups among only postmenopausal women (Table III). Postmenopausal women had greater weight gain compared to premenopausal women and women who became postmenopausal after diagnosis for all age groups. When examining weight gain by age groups among only postmenopausal women, we observed a significant trend of greater weight gain among younger postmenopausal women compared to older postmenopausal women, p for trend = .0020 (Table III).
Table II.
Weight change (kg) from baseline assessment to assessment conducted during year three following diagnosis in HEAL breast cancer survivors (N = 514).
| N | Unadjusted | Adjusted1 | |||
|---|---|---|---|---|---|
| Mean ± SE | % Change ± SE | Mean ± SE | % Change ± SE | ||
| Disease Stage | |||||
| In Situ | 127 | 1.3 ± 0.4 | 1.8% ± 0.6 | 0.9 ± 0.5 | 1.3% ± 0.7 |
| Stage I | 294 | 1.4 ± 0.3 | 2.3% ± 0.4 | 1.7 ± 0.3 | 2.5% ± 0.4 |
| Stage II – IIIA | 93 | 3.1 ± 0.5* Φ | 4.5% ± 0.7* Φ | 3.2 ± 0.5* Φ | 4.5% ± 0.7* Φ |
| P for trend | .0036 | .0031 | .0039 | .0037 | |
| Treatment | |||||
| Surgery only | 156 | 1.5 ± 0.4 | 2.3% ± 0.5 | 2.0 ± 0.5 | 3.0 ± 0.6 |
| Surgery + Radiation | 217 | 1.1 ± 0.3 | 1.6% ± 0.4 | 1.5 ± 0.3 | 3.2 ± 0.4 |
| Any Chemotherapy | 141 | 3.0 ± 0.4* Φ | 4.3% ± 0.5* Φ | 2.0 ± 0.5 | 3.0 ± 0.7 |
| Age Group | |||||
| 40 – 49 years | 142 | 2.8 ± 0.4 | 4.3% ± 0.5 | 3.7 ± 0.5 | 5.6% ± 0.7 |
| 50 – 59 years | 194 | 2.1 ± 0.3 | 2.9% ± 0.5* | 1.8 ± 0.3 | 2.5% ± 0.5* |
| 60 + years | 178 | 0.5 ± 0.3* Φ | 0.9 ± 0.5 * Φ | 0.3 ± 0.4* Φ | 0.4% ± 0.6 * Φ |
| P for trend | .0001 | .0001 | .0001 | .0001 | |
| Menopausal Status | |||||
| Premenopausal | 84 | 1.6 ± 0.5 | 2.4% ± 0.7 | 0.3 ± 0.6 | 0.3% ± 0.8 |
| Pre to Postmenopausal | 77 | 3.0 ± 0.5* | 4.1% ± 0.8 | 1.5 ± 0.6 | 2.0% ± 0.8 |
| Postmenopausal | 353 | 1.5 ± 0.2 Φ | 2.3% ± 0.4 Φ | 2.2 ± 0.3* | 3.3% ± 0.4* |
| Tamoxifen Use | |||||
| No Tamoxifen | 267 | 1.8 ± 0.3 | 2.7% ± 0.4 | 1.9 ± 0.3 | 2.8% ± 0.4 |
| Tamoxifen | 247 | 1.7 ± 0.3 | 2.4% ± 0.4 | 1.7 ± 0.3 | 2.4 % ± 0.4 |
| Body Mass Index | |||||
| < 25.0 kg/m2 | 239 | 1.7 ± 0.3 | 2.9% ± 0.4 | 1.7 ± 0.3 | 3.0% ± 0.4 |
| 25.0 – 29.9 kg/m2 | 167 | 1.5 ± 0.4 | 2.1% ± 0.5 | 1.7 ± 0.3 | 2.4% ± 0.5 |
| ≥ 30.0 kg/m2 | 108 | 2.2 ± 0.4 | 2.5% ± 0.6 | 1.8 ± 0.4 | 2.0% ± 0.6 |
| P for trend | .34 | .60 | .92 | .20 | |
| Ethnicity | |||||
| Non-Hispanic White | 436 | 1.9 ± 0.2 | 2.7% ± 0.3 | 1.9 ± 0.2 | 2.8% ± 0.3 |
| Hispanic White | 78 | 1.0 ± 0.5 | 1.7% ± 0.7 | 1.1 ± 0.5 | 1.9% ± 0.8 |
| Change in total caloric intake | |||||
| < −270 kcals | 113 | 1.1 ± 0.4 | 1.6% ± 0.6 | 1.0 ± 0.4 | 1.5% ± 0.6 |
| −270 to 100 kcals | 113 | 2.7 ± 0.4 | 3.8% ± 0.6* | 3.0 ± 0.4* | 4.0% ± 0.6* |
| > 100 kcals | 112 | 2.1 ± 0.4 | 3.2% ± 0.6 | 2.1 ± 0.4 | 3.2% ± 0.6* |
| P for trend | .14 | .072 | .083 | .040 | |
| Change in sports hrs/wk | |||||
| ≤ 0 hr/wk | 204 | 1.9 ± 0.3 | 2.7% ± 0.5 | 2.2 ± 0.3 | 3.2% ± 0.5 |
| 0.1 – 1.5 hr/wk | 155 | 2.1 ± 0.4 | 3.0% ± 0.6 | 1.9 ± 0.4 | 2.8% ± 0.5 |
| ≥ 1.5 hr/wk | 155 | 1.3 ± 0.4 | 2.0% ± 0.5 | 1.2 ± 0.3* | 1.8% ± 0.5* |
| P for trend | .18 | .29 | .032 | .048 | |
Adjusted for disease stage, treatment, study site, smoking status, body mass index, tamoxifen use, age, menopausal status, change in total physical activity, change in total caloric intake, time from diagnosis to baseline interview, months from completing treatment, ethnicity, education, completed treatment, family history of breast cancer, family history of type 2 diabetes, physician-diagnosed type 2 diabetes, cardiovascular disease.
significantly different from first level and
significantly different from second level using Tukey’s Honestly Significant Difference with overall level of statistical significance constrained at 0.05.
Table III.
Weight change from baseline assessment to assessment conducted during year three following diagnosis in breast cancer survivors (N = 514).
| N | Unadjusted | Adjusted1 | |||
|---|---|---|---|---|---|
| Mean ± SE | % Change ± SE | Mean ± SE | % Change ± SE | ||
| 40 – 49 years | |||||
| Premenopausal | 72 | 1.7 ± 0.6 | 2.6% ± 0.9 | 1.8 ± 0.6 | 2.6% ± 0.9 |
| Pre to Postmenopausal | 46 | 3.4 ± 0.8 | 4.8% ± 1.1 | 3.1 ± 0.8 | 4.5% ± 1.1 |
| Postmenopausal | 24 | 5.2 ± 1.0* | 8.4% ± 1.5* Φ | 5.7 ± 1.1* | 9.2% ± 1.6* Φ |
| 50– 59 years | |||||
| Premenopausal | 12 | 0.8 ± 1.3 | 1.6% ± 1.8 | 0.1 ± 1.4 | 0.4% ± 2.0 |
| Pre to Postmenopausal | 31 | 2.4 ± 0.8 | 3.0% ± 1.1 | 1.7 ± 0.9 | 2.1% ± 1.3 |
| Postmenopausal | 151 | 2.1 ± 0.4 | 2.9% ± 0.5 | 2.3 ± 0.4 | 3.2% ± 0.5 |
| 60 + years | |||||
| Premenopausal | 0 | NA | NA | NA | NA |
| Pre to Postmenopausal | 0 | NA | NA | NA | NA |
| Postmenopausal | 178 | 0.5 ± 0.3 | 0.9% ± 0.4 | 0.6 ± 3.0 | 1.0% ± 0.4 |
| Age (postmenopausal women only) | |||||
| 40 – 49 years | 24 | 5.2 ± 1.4 | 8.4% ± 1.4 | 5.2 ± 1.4 | 8.6% ± 1.4 |
| 50 – 59 years | 151 | 2.1 ± 0.4* | 2.9% ± 0.6* | 2.1 ± 0.4* | 2.9% ± 0.6* |
| 60 – 69 years | 116 | 0.8 ± 0.4*Φ | 1.2% ± 0.6* | 1.0 ± 0.4* | 1.4% ± 0.6* |
| 70 – 79 years | 47 | −0.1 ± 0.6*Φ | 0.1% ± 0.9* Φ | 0.4 ± 0.7* | 0.5% ± 1.0* |
| 80 + years | 15 | 0.5 ± 1.1* | 1.0% ± 1.6* | 0.5 ± 1.2* | 0.8% ± 1.7* |
| P for trend | .006 | .0002 | .002 | .0003 | |
Adjusted for disease stage, treatment, study site, smoking status, body mass index, tamoxifen use, age, menopausal status, change in total physical activity, change in total caloric intake, time from diagnosis to baseline interview, months from completing treatment, ethnicity, education, completed treatment, family history of breast cancer, family history of type 2 diabetes, physician-diagnosed type 2 diabetes, cardiovascular disease
significantly different from first level;
significantly different from second level using Tukey’s Honestly Significant Difference with overall level of statistical significance constrained at 0.05.
In unadjusted analyses, we observed greater weight gain among women receiving chemotherapy than women receiving just surgery or surgery plus radiation (p < .05). However, when we adjusted for potential confounders, weight gain was no longer greater among women receiving chemotherapy compared to the other two treatment groups. To determine which variables were the strongest confounders in the treatment and weight change association, we analyzed the treatment data by adding each adjustment variable one at a time as well as by examining the effect of treatment on weight change stratified by each adjustment variable. No one variable significantly changed the treatment – weight change association. However, when we stratified the analyses by menopausal status, in postmenopausal women we observed nonsignificantly greater weight gain among women receiving chemotherapy than women receiving just surgery or surgery plus radiation, but not among premenopausal women or women who became postmenopausal after diagnosis (data not shown). Further, when we stratified the analyses by change in sports/recreational physical activity from baseline to within 3 years after diagnosis, in women who did not increase their physical activity from baseline to within 3 years after diagnosis, we observed greater weight gain among women receiving chemotherapy than women receiving just surgery or surgery plus radiation (p = .061 and p = .048, respectively), but not among women who increased their activity from baseline to within 3 years after diagnosis (data not shown). This latter group actually had less weight gain among those receiving chemotherapy compare to the two other treatment groups (p < .05). Thus, it appears that menopausal status and physical activity had the strongest effect on the treatment and weight change association. Lastly, we also examined the effect of type of chemotherapy (i.e., those taking anthracycline-based chemotherapy vs. those not taking anthracycline-based chemotherapy) on change in body weight. In unadjusted and adjusted analyses, we observed similar amounts of weight gain in both chemotherapy groups (p = .68).
No significant associations were observed between changes in weight and family history of breast cancer, family history of type 2 diabetes, physician-diagnosed type 2 diabetes, cardiovascular disease, and education (data not shown).
We observed a statistically significant trend of increasing gains in body fat with decreasing categories of BMI (p for trend = .050) and sports/recreational physical activity from baseline to within 3 years after diagnosis (p for trend = .051) (Table IV). The trend of increasing gains in body fat associated with decreasing age approached significance (p for trend = .061). No statistically significant associations were observed between changes in body fat and disease stage, treatment, menopausal status, tamoxifen use, ethnicity, and change in total caloric intake.
Table IV.
Percent body fat change from baseline assessment to assessment conducted during year three following diagnosis in HEAL breast cancer survivors (N = 132).
| N | Unadjusted | Adjusted1 | |||
|---|---|---|---|---|---|
| Mean ± SE | % Change ± SE | Mean ± SE | % Change ± SE | ||
| Disease Stage | |||||
| In Situ | 28 | 1.1 ± 0.7 | 4.5% ± 2.7 | 1.3 ± 0.8 | 4.6% ± 2.0 |
| Stage I | 85 | 2.1 ± 0.4 | 8.0% ± 1.6 | 2.4 ± 0.4 | 9.0% ± 1.5 |
| Stage II – IIIA | 19 | 3.2 ± 0.9 | 10.3% ± 3.3 | 1.8 ± 1.0 | 5.8% ± 3.7 |
| P for trend | .078 | .18 | .68 | .82 | |
| Treatment | |||||
| Surgery only | 39 | 1.9 ± 0.6 | 7.0% ± 2.3 | 2.2 ± 0.7 | 8.4 ± 2.6 |
| Surgery + Radiation | 62 | 1.6 ± 0.5 | 6.7% ± 1.9 | 2.0 ± 0.5 | 8.3 ± 1.8 |
| Any Chemotherapy | 31 | 3.2 ± 0.7 | 10.0% ± 2.6 | 2.2 ± 1.0 | 5.6 ± 3.5 |
| Age Group | |||||
| 40 – 49 years | 36 | 3.3 ± 0.6 | 11.5% ± 2.4 | 3.4 ± 0.8 | 10.3% ± 2.9 |
| 50 – 59 years | 47 | 2.1 ± 0.6 | 8.5% ± 2.1 | 1.9 ± 0.6 | 8.4% ± 2.1* |
| 60 + years | 49 | 1.2 ± 0.5* | 3.8 ± 2.0 * | 1.3 ± 0.6 | 5.2% ± 2.2 |
| P for trend | .013 | .017 | .061 | .21 | |
| Menopausal Status | |||||
| Premenopausal | 21 | 2.5 ± 0.8 | 10.9% ± 3.1 | 2.0 ± 0.9 | 5.0% ± 3.9 |
| Pre to Postmenopausal | 23 | 3.4 ± 0.8 | 12.6% ± 3.0 | 2.8 ± 0.9 | 8.4% ± 3.4 |
| Postmenopausal | 88 | 1.6 ± 0.4 | 5.5% ± 1.5 | 1.9 ± 0.4 | 8.1% ± 1.7 |
| Tamoxifen Use | |||||
| No Tamoxifen | 77 | 2.1 ± 0.4 | 8.7% ± 1.7 | 1.9 ± 0.4 | 7.5% ± 1.6 |
| Tamoxifen | 55 | 1.9 ± 0.5 | 6.0% ± 2.0 | 2.3 ± 0.5 | 7.9 % ± 1.9 |
| Body Mass Index | |||||
| < 25.0 kg/m2 | 57 | 3.3 ± 0.5 | 13.9% ± 1.8 | 3.2 ± 0.6 | 13.3% ± 2.0 |
| 25.0 – 29.9 kg/m2 | 52 | 1.1 ± 0.5 | 2.9% ± 1.9* | 1.2 ± 0.6* | 3.5% ± 2.0* |
| ≥ 30.0 kg/m2 | 23 | 1.1 ± 0.8* | 2.4% ± 2.8* | 1.2 ± 0.9* | 3.2% ± 3.1* |
| P for trend | .019 | .00080 | .050 | .010 | |
| Ethnicity | |||||
| Non-Hispanic White | 105 | 2.2 ± 0.4 | 8.4% ± 1.4 | 2.0 ± 0.4 | 8.0% ± 1.3 |
| Hispanic White | 27 | 1.3 ± 0.7 | 4.3% ± 2.8 | 2.2 ± 0.8 | 6.4% ± 2.8 |
| Change in total caloric intake | |||||
| < −200 kcals | 28 | 0.9 ± 0.8 | 3.9% ± 3.0 | 1.0 ± 0.9 | 3.9% ± 3.0 |
| −200 to 50 kcals | 28 | 3.1 ± 0.8 | 12.7% ± 3.0* | 3.5 ± 0.9 | 12.6% ± 3.0* |
| > 50 kcals | 28 | 2.4 ± 0.8 | 7.3% ± 3.0 | 1.9 ± 0.9 | 7.1% ± 3.0 |
| P for trend | .21 | .42 | .52 | .49 | |
| Change in sports hrs/wk | |||||
| 0 hr/wk | 44 | 3.1 ± 0.6 | 12.1% ± 2.2 | 2.9 ± 0.6 | 11.12% ± 2.2 |
| 0.1 – 1.5 hr/wk | 44 | 1.5 ± 0.6* | 4.2% ± 2.2* | 2.0 ± 0.6 | 6.8% ± 2.1 |
| ≥ 1.5 hr/wk | 44 | 1.6 ± 0.6* | 6.5% ± 2.2 | 1.3 ± 0.6 | 5.2% ± 2.0* |
| P for trend | .051 | .067 | .051 | .047 | |
Adjusted for disease stage, treatment, study site, smoking status, body mass index, tamoxifen use, age, menopausal status, change in total physical activity, change in total caloric intake, time from diagnosis to baseline interview, months from completing treatment, ethnicity, education, completed treatment, family history of breast cancer, family history of type 2 diabetes, physician-diagnosed type 2 diabetes, cardiovascular disease.
significantly different from first level;
significantly different from second level using Tukey’s Honestly Significant Difference with overall level of statistical significance constrained at 0.05.
DISCUSSION
On average, breast cancer patients in our study gained 1.7 kg of weight and 2.1% body fat over 2 years. More than 68% and 74% of the women experienced gains in weight and percent body fat, respectively. The mean increases for those who gained weight and percent body fat were 3.9 kg and 3.6%, with a range of 0.1 kg to 27.0 kg and 0.1% to 15.0%. The weight gain that we observed is comparable to that reported by others in women with recently diagnosed breast cancer. Goodwin et al (4) reported that 84% of 535 breast cancer patients gained weight in the first year after diagnosis, with a mean weight gain of 1.6 kg overall. In a study by Rock et al (1), 60% of 1116 women enrolled in the WHEL Study reported weight gain from one year before diagnosis to within four years after diagnosis. The mean weight gain was 2.7 kg.
The weight gain we and others have observed in breast cancer survivors is larger than that observed by others in healthy women. Williamson et al (32) studied weight gain over a 10-year period in the 1970’s in 6,135 women who participated in the NHANES I Study. Over 10 years, women aged 45 to 55 years gained only 0.79 kg (0.08 kg/yr), whereas those aged 55 years and older lost weight. Our findings cannot be compared directly with the NHANES I study, however, women with breast cancer may experience a greater weight gain than women in the general population.
The effects of weight gain on breast cancer recurrence have been debated in the literature. Some (7–9), but not all (33–37) investigators have associated weight gain with an earlier disease recurrence. Camoriano et al (8) followed 646 breast cancer patients for a median of 6.6 years and found that premenopausal women who gained more than 5.9 kg were 1.5 times more likely to relapse and 1.6 times more likely to die of their breast cancer than were women gaining less weight.
Although it remains to be determined whether post-diagnosis weight gain influences risk for progressive disease, it is known that weight gain adversely affects risk for cardiovascular disease, hypertension, and diabetes (15–17). Furthermore, many studies have identified obesity as an important negative prognostic factor for breast cancer survival (10–14). In a review by Chlebowski et al (10), 17 of 26 studies found increased weight to be a significant risk factor for recurrent disease and decreased survival; seven studies produced null findings; and two studies found an inverse association between weight and recurrence. In the studies that found a significant positive association between overweight and progressive disease, women categorized in the higher vs. lower levels of obesity exhibited a 30% to 540% increased risk of death. Data from the National Surgical Adjuvant Breast and Bowel Project in 3,385 women indicated that while obesity did not increase risk for breast cancer recurrence or death, obesity did increase risk of overall death, risk of other cancers, and death from cardiac disease (38). It is currently unknown whether post-diagnosis weight reduction modifies the relationship between obesity and breast cancer recurrence and mortality.
Several mechanisms have been proposed to explain the adverse effect of adiposity and weight gain on breast cancer prognosis. One mechanism rests on greater peripheral conversion of androstenedione to estradiol and inhibition of synthesis of sex hormone binding globulin with an increase in free estradiol which stimulates neoplastic cells (39), especially in postmenopausal women (23). We have previously reported an association between increased adiposity and increased concentrations of estrone, estradiol, and free estradiol in the HEAL cohort of breast cancer survivors (23). Another mechanism relates to insulin and IGF-1 and the interactions of these hormones with adiposity (40). Insulin and IGFs exhibit mitogenic effects that influence both premalignant and cancerous stages of cell growth. Both insulin and IGF-1 stimulate the synthesis of sex steroids, and thus, their cancer-promoting effects in the progression of breast cancer may be mediated by an effect on sex hormones. Another explanation for poorer survival may be associated with obese women failing to respond to treatment as a result of the common practice of chemotherapy capping at a body surface area of 2 m2, which may offer suboptimal treatment benefit (41).
In our study, higher disease stage, being postmenopausal, and less participation in physical activity were significantly related to gains in body weight. Receiving chemotherapy was also associated with greater weight gain, however this observation was limited to postmenopausal women and women who did not increase their physical activity from baseline to within 3 years after diagnosis. Goodwin et al. (4) examined factors associated with weight gain during the subsequent year after diagnosis in 535 newly diagnosed breast cancer survivors. In multivariate analysis, onset of menopause and administration of chemotherapy were independent predictors of weight gain.
A study by Demark-Wahnefried studied energy balance over the first year after breast cancer diagnosis in 53 premenopausal women (2). Weight gain during chemotherapy was associated with an increase in fat mass and decrease in lean body mass, a pattern consistent with sarcopenic obesity. This form of obesity is associated with reduced physical activity, aging, and menopause. A significantly lower level of physical activity throughout the year of observation was demonstrated. The authors conclude that reduced physical activity is the primary factor responsible for weight gain during chemotherapy for breast cancer. Demark-Wahnefried et al’s results are also consistent with a report by Rock et al (1) stating that physical activity predicted weight stability in 1116 breast cancer survivors participating in the WHEL Study.
Despite strong evidence suggesting that regular physical activity can protect against gains in body fat and weight, only 32% of breast cancer survivors enrolled in the HEAL Study engaged in the recommended level of physical activity defined as 150 min per week of moderate- to vigorous-intensity physical activity (24). This percentage is similar to the proportion of healthy U.S. women (27%) meeting the current recommendation (29). Recently, we reported that women diagnosed with breast cancer were significantly less physically active within their first year after diagnosis than they were one year before diagnosis (22). While physical activity levels reported three years after diagnosis increased to pre-diagnosis levels for approximately 50% of the sample, this was mostly limited to non-obese women (24). Exercise interventions and physical activity programs focused on increasing physical activity among breast cancer survivors with the ultimate goal of testing the effects on prognosis are needed. Previous research has also shown beneficial effects of dietary interventions on breast cancer prognosis in breast cancer survivors (42,43). Studies examining the combination of diet and physical activity on prognosis are also needed.
The HEAL Study has several strengths. It is one of a handful that has examined changes in measured body weight for a relatively long follow-up period, and the largest study reporting changes in body fat measured via DEXA. However, a limitation of our study is that we were unable to measure weight and percent body fat at other time points (e.g., within the second year after diagnosis). Women may have initially gained and then lost weight during this time. Thus, we are unable to report the maximal gains in body weight and body fat within three years after a breast cancer diagnosis. Another limitation of the study is that some women were recruited into the study while undergoing treatment, while others had not begun treatment or had completed treatment. Some women may have already experienced changes in weight or body fat after diagnosis, but prior to enrollment in the study. Lastly, our sample was highly educated and mostly non-Hispanic White, therefore, we cannot be sure that these findings pertain to all breast cancer survivors.
Weight gain is a concern of many women after a diagnosis of breast cancer. In a community-based study (44), the majority of breast cancer survivors were dissatisfied with their weight and stated that they were ready to take steps necessary to reduce their weight. A study conducted among 531 breast cancer survivors found that 52% wanted nutritional guidance at the time of diagnosis or soon after, although few reported having ever received dietary recommendations from their physicians (45).
In conclusion, over two-thirds of women in our study gained weight and body fat within the first three years after a diagnosis of breast cancer, and to a greater degree than that previously reported in studies of healthy women. Weight and body fat gains were greatest in women who reported less participation in physical activity. Since higher levels of body fat are associated with increased breast cancer recurrence and decreased survival, it is imperative that research be conducted on the prognostic effect of physical activity and weight loss in overweight and obese breast cancer survivors.
ACKNOWLEDGEMENTS
This study was supported through NCI contracts N01-CN-75036-20, NO1-CN-05228, NO1-PC-67010, and training grant T32 CA09661. A portion of this work was conducted through the Clinical Research Center at the University of Washington and supported by the National Institutes of Health, Grant M01-RR-00037. Data collection for the Women’s CARE Study at the University of Southern California was supported by contract N01-HD-3-3175 from the National Institute of Child Health and Human Development and patient identification was supported in part by 050Q-8709-S1528 from the California Department of Health Services.
REFERENCES
- 1.Rock C, Flatt S, Newman V, et al. Factors associated with weight gain in women after diagnosis of breast cancer. JADA. 1999;99:1212–1218. doi: 10.1016/s0002-8223(99)00298-9. [DOI] [PubMed] [Google Scholar]
- 2.Demark-Wahnefried W, Peterson B, Winer E, et al. Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. JCO. 2001;19(9):2381–2389. doi: 10.1200/JCO.2001.19.9.2381. [DOI] [PubMed] [Google Scholar]
- 3.McInnes J, Knobf M. Weight gain and quality of life in women treated with adjuvant chemotherapy for early-stage breast cancer. ONF. 2001;28(4):1–11. [PubMed] [Google Scholar]
- 4.Goodwin P, Ennis M, Pritchard K, et al. Adjuvant treatment and onset of menopause predict weight gain after breast cancer diagnosis. JCO. 1999;17(1):120–129. doi: 10.1200/JCO.1999.17.1.120. [DOI] [PubMed] [Google Scholar]
- 5.Aslani A, Smith R, Allen B, Pavlakis N, Levi J. Changes in body composition during breast cancer chemotherapy with the CMF-regimen. Breast Cancer Res Treat. 1999;57:285–290. doi: 10.1023/a:1006220510597. [DOI] [PubMed] [Google Scholar]
- 6.Demark-Wahnefried W, Rimer B, WIner E. Weight gain in women diagnosed with breast cancer. JADA. 1997;97:519–526. doi: 10.1016/s0002-8223(97)00133-8. [DOI] [PubMed] [Google Scholar]
- 7.Chlebowski RT, Weiner JM, Reynolds R, et al. Long-term survival following relapse after 5-FU but not CMF adjuvant breast cancer therapy. BCRT. 1986;7:23–29. doi: 10.1007/BF01886732. [DOI] [PubMed] [Google Scholar]
- 8.Camoriano JK, Loprinzi CL, Ingle JN, et al. Weight change in women treated with adjuvant therapy or observed following mastectomy for node positive breast cancer. JCO. 1990;8:1327–1334. doi: 10.1200/JCO.1990.8.8.1327. [DOI] [PubMed] [Google Scholar]
- 9.Bonomi P, Bunting N, Fishman D, et al. Weight gain during adjuvant chemotherapy or hormone-chemotherapy for stage II breast cancer evaluated in relation to disease free survival. BCRT. 1985;4:339. (abstr) [Google Scholar]
- 10.Chlebowski R, Aiello E, McTiernan A. Weight loss in breast cancer patient management. J Clin Oncol. 2002;20:1128–1143. doi: 10.1200/JCO.2002.20.4.1128. [DOI] [PubMed] [Google Scholar]
- 11.Boyd NF, Campbell JE, Germanson T, et al. Body weight and prognosis in breast cancer. JNCI. 1981;67:785–789. [PubMed] [Google Scholar]
- 12.Goodwin PJ, Ennis M, Pritchard KI, et al. Fasting insulin and outcome in early-stage breast cancer: Results of a prospective cohort study. JCO. 2002;20:42–51. doi: 10.1200/JCO.2002.20.1.42. [DOI] [PubMed] [Google Scholar]
- 13.Kumar NB, Cantor A, Allen K, et al. Android obesity at diagnosis and breast carcinoma survival: Evaluation of the effects of anthropometric variables at diagnosis, including body composition and body fat distribution and weight gain during lifespan, and survival from breast carcinoma. Cancer. 2000;88:2751–2757. doi: 10.1002/1097-0142(20000615)88:12<2751::aid-cncr13>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 14.Newman SC, Miller AB, Howe GR. A study of the effect of weight and dietary fat on breast cancer survival time. Am J Epidemiol. 1986;123:767–774. doi: 10.1093/oxfordjournals.aje.a114305. [DOI] [PubMed] [Google Scholar]
- 15.Willett WC, Manson JE, Stampfer MJ, et al. Weight, weight change, and coronary heart disease in women: risk within the ‘normal’ weight range. JAMA. 1995;273:461–465. doi: 10.1001/jama.1995.03520300035033. [DOI] [PubMed] [Google Scholar]
- 16.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Health CW., Jr Body mass index and mortality in a prospective cohort of US adults. NEJM. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 17.Kopelman P. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 18.Aziz NM. Long-term survivorship: late effects. In: Berger AM, Portenoyu RK, Weissman DE, editors. Principles and Practice of Palliative Care and Supportive Oncology. 2nd Ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2002. pp. 1019–1033. [Google Scholar]
- 19.Ganz PA. Cancer Survivors: Physiologic and psychosocial outcomes. Alexandria, VA: American Soceity of Clinical Oncology; 1998. pp. 118–123. [Google Scholar]
- 20.Harvie M, Campbell I, Baildman A, Howell A. Energy balance in early breast cancer patients receiving adjuvant chemotherapy. Breast Cancer Res Treat. 2004;83:201–210. doi: 10.1023/B:BREA.0000014037.48744.fa. [DOI] [PubMed] [Google Scholar]
- 21.Freedman R, Aziz N, Albanes D, et al. Weight and body composition changes during and after adjuvant chemotherapy in women with breast cancer. J Clin Endocrinol Metab. 2004;89:2248–2253. doi: 10.1210/jc.2003-031874. [DOI] [PubMed] [Google Scholar]
- 22.Irwin ML, Crumley D, McTiernan A, et al. Physical activity levels before and after a diagnosis of breast carcinoma: The Health, Eating, Activity, and Lifestyle (HEAL) Study. Cancer. 2003;97:1746–1757. doi: 10.1002/cncr.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McTiernan A, Rajan B, Tworoger S, et al. Adiposity and sex hormones in postmenopausal breast cancer survivors. J Clin Oncol. 2003 May 15;21(10):1961–1966. doi: 10.1200/JCO.2003.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irwin ML, McTiernan A, Bernstein L, et al. Physical activity levels among breast cancer survivors. Med Sci Sports Exerc. In Press. [PMC free article] [PubMed] [Google Scholar]
- 25.American Joint Committee on Cancer. AJCC Cancer Staging Handbook. Philadelphia, PA: Lippincott-Raven Publishers; 1998. [Google Scholar]
- 26.National Institutes of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults – the evidence report. Obesity Res. 1998;6 Suppl 2:51S–209S. [PubMed]
- 27.Kriska A. Modifiable activity questionnaire. Med Sci Sports Exer. 1997;29:S73–S78. [Google Scholar]
- 28.Ainsworth B, Haskell W, Whitt M, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exer. 2000;32:S498–S516. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 29.U.S. Department of Health and Human Services. Physical Activity and Health: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; 1996. [Google Scholar]
- 30.National Cancer Institute. The SEER program code manual. Bethesda (MD): Cancer Statistics Branch, Surveillance Program, Division of Cancer Prevention and Control. National Cancer Institute, U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health; 1992 June; 1992 NIH Pub. No. 92-1999.
- 31.Patterson R, Kristal A, Tinker L, et al. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9(3):178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 32.Williamson DF, Kahn HS, Remington PL, et al. The 10-year incidence of overweight and major weight gain in US adults. Arch Intern Med. 1990;150:665–672. [PubMed] [Google Scholar]
- 33.Levine EG, Raczynski JM, Carpenter JT. Weight gain in women with breast cancer adjuvant treatment. Cancer. 1991;67:1954–1959. doi: 10.1002/1097-0142(19910401)67:7<1954::aid-cncr2820670722>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 34.Heasman KZ, Sutherland HJ, Campbell JA, Elhakim T, Boyd NF. Weight gain during adjuvant chemotherapy for breast cancer. Breast Cancer Res Treat. 1985;5:195–200. doi: 10.1007/BF01805994. [DOI] [PubMed] [Google Scholar]
- 35.Goodwin PJ, Panzarella T, Boyd NF. Weight gain in women with localized breast cancer-a descriptive study. Breast Cancer Res Treat. 1988;11:59–66. doi: 10.1007/BF01807559. [DOI] [PubMed] [Google Scholar]
- 36.Goodwin PJ, Ennis M, Pritchard KI, et al. Adjuvant treatment and onset of menopause predict weight gain after breast cancer diagnosis. J Clin Oncol. 1999;17:120–129. doi: 10.1200/JCO.1999.17.1.120. [DOI] [PubMed] [Google Scholar]
- 37.Costa LJ, Varella PC, del Giglio A. Weight changes during chemotherapy for breast cancer. Sao Paulo Med J. 2002;120:113–117. doi: 10.1590/S1516-31802002000400005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dignam J, Wieand K, Johnson K, et al. Obesity, tamoxifen use, and outcomes in women with estrogen receptor-positive early-stage breast cancer. JNCI. 2003;95(19):1467–1476. doi: 10.1093/jnci/djg060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaye SA, Folsom AR, Soler JT, et al. Associations of body mass and fat distribution with sex hormone concentrations in postmenopausal women. Int J Epidemiol. 1991;20:151–156. doi: 10.1093/ije/20.1.151. [DOI] [PubMed] [Google Scholar]
- 40.Yu H, Rohan T. Role of insulin-like growth factor family in cancer development and progression. JNCI. 2000;92:1472–1489. doi: 10.1093/jnci/92.18.1472. [DOI] [PubMed] [Google Scholar]
- 41.Georgiadis MS, Steinberg SM, Hankins LA, Ihde DC, Johnson BE. Obesity and therapy-related toxicity in patients treated for small-cell lung cancer. JNCI. 1995;87:361–366. doi: 10.1093/jnci/87.5.361. [DOI] [PubMed] [Google Scholar]
- 42.Rock CL, Demark-Wahnefried W. Nutrition and survival after the diagnosis of breast cancer: a review of the evidence. J Clin Oncol. 2002;20(14):3302–3316. doi: 10.1200/JCO.2002.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rock CL, Demark-Wahnefried W. Can lifestyle modification increase survival in women diagnosed with breast cancer. J Nutr. 2002;132:3504S–3509S. doi: 10.1093/jn/132.11.3504S. [DOI] [PubMed] [Google Scholar]
- 44.Jones LW, Courneya KS. Exercise discussions during cancer treatment consultations. Cancer Pract. 2002;10(2):66–74. doi: 10.1046/j.1523-5394.2002.102004.x. [DOI] [PubMed] [Google Scholar]
- 45.Demark-Wahnefried W, Peterson B, McBride C, Lipkus I, Clipp E. Current health behaviors and readiness to pursue lifestyle changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer. 2000;88:674–684. [PubMed] [Google Scholar]