Abstract
Objectives
To further define the role of sarcomere mutations in DCM and associated clinical phenotypes.
Background
Mutations in several contractile proteins contribute to DCM, but definitive evidence for the roles of most sarcomere genes remains limited by the lack of robust genetic support.
Methods
Direct sequencing of 6 sarcomere genes was performed on 334 probands with DCM. A novel D230N missense mutation in the gene encoding α-tropomyosin (TPM1) was identified. Functional assessment was performed using an in vitro reconstituted sarcomere complex to evaluate ATPase regulation and Ca2+ affinity as correlates of contractility.
Results
TPM1 D230N segregated with DCM in two large unrelated families. This mutation altered an evolutionarily conserved residue and was absent in >1000 control chromosomes. In vitro studies demonstrated major inhibitory effects on sarcomere function with reduced Ca2+-sensitivity, maximum activation, and Ca2+ affinity compared to wildtype TPM1. Clinical manifestations ranged from decompensated heart failure or sudden death in those presenting early in life, to asymptomatic left ventricular dysfunction in those diagnosed during adulthood. Notably, several affected infants had remarkable improvement.
Conclusions
Genetic segregation in 2 unrelated families and functional analyses conclusively establish a pathogenic role for TPM1 mutations in DCM. In vitro results demonstrate contrasting effects of DCM and HCM mutations in TPM1, suggesting that specific functional consequences shape cardiac remodeling. Along with prior reports, our data support a distinctive, age-dependent phenotype with sarcomere-associated DCM where presentation early in life is associated with severe, sometimes lethal, disease. These observations have implications for the management of familial DCM.
Keywords: cardiomyopathy, heart failure, genetics
INTRODUCTION
Dilated cardiomyopathy (DCM) is an important cause of heart failure (HF) and a leading indication for heart transplantation in children (1) and adults (2). Over the past decade, there has been increasing recognition of the important contribution of genetic etiologies in causing “idiopathic” DCM with family studies suggesting that 30–50% of disease is inherited (3,4). However, clinical manifestations may be highly variable and obscure identification of familial disease.
In contrast to hypertrophic cardiomyopathy (HCM), where sarcomere mutations cause the majority of disease (5), the genetics of DCM are more diverse and not as well defined. Mutations in a broad spectrum of genes have been implicated, including those encoding sarcomere proteins, components of the cytoskeleton, and mitochondrial proteins (3,6). Through comprehensive sequence analyses of sarcomere genes in 334 DCM probands we identified 2 unrelated, multi-generational families with DCM that shared a variant in the gene encoding α-tropomyosin (TPM1). α-Tropomyosin is an α-helical, coiled-coil homodimeric thin filament protein which participates in the Ca2+-regulation of contraction and acto-myosin interaction. While TPM1 mutations are known causes of HCM, their pathogenicity in DCM has been inconclusive, as they have previously been identified only in individual patients (7).
We report genetic segregation of a TPM1 mutation and its in vitro functional consequences on sarcomere function. Together these data provide strong evidence that TPM1 mutations are pathogenic in DCM and extends knowledge about the pathogenesis and clinical course associated with sarcomere gene mutations.
METHODS
Genetic Analysis
Genomic DNA was isolated from blood using standard methods (6) in probands with DCM. Direct DNA sequence analysis of all coding regions and intron/exon boundaries was performed in 6 sarcomere genes: myosin binding protein-C (MYBPC3); β-myosin heavy chain (MYH7); cardiac troponin-T (TNNT2); cardiac troponin-I (TNNI3); α-tropomyosin (TPM1); α-actin (ACTC1).
To calculate the statistical likelihood that a genetic variant was associated with disease, lod scores were calculated using Vitesse (version 2.0) for Mac/PC, assuming 80% DCM penetrance at 30 years and an allele frequency=0.005. Haplotype analysis was performed to determine family relatedness by characterizing flanking single nucleotide polymorphisms (SNPs) in relevant families.
Actin-Tropomyosin-activated Myosin ATPase Assay
Bacterial expression constructs in pMW172 encoding human wildtype and D230N (mutant) Ala-Ser-α-tropomyosin were created by two-step PCR site-directed mutagenesis. The Ala-Ser N-terminal addition to α-tropomyosin compensates for the absence of N-terminal acetylation in the bacterially produced peptide (8). Mutant or wildtype Ala-Ser-α-tropomyosin were expressed with wildtype troponin I, troponin T, troponin C in BL21 (DES) pLysS E. coli cells and subsequently purified according to established protocols (9). Actin and myosin subfragment-1 (S-1) were obtained from rabbit skeletal muscle by standard procedures (10). Wildtype cardiac troponin complex was reconstituted from individual subunits using stepwise dialysis and gel filtration as previously described (11). Thin filaments were reconstituted at an actin, Ala-Ser-α-tropomyosin, and troponin ratio of 7:1:1 respectively.
Assays were carried out as previously described using 0.5 µM myosin S-1 and thin filaments reconstituted using 3.5 µM actin, 0.5 µM tropomyosin, and 0.5 µM troponin in 50 mM KCl, 5 mM PIPES, 3.87 mM MgCl2, 0.25 mM dithiothreitol, pH 7.0, at 37 °C. The free Ca2+ concentration was set using 1 mM EGTA and the appropriate concentration of CaCl2 as previously described (11). Phosphate release was determined colorimetrically by standard protocols.
Measurement of Ca2+ affinity using IAANS troponin
Thin filament Ca2+ affinity was measured using 2-[4'-(iodoacetamido)aniline]-naphthalene-6-sulfonate (IAANS) label bound to Cys35 of recombinant human troponin C (12). This acts as a reporter of Ca2+ binding to its low affinity site (site II) (12). Thin filaments were reconstituted with 21µM actin, 3µM Ala-Ser-α-tropomyosin and 3µM IAANS troponin. The final buffer concentration of EGTA was 1mM and the free Ca2+ concentration was set using the appropriate concentration of CaCl2 as previously described (13). Steady state fluorescence measurements (excitation 325nm, emission 455nm) were made using a RF-1501 spectrofluorometer (Shimadzu, Japan) at 22°C. The change in fluorescence (ΔF) was monitored as the Ca2+ was titrated with final ΔF values adjusted for the difference in assay mix volume following each incremental addition of 10mM CaCl2. The adjusted and normalized ΔF was plotted as a function of Ca2+ concentration and the resultant curves fitted to the Hill equation, a measure of the cooperativity of binding between Ca2+ and the thin filament.
Clinical Evaluation
Families with apparent familial DCM were recruited for genetic research. Family members were evaluated through history, physical examination, electrocardiography, and echocardiography. Echocardiographic dimensions were represented as Z-scores for subjects less than 16 years of age and LV end-diastolic internal diameter (LVIDD) Z-score greater than 2.0 was considered enlarged. The LV ejection fraction (LVEF) was calculated using the modified Simpson’s method. Individuals were considered affected with DCM if they had any of the following: clinical HF with LV enlargement or systolic dysfunction, asymptomatic LV enlargement (LV dimensions >reference normal dimensions in adults (13) or z-score >2 in children) or asymptomatic LV systolic dysfunction (LVEF <55%). Clinical information on deceased subjects was obtained from their relatives and medical records whenever possible. All subjects provided informed consent in accordance with the guidelines of the University of Chicago and Brigham and Women’s Hospital Human Subjects Committees.
Statistics
SAS, version 9.1 (SAS Institute, Cary, NC), was used to generate Kaplan-Meier curves to describe disease penetrance and event-free survival. Statistically significant differences in Ca2+ affinity and myosin ATPase activity were determined using an unpaired Students t test (InStat, GraphPad Software), with significance values defined as P<0.05.
RESULTS
Genetic Analysis
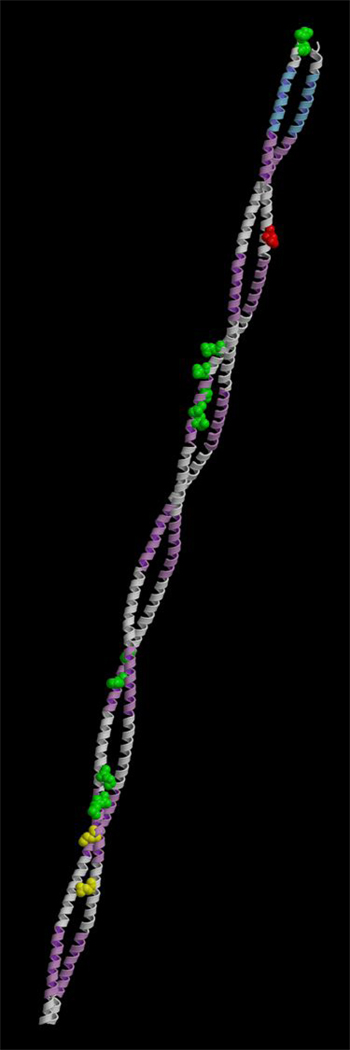
334 individuals with DCM underwent sarcomere gene sequence analysis. A G>A substitution at residue 688 in the gene encoding α-tropomyosin (TPM1) was identified in 2 probands with familial disease (supplementary figure). This variant is predicted to substitute a highly conserved, negatively charged aspartate residue (D, position 230; supplementary table) with a neutral asparginine (N) on the surface of tropomyosin (14) (Figure 1).
Figure 1. Molecular Model of α-Tropomyosin.
α-tropomyosin is an alpha-helical coiled-coil which interacts with actin (violet functional domains) and troponin T (blue). The locations of mutations associated with DCM (yellow) and HCM (green) are indicated. The D230N mutation is shown in red.
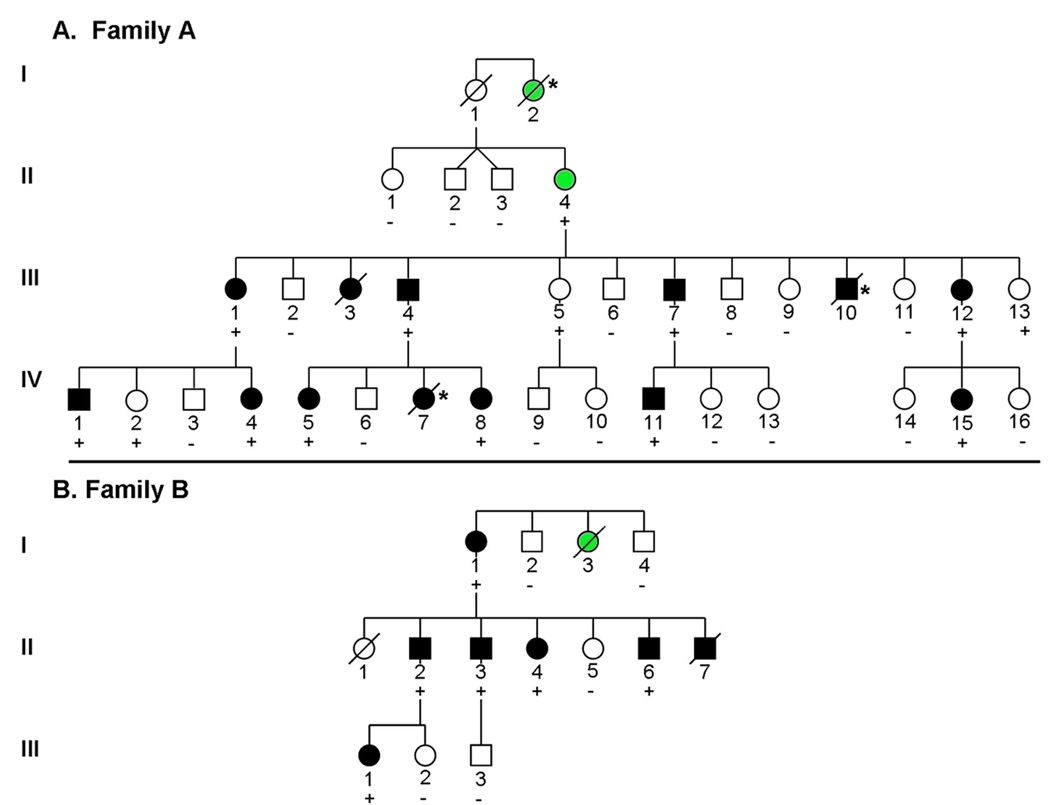
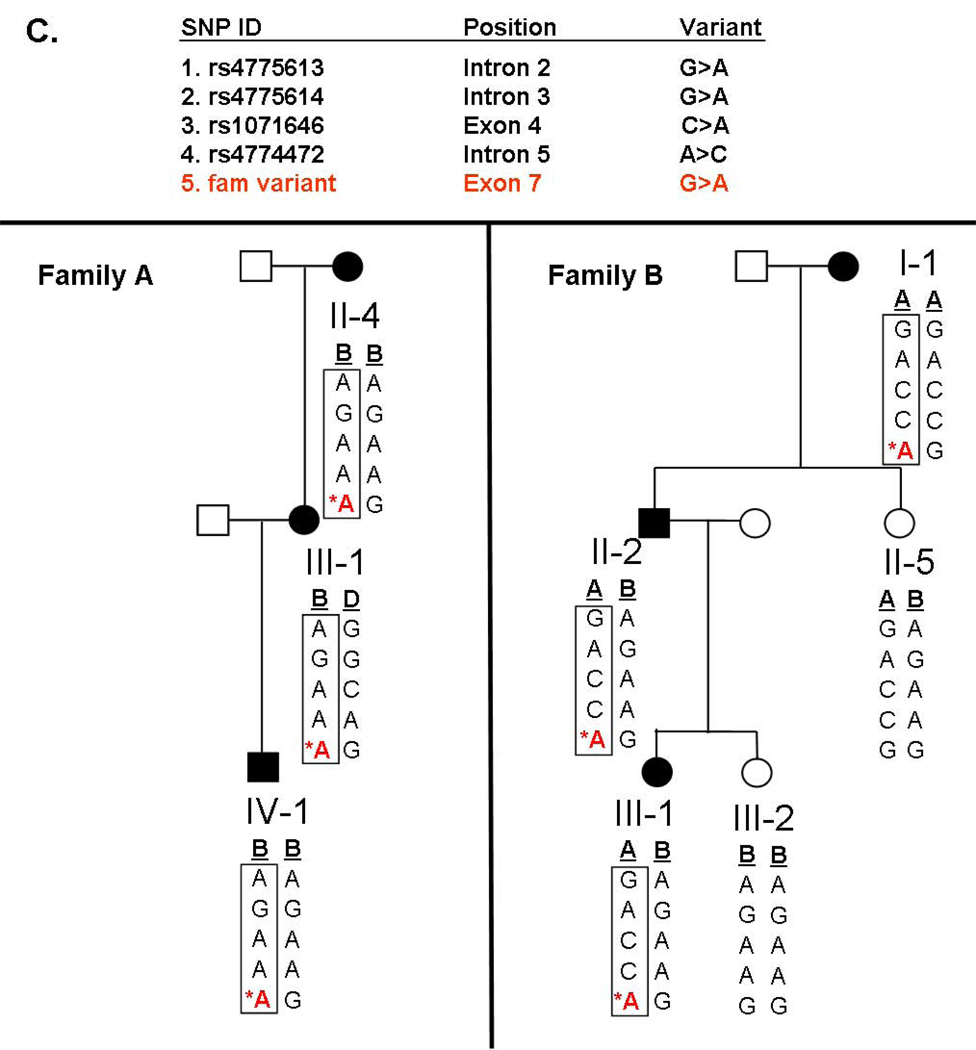
TPM1 D230N segregated with DCM in 2 large Caucasian families (Figure 2A and B). Haplotype analysis was performed by characterizing flanking SNPs; found to occur at frequencies of 24% and 52% respectively in the general population (www.hapmap.org). Disease haplotypes were different in the 2 families (Figure 2C), indicating that the families were unrelated and that TPM1 D230N arose independently in each family.
Figure 2. Pedigrees and Haplotype Analysis of Families with Autosomal Dominant DCM due to D230N Mutation in α-Tropomyosin.
A., B. Pedigrees are shown for 2 unrelated families with DCM. Squares indicate males, circles females, black symbols DCM, open symbols unaffected, green symbols uncertain clinical status, slashes deceased individuals. Genotype results are indicated by (+) = D230N present, (−) =mutation absent. * = sudden cardiac death.
C. Haplotype analysis indicates that the families are unrelated and that the D230N (688G>A) TPM1 mutation arose independently in these families. Several family members from each kindred were genotyped at four loci in the TPM1 gene (rs4775613, rs4775614, rs1071646, rs4774472), comprising 4 common haplotypes in Caucasians (A, B, C, D) as described by HapMap (www.hapmap.org). The D230N mutation is shown in red with *.
The TPM1 D230N variant was present in all affected individuals in Families A and B and absent from 21 of 25 unaffected adult family members as well as >1000 unrelated Caucasian control chromosomes. The combined calculated lod score was 5.22. Therefore, we concluded that TPM1 D230N caused DCM in these 2 unrelated families.
In vitro α-tropomyosin functional studies
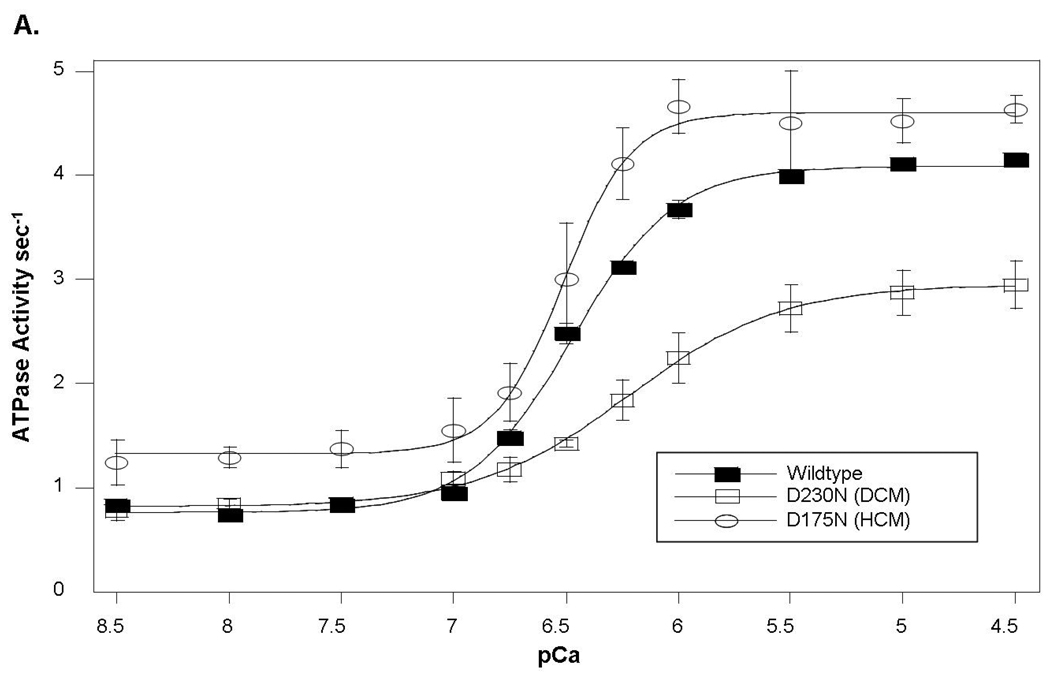
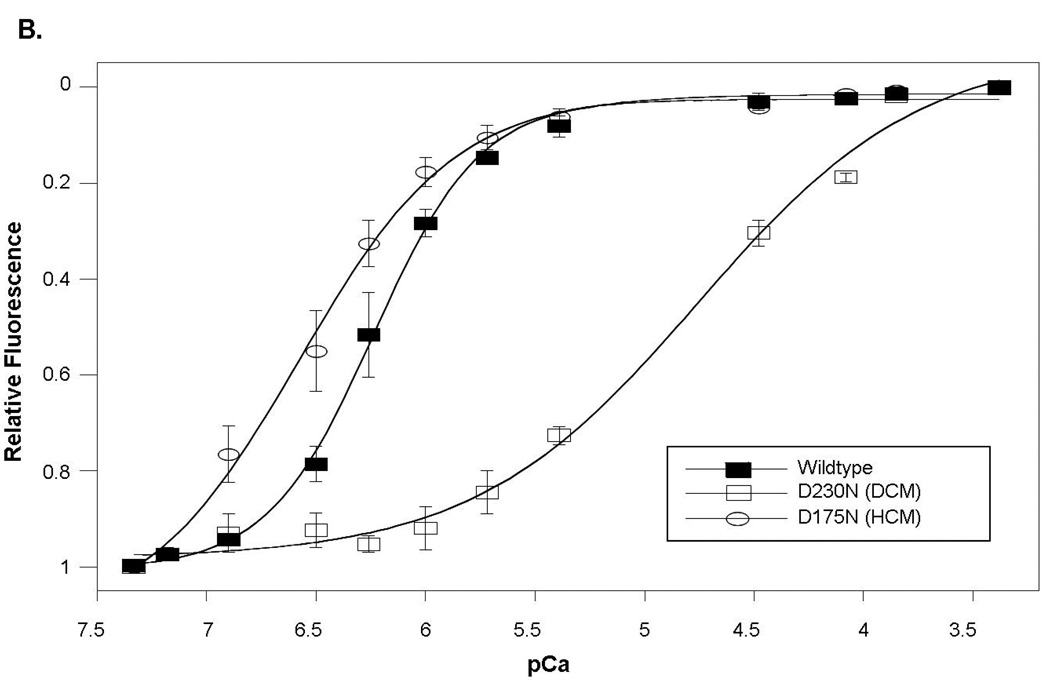
To investigate the functional impact of TPM1 D230N, Ca2+- regulation of actin-tropomyosin-activated myosin S-1 ATPase was evaluated as an in vitro correlate of contraction. Thin filaments reconstituted with wildtype Ala-Ser-α-tropomyosin had a maximally activated rate of ATP-turnover of 4.08±0.01 sec−1. The concentration of Ca2+ at half-maximal ATP-turnover (pCa50) was 6.47±0.02 (n=5) (Figure 3A). In contrast, thin filaments reconstituted using D230N Ala-Ser-α-tropomyosin produced a lower maximum ATPase turnover rate (2.95±0.11 sec−1, n=3, p<0.001) and reduced Ca2+-sensitivity (pCa50=6.20±0.04, n=3, p<0.05) (Figure 3A). Filaments reconstituted with a 1:1 mixture of wildtype:D230N Ala-Ser-α-tropomyosin (the expected ratio in situ) also showed a depressed maximally activated rate (3.26±0.21 sec−1, p<0.001) and Ca2+-sensitivity (pCa50=6.20±0.07, n=3, p<0.05). The maximally inhibited rate at pCa8.5 (0.75±0.02 sec−1) was not significantly affected by the presence of the mutation.
Figure 3. Functional Properties of the D230N Mutation in α-Tropomyosin.
In vitro functional analyses of the D230N mutant α-tropomyosin show that at any activating-level of [Ca2+], the D230N mutation resulted in diminished sarcomere function and calcium affinity.
A. Ca2+ sensitivity of thin filament regulation of actin-tropomyosin-activated myosin ATPase activity. Compared to wildtype, maximal ATPase turnover and pCa50 were significantly reduced (p<0.05) in the DCM mutant α-tropomyosin (D230N). In contrast, both parameters were significantly increased (p<0.05) in the HCM mutant protein (D175N).
B. Ca2+ binding to the thin filament causes a decrease in spectrofluorescent intensity. The Ca2+ affinity of reconstituted thin filaments containing D230N α-tropomyosin DCM-mutant was significantly reduced (p<0.001) compared to wildtype α-tropomyosin. In contrast the D175N HCM-mutant was associated with significantly increased Ca2+ affinity (p<0.05).
The functional impact of this TPM1 D230N mutation is strikingly different than a TPM1 mutation that causes HCM (D175N) (15). Thin filaments reconstituted with the HCM-mutant protein resulted in regulation with higher Ca2+-sensitivity (pCa50=6.58±0.04, n=3, p<0.05) and increased maximum ATP turnover (4.55±0.09 sec−1, n=3, p<0.001) relative to wildtype tropomyosin (Figure 3A).
To determine whether the observed decrease in Ca2+-sensitivity of contractility was due to an actual change in Ca2+ affinity rather than an apparent change caused by altered troponin-tropomyosin switching, thin filaments were reconstituted using actin, IAANS-labeled troponin C, and wildtype, D230N, or a 1:1 wildtype:DCM-mutant mixture tropomyosin. Wildtype thin filaments bound Ca2+ with a pCa50 of 6.24±0.02 with nH of 1.74±0.12 (Figure 3B). In contrast, there was a dramatic decrease in the affinity of filaments containing D230N (pCa50=4.76±0.09 p<0.001) and the Hill coefficient was also significantly reduced (nH=0.85±0.10 p<0.001) (Figure 3B), indicating decreased cooperativity of calcium binding. Experiments using the 1:1 wildtype:DCM-mutant mixture also showed reduced Ca2+ affinity (pCa50=5.54±0.08) and lower cooperativity (nH=1.04±0.23 p<0.001) (data not shown in figure). In contrast, thin filaments reconstituted with the HCM mutant protein were found to have significantly increased Ca2+ affinity compared to wildtype tropomyosin (pCa50=6.57±0.09 p<0.05), consistent with the increased Ca2+-sensitivity observed in the ATPase assay (Figure 3B).
Clinical Features of TPM1 D230N
The clinical manifestations and course of DCM in Families A and B are summarized in the table. No family members with TPM1 D230N had evidence of cardiac conduction disease, and with one exception, none had evidence of skeletal myopathy. Abnormal cardiac dimensions and contractile parameters were identified in 16 of 20 mutation carriers, indicating a penetrance of 80% for DCM in the 2 families. As seen in Figure 4, development of DCM occurred over a wide spectrum of ages. However, there were striking differences in clinical course based on age of presentation, with adverse outcomes occurring early in life (Figure 4).
Table 1.
Clinical Characteristics of Affected Family Members Based on Severity of Clinical Presentation
| Family | Pedigree | Age at Diagnosis*, years. |
Current Age or age at death† |
D230N | Sex | Clinical Features |
EF (%) |
LVIDD (cm)/(Z score)†† |
Comments | |
|---|---|---|---|---|---|---|---|---|---|---|
| NYHA > II | A | I-2 | - | 3† | NA | F | SCD | - | - | No symptoms prior to SCD. No autopsy performed. |
| A | III-3 | 13 | 13† | NA | F | NYHA IV HF Death |
- | - | Died of multi-system organ failure during heart failure hospitalization. |
|
| A | III-10 | 13 mos. | 13 mos.† |
NA | M | SCD | - | - | No symptoms prior to SCD. Autopsy notable for LV dilation. |
|
| A | IV-1 | 7 | 26 | + | M | NYHA IV Txp |
9 | 8.6 | Diagnosed during family screening and became progressively symptomatic culminating in cardiac transplantation at 18 years. |
|
| A | IV-5 | 5 mos. | 28 | + | F | NYHA IV With improvement |
27 | 4.2 (13.2) |
Severe heart failure as an infant with improvement after medical therapy (LVEF now 50%). |
|
| A | IV-7 | 6 mos. | 6 mos.† | NA | F | SCD | - | - | SCD at age 6 mos without prior symptoms. Autopsy reported LV dilation. |
|
| A | IV-8 | 5 mos. | 21 | + | F | NYHA IV With improvement |
29 | 4.5 (15.2) |
Severe heart failure as an infant with improvement after medical therapy (EF now 58%). |
|
| B | I-1 | 56 | 64 | + | F | VT | 25 | 5.9 | Presented with symptomatic VT and severe DCM which improved with medical therapy (EF now 40%). |
|
| B | II-6 | 20 | 24 | + | M | NYHA IV Txp |
<20 | - | Cardiac transplantation at age 20. | |
| B | II-7 | 9 | 12† | NA | M | NYHA IV HF Death |
- | - | Presented with DCM at 9 yrs and died of HF at 12 yrs. Also had congenital cataracts and skeletal weakness. |
|
| B | III-1 | 10 wks. | 8 | + | F | NYHA IV | 15 | 4.8 (15.6) |
Severe heart failure as an infant with improvement after medical therapy (LVEF now 46%). |
|
| NYHA ≤ II | A | II-4 | - | 71 | + | F | NYHA I | 49 | 4.6 | Asymptomatic. Mildly reduced LVEF identified after myocardial infarction at 71 years. |
| A | III-1 | 54 | 57 | + | F | NYHA I | 40 | 5.5 | Asymptomatic but LVEF declined during follow-up from 52% to 40%. |
|
| A | III-4 | 47 | 53 | + | M | NYHA II | 50 | 6.4 | Minimal exercise intolerance developed at age 48. |
|
| A | III-5 | - | 53 | + | F | NYHA I | 56 | 4.8 | Asymptomatic without evidence of LV enlargement or dysfunction. |
|
| A | III-7 | 45 | 50 | + | M | NYHA II | 50 | 5.7 | Minimal exercise intolerance developed at age 47. |
|
| A | III-12 | 40 | 44 | + | F | NYHA II | 50 | 5.6 | Palpitations and mild LV enlargement/systolic dysfunction without exercise limitation. |
|
| A | III-13 | - | 42 | + | F | NYHA I | 55 | 4.7 | Asymptomatic without evidence of LV enlargement or dysfunction. |
|
| A | IV-2 | - | 23 | + | F | NYHA I | 59 | 4.8 | Asymptomatic without evidence of LV enlargement or dysfunction. |
|
| A | IV-4 | 16 | 19 | + | F | NYHA I | 57 | 5.6 | Asymptomatic with mild left ventricular enlargement. |
|
| A | IV-11 | 22.0 | 24 | + | M | NYHA I | 40 | 5.5 | Asymptomatic but LVEF declined during follow-up from 50% to 40% |
|
| A | IV-15 | 8.0 | 11 | + | F | NYHA I | 51 | 4.2 | Asymptomatic with mildly reduced LVEF. |
|
| B | II-2 | 30 | 38 | + | M | NYHA I | 45 | 5.1 | Asymptomatic with mildly reduced LVEF. |
|
| B | II-3 | 32 | 36 | + | M | NYHA I | 35 | 6.4 | Asymptomatic but moderately reduced LVEF that declined during follow-up from 42% to 35% with concomitant 5 mm increase in LVIDD. |
|
| B | II-4 | 31 | 34 | + | F | NYHA I | 39 | 5.6 | Asymptomatic but LVEF declined after pregnancy from 48% to 39%, normalizing with medical therapy (55%). |
No age provided for subjects without evidence of DCM;
Age at Death;
LVIDD = LV end diastolic diameter, Z-score calculated for children less than 16 years, NA = Not available; LVEF = LV ejection fraction; SCD = sudden cardiac death, Txp = cardiac transplantation; VT = ventricular tachycardia; HF = Heart Failure
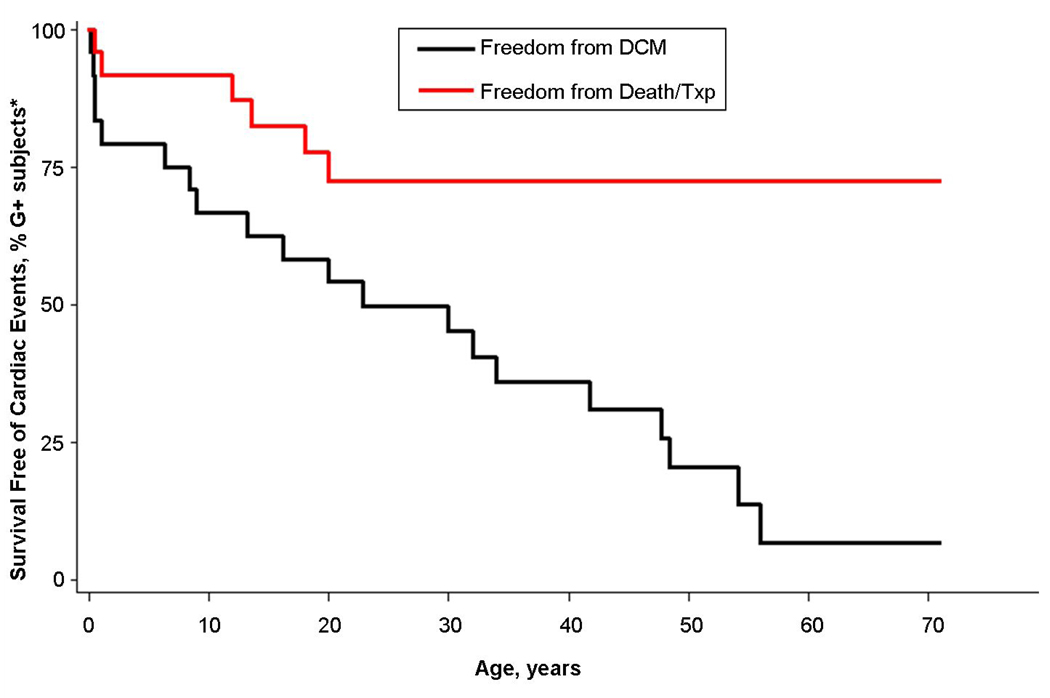
Figure 4. Clinical Outcomes in Carriers of the D230N α-Tropomyosin Mutation.
The presence of TPM1 D230N is associated with a high risk for developing DCM, but over a wide spectrum of ages, ranging from infancy to the 6th decade (Black curve). DCM includes both symptomatic and asymptomatic left ventricular systolic dilation and/or dysfunction. Severe clinical outcomes, cardiac death and transplantation, were confined to young family members and distinctly absent from those presenting in adulthood (red curve). * Individuals who suffered cardiac death prior to genotyping were assumed to be mutation carriers. G+ = mutation carriers.
Family A
Family A was notable for severe HF and sudden death in young children. Individuals I-2, III-10 and IV-7 died suddenly at ages 3 years, 13 months, and 5 months respectively, without prior evidence of heart disease. Two siblings, IV-5 and IV-8, presented with DCM and advanced HF at 5 months of age (LVEF 27% and 29%), and were given the presumptive diagnosis of myocarditis. In IV-5, adenovirus DNA was detected by polymerase chain reaction on endomyocardial biopsy. Both had substantial recovery of LV systolic function and resolution of symptoms after receiving standard medical care at the time of presentation (digoxin and furosemide). Notably IV-8, now age 21, participates in marathons.
Individuals III-3 and IV-1 developed end-stage HF refractory to medical therapy as teenagers. III-3 presented at age 13 with refractory HF, underwent mitral and tricuspid valve replacement for functional regurgitation and died weeks later of multisystem organ failure before genetic testing. IV-1 was asymptomatic when diagnosed with DCM at 7 years during family screening, but developed severe HF at age 17 and underwent transplant at 18 years of age. Histologic examination of his explanted heart revealed non-specific changes consistent with DCM, without myocyte disarray, marked fibrosis or inflammation to suggest either end stage HCM or myocarditis.
By contrast, 4 mutation carriers who presented in adulthood had mild clinical courses. Individuals III-4, III-7 and III-12 developed mild exercise intolerance in their 5th decade. Clinical studies revealed mild to moderate LV systolic dysfunction and enlargement and symptoms resolved with medical management. II-6 was well prior to an inferior myocardial infarction at 71 years. She was subsequently found to have mild LV systolic dysfunction (LVEF 49%) and remains asymptomatic.
Four mutation carriers (individuals III-1, IV-4, IV-11, IV-15; ages 54, 16, 22, and 8 years respectively), had asymptomatic LV systolic dysfunction and/or enlargement. Three adult mutation carriers (III-5, III-13, IV-2), were free of symptoms and had normal LV size and systolic function.
Family B
Young members of this family also presented with severe HF. Individual III-1 developed failure to thrive at 10 weeks of age and echocardiography revealed severe LV dilation (LVIDD Z-score 15.6) and systolic dysfunction (LVEF 15%). Transplantation was considered, but she had substantial recovery with basic medical therapy. By age 8 years, she was asymptomatic with only mild LV systolic dysfunction (LVIDD Z-score 1.3, LVEF 46%). At age 20, II-6 underwent cardiac transplantation shortly after presenting with refractory HF symptoms and severe LV systolic dysfunction (LVEF < 20%)
Individual II-7 died of HF at 12 years, prior to genetic testing. In addition to DCM, he had congenital cataracts and skeletal muscle weakness. Post mortem examination showed cardiomegaly with marked endocardial and interstitial fibrosis. Skeletal muscle analyses showed chronic myopathic changes but normal dystrophin staining, and no inflammation, evidence of storage disease, tissue-specific atrophy, nor fiber type grouping.
As in Family A, the clinical course of mutation carriers in Family B identified in adulthood was far less severe. I-1 had unexplained syncope at age 56 years. Cardiac studies revealed ventricular tachycardia and DCM (LVEF 25%) which improved with medical management. Three other adults (II-2, II-3, II-4, ages 38, 36 and 33 years, respectively) had asymptomatic LV systolic dysfunction. After an uncomplicated pregnancy, II-4 had mild further decline in LVEF from 48% to 39%, improving to 55% with institution of an ACE inhibitor.
DISCUSSION
We identified a D230N missense mutation in TPM1 as the cause of DCM in two unrelated, multigenerational families. Unlike previously reported TPM1 variants (E45K E40K) associated with DCM in isolated individuals (7), we demonstrate that dominant transmission of TPM1 D230N segregates with disease and deleteriously impacts in vitro assays of contractility, providing definitive evidence that TPM1 mutations cause DCM. Furthermore, these functional studies demonstrated that mutations in the same genes have different effects that may influence whether a phenotype of dilated or hypertrophic cardiomyopathy develops. The clinical profile of these families suggests a pattern for sarcomeric DCM in which clinical outcomes differ dramatically based on age of presentation.
Divergent functional consequences of sarcomere mutations may shape different patterns of cardiac remodeling
Sarcomere mutations were initially characterized as the cause of hypertrophic cardiomyopathy, but, as demonstrated in this study, can also cause DCM (6,16). The molecular mechanisms which determine whether a dilated or hypertrophic phenotype develops have not been clearly elucidated. Mutation location does not appear to be critical. As shown in Figure 1, mutations which cause HCM and DCM are closely interspersed and in the same functional domains in tropomyosin. However, the in vitro functional consequences of these mutations differ. Consistent with prior reports evaluating DCM mutations in thin filament proteins (12), our results demonstrate that D230N alters Ca2+-regulation in tropomyosin by reducing Ca2+-sensitivity, maximum activation, and Ca2+ affinity. Experimental models utilizing mechanically-loaded cardiac muscle fibers have also shown a Ca2+-desensitizing effect of thin filament mutations (17). Collectively, these DCM mutations are predicted to produce a muscle intrinsically capable of producing less force at any activating Ca2+ concentration. LV dilation may represent a compensatory mechanism to maintain stroke volume in the setting of reduced contractility due to decreased force production and/or calcium affinity associated with the TPM1 mutation. Activation of the neurohormonal axis may also occur, culminating in progressive cardiac failure.
Notably, these functional changes are opposite to those observed for sarcomere mutations that ultimately give rise to HCM in which calcium sensitivity is enhanced and predicted to increase contractility (12). Figure 3 illustrates this contrast. Maximum ATPase activity, Ca2+-sensitivity, and affinity are decreased with the D230N DCM TPM1 mutation but increased with the D175N HCM TPM1 mutation. Moreover, prior biophysical studies examining the thick filament demonstrated a similar pattern. Force generation and ATPase activity are decreased in myosin heavy chain mutations associated with DCM, but increased in HCM-associated mutations (18). Collectively these results suggest that fundamental differences in the functional consequences of sarcomere mutations may underlie the very disparate patterns of remodeling seen in DCM and HCM, despite an apparently common genetic etiology. We postulate that sarcomere mutations that compromise force generation may lead to a dilated phenotype, whereas a hypertrophic phenotype may arise from mutations that increase force generation.
A distinctive age-dependent phenotypic profile of sarcomere mutation DCM
Consistent with prior descriptions of DCM caused by sarcomere mutations, the clinical profiles of our families differ from both HCM caused by sarcomere mutations and DCM of other genetic etiologies, neither of which are characterized by severe disease in early childhood (19,20). As illustrated in Figure 4, marked age-dependent differences in outcomes were observed in our two families with TPM1 mutations. Presentation early in life, from infancy to adolescence, was not uncommon and was associated with severe, sometimes lethal outcomes, including SCD and refractory HF leading to death or transplantation. In contrast, a mild course was seen in relatives diagnosed as adults.
The pattern of severe childhood but mild adult-onset disease seen with the TPM1 D230N mutation is similar to that reported with two other sarcomere genes, MYH7 and TNNT2 (6,16,21). Affected members of these families also demonstrated marked LV dysfunction and HF very early in life, with either striking recovery or progression to death or transplantation, while those identified in adulthood generally had mild disease. This clinical profile differs meaningfully from that seen in other genetic causes of DCM, such as that caused by lamin A/C or phospholamban mutations where manifestations typically do not develop until adulthood, and are progressive (19,22) The mechanisms underlying the different clinical course in children and adults have not been defined, however, recognizing this pattern as a feature of sarcomere mutation DCM is important for appropriate family evaluation and intervention.
Moreover, although early presentation with DCM was typically severe, there was potential for remarkable improvement, as 3 of 5 affected infants with the TPM1 mutation had striking recovery of LV function. We speculate that the underlying TPM1 mutation may confer susceptibility to myocardial injury due to viral infections (e.g. myocarditis) or systemic illness that could account for the initially dramatic clinical presentations. Factors leading to the marked improvement in a subset of these children are less clear and unlikely to be related solely to receiving basic medical therapy. However, further elucidation of the mechanisms which allowed recovery in this primary genetic cardiomyopathy may provide important insights regarding the pathogenesis and management of more common secondary forms of DCM and heart failure.
Conclusions and Clinical Implications
We present robust evidence that mutations in TPM1 cause DCM and further characterization of disease pathogenesis and clinical course. In vitro functional studies provide insight into the phenotypic development of cardiomyopathy. Although sarcomere mutations are a common cause of both hypertrophic and dilated cardiomyopathy, the functional consequences appear markedly different in these two diseases, with opposite effects on calcium affinity, sensitivity, and contractility. These fundamental differences may play an important role in shaping the type of cardiac remodeling that arises.
The pattern emerging in these and other DCM families suggests that sarcomere mutations may result in a distinctive, age-dependent clinical profile. Early presentation is associated with severe, sometimes lethal disease, although with potential for substantial recovery. In contrast, presentation in adulthood is generally benign. These observations have several important clinical implications. In pediatric-onset HF, inherited causes of cardiomyopathy are often overlooked in favor of a presumptive diagnosis of myocarditis (23), particularly if there is dramatic clinical improvement. However, in these two families, childhood disease was caused by the TPM1 mutation. This highlights the importance of considering genetic etiologies in new onset DCM in children, and the need to consider at-risk family members. Furthermore, these findings have relevance for screening families with DCM. Current guidelines for family screening in HCM recommend that formal evaluation typically begins in adolescence (24). However, the severe disease manifestations seen in young children with DCM suggest that the identification of a sarcomere mutation should prompt aggressive screening for DCM in all first degree relatives, starting early in life.
Supplementary Material
DNA sequence traces from an individual with DCM show the single base pair change (arrow) underlying the D230N missense mutation. Wildtype sequence from a healthy relative without the familial mutation is shown for comparison.
ACKNOWLEDGEMENTS
The authors are grateful for the participation of these families.
FUNDING SOURCES
Support for these studies comes from grants from the NIH (NL, CYH, JGS, CES), HHMI (CES) the Leducq Foundation (JGS, CES), the Doris Duke Charitable Foundation (EM), and the British Heart Foundation (HW, PR, CR).
SELECTED ABBREVIATIONS
- DCM
Dilated Cardiomyopathy
- HCM
Hypertrophic Cardiomyopathy
- HF
Heart Failure
- LVEF
Left Ventricular Ejection Fraction
- SCD
Sudden Cardiac Death
- SNP
Single Nucleotide Polymorphism
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
The authors have no conflicts of interest to report.
REFERENCES
- 1.Boucek MM, Aurora P, Edwards LB, et al. Registry of the International Society for Heart and Lung Transplantation: Tenth Official Pediatric Heart Transplantation Report--2007. The Journal of Heart and Lung Transplantation. 2007;26:796–807. doi: 10.1016/j.healun.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Taylor DO, Edwards LB, Boucek MM, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult heart transplant report--2007. J Heart Lung Transplant. 2007;26:769–781. doi: 10.1016/j.healun.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Burkett EL, Hershberger RE. Clinical and genetic issues in familial dilated cardiomyopathy. J Am Coll Cardiol. 2005;45:969–981. doi: 10.1016/j.jacc.2004.11.066. [DOI] [PubMed] [Google Scholar]
- 4.Mahon NG, Murphy RT, MacRae CA, Caforio AL, Elliott PM, McKenna WJ. Echocardiographic evaluation in asymptomatic relatives of patients with dilated cardiomyopathy reveals preclinical disease. Ann Intern Med. 2005;143:108–115. doi: 10.7326/0003-4819-143-2-200507190-00009. [DOI] [PubMed] [Google Scholar]
- 5.Ho CY, Seidman CE. A Contemporary Approach to Hypertrophic Cardiomyopathy. Circulation. 2006;113:e858–e862. doi: 10.1161/CIRCULATIONAHA.105.591982. [DOI] [PubMed] [Google Scholar]
- 6.Kamisago M, Sharma SD, DePalma SR, et al. Mutations in Sarcomere Protein Genes as a Cause of Dilated Cardiomyopathy. N Engl J Med. 2000;343:1688–1696. doi: 10.1056/NEJM200012073432304. [DOI] [PubMed] [Google Scholar]
- 7.Olson TM, Kishimoto NY, Whitby FG, Michels VV. Mutations that Alter the Surface Charge of Alpha-tropomyosin are Associated with Dilated Cardiomyopathy. Journal of Molecular and Cellular Cardiology. 2001;33:723–732. doi: 10.1006/jmcc.2000.1339. [DOI] [PubMed] [Google Scholar]
- 8.Monteiro PB, Lataro RC, Ferro JA, Reinach Fde C. Functional alpha-tropomyosin produced in Escherichia coli. A dipeptide extension can substitute the amino-terminal acetyl group. J Biol Chem. 1994;269:10461–10466. [PubMed] [Google Scholar]
- 9.Redwood C, Lohmann K, Bing W, et al. Investigation of a truncated cardiac troponin T that causes familial hypertrophic cardiomyopathy: Ca(2+) regulatory properties of reconstituted thin filaments depend on the ratio of mutant to wildtype protein. Circ Res. 2000;86:1146–1152. doi: 10.1161/01.res.86.11.1146. [DOI] [PubMed] [Google Scholar]
- 10.Pardee JD, Spudich JA. Purification of muscle actin. Methods Enzymol. 1982;85(Pt B):164–181. doi: 10.1016/0076-6879(82)85020-9. [DOI] [PubMed] [Google Scholar]
- 11.Robinson P, Mirza M, Knott A, et al. Alterations in thin filament regulation induced by a human cardiac troponin T mutant that causes dilated cardiomyopathy are distinct from those induced by troponin T mutants that cause hypertrophic cardiomyopathy. J Biol Chem. 2002;277:40710–40716. doi: 10.1074/jbc.M203446200. [DOI] [PubMed] [Google Scholar]
- 12.Robinson P, Griffiths PJ, Watkins H, Redwood CS. Dilated and Hypertrophic Cardiomyopathy Mutations in Troponin and {alpha}-Tropomyosin Have Opposing Effects on the Calcium Affinity of Cardiac Thin Filaments. Circ Res. 2007;101:1266–1273. doi: 10.1161/CIRCRESAHA.107.156380. [DOI] [PubMed] [Google Scholar]
- 13.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Whitby FG, Phillips GN., Jr Crystal structure of tropomyosin at 7 Angstroms resolution. Proteins. 2000;38:49–59. [PubMed] [Google Scholar]
- 15.Thierfelder L, Watkins H, MacRae C, et al. Alpha-tropomyosin and cardiac troponin T mutations cause familial hypertrophic cardiomyopathy: a disease of the sarcomere. Cell. 1994;77:701–712. doi: 10.1016/0092-8674(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 16.Mogensen J, Murphy RT, Shaw T, et al. Severe disease expression of cardiac troponin C and T mutations in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2004;44:2033–2040. doi: 10.1016/j.jacc.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 17.Morimoto S, Lu QW, Harada K, et al. Ca(2+)-desensitizing effect of a deletion mutation Delta K210 in cardiac troponin T that causes familial dilated cardiomyopathy. Proc Natl Acad Sci U S A. 2002;99:913–918. doi: 10.1073/pnas.022628899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Debold EP, Schmitt JP, Patlak JB, et al. Hypertrophic and dilated cardiomyopathy mutations differentially affect the molecular force generation of mouse alpha-cardiac myosin in the laser trap assay. Am J Physiol Heart Circ Physiol. 2007;293:H284–H291. doi: 10.1152/ajpheart.00128.2007. [DOI] [PubMed] [Google Scholar]
- 19.Pasotti M, Klersy C, Pilotto A, et al. Long-term outcome and risk stratification in dilated cardiolaminopathies. J Am Coll Cardiol. 2008;52:1250–1260. doi: 10.1016/j.jacc.2008.06.044. [DOI] [PubMed] [Google Scholar]
- 20.Maron BJ, Casey SA, Poliac LC, Gohman TE, Almquist AK, Aeppli DM. Clinical Course of Hypertrophic Cardiomyopathy in a Regional United States Cohort. JAMA. 1999;281:650–655. doi: 10.1001/jama.281.7.650. [DOI] [PubMed] [Google Scholar]
- 21.Villard E, Duboscq-Bidot L, Charron P, et al. Mutation screening in dilated cardiomyopathy: prominent role of the beta myosin heavy chain gene. Eur Heart J. 2005;26:794–803. doi: 10.1093/eurheartj/ehi193. [DOI] [PubMed] [Google Scholar]
- 22.Schmitt JP, Kamisago M, Asahi M, et al. Dilated Cardiomyopathy and Heart Failure Caused by a Mutation in Phospholamban. Science. 2003;299:1410–1413. doi: 10.1126/science.1081578. [DOI] [PubMed] [Google Scholar]
- 23.Towbin JA, Lowe AM, Colan SD, et al. Incidence, Causes, and Outcomes of Dilated Cardiomyopathy in Children. JAMA. 2006;296:1867–1876. doi: 10.1001/jama.296.15.1867. [DOI] [PubMed] [Google Scholar]
- 24.Hershberger RE, Lindenfeld J, Mestroni L, Seidman CE, Taylor MR, Towbin JA. Genetic evaluation of cardiomyopathy--a Heart Failure Society of America practice guideline. J Card Fail. 2009;15:83–97. doi: 10.1016/j.cardfail.2009.01.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DNA sequence traces from an individual with DCM show the single base pair change (arrow) underlying the D230N missense mutation. Wildtype sequence from a healthy relative without the familial mutation is shown for comparison.