Abstract
Background
Multiple methods for non-invasive measurement of cardiac output (CO) and stroke volume (SV) exist. Their comparative capabilities are not clearly established.
Methods
Healthy human subjects (n=21) underwent central hypovolaemia through progressive lower body negative pressure (LBNP) until the onset of presyncope, followed by termination of LBNP, to simulate complete resuscitation. Measurement methods were electrical bioimpedance (EBI) of the thorax and three measurements of CO and SV derived from the arterial blood pressure (ABP) waveform: the Modelflow (MF) method, the long-time interval (LTI) method, and pulse pressure (PP). We computed areas under receiver-operating characteristic curves (ROC AUCs) for the investigational metrics, to determine how well they discriminated between every combination of LBNP levels.
Results
LTI and EBI yielded similar reductions in SV during progressive hypovolaemia and resuscitation (correlation coefficient 0.83) with ROC AUCs for distinguishing major LBNP (−60 mm Hg) vs resuscitation (0 mm Hg) of 0.98 and 0.99, respectively. MF yielded very similar reductions and ROC AUCs during progressive hypovolaemia, but after resuscitation, MF-CO did not return to baseline, yielding lower ROC AUCs (ΔROC AUC range, −0.18 to −0.26, P<0.01). PP declined during hypovolaemia but tended to be an inferior indicator of specific LBNP levels, and PP did not recover during resuscitation, yielding lower ROC curves (P<0.01).
Conclusions
LTI, EBI, and MF were able to track progressive hypovolaemia. PP decreased during hypovolaemia but its magnitude of reduction underestimated reductions in SV. PP and MF were inferior for the identification of resuscitation.
Keywords: arterial pressure, measurement; blood, loss; cardiovascular system, responses; equipment, finapres; monitoring, cardiopulmonary
Key points.
Controlled application of lower body negative pressure simulated hypovolaemia and resuscitation in human subjects.
Thoracic electrical bioimpedance, and arterial blood pressure (ABP) analysis by the Long Time Interval method, were the most discriminatory measurements.
The Modelflow method of ABP analysis effectively tracked hypovolaemia, but underestimated resuscitation.
Pulse pressure tracked hypovolemia and resuscitation, but underestimated changes in stroke volume.
Progressive haemorrhage is a serious cause of hypotension and shock, causing morbidity and mortality in diverse patient populations, including trauma casualties, surgical patients, and patients treated with anticoagulation. Of course, the earlier haemorrhage is detected, the greater the opportunity exists for caregivers to administer volume replacement or perform a haemostatic intervention. Moreover, it is important to assess the efficacy of volume resuscitation, for example, to discriminate between patients in whom aggressive volume therapy is failing so operative management may be necessary, vs patients who are being successfully treated with volume therapy and may even be at risk for over-resuscitation. However, conventional vital signs, such as arterial blood pressure (ABP)1,2 and heart rate (HR),2–4 have been criticized as being imprecise indicators of hypovolaemia. Therefore, there is wide interest in novel non-invasive methods for detecting and quantifying intravascular volume loss and endpoints of resuscitation.2,5
In theory, monitoring cardiac output (CO) and stroke volume (SV) could offer a more accurate assessment of circulatory state than conventional vital signs. There are several CO measurement methods that use non-invasive instrumentation and might be useful for a broad patient population. Several of these methods estimate changes in CO by analysis of the ABP waveform; peripheral ABP is routinely measured in intensive care unit (ICU) patients via arterial catheters, and FDA-approved devices such as the Finometer can measure peripheral ABP non-invasively in non-critically ill populations.6 The Modelflow algorithm (CO-MF and SV-MF, from Finapres Medical Systems, Amsterdam, The Netherlands) computes CO by applying each beat of the ABP waveform to a three-element non-linear arterial model.7 Over 100 yr ago, it was suggested that pulse pressure (PP) alone can serve as a quantitative correlate of SV (SV-PP), and thus, the product of PP and HR (CO-PP*HR) would reveal relative changes in CO.8 There are several contemporary, commercially available CO-from-ABP methods that rely on PP as a surrogate for SV; the basis for the FloTrac method (Edwards Lifesciences, Irvine, CA, USA) ‘is the physiological premise that PP is proportional to stroke volume’,9 whereas the PulseCO method (LiDCO Ltd, London, UK) uses an autocorrelation pulse power calculation10 that is largely a function of PP. More recently, a different analytic methodology for estimating CO from ABP was developed, the long-time interval (CO-LTI and SV-LTI), which estimates CO by analysing a continuous ABP waveform and extracting information from the inter-beat or beat-to-beat variations.11 The motivation for the LTI method was to develop an algorithm that would not be affected by the wave reflections and transmission phenomena that shape individual pulses. Finally, electrical bioimpedance cardiography (CO-EBI and SV-EBI) estimates CO by correlating changes in thoracic impedance with changes in thoracic blood volume due to cardiac filling and ejection.12–14 EBI requires a set of chest electrodes attached to the patient and a specialized apparatus that applies a small current across the chest and measures the thoracic impedance.
Each of the aforementioned non-invasive CO methods offers a potential tool for clinicians to carefully monitor the circulatory state of patients at risk of haemorrhage. In this investigation, we studied these methods applied to healthy subjects undergoing progressive lower body negative pressure (LBNP), a well-established experimental model of central hypovolaemia.15 We assessed how well the investigational methods detected progressive hypovolaemia and discriminated between hypovolaemia and the restoration of central volume. This report substantially expands upon a preliminary set of results reported in conference proceedings.16 The investigation is valuable for two primary reasons. First, it provides a comparison between alternative CO methods simultaneously applied to a consistent set of subjects; this design eliminates the ambiguity when comparing results from disparate patient populations and clinical settings.17,18 Secondly, clinical trials of CO monitoring are complicated by the absence of a perfect reference measure: uncertainty about the outcome of interest, the ‘true’ CO, is therefore always a source of error. Here, we used a laboratory procedure to induce standardized circulatory disturbances, so that the circulatory states of the subjects were carefully controlled, enhancing the validity of this comparative study.
Methods
Lower body negative pressure
The study was approved by the Institutional Review Board for the use of human subjects at the Brooke Army Medical Center at Fort Sam Houston, TX, USA. Twenty-one healthy, normotensive subjects aged 27–52 yr with no chronic cardiopulmonary medical condition underwent the investigational protocol. In addition, female subjects underwent an initial urine test before experimentation to ensure that they were not pregnant. Subjects maintained their normal sleep pattern, refrained from exercise, and abstained from caffeine and other autonomic stimulants. During an orientation session that preceded each experiment, all subjects received a verbal briefing and a written description of all procedures and risks associated with the experiments and were made familiar with the laboratory, the protocol, and procedures. Subjects gave written informed voluntary consent to participate in the experiments.
LBNP was used in the present investigation as a highly reproducible experimental tool to induce loss of central blood volume in humans, thereby simulating haemorrhage.15 Subjects were placed in the supine position and secured in the LBNP chamber using a neoprene skirt designed to form an airtight seal between the subject and the chamber. The application of negative pressure to the lower body (below the iliac crest) results in a redistribution of blood away from the upper body to the lower extremities and pelvis. Subjects underwent an LBNP protocol consisting of a 5 min baseline period, followed by sequential exposure to −15, −30, −45, −60, −70, and −80 mm Hg decompression for 5 min each.
Not all subjects were exposed to all levels of LBNP. Termination of LBNP was based on a precipitous reduction in systolic ABP of more than 15 mm Hg coincident with presyncopal signs and symptoms such as bradycardia, nausea, dizziness, or lightheadedness. Upon the presence of these signs and symptoms (i.e. haemodynamic decompensation), LBNP was released and the pressure within the chamber immediately returned to atmospheric pressure (0 mm Hg).
After cessation of LBNP and a transition interval to allow for a return of fluid sequestered in the lower body, data were collected for an additional 5 min (‘recovery’), which simulated complete volume resuscitation of hypovolaemic patients (intravascular fluid sequestered by LBNP is immediately returned upon cessation of LBNP, and most oedema is resorbed within minutes after cessation of LBNP).19
Measurements
Continuous HR was measured with a four-lead ECG with lead II configuration. Beat-to-beat SV was measured non-invasively using CO-EBI (HIC-2000; Bio-impedance Technology, Inc., Chapel Hill, NC, USA). This technique is based on the resistance changes in the thorax to a low-intensity (4 mA), high-frequency (70 kHz) alternating current applied to the thorax by two surface electrodes placed at the root of the neck and two surface electrodes placed at the xiphoid process at the mid-axillary line. SV was computed, according to the Kubicek equation, as a function of Z0, the baseline thoracic impedance; dZ/dt, the change in impedance over time; ρ, the average electrical resistivity of blood at 100 kHz (150 Ω cm); L, the mean distance between the two inner electrodes in centimetres; and T, the ventricular ejection time in seconds, as measured from the dZ/dt and ECG waveforms.12 CO-EBI was taken as the product of SV-EBI and HR.
Continuous non-invasive ABP was measured using the Finometer (Finapres Medical Systems, Amsterdam, The Netherlands) which uses the volume clamp method of Penaz.20 All continuous waveform data were sampled at 500 Hz and were recorded directly to the computer with commercial hardware and software (WINDAQ; Dataq Instruments, Akron, OH, USA). The Finometer also automatically outputs SV and CO estimated from the ABP waveform using the CO-MF method.7,21 These data were recorded directly to a data acquisition system on a beat-by-beat basis and subsequently converted to an Excel spreadsheet file for analysis.
Additional data processing
Offline, the continuous ABP data were processed using software routines implemented in Matlab (Mathworks, Natick, MA), to compute CO-LTI11 and CO-PP*HR (where PP was determined as the difference between systolic pressure and diastolic pressure). Each of the metrics (CO-EBI, CO-MF, CO-LTI, CO-PP*HR) was normalized by its baseline value for each subject. The reported metrics therefore represent relative or per cent values with respect to baseline. Group means (%) were computed for each metric, using the average of all subjects’ values, for each level of decompression, and the recovery. SV-LTI was computed by dividing CO-LTI by HR.
In order to quantify the relationships between the metrics based on ABP waveform analysis, we performed pair-wise correlation coefficient analysis for those investigational metrics vs EBI metrics.
Detection of progressive hypovolaemia and discrimination between hypovolaemia and its resolution
We compared how well the metrics could distinguish between any two levels of LBNP. Specifically, for every combination of LBNP levels for which there were data for all subjects (i.e. −15, −30, −45, −60 mm Hg, and recovery), we computed receiver-operating characteristic (ROC) curves for the investigational metrics and used the area under the ROC curve (AUC) as our performance metric.
The ROC AUC quantifies how well two different LBNP levels were distinguished by an investigational metric. If all the measurements at −30 mm Hg, across all subjects, were lower than all measurements from −15 mm Hg, then that hypothetical metric would yield an AUC of 1.00 (indicating a perfect ability to discriminate between the two states). Conversely, if the distribution of measurements obtained from subjects while at −30 mm Hg was perfectly overlapped with the measurements from −15 mm Hg, then that investigational metric would yield an AUC of 0.50 (indicating no utility for discriminating between the two LBNP levels). One broader way of interpreting the AUC is as follows: AUC of 0.50 implies that the probability that two individuals, one drawn from each class (i.e. equal pre-test probability), will be accurately classified 50% of the time, whereas an AUC of 1.00 implies classification will be accurate 100% of the time.22 In this investigation, the level of statistical significance of the difference between ROC AUCs of two given metrics was determined using the Hanley–McNeil23 method for paired data.
Discrimination of tolerant vs non-tolerant subjects
All subjects tolerated LBNP to −60 mm Hg for at least 90 s (which was needed to compute CO-LTI). Eight out of 21 subjects were unable to withstand LBNP of −70 mm Hg for at least 90 s (i.e. experienced presyncope before the initial 90 s of −70 mm Hg of LBNP), so these subjects were termed the ‘low-tolerant’ subjects. The remaining 13 subjects tolerated −70 mm Hg for at least 90 s so these subjects were termed the ‘high-tolerant’ subjects (and nine of the ‘high-tolerant’ subjects also tolerated −80 mm Hg for at least 90 s, as per the progression of decompression in the experimental protocol). We tested whether the investigational CO and SV metrics, as measured at −60 mm Hg, would discriminate between high- and low-tolerant subjects. We calculated ROC curves and tested for differences using the Hanley–McNeil23 method for paired data.
Results
Detection of progressive hypovolaemia
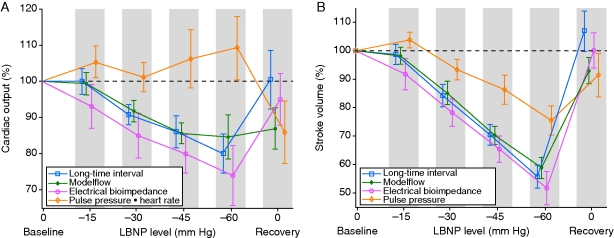
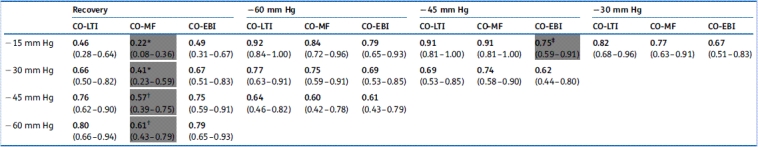
The group means of CO-EBI, CO-LTI, CO-MF, and CO-PP*HR are shown in Figure 1(a). CO-PP*HR did not decline with increasing LBNP. Indeed, on average, CO-PP*HR increased even as LBNP progressed to −60 mm Hg. Because CO-PP*HR failed to provide the most rudimentary indication of progressive central hypovolaemia during our protocol, we do not report further results concerning CO-PP*HR. CO-LTI, CO-MF, and CO-EBI decreased during progressive decompression. These methods were statistically similar in distinguishing between decompression levels of −15, −30, −45, and −60 mm Hg, as determined by ROC AUC analysis (Table 1).
Fig 1.
(a) CO metrics and (b) SV metrics. Group means of subjects (n=21) for the investigative metrics through progressive levels of LBNP decompression, expressed as per cent of subject's baseline value. Vertical positions of data points are staggered to display non-overlapping 95% CIs. After 60 mm Hg, some subjects progressed to −70 and −80 mm Hg before their recovery; see text for details.
Table 1.
Discrimination between different levels of LBNP using cardiac output metrics. ROC AUCs (95% CI) for discrimination between different levels of LBNP in 21 healthy subjects progressing from baseline through four levels of LBNP and then to recovery, that is, cessation of LBNP. Results for relative changes in CO-LTI (cardiac output by long-time interval method), CO-MF (cardiac output by the Modelflow method), and CO-EBI (cardiac output by electrical bioimpedance) are reported. CO-PP*HR (cardiac output by the product of pulse pressure and heart rate) was excluded from analysis because, paradoxically, it increased throughout decompression. Grey box indicates ROC AUC significantly less than at least one other investigational CO metric (by the Hanley-McNeil test for paired data). *CO-MF significantly less than CO-LTI (P<0.001) and CO-EBI (P<0.001); †CO-MF significantly less than CO-LTI (P<0.01) and CO-EBI (P<0.05); ‡CO-EBI significantly less than CO-MF (P<0.05)
 |
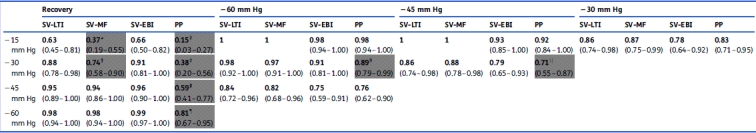
Group means of SV-EBI, SV-LTI, SV-MF, and SV-PP are shown in Figure 1(b). SV-LTI, SV-MF, and SV-EBI also tracked LBNP during progressive decompression (Table 2). These methods were statistically similar in distinguishing between decompression levels of −15, −30, −45, and −60 mm Hg, as determined by ROC AUC analysis. PP also declined, but not as reliably, and it yielded lower AUCs than the other metrics, sometimes significantly less so.
Table 2.
Discrimination between different levels of LBNP using SV metrics. ROC AUCs (95% CI) for discrimination between different levels of LBNP in 21 healthy subjects progressing from baseline through four levels of LBNP and then to recovery, that is, cessation of LBNP. Results for relative changes in SV-LTI (stroke volume by long-time interval method), SV-MF (stroke volume by the Modelflow method), SV-EBI (stroke volume by electrical bioimpedance), and PP (pulse pressure as measured by the Finometer) are reported. Grey box indicates ROC AUC significantly less than at least one other investigational SV metric (by the Hanley-McNeil test for paired data). *SV-MF significantly less than SV-LTI (P<0.0001) and SV-EBI (P<0.01); †SV-MF significantly less than SV-LTI (P<0.01) and SV-EBI (P<0.05); ‡PP significantly less than SV-LTI (P<0.0001), SV-MF (P<0.01), and SV-EBI (P<0.0001); ¶PP significantly less than SV-LTI (P<0.01), SV-MF (P<0.01), and SV-EBI (P<0.01); §PP significantly less than SV-LTI (P<0.05); ||PP significantly less than SV-LTI (P<0.05) and SV-MF (P<0.05)
 |
The correlation coefficients for the CO metrics relative to CO-EBI were CO-LTI:CO-EBI 0.64 (95% CI 0.51–0.74) and CO-MF:CO-EBI 0.52 (95% CI 0.36–0.65).
The correlation coefficients for the SV metrics relative to SV-EBI were, from highest to lowest, SV-LTI:SV-EBI 0.83 (95% CI 0.76–0.88), SV-MF:SV-EBI 0.77 (95% CI 0.69–0.85), and SV-PP:SV-EBI 0.60 (95% CI 0.46–0.71). Note that a greater range of SV metrics was observed, vs the narrower range in CO, as seen in Figure 1. Accordingly, correlations between SV metrics tended to be greater than between CO metrics.
Discrimination between hypovolaemia and its resolution
CO-LTI and CO-EBI tracked decompression (Fig. 1); then, during recovery, they returned to a near baseline level. CO-MF tracked decompression but, during recovery, CO-MF returned to only 87% of baseline, which was comparable with the average CO-MF measured between −30 and −45 mm Hg of LBNP.
For discrimination between LBNP and recovery, CO-LTI was quite similar to CO-EBI in terms of ROC AUCs: their mean difference in ROC AUC was −0.00 (sd 0.01). Both were superior to CO-MF for discriminating between hypovolaemia and euvolaemia (i.e. termination of LBNP, which was our simulation of complete resuscitation). This difference was statistically significant for any level of LBNP (Table 1).
In all comparisons, for all modalities, any SV metric was more discriminatory (i.e. higher ROC AUCs) than the corresponding CO metric. Comparing different SV metrics, SV-LTI and SV-EBI were similar in terms of ROC AUCs for discrimination between LBNP and recovery: their mean difference in ROC AUC was −0.01 (sd 0.01). At two LBNP levels, both were superior to SV-MF for discriminating between hypovolaemia and its resolution (Table 2), and for all LBNP levels, both were superior to SV-PP.
Discrimination between high- and low-tolerant subjects
The best discriminator between high- and low-tolerant subjects (i.e. which subjects were most at risk of haemodynamic decompensation with any additional LBNP) was assessing which subjects had the largest reductions in SV-LTI at −60 mm Hg (Table 3). ROC analysis of SV-LTI yielded an AUC=0.86, where preservation of SV-LTI was associated with high-tolerant subjects and lower SV-LTI was associated with low-tolerant subjects. The ROC AUC for SV-LTI was significantly greater than SV-EBI and SV-MF, although non-significantly better than SV-PP (Table 3). Interestingly, CO-MF yielded an ROC AUC of 0.40, which means that preservation of CO-MF was paradoxically associated with low-tolerant subjects.
Table 3.
Discrimination between high- and low-tolerant LBNP subjects using SV and CO metrics at −60 mm Hg. ROC AUCs (95% CI) for CO and SV metrics at −60 mm Hg, as discriminators between 13 high-tolerant subjects who subsequently endured at least −70 mm Hg LBNP, vs eight low-tolerant subjects who could not endure LBNP of −70 mm Hg. LTI, long-time interval method; MF, Modelflow method; EBI, electrical bioimpedance; PP, pulse pressure as measured by the Finometer. Grey box: statistically significant ROC AUC by the Hanley–McNeil test for paired data. *SV-MF significantly less than SV-LTI (P<0.01); †CO-MF significantly less than CO-LTI (P<0.05); ‡SV-EBI significantly less than SV-LTI (P<0.05)
 |
Discussion
In this study, we used a carefully controlled laboratory procedure, LBNP, to induce, then resolve, a standardized circulatory disturbance, and we then directly compared the diagnostic capabilities and limitations of four non-invasive CO and SV measurement modalities. LTI and EBI, based on the waveform analysis and thoracic bioimpedance, respectively, yielded CO and SV ROC AUCs which were similar and diagnostically promising. Throughout progressive hypovolaemia, the third investigative modality, MF, was diagnostically similar to both LTI and EBI. During progressive hypovolaemia, PP also declined, but not as reliably, and it trended towards lower AUCs than the other metrics. The reduction in PP underestimated the reduction in SV; this was evidenced by the finding that CO-PP*HR yielded a paradoxical increase throughout progressive LBNP. After simulated resuscitation, (i.e. termination of LBNP), both MF and PP remained reduced, with inferior ability to discriminate between ongoing hypovolaemia (e.g. −30 or −45 mm Hg of LBNP, as in Table 1) and euvolaemia.
It appears that two of the investigational methods, CO-EBI and CO-LTI, would be preferable for monitoring subjects who receive resuscitation for their hypovolaemia. In terms of practical requirements, CO-EBI measurement involves electrode placement on the neck and chest, which is sometimes precluded by surgical wounds, injury, or both (e.g. burns), and CO-EBI may not perform as well in critically ill patients vs healthy research subjects, because of errors associated with abnormal thoracic anatomy (e.g. post-pneumonectomy) or intrathoracic fluid (e.g. pleural effusions, pulmonary oedema, acute respiratory distress syndrome).12,13,14 LTI requires an ABP waveform, which can be measured via an indwelling arterial catheter and can also be measured using a non-invasive finger-cuff apparatus, for example, the Finometer (used in this study), which uses the volume-clamp method of Penaz.
Clinically, it would be most useful to monitor a circulatory metric that is an accurate indicator of impending cardiovascular collapse. Accordingly, we assessed which metrics gave the best indication that subjects would prove unable to tolerate the highest levels of LBNP. We found that protection of SV-LTI at −60 mm Hg was a strong predictor (ROC AUC 0.86) of high tolerance to the deepest levels of LBNP (see the Methods section, and Table 3, for details). The ROC AUC for SV-LTI was significantly higher than SV-EBI (AUC 0.61) and SV-MF (AUC 0.54). In general, SV metrics were more predictive of tolerance than CO metrics. Paradoxically, CO-MF yielded an AUC of <0.50, meaning that the biggest reductions in CO-MF at −60 mm Hg were associated with the most tolerant subjects, which is counter-intuitive (and also inconsistent with prior findings).24,25 Overall, the superior ability of SV-LTI to identify which subjects would prove intolerant of higher levels of LBNP suggests that it may be a more valid assessment of circulatory status compared with the alternatives.
It is interesting to consider why LTI vs MF, both based on the analysis of the ABP waveform, had different diagnostic performances. CO-MF, with its underlying three-element model, is an example of a ‘lumped parameter’ model, in which a sprawling, complex system—in this case, the arterial tree—is represented by a set of simple elements intended to capture the essential behaviour of the actual system. (The analytic model implicitly assumed when PP is taken as a surrogate for SV is another example of a lumped parameter model.)26 Yet pressure pulses are shaped by many other factors. For instance, different frequency components of a pressure pulse travel down an artery at different velocities, and pulses are reflected backwards from arterial bifurcations and terminal locations.27 As a result of these so-called transmission effects, ABP amplitude (i.e. PP) and shape are quite varied in different arterial locations, which is not considered by simple lumped parameter models. We found that both PP and CO-MF remained reduced during recovery, so that these measures did not reliably indicate when blood volume was restored. We speculate that, perhaps, the simple model upon which the CO-MF method is based was not flexible enough to account for the effects that altered the shape and reduced the amplitude of ABP in recovery.
LTI was developed to address the transmission effects that shape individual pressure pulses.11 When ABP is analysed over longer time scales that span multiple beats, there are fewer factors that affect the elevations and reductions of ABP: fluctuations in ABP over longer timescales are purely a function of cardiac ejection plus the arterial tree's net compliance and its total peripheral vascular resistance (PVR). The reason for this is that over longer time scales, the transmission effects become negligible (just as when a pebble falls in a pond, the resultant splash waves settle out within a minute, so does the to-and-fro of pulses and their reflections within the arterial tree). LTI uses a mathematical technique that analyses a sequence of heartbeats from the measured ABP and estimates the contribution of each individual heartbeat. Specifically, the algorithm generates a theoretical estimate of the ABP waveform that would be generated by one single isolated heartbeat. The LTI method is based on a very well-known engineering technique called system identification.11 In the later portions of that theoretical ABP waveform, the reflected waves and transmission effects have faded away, which is consistent with real-world physics where indeed reflected waves and transmission effects diminish over time. Those later portions, where the waveform is a function of PVR and arterial compliance, are used to estimate PVR, and the ratio of MAP to PVR yields CO. In theory, LTI-CO could therefore be confounded by changes in arterial compliance (the method makes the assumption that changes in arterial compliance are negligible), but reports to date suggest that this is not a major source of error.11,28 In this report, LTI performed quite comparably with a very different measurement modality, EBI. One potential limitation is that LTI metrics will be slower to indicate changes in CO and SV that occur quickly, that is, within the span of several seconds. Also, beat-to-beat changes cannot be resolved. The LTI method is not commercially available presently.
PP has value in the diagnosis of progressive hypovolaemia (Table 2), although it was consistently less diagnostic than SV-LTI and SV-EBI, sometimes significantly less so. We found that PP and SV were correlated, which is an expected finding that has been previously noted in other reports (e.g. high correlation of variability of PP and SV during major abdominal surgery).29 At the same time, it is important to appreciate that the magnitude of reduction in PP underestimated the reduction in SV, that is, they were correlated, but not strictly proportional. This is highlighted by the fact that PP*HR yields an estimate of CO that paradoxically increases throughout progressive LBNP. Additionally, after recovery, PP again appears to underestimate the recovered SV. Our findings are consistent with a prior investigation on haemodynamic changes from postural change, which reported ‘a greater postural fall in stroke index than the corresponding change in pulse pressure.’30
Our results suggest that any algorithm that assumes PP is a quantitative surrogate for SV, for example, the FloTrac method9 and the PulseCO,10 will need to offer substantial compensation, as we find that those two parameters are not proportional and we speculate that our findings explain why certain PP-based algorithms have shown inconsistent reliability in some clinical reports.31 Sun and colleagues reported on a variation of CO-PP*HR proposed by Liljestrand and Zander32 (in which PP*HR was scaled by MAP to adjust for arterial compliance). In Sun's33 report, this method performed well in an ICU population consisting of older patients with relatively less dynamic changes in PVR. However, in our data set, PP*HR/MAP failed to decline with progressive LBNP (data not shown). Another key implication is that CO-from-ABP algorithms do not necessarily perform equally well in different populations under different conditions, which re-emphasizes the need for direct comparative evaluations in varied subject populations.17,18
There are several limitations to this study. First, the study of healthy subjects in laboratory conditions may not be strictly equivalent to clinical use. For instance, there may be more measurement error in actual clinical use, or other confounding factors, such as vasopressor infusion. However, this study design provided unambiguous outcomes impossible to accomplish through clinical trials, so such controlled laboratory studies may be quite complementary to clinical ‘real world’ investigations.
Secondly, our ABP was measured using the Finometer. It is possible that waveform analysis of ABP measured by an indwelling arterial catheter might behave differently. Therefore, our results may not necessarily generalize to patients with an indwelling ABP. On the other hand, the Finometer has been shown to provide a valid measurement of ABP.34 Moreover, a truly non-invasive method of monitoring CO could be quite useful in the management of the majority of hospitalized patients without invasive lines, and this report illustrates certain capabilities and limitations of several different non-invasive alternatives.
In conclusion, we found that CO and SV measured by LTI, EBI, and MF, all tracked progressive hypovolaemia. SV-PP also declined, but this reduction in PP underestimated the reduction in SV. Hence, CO-PP*HR yielded a paradoxical increase during progressive LBNP. After restoration of circulating volume, CO and SV by LTI and EBI were able to distinguish between ongoing hypovolaemia and resuscitation, whereas the MF and PP metrics were significantly less discriminatory. These results may have serious implications for the utility of non-invasive CO and SV measurements to track progressive bleeding and effective fluid resuscitation, especially those that assume proportionality between PP and SV.
Disclaimer
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the US Army or of the US Department of Defense. This paper has been approved for public release with unlimited distribution.
Conflict of interest
In 2008, A.R. served on the General Electric Healthcare (GE) customer advisory board. The company sells monitoring equipment, but the author is unaware of any relationship or direct competition with the technology from this manuscript.
Funding
This work was supported by the United States Army MRMC Grant W23RYX6023N603 and 604 and by NHLBI Grant HL-080568.
Acknowledgements
The authors thank the subjects for their time and cheerful cooperation and Gary Muniz for his engineering and technical assistance during the experiments.
References
- 1.Parks JK, Elliott AC, Gentilello LM, Shafi S. Systemic hypotension is a late marker of shock after trauma: a validation study of Advanced Trauma Life Support principles in a large national sample. Am J Surg. 2006;192:727–31. doi: 10.1016/j.amjsurg.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 2.Convertino VA, Ryan KL, Rickards CA, et al. Physiological and medical monitoring for en route care of combat casualties. J Trauma. 2008;64:S342–53. doi: 10.1097/TA.0b013e31816c82f4. [DOI] [PubMed] [Google Scholar]
- 3.Snyder HS, Dresnick SJ. Lack of tachycardic response to hypotension in penetrating abdominal injuries. J Emerg Med. 1989;7:335–9. doi: 10.1016/0736-4679(89)90294-1. [DOI] [PubMed] [Google Scholar]
- 4.Victorino GP, Battistella FD, Wisner DH. Does tachycardia correlate with hypotension after trauma? J Am Coll Surg. 2003;196:679–84. doi: 10.1016/S1072-7515(03)00128-5. [DOI] [PubMed] [Google Scholar]
- 5.Porter JM. The search for an optimal end point of resuscitation. J Trauma. 2000;48:360. doi: 10.1097/00005373-200002000-00039. [DOI] [PubMed] [Google Scholar]
- 6.Elvan-Taspinar A, Uiterkamp LA, Sikkema JM, et al. Validation and use of the Finometer for blood pressure measurement in normal, hypertensive and pre-eclamptic pregnancy. J Hypertens. 2003;21:2053–60. doi: 10.1097/00004872-200311000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol. 1993;74:2566–73. doi: 10.1152/jappl.1993.74.5.2566. [DOI] [PubMed] [Google Scholar]
- 8.Erlanger J, Hooker DR. An experimental study of blood pressure and of pulse-pressure in man. Johns Hopkins Hosp Rep. 1904;12:145. [Google Scholar]
- 9.Edwards Lifesciences. Available from http://www.edwards.com/sitecollectionimages/products/mininvasive/ar04099.pdf. (accessed July 14, 2010)
- 10.Rhodes A, Sunderland R. Arterial pulse power analysis: the LiDCO plus System. In: Pinsky MR, Payen D, editors. Functional Hemodynamic Monitoring. Berlin: Springer-Verlag; 2005. [Google Scholar]
- 11.Mukkamala R, Reisner AT, Hojman HM, Mark RG, Cohen RJ. Continuous cardiac output monitoring by peripheral blood pressure waveform analysis. IEEE Trans Biomed Eng. 2006;53:459–67. doi: 10.1109/TBME.2005.869780. [DOI] [PubMed] [Google Scholar]
- 12.Kubicek WG, Patterson RP, Witsoe DA. Impedance cardiography as a noninvasive method of monitoring cardiac function and other parameters of the cardiovascular system. Ann NY Acad Sci. 1970;170:724–32. [Google Scholar]
- 13.Critchley LA. Impedance cardiography. The impact of new technology. Anaesthesia. 1998;53:677–84. doi: 10.1046/j.1365-2044.1998.437-az0550.x. [DOI] [PubMed] [Google Scholar]
- 14.Bloch KE. Impedance and inductance monitoring of cardiac output. In: Tobin MJ, editor. Principles and Practice of Intensive Care Monitoring. New York: McGraw-Hill; 1998. pp. 915–30. [Google Scholar]
- 15.Cooke WH, Ryan KL, Convertino VA. Lower body negative pressure as a model to study progression to acute hemorrhagic shock in humans. J Appl Physiol. 2004;96:1249–61. doi: 10.1152/japplphysiol.01155.2003. [DOI] [PubMed] [Google Scholar]
- 16.Reisner AT, Xu D, Ryan KL, Convertino VA, Mukkamala R. Comparison of cardiac output monitoring methods for detecting central hypovolemia due to lower body negative pressure. Conf Proc IEEE Eng Med Biol Soc. 2007:955–8. doi: 10.1109/IEMBS.2007.4352450. [DOI] [PubMed] [Google Scholar]
- 17.Heldt T. Continuous blood pressure-derived cardiac output monitoring—should we be thinking long term? J Appl Physiol. 2006;101:373–4. doi: 10.1152/japplphysiol.00502.2006. [DOI] [PubMed] [Google Scholar]
- 18.van Lieshout JJ, Jansen JR. Continuous cardiac output monitoring by blood pressure analysis. J Appl Physiol. 2007;102:826. doi: 10.1152/japplphysiol.00951.2006. author reply 7. [DOI] [PubMed] [Google Scholar]
- 19.Lundvall J, Bjerkhoel P, Edfeldt H, Ivarsson C, Lanne T. Dynamics of transcapillary fluid transfer and plasma volume during lower body negative pressure. Acta Physiol Scand. 1993;147:163–72. doi: 10.1111/j.1748-1716.1993.tb09485.x. [DOI] [PubMed] [Google Scholar]
- 20.Drzewiecki G. Noninvasive arterial blood pressure and mechanics. In: Bronzino JD, editor. The Biomedical Engineering Handbook. Boca Raton: IEEE Press; 2000. pp. 71-1–16. [Google Scholar]
- 21.Jellema WT, Wesseling KH, Groeneveld AB, Stoutenbeek CP, Thijs LG, van Lieshout JJ. Continuous cardiac output in septic shock by simulating a model of the aortic input impedance: a comparison with bolus injection thermodilution. Anesthesiology. 1999;90:1317–28. doi: 10.1097/00000542-199905000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 23.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–43. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 24.Convertino VA, Sather TM. Effects of cholinergic and beta-adrenergic blockade on orthostatic tolerance in healthy subjects. Clin Auton Res. 2000;10:327–36. doi: 10.1007/BF02322256. [DOI] [PubMed] [Google Scholar]
- 25.Sather TM, Goldwater DJ, Montgomery LD, Convertino VA. Cardiovascular dynamics associated with tolerance to lower body negative pressure. Aviat Space Environ Med. 1986;57:413–9. [PubMed] [Google Scholar]
- 26.Mark RG. Cardiovascular Mechanics. Cambridge: MIT OCW; 2004. [Google Scholar]
- 27.Nichols WW, O'Rourke MF. McDonald's Blood Flow in Arteries. New York: Oxford University Press; 1998. Contours of pressure and flow waves in arteries; pp. 170–200. [Google Scholar]
- 28.Lu Z, Mukkamala R. Continuous cardiac output monitoring in humans by invasive and noninvasive peripheral blood pressure waveform analysis. J Appl Physiol. 2006;101:598–608. doi: 10.1152/japplphysiol.01488.2005. [DOI] [PubMed] [Google Scholar]
- 29.Derichard A, Robin E, Tavernier B, et al. Automated pulse pressure and stroke volume variations from radial artery: evaluation during major abdominal surgery. Br J Anaesth. 2009;103:678–84. doi: 10.1093/bja/aep267. [DOI] [PubMed] [Google Scholar]
- 30.Alfie J, Waisman GD, Galarza CR, Cámera MI. Contribution of stroke volume to the change in pulse pressure pattern with age. Hypertension. 1999;34:808–12. doi: 10.1161/01.hyp.34.4.808. [DOI] [PubMed] [Google Scholar]
- 31.Eleftheriadis S, Galatoudis Z, Didilis V, et al. Variations in arterial blood pressure are associated with parallel changes in FlowTrac/Vigileo-derived cardiac output measurements: a prospective comparison study. Crit Care. 2009;13:R179. doi: 10.1186/cc8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liljestrand G, Zander E. Vergleichen die bestimmungen des minutenvolumens des herzens beim menschen mittels der stichoxydulmethode und durch blutdruckmessung. Ztschr ges exper med. 1928;59:105–22. [Google Scholar]
- 33.Sun JX, Reisner AT, Saeed M, Heldt T, Mark RG. The cardiac output from blood pressure algorithms trial. 2009;37:72–80. doi: 10.1097/CCM.0b013e3181930174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guelen I, Westerhof BE, Van Der Sar GL, et al. Finometer, finger pressure measurements with the possibility to reconstruct brachial pressure. Blood Press Monit. 2003;8:27–30. doi: 10.1097/00126097-200302000-00006. [DOI] [PubMed] [Google Scholar]