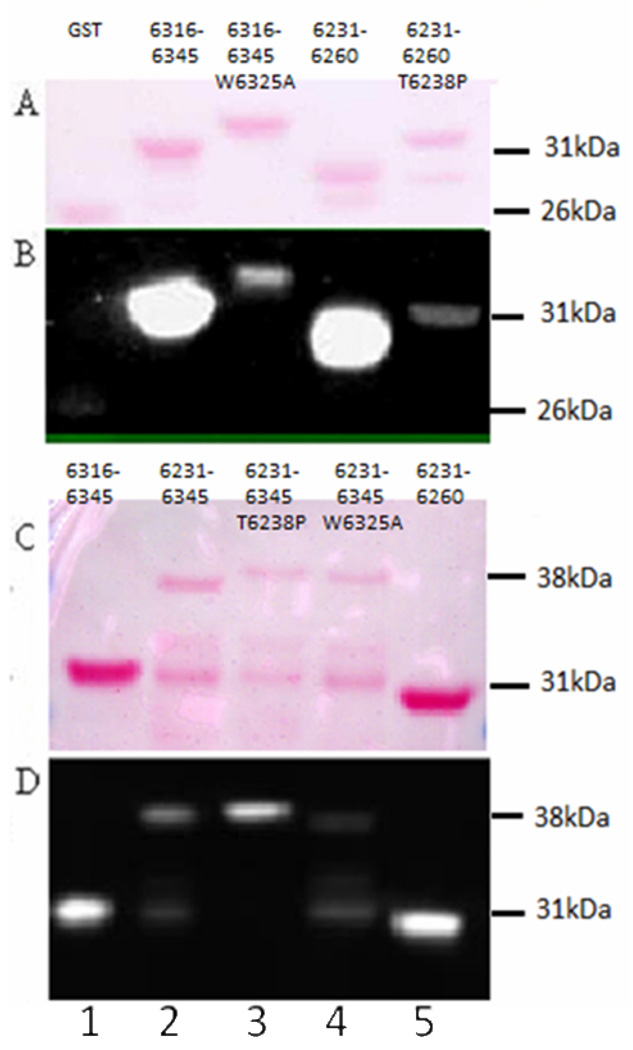
Fig. 5. Residues 6316–6345 of obscurin dominate binding to sAnk1 when both binding sites are present.
All GST constructs following transfer to nitrocellulose are shown in panel A and C with a Ponceau Red stain, which show equal loading. Panels B and D show the results of overlay assays of these nitrocellulose blots. Mutations of W6325 of Obsc6316–6345 to alanine (A,B; Lane 3, GST-Obsc6316–6345 W6325A) and T6328 of Obsc6231–6260 to proline (A,B; Lane 5, GST-Obsc6231–6260T6328P) completely ablate binding of the individual sites to MBP-sAnk129–155 in blot overlays and are barely greater than GST vector alone (A,B; Lane 1). Binding of WT constructs are shown in the adjacent lanes: Lane 2 (GST-Obsc6316–6345), Lane 4 (GST-Obsc6231–6260). These bands run at 31–32 kDa, consistent with the size of the GST fusion constructs. This developed blot in panel B was intentionally overexposed to show the dramatic nature of the inhibition by the mutants which is completely undetectable at low exposures. The GST-Obsc6231–6345 construct, which contains both binding sequences, is difficult to purify and is unstable, so only a small amount runs at the appropriate molecular mass of 38 kDa (C,D; Lane 2); a breakdown product, which retains the binding site within residues 6231–6260, is present at 31 kDa and also binds MBP-sAnk129–155. Mutation of W6325 of GST-Obsc6231–6345 to A (Lane 4: GST-Obsc6231–6345W6325A) reduces binding of the 38kDa band to MBP-sAnk1 almost completely (compare to Lane 2). Mutation of T6328 of GST-Obsc6231–6345 to P (lane 3, GST-Obsc6231–6345T6328P) does not inhibit binding of the 38kDa band to MBP-sAnk129–155 and may in fact enhance it (compared to Lanes 2 and 4). The 31 kDa breakdown product of GST-Obsc6231–6345T6328P does not bind to MBP-sAnk129–155, however, consistent with the effects of this mutation on GST-Obsc6231–6260. Both individual sites, GST-Obsc6316–6345 (Lane 1) and GST-Obsc6231–6260 (Lane 5), like panels A and B bind MBP-sAnk1. This experiment was repeated 5 times, with the same results.