Abstract
Histologically, Schmorl's nodes are defined as the loss of nuclear material through the cartilage plate, growth plate, and end plate into the vertebral body. Most Schmorl's nodes are asymptomatic, although there are some reports of symptomatic Schmorl's nodes, which should be treated similarly to vertebral compression fractures, with conservative treatment as the first choice. We report the case that we reduced the pain by blocking the ramus communicans nerve in a patient with Schmorl's node.
Keywords: ramus communicans nerve, Schmorl's node
Since the original report by Schmorl in 1927, Schmorl's nodes, which are defined histologically as a loss of nuclear material through the cartilage plate, growth plate, and end plate into the vertebral body, have been considered common thoracolumbar lesions [1]. Most Schmorl's nodes are asymptomatic [2]. There are, however, some reports of symptomatic Schmorl's nodes [3]. Although there are several attractive theories pertaining to the mechanism of onset of Schmorl's nodes, their true etiology is unknown [1]. MRI findings in symptomatic Schmorl's node indicate an inflammatory response with bone marrow edema [4]. The inflammatory response in the vertebral body marrow seems to be induced by intraosseous fracture with herniation of the disc material. Symptomatic Schmorl's nodes should be treated similarly to vertebral compression fractures, with conservative treatment as the first choice [4]. One study examined the usefulness of a ramus communicans nerve block in patients suffering from back pain due to vertebral compression fractures [5]. In a patient with Schmorl's node, we reduced the pain by blocking the rami communicans nerve. We report our case here.
CASE REPORT
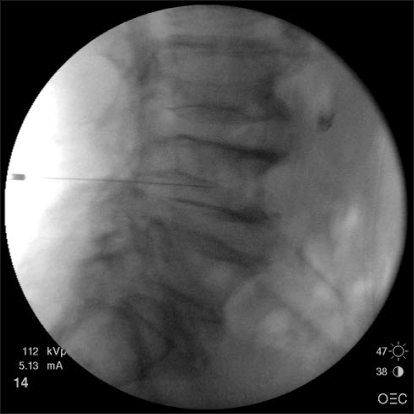
An 82-year-male presented with severe pain in the low back and both buttocks that was exacerbated by lumbar motion for 3 days. The intensity of the pain was 9/10 on a visual analogue scale (VAS). He had neither history of injury nor significant exertional activity. The pain was relieved when he rested in bed, and aggravated by weight loading or motion. The physical examination revealed a reduction in all back movements and tenderness over the L4 spinous process. The patient had straight-leg-raising restriction of 70° in his right leg and 75° in his left leg. Neither sensory nor motor deficit of his lower extremities was apparent. The laboratory values were normal, including the erythrocyte sedimentation rate and C-reactive protein. Plain radiographs showed hyperostosis in the lumbar spine (Fig. 1) and magnetic resonance imaging (MRI) revealed a Schmorl's node of L4 with adjacent marrow edema (Fig. 2). The spinal canal was narrow at the L3-4 and L4-5 levels. We performed nerve blocks on the L4 ramus communicans nerve. With the patient prone on the operating table, a 22-gauge spinal needle (Spinocan®, B.brown, Germany) was directed underneath the pedicle to rest slightly anterior to the superior aspect of the neural foramen. The needle was advanced until it was positioned at the inferior third aspect of the vertebral body under lateral fluoroscopic view. With the needle in the proper position, a mixture of 0.5 ml of 2% mepivacaine and 1.5 ml of contrast medium was injected to confirm the absence of intravascular, somatic nerve root, or epidural placement (Fig. 3). Next, a mixture of 2 ml of 1% mepivacaine and 10 mg of triamcinolone was injected on each side. The nerve blocks were performed once a week for two consecutive weeks. His pain improved dramatically immediately after the nerve block (VAS score 2/10); there were no serious procedure-related complications. At the 1-month follow-up, the patient had slight back discomfort (VAS score 2/10).
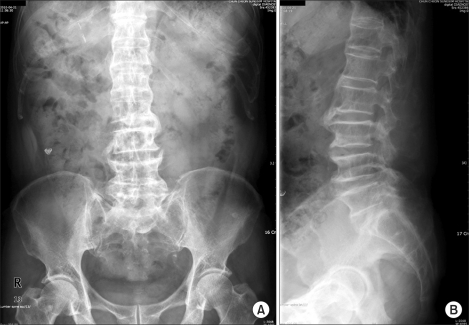
Fig. 1.
Plain radiographs showing hyperostosis with marked bone spurs at all lumbar segments and a bone bridge at L2-3. (A) Anteroposterior view, (B) Lateral view.
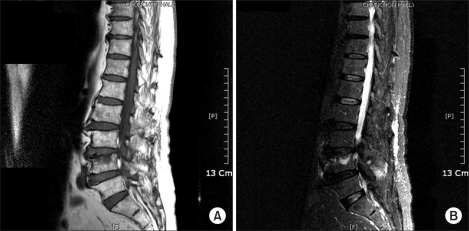
Fig. 2.
Magnetic resonance imaging of the lumbar spine shows a Schmorl's node at L4 with adjacent marrow edema. (A) Sagittal T1-weighted MRI. (B) Sagittal T2-weighted fat-suppression MRI.
Fig. 3.
Fluoroscopic image during the rami communicans nerve block (lateral view).
DISCUSSION
Schmorl first described cartilaginous nodal herniation of the disc into an adjacent vertebral body in 1927 [6]. The reported incidence ranges from 2-76% [6], with male predominance [7]. The majority of authors report that typical Schmorl's nodes are usually asymptomatic [8]. However, an acute or inflamed Schmorl's node may be symptomatic and clinically significant [9].
Schmorl's nodes are most commonly located at the middle third of the inferior endplate, near the thoracolumbar junction [7]. Schmorl's nodes occur when the cartilaginous endplate of the vertebral body has been disrupted [10] by an intrinsic abnormality of the endplate itself such as indentations left by the regression of the chorda dorsalis, ossification gaps, vascular channels, Scheuermann's disease or by alterations in the subchondral bone itself such as osteomalacia, hyperparathyroidism, Paget's disease, infection, neoplasm, trauma or mechanical overuse, Scheuermann's disease, and osteoporosis. Such weakening of the endplate [10] is not a necessary prerequisite for extrusion and is thought to be present as an underlying cause only in a small percentage of Schmorl's node cases. Most Schmorl's nodes form after axial-loading trauma results in the preferential extrusion of nuclear material through the vertebral endplate, rather than through an intact, normal annulus fibrosus [8].
Schmorl's nodes are commonly seen on radiographs or at autopsy. In most cases, it is a radiographic or autopsy diagnosis. Clinically, they are usually asymptomatic. They are difficult to diagnose in the acute stage. The demonstration of disc prolapse by radiography is usually possible only after the bony reaction of the vertebral body has developed an osseous sclerotic bone casting [4]. MRI has contributed to rapid improvements in the fundamental understanding of the bone marrow and its anatomy and physiology [11]. In all symptomatic cases, the vertebral body marrow surrounding the Schmorl's node had low signal intensity on T1-weighted images and high signal intensity on T2-weighted images [4]. In our case, the Schmorl's node was not apparent on plain radiographs. The vertebral body marrow surrounding the Schmorl's node was seen as low signal intensity on T1-weighted images and high signal intensity on T2-weighted images, so we made an early diagnosis of a symptomatic Schmorl's node.
The principal branches of the lumbar sympathetic trunks are the rami communicantes to the lumbar ventral rami. White rami communicantes are distributed to the L1 and L2 ventral rami, and grey rami communicantes are distributed to every lumbar ventral ramus. The number of rami communicantes to each lumbar nerve varies from one to three, and exceptionally may be as high as five. In general, the rami communicantes reach the ventral rami by passing through the tunnels deep to the psoas muscle that lie along the concave lateral surfaces of the lumbar vertebral bodies. These tunnels direct them to the lower borders of the transverse processes where the rami communicantes join the ventral rami just outside the intervertebral foramina [12].
The source of the nerve endings in the lumbar discs and vertebral bodies, are two extensive microscopic plexuses of nerve that accompany the anterior and posterior longitudinal ligaments. The anterior plexus bridges the two lumbar sympathetic trunks and covers the anterior longitudinal ligament. It is formed by branches of the sympathetic trunks and branches from the proximal ends of the grey rami communicantes. The posterior plexus is derived from the sinuvertebral nerves and accompanies the posterior longitudinal ligament. The anterior and posterior plexuses are connected around the lateral aspects of the vertebral bodies and discs by way of a lateral plexus that is formed by branches of the grey rami communicantes. The anterior and posterior plexuses supply superficial branches that innervate the periosteum of the vertebral bodies, and long penetrating branches that enter the intervertebral discs and vertebral bodies. Through these branches the vertebral bodies and intervertebral discs are innervated around their entire circumference [12].
This type of nerve block was initiated from the basis of the observation of the course of gray ramus communicans, containing unmyelinated postganglionic fibers which rejoin dorsal and ventral rami, distributing around vertebral body wall and coursing into anterior disc. It is known that gray ramus communicans nerve provides the greatest source of disc innervations and vertebral column [13]. With respect to complications, Chandler et al. [5] reported potential risks, including infection, bowel puncture, intravascular injection, somatic nerve root trauma, intrathecal or epidural injection, kidney puncture with a far lateral approach and pneumothorax in thoracic approach. However, there was no such serious complication in our case.
MRI of patients with symptomatic Schmorl's nodes has demonstrated inflammation and edema in the vertebral body, localized to the area around the Schmorl's node. Symptomatic Schmorl's nodes represent a fresh intraosseous fracture in the vertebral body. Inflammatory change in the vertebral body marrow induced by intraosseous fracture and some biological reaction to the intraspongious disc materials might cause pain. After the fracture has healed and the inflammation subsided, the Schmorl's node would be asymptomatic in analogy with an old vertebral compression fracture. Symptomatic Schmorl's nodes should be treated similarly to vertebral compression fracture, and conservative treatment is the first choice. If conservative treatment fails, surgery with anterior interbody fusion might be indicated [4]. In our case, the Schmorl's node was treated similarly to a vertebral compression fracture, and we successfully reduced the pain after blocking the ramus communicans nerve. The pain fell to a VAS score of 2, and the patient was discharged 1 week after the block.
Most Schmorl's nodes are asymptomatic, although acute or inflammatory Schmorl's nodes are painful and clinically significant. Some patients suffer from disabling pain due to Schmorl's nodes, despite conservative treatment. A rami communicans nerve block is one treatment modality for patients with back pain due to a Schmorl's node.
References
- 1.Hasegawa K, Ogose A, Morita T, Hirata Y. Painful Schmorl's node treated by lumbar interbody fusion. Spinal Cord. 2004;42:124–128. doi: 10.1038/sj.sc.3101506. [DOI] [PubMed] [Google Scholar]
- 2.Tsuji H, Yoshioka T, Sainoh H. Developmental balloon disc of the lumbar spine in healthy subjects. Spine. 1985;10:907–911. doi: 10.1097/00007632-198512000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, Takata K. A large painful Schmorl's node: a case report. J Spinal Disord. 1994;7:77–81. doi: 10.1097/00002517-199407010-00011. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K, Miyazaki T, Ohnari H, Takino T, Tomita K. Schmorl's nodes and low-back pain. Analysis of magnetic resonance imaging findings in symptomatic and asymptomatic individuals. Eur Spine J. 1995;4:56–59. doi: 10.1007/BF00298420. [DOI] [PubMed] [Google Scholar]
- 5.Chandler G, Dalley G, Hemmer J, Jr, Seely T. Gray ramus communicans nerve block: novel treatment approach for painful osteoporotic vertebral compression fracture. South Med J. 2001;94:387–393. [PubMed] [Google Scholar]
- 6.Walters G, Coumas JM, Akins CM, Ragland RL. Magnetic resonance imaging of acute symptomatic Schmorl's node formation. Pediatr Emerg Care. 1991;7:294–296. doi: 10.1097/00006565-199110000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Hauger O, Cotten A, Chateil JF, Borg O, Moinard M, Diard F. Giant cystic Schmorl's nodes: imaging findings in six patients. AJR Am J Roentgenol. 2001;176:969–972. doi: 10.2214/ajr.176.4.1760969. [DOI] [PubMed] [Google Scholar]
- 8.Fahey V, Opeskin K, Silberstein M, Anderson R, Briggs C. The pathogenesis of Schmorl's nodes in relation to acute trauma. An autopsy study. Spine. 1998;23:2272–2275. doi: 10.1097/00007632-199811010-00004. [DOI] [PubMed] [Google Scholar]
- 9.Stäbler A, Bellan M, Weiss M, Gärtner C, Brossmann J, Reiser MF. MR imaging of enhancing intraosseous disk herniation (Schmorl's nodes) AJR Am J Roentgenol. 1997;168:933–938. doi: 10.2214/ajr.168.4.9124143. [DOI] [PubMed] [Google Scholar]
- 10.Wagner AL, Murtagh FR, Arrington JA, Stallworth D. Relationship of Schmorl's nodes to vertebral body endplate fractures and acute endplate disk extrusions. AJNR Am J Neuroradiol. 2000;21:276–281. [PMC free article] [PubMed] [Google Scholar]
- 11.Vogler JB, 3rd, Murphy WA. Bone marrow imaging. Radiology. 1988;168:679–693. doi: 10.1148/radiology.168.3.3043546. [DOI] [PubMed] [Google Scholar]
- 12.Bogduk N. Clinical anatomy of the lumbar spine and sacrum. 3rd ed. New York: Bath press Ltd; 1997. pp. 127–143. [Google Scholar]
- 13.Tae HS, Kim SD, Park JY, Kim SH, Lim DJ, Suh JK. Gray ramus communicans nerve block: a useful therapeutic adjuvant for painful osteoporotic vertebral compression fracture. J Korean Neurosurg Soc. 2003;34:505–508. [Google Scholar]