Abstract
Background
A cause of suboptimal accuracy in amperometric glucose sensors is the presence of a background current (current produced in the absence of glucose) that is not accounted for. We hypothesized that a mathematical correction for the estimated background current of a commercially available sensor would lead to greater accuracy compared to a situation in which we assumed the background current to be zero. We also tested whether increasing the frequency of sensor calibration would improve sensor accuracy.
Methods
This report includes analysis of 20 sensor datasets from seven human subjects with type 1 diabetes. Data were divided into a training set for algorithm development and a validation set on which the algorithm was tested. A range of potential background currents was tested.
Results
Use of the background current correction of 4 nA led to a substantial improvement in accuracy (improvement of absolute relative difference or absolute difference of 3.5–5.5 units). An increase in calibration frequency led to a modest accuracy improvement, with an optimum at every 4 h.
Conclusions
Compared to no correction, a correction for the estimated background current of a commercially available glucose sensor led to greater accuracy and better detection of hypoglycemia and hyperglycemia. The accuracy-optimizing scheme presented here can be implemented in real time.
Introduction
For the management of diabetes, none of the currently available continuous glucose monitors is approved in the United States for replacing standard capillary glucose monitoring.1 Although the reliability of the continuous monitors is generally considered to be good, one of the causes of suboptimal accuracy is the presence of a background current, i.e., a current produced in the absence of glucose. The source of a sensor background current in vivo is typically the presence of endogenous compounds, such as ascorbic acid and uric acid, and exogenous compounds, such as acetaminophen. These compounds are oxidized and thus generate current, in the presence of positive polarizing biases >500 mV at an indicating electrode. This oxidation can be minimized by a membrane that blocks the transfer of such compounds to the electrode2 or reducing the polarizing bias such as in the Abbott Diabetes Care (Alameda, CA) Navigator® sensor.3 Suboptimal accuracy of glucose sensors in the hypoglycemic range is of particular concern, as missed hypoglycemia can be dangerous and is common in persons with hypoglycemic unawareness. One method of determining the background current is to measure sensor output at two or more different glucose levels and find the y-intercept at zero glucose. Although theoretically attractive, this two-point method leads to poorer accuracy than a one-point method because of greater measurement uncertainty.4,5 It is not possible to directly measure background current at zero glucose in vivo. Nonetheless, in vivo background current can be estimated by extrapolation during analysis of sensor current data obtained over a wide range of blood glucose values.
In this study, we were interested in the background current that optimized sensor accuracy and detection of hypoglycemia/hyperglycemia. We were also interested in whether calibration frequency would affect accuracy and whether background current correction improves sensitivity for hypoglycemia.
Materials and Methods
General study design
In this report, a total of 20 sensor datasets were analyzed, obtained during a study investigating the use of a closed-loop system for automated insulin and glucagon delivery, the data for which have been submitted as a separate manuscript.6 These sets came from 10 experiments in seven human subjects with type 1 diabetes who were over 18 years of age, each of whom wore two sensors in each experiment. Patients who were pregnant or had cardiovascular, cerebrovascular, kidney disease, or liver disease or any other uncontrolled medical condition were excluded. Other exclusion criteria included oral or parenteral corticosteroid use, immunosuppressant use, visual or physical impairments that prevented the use of a continuous glucose monitoring device, allergy to insulin or glucagon, hypoglycemia unawareness, hospitalization within the past 2 years for severe hypoglycemia, serum insulin antibody titer that exceeded 100 μU/mL, or requirement of greater than 200 units of insulin/day. Exclusion criteria were chosen to ensure a relatively healthy, nonpregnant, type 1 diabetes population, free from end-organ damage. Participants were asked to avoid ingesting ascorbic acid and acetaminophen from 24 h before to the end of the study, in order to minimize confounding due to their potential to increase background current. Study duration was either 28 h or 9 h (mean study duration was 20 ± 2 h). Subjects were inpatients at a clinical research center at the Oregon Clinical and Translational Research Institute of the Oregon Health and Science University, Portland, OR, for the duration of the studies. The research protocol was approved by the Oregon Health and Science University Institutional Review Board, and all subjects provided written informed consent. Permission to carry out these studies was granted by the U.S. Food and Drug Administration (Investigational Device Exemption number G080130). Regarding the different study durations, there was a need to carry out studies of short duration (9 h) in addition to the longer studies, as requested by the Food and Drug Administration.
Age was 41.3 ± 5.8 years, and duration of diabetes was 15.3 ± 4.5 years. Hemoglobin A1c was 8.2 ± 0.5%, and body mass index was 30.1 ± 2.6 kg/m2. Two unmodified Medtronic Minimed® (Northridge, CA) Guardian® continuous glucose monitors were worn by each subject during each study for a total of 20 datasets. Insertion site was limited to the abdomen and flanks, using the proprietary insertion kit provided by the manufacturer. Data analysis was carried out using sensor interstitial current values (in nA) that were downloaded from this device. The glucose values calculated by the manufacturer and displayed during the studies were compared to values calculated by our algorithm in which a background current correction was utilized.
Arterialized venous blood glucose values were drawn every 10 min, and sensor data (interstitial sensor current and displayed values for sensor glucose) were obtained every 5 min. Blood was obtained from an intravenous catheter placed in a forearm vein, warmed with a heating pad to keep the venous blood arterialized.7 Blood glucose values were measured by the HemoCue® 201 blood glucose analyzer (HemoCue AB, Ängelholm, Sweden). Each venous glucose value used as a reference value for sensor accuracy assessment was the mean of two separate blood samples obtained at each time point. The coefficient of variation for the duplicates was 1.9%, indicating a high degree of precision of the device.
The data from this study were divided into two datasets: a training set, used for parameter determination and algorithm development, and a validation set, used to assess algorithm accuracy. For the training set, four 28-h and four 9-h study datasets were obtained from early studies. The validation studies comprised eight 28-h and four 9-h study datasets obtained later.
The training dataset comprised a total of 1,015 sensor–reference pairs, while the validation data set comprised 1,776 pairs. During assessment of the effect of calibration frequency on accuracy, if the rate of change of estimated glucose was more than 1.0 mg/dL/min over the previous 10 min, calibration was postponed until the slope fell below this criterion. Sensor values obtained at the time of calibration were not used in accuracy assessment because they are by definition perfectly accurate in one-point calibrations such as these. All other reference–sensor pairs were included in the analysis. No data were omitted, and the training and validation datasets were mutually exclusive (data from any particular subject were part of one or the other, but not both). In this study, two methods were tested in an effort to improve sensor accuracy: (1) measurement and correction for background current and (2) varying the frequency of sensor calibration.
Mathematical analysis
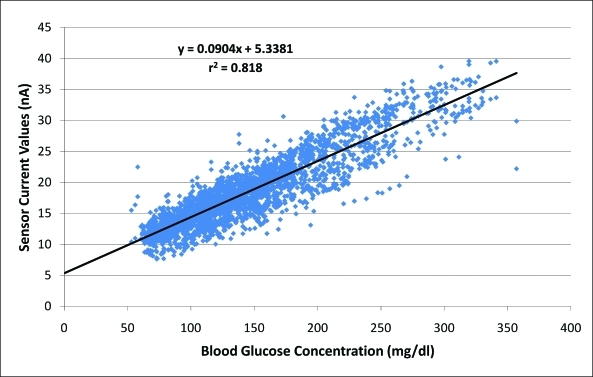
In order to estimate the background current of the sensors, the interstitial current values from all the human studies were plotted against the arterialized venous glucose values from 10 min before. Using linear regression (r = 0.9, r2 = 0.818) (Fig. 1), a y-intercept value of 5.3 was obtained. The average of individual sensor background estimates in all datasets was also measured and was also found to be 5.3 nA, whereas the average of the individual estimates in the training and validation datasets were 5.2 and 5.4 nA, respectively. Based on these findings, background current values between 0 nA and 6.5 nA at 0.5-nA intervals were tested to optimize accuracy (an upper limit of 6.5 nA was chosen as the lowest measured sensor currents were ∼7 nA). Correction for background current was then performed by its subtraction from interstitial current values, in order to arrive at a glucose current, as follows:
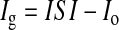 |
FIG. 1.
In order to estimate background current in amperometric glucose sensors in persons with type 1 diabetes, interstitial current value for each data pair is shown as a function of arterialized venous glucose concentration from 10 minutes prior. As the best-fit line is extrapolated to a glucose level of zero, the background current is seen to be approximately 5–6 nA. Color images available online at www.liebertonline.com/dia.
where Ig is the true glucose current, ISI is the interstitial current from the sensor download, and Io is the calculated background current. The background current corrections were carried out both during calibration and during evaluation.
After Ig values were obtained, a one-point calibration was performed by comparing Ig to blood glucose values (using values from 10 min before so as to compensate for sensor time delay). Calibration provided a sensitivity value (in nA/mg/dL) that allowed glucose currents to be converted to sensed glucose values:
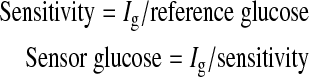 |
An initial calibration was performed in all cases at 10 min, followed by repeat calibration at one of three frequencies: every 12 h (Q12 h), every 8 h (Q8 h), or every 4 h (Q4 h). To avoid confounding from differing calibration times, when the manufacturer's displayed value during the study was compared to the use of a background current correction, the sensor datasets were calibrated at exactly the same times as the sensors in the actual human study (average of every 8.6 h).
The time delay value that resulted in greatest accuracy improvement was determined by Poincaré-type plotting of reference and sensor glucose values delayed by 0 through 30 min at 5-min intervals, as described previously,8 after normalizing reference and sensor glucose values with a logarithmic transformation.9
The primary metric of sensor accuracy was the mean absolute relative difference (MARD) for glucose values >75 mg/dL and the mean absolute difference (MAD) for values ≤75 mg/dL, as recommended by a consensus statement regarding sensor accuracy measurement10:
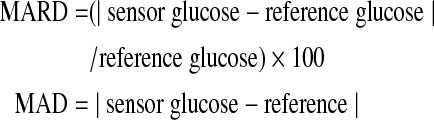 |
As with calibration, sensor values were compared to reference values from 10 min earlier, in order to reduce error due to estimated sensor lag. Averaged results were expressed as a composite of MARD and MAD values, each unweighted, so that values for each were considered equally.
For the sensor–reference pairs of the validation dataset, we also investigated the effect of differing background currents (from 0 to 6.5 nA, in increments of 0.5 nA) on the ability of the sensors to correctly discern hypoglycemia, defined as reference glucose ≤70 mg/dL. In this analysis, we measured sensitivity and specificity and defined a receiver-operator characteristic curve: (1–specificity) versus sensitivity.
Statistical analysis
For all parametric data, Pearson's t test was used for statistical analysis, paired or unpaired as appropriate. For all nonparametric data, a Wilcoxon rank test was used, paired or unpaired as appropriate. A P value of ≤0.05 determined statistical significance. Microsoft (Redmond, WA) Excel and MATLAB (MathWorks, Natick, MA) software were used for data analysis.
Results
Algorithm development using training dataset
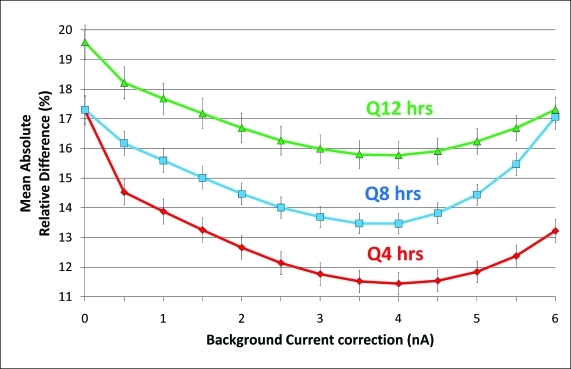
Using the training set, different background currents and calibration frequencies were tested to assess their effect on glucose sensor error (MARD/MAD). This analysis showed that MARD/MAD values were minimized at a background current of 4 nA and were significantly improved compared to a background current of 0 nA (Fig. 2). In addition, these metrics showed somewhat better accuracy as the frequency of calibration increased. The lowest error was obtained by using a background current of 4 nA and a calibration frequency of Q4 h (Figs. 2 and 3). Accuracy tended to be greater as calibration frequency increased: a calibration frequency of Q4 h was significantly better than either Q8 h or Q12 h at the same background current. Similarly, median ARD values also showed the greatest accuracy at a background current of 4 nA and at a calibration frequency of Q4 h (data not shown).
FIG. 2.
Comparison of error (mean absolute relative difference [MARD] and mean absolute difference [MAD] values ± SEM) by background current correction and calibration frequency for the training dataset in subjects with type 1 diabetes. Usage of the appropriate background current correction significantly minimized MARD/MAD at all calibration frequencies. At the appropriate background current correction of 4 nA, a calibration frequency of Q4 h improved MARD/MAD over both Q12 h and Q8 h. Color images available online at www.liebertonline.com/dia.
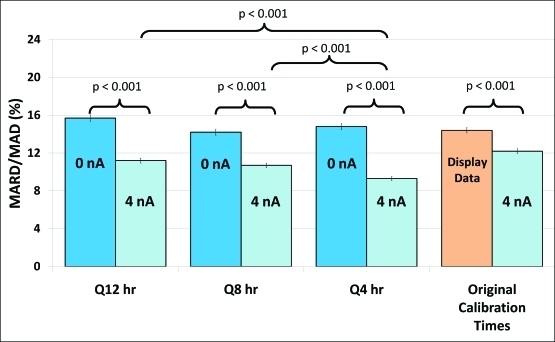
FIG. 3.
Comparison of error (mean absolute relative difference [MARD] and mean absolute difference [MAD] values ± SEM) by background current correction and calibration frequency for the validation dataset in subjects with type 1 diabetes. Usage of a 4 nA background current correction significantly improved MARD/MAD at all calibration frequencies. At this background current correction, a calibration frequency of Q4 h improved MARD/MAD over both Q12 h and Q8 h. Color images available online at www.liebertonline.com/dia.
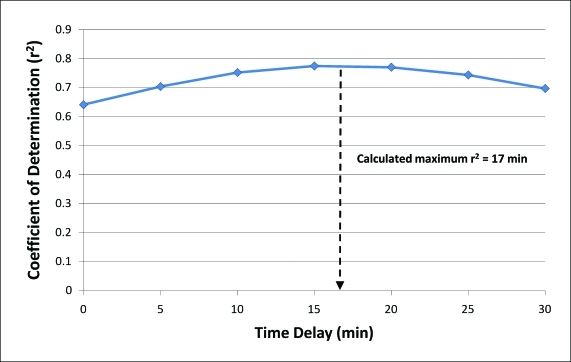
In terms of time delay analysis, maximum improvement in the coefficient of determination (r2) between sensor and reference pairs was noted at an average time delay of 17 minutes for all data pairs (Fig. 4). Initially, we also calculated a time delay estimation based on rate of change of prior sensor glucose as a means to optimize, in real time, the sensor readings. However, after comparison with results from the Poincaré-type plots, it was determined that this simple method would be susceptible to errors from noisy signals (without using any filtering methods) and from calibration errors (which could erroneously alter sensor rate of change). The result of this time delay correction was therefore not used in the final analysis.
FIG. 4.
Assessment of correlation between reference and sensor glucose pairing with application of sequential time delays at 5-min intervals from 0 to 30 min. Maximum r2 value (used as the measure of goodness of fit) was found at 15 min. Using the curve-fitting equation, the calculated delay leading to the greatest accuracy was 17 min. Color images available online at www.liebertonline.com/dia.
In summary, training data analysis showed that the optimal parameters for sensor accuracy were obtained with the use of a background current of 4 nA and a calibration frequency of Q4 h. The accuracy during the more convenient calibration frequency of Q8 h was nearly as good as during Q4 h.
Algorithm testing using validation dataset
The parameters obtained with the training set were then applied to the validation set. Figure 3 shows comparisons of MARD/MAD values at 0 nA and 4 nA for calibration frequencies of Q12 h, Q8 h, and Q4 h. A background current correction of 4 nA led to substantial and statistically significant improvements in MARD/MAD values at all calibration frequencies (P < 0.001 for all comparisons). The greatest improvement in accuracy was found with the background current correction, which tended to reduce MARD/MAD by 3.5–5.5 units. Using a background current of 4 nA, an increase in calibration frequency from Q8 h to Q4 h led to an additional modest improvement of about 1.4 MARD units.
We also performed an accuracy comparison between glucose values displayed by the manufacturer versus usage of background current correction in the validation dataset. Calibrations for the 4 nA datasets were performed at exactly the same times as the original calibration times (mean calibration interval, 8.6 h). Figure 3 shows that a background current correction of 4 nA leads to a lower MARD/MAD compared to the originally displayed data (12.0% vs. 14.2%, P < 0.001).
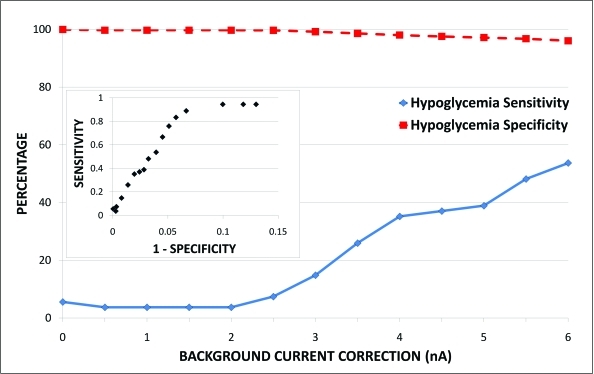
The effects of background current correction on hypoglycemia detection using the validation dataset are shown in Figure 5. With increasing background current correction, especially between 3 and 5 nA, sensitivity for hypoglycemia improved markedly with only a minimal loss of specificity. Analysis of sensitivity and specificity for detection of hyperglycemia revealed similar results (data not shown).
FIG. 5.
Evaluation of hypoglycemia detection at different background current correction values. Sensitivity for hypoglycemia improves considerably between 3 and 5 nA without significant loss of specificity. (Inset) Receiver-operator characteristic curve for hypoglycemia detection at differing background current corrections. Color images available online at www.liebertonline.com/dia.
Discussion
In this study, we used 20 continuous glucose monitoring sensor datasets obtained in subjects with type 1 diabetes to examine the effect of different background currents and calibration frequency on sensor accuracy. We found that, compared to a background current of zero, the use of a non-zero background current yielded a clear benefit in sensor accuracy. This benefit was seen both with analysis of post hoc processed data in which differing background currents were compared and also when directly compared to the manufacturer's displayed data. The magnitude of this improvement was quite substantial, approximately 3.5–5.5 MARD units, depending on calibration frequency. There are several sources of background current in amperometric glucose sensors. One is the presence of certain non-glucose compounds that diffuse from the interstitial fluid to the indicating electrode where they are oxidized, leading to a current that masquerades as a glucose current. A number of materials have been used in the construction of sensors for the purpose of eliminating such interferents, including Nafion® (DuPont, Wilmington, DE),11 other charged membranes,12 cellulose, sulfone-type polymers,2 phenylenediamine,13,14 or other electron shuttle mediators.15,16 Many of these mediators allow electrode polarization at a voltage low enough to minimize interferent oxidation. Despite the multitude of methods that have been proposed, it is often difficult to completely eliminate the effect of these oxidizable compounds. One explanation is that, in glucose oxidase-based sensors, common interferents such as ascorbic acid, uric acid, and acetaminophen, unlike glucose, do not require enzymatic conversion to be measured at an anode. For this reason, even small amounts of these compounds are efficiently oxidized, thus creating a relatively large signal. Another potentially problematic issue is the fact that slight alterations in chemical handling or manufacturing procedures can impact the effectiveness of layers designed to prevent passage of interfering compounds. It should be noted that interference is much less of an issue for sensors that are not positively polarized, such as those that exploit the reduction of oxygen as opposed to the oxidation of an electron shuttle or peroxide.17
Compared to a background current of zero, we found that a widely used commercial amperometric glucose sensor functioned with greater accuracy with a background current of 4 nA. This background current was accounted for during the process of calibration and during calculation of glucose in unknowns. This finding suggests that near-perfect physical exclusion of interfering compounds by the sensor may not be necessary. Instead, if it can be measured and accounted for in human pilot studies, mathematical correction of background current may be sufficient to optimize accuracy. Of course, if the magnitude of the background current is quite large compared to the glucose current (not the case in this study), the effect of measurement noise might prevent such a correction from being successful. Background current correction between 3 and 5 nA was also found to optimize detection of hypoglycemia. This range led to much better hypoglycemia detection sensitivity than in a lower range of background current, with only a minimal loss of specificity and positive predictive value.
We also addressed the benefit of calibration frequency, knowing that recalibration helps to compensate for sensor drift. When a very accurate blood glucose reference measuring instrument is used, the process of recalibration returns the sensor glucose value to the blood glucose value (unless prior sensed glucose or blood glucose data are also taken into account, which was not the case here). Compared to the adjustment for background current, the benefit of increasing calibration frequency in our study was modest. In the validation dataset, there was no significant benefit of increasing frequency from every 12 h to every 8 h; only when the frequency was increased further to every 4 h, with background current correction, was a benefit appreciated, and, even then, the benefit was small. Such a finding suggests that the sensors under study were relatively stable and did not undergo rapid drift. In addition to the process of recalibration, there are other methods that can improve the fidelity of the sensor signal by mathematically filtering out noise. Clarke and Kovatchev18 and the Rensselaer group19 have proposed such methods, which might help to minimize the need for frequent recalibration. Review of the Medtronic patent suggests variable background current correction, based upon the magnitude of the sensitivity.20
There is a general agreement that the signals from subcutaneously implanted glucose sensors lag behind blood values, although the estimates of the lag magnitude are dependent on the particular device. The causes of lag include the physiologic duration required for glucose to reach interstitial fluid from blood (likely very short21), the time required for glucose to pass through sensor membranes, and delays that result from data smoothing and filtering. Kovatchev et al.8 discussed in some detail each of these sources of lag and found that the lag of the Abbott Navigator sensor, typically in the 12–17 min range, was greater during rising glucose than during falling glucose. Kamath et al.22 found a somewhat shorter lag duration in their recent study of the DexCom™ (San Diego, CA) Seven® Plus sensor. A recent report from Medtronic found that software processing and filtering can lead to an apparent lag, although such a delay cannot be considered a true physical lag.23 Medtronic uses a “Wiener filter” for compensation of sensor lag,24 although details are not provided. The autoregression techniques used by the Reifman/Gani group appear to be a promising method of minimizing sensor inaccuracy and possibly lag.25 Reported Medtronic lag times include 4.0 ± 1.0 min during glucose changes,26 up to 18 min during insulin-induced hypoglycemia,27 and up to 12 min during normalization of glucose after administration of exogenous insulin.28
An interesting result of our study was that error from an incorrect background current can sometimes have the appearance of a time lag. We found that before the background current correction was carried out, inspection of the time series curve (reference and sensor glucose vs. time) often suggested a marked lag, especially under certain conditions such as rising glucose after meals. In contrast, after this correction was carried out, the time series curves often suggested a much smaller lag. Consider the situation during which background current is underestimated during calibration at a low or normal glucose level. As glucose rises, the calibration error will lead to an apparent lag of sensor glucose behind the reference blood glucose. However, if the data are analyzed with the correct background current, the sensor glucose values rise appropriately with the meal, and the apparent “lag” is reduced. For this reason, before estimation of sensor lag, it is important to measure glucose with the correct background current.
In order to determine the background current in this study, we used data collected at many glucose levels and extrapolated the current versus glucose data line to the y-intercept at zero glucose. Of course, calibrating at many glucose levels for patients in the hospital or outpatient setting is impractical as a means of determining background current, and we acknowledge that, in some ways, the research setting with a highly accurate glucose monitor gives us an unfair advantage when we compare sensor accuracy to values typically obtained during unsupervised use by outpatients. Ideally, the background current for each sensor would be measured in human studies in order to optimize the algorithm used to transform electrical current into an estimated glucose value. We believe that determination of background current is more accurately determined during such in vivo testing rather than during in vitro testing. Humans possess oxidizable interfering compounds that enter into the interstitial fluid (perhaps even many that have not yet been identified), and for this reason it may well be impossible to determine background current from in vitro studies.
In conclusion, we found that with an amperometric glucose sensor commonly used in clinical settings, accuracy was markedly improved by the use of a correction for background current. This correction can be carried out in human studies by obtaining in vivo preliminary training sensor data accompanied by corresponding reference glucose values. When tested in a validation study, the background current correction was robust. We also found improvements in accuracy, although lesser in magnitude, with an increase in calibration frequency. In some cases, sensor lag can appear falsely high when background current is underestimated. The accuracy-optimizing scheme presented here can be implemented in real time.
Acknowledgments
This work was supported by grants from the Juvenile Diabetes Research Foundation (Artificial Pancreas Consortium), Good Samaritan Foundation (Portland, OR), and the National Institutes of Health (NIH) (grant T32 DK 007674). We thank Jillian Hansen for her technical support and Dr. Kevin Yuen for his assistance. We also thank the staff and research subjects who carried out these studies at the Oregon Clinical and Translational Research Institute, which is supported by grant UL1 RR024140 from the National Center for Research Resources, a component of the NIH, and NIH Roadmap for Medical Research.
Author Disclosure Statement
No competing financial interests exist for any author.
References
- 1.U.S. Food and Drug Administration. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm074293.htm. [Jul 16;2010 ]. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm074293.htm
- 2.Ward WK. Jansen LB. Anderson E. Reach G. Klein JC. Wilson GS. A new amperometric glucose microsensor: in vitro and short-term in vivo evaluation. Biosens Bioelectron. 2002;17:181–189. doi: 10.1016/s0956-5663(01)00268-8. [DOI] [PubMed] [Google Scholar]
- 3.Feldman B. Brazg R. Schwartz S. Weinstein R. A continuous glucose sensor based on wired enzyme technology—results from a 3-day trial in patients with type 1 diabetes. Diabetes Technol Ther. 2003;5:769–779. doi: 10.1089/152091503322526978. [DOI] [PubMed] [Google Scholar]
- 4.Choleau C. Klein JC. Reach G. Aussedat B. Demaria-Pesce V. Wilson GS. Gifford R. Ward WK. Calibration of a subcutaneous amperometric glucose sensor implanted for 7 days in diabetic patients. Part 2. Superiority of the one-point calibration method. Biosens Bioelectron. 2002;17:647–654. doi: 10.1016/s0956-5663(01)00304-9. [DOI] [PubMed] [Google Scholar]
- 5.Choleau C. Klein JC. Reach G. Aussedat B. Demaria-Pesce V. Wilson GS. Gifford R. Ward WK. Calibration of a subcutaneous amperometric glucose sensor. Part 1. Effect of measurement uncertainties on the determination of sensor sensitivity and background current. Biosens Bioelectron. 2002;17:641–646. doi: 10.1016/s0956-5663(01)00306-2. [DOI] [PubMed] [Google Scholar]
- 6.Castle JR. Engle JM. El Youssef J. Massoud RG. Yuen KC. Kagan R. Ward WK. Novel use of glucagon in a closed-loop system for prevention of hypoglycemia in type 1 diabetes. Diabetes Care. 2010;33:1282–1287. doi: 10.2337/dc09-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu D. Moberg E. Kollind M. Lins PE. Adamson U. Macdonald IA. Arterial, arterialized venous, venous and capillary blood glucose measurements in normal man during hyperinsulinaemic euglycaemia and hypoglycaemia. Diabetologia. 1992;35:287–290. doi: 10.1007/BF00400932. [DOI] [PubMed] [Google Scholar]
- 8.Kovatchev BP. Shields D. Breton M. Graphical and numerical evaluation of continuous glucose sensing time lag. Diabetes Technol Ther. 2009;11:139–143. doi: 10.1089/dia.2008.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kovatchev BP. Cox DJ. Gonder-Frederick LA. Clarke W. Symmetrization of the blood glucose measurement scale and its applications. Diabetes Care. 1997;20:1655–1658. doi: 10.2337/diacare.20.11.1655. [DOI] [PubMed] [Google Scholar]
- 10.CLSI document POCT05-A. Wayne, PA: Clinical and Laboratory Standards Institute; 2008. Performance Metrics for Continuous Interstitial Glucose Monitoring; Approved Guideline. [Google Scholar]
- 11.Yu B. Moussy Y. Moussy F. Coil-type implantable glucose biosensor with excess enzyme loading. Front Biosci. 2005;10:512–520. doi: 10.2741/1547. [DOI] [PubMed] [Google Scholar]
- 12.Vaidya R. Wilkins E. Use of charged membranes to control interference by body chemicals in a glucose biosensor. Med Eng Phys. 1994;16:416–421. doi: 10.1016/1350-4533(90)90008-v. [DOI] [PubMed] [Google Scholar]
- 13.Osborne PG. Hashimoto M. Chemical polymerization of m-phenylenediamine, in the presence of glucose oxidase, produces an enzyme-retaining electrooxidisable polymer used to produce a biosensor for amperometric detection of glucose from brain dialysate. Analyst. 2004;129:759–765. doi: 10.1039/b403035d. [DOI] [PubMed] [Google Scholar]
- 14.Jing-Juan X. Hong-Yuan C. Amperometric glucose sensor based on coimmobilization of glucose oxidase and poly(p-phenylenediamine) at a platinum microdisk electrode. Anal Biochem. 2000;280:221–226. doi: 10.1006/abio.2000.4502. [DOI] [PubMed] [Google Scholar]
- 15.Mao F. Mano N. Heller A. Long tethers binding redox centers to polymer backbones enhance electron transport in enzyme “Wiring” hydrogels. J Am Chem Soc. 2003;125:4951–4957. doi: 10.1021/ja029510e. [DOI] [PubMed] [Google Scholar]
- 16.Jeong RA. Hwang JY. Joo S. Chung TD. Park S. Kang SK. Lee WY. Kim HC. In vivo calibration of the subcutaneous amperometric glucose sensors using a non-enzyme electrode. Biosens Bioelectron. 2003;19:313–331. doi: 10.1016/s0956-5663(03)00219-7. [DOI] [PubMed] [Google Scholar]
- 17.Makale MT. Chen PC. Gough DA. Variants of the tissue-sensor array window chamber. Am J Physiol Heart Circ Physiol. 2005;289:H57–H65. doi: 10.1152/ajpheart.01001.2004. [DOI] [PubMed] [Google Scholar]
- 18.Clarke WL. Kovatchev B. Continuous glucose sensors: continuing questions about clinical accuracy. J Diabetes Sci Technol. 2007;1:669–675. doi: 10.1177/193229680700100510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuure-Kinsey M. Palerm CC. Bequette BW. A dual-rate Kalman filter for continuous glucose monitoring. Conf Proc IEEE Eng Med Biol Soc. 2006;1:63–66. doi: 10.1109/IEMBS.2006.260057. [DOI] [PubMed] [Google Scholar]
- 20.Mastrototaro J, inventor; Gross T, inventor; Shin J, inventor. Medtronic, Inc., assignee: A method of calibrating glucose monitor data. U.S. Patent 6424847. Jul 23, 2002.
- 21.Wientjes KJ. Schoonen AJ. Determination of time delay between blood and interstitial adipose tissue glucose concentration change by microdialysis in healthy volunteers. Int J Artif Organs. 2001;24:884–889. [PubMed] [Google Scholar]
- 22.Kamath A. Mahalingam A. Brauker J. Analysis of time lags and other sources of error of the DexCom SEVEN continuous glucose monitor. Diabetes Technol Ther. 2009;11:689–695. doi: 10.1089/dia.2009.0060. [DOI] [PubMed] [Google Scholar]
- 23.Keenan DB. Mastrototaro JJ. Voskanyan G. Steil GM. Delays in minimally invasive continuous glucose monitoring devices: a review of current technology. J Diabetes Sci Technol. 2009;3:1207–1214. doi: 10.1177/193229680900300528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bequette BW. Continuous glucose monitoring: real-time algorithms for calibration, filtering, and alarms. J Diabetes Sci Technol. 2010;4:404–418. doi: 10.1177/193229681000400222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gani A. Gribok AV. Lu Y. Ward WK. Vigersky RA. Reifman J. Universal glucose models for predicting subcutaneous glucose concentration in humans. IEEE Trans Inf Technol Biomed. 2010;14:157–165. doi: 10.1109/TITB.2009.2034141. [DOI] [PubMed] [Google Scholar]
- 26.Steil GM. Rebrin K. Mastrototaro J. Bernaba B. Saad MF. Determination of plasma glucose during rapid glucose excursions with a subcutaneous glucose sensor. Diabetes Technol Ther. 2003;5:27–31. doi: 10.1089/152091503763816436. [DOI] [PubMed] [Google Scholar]
- 27.Steil GM. Rebrin K. Hariri F. Jinagonda S. Tadros S. Darwin C. Saad MF. Interstitial fluid glucose dynamics during insulin-induced hypoglycaemia. Diabetologia. 2005;48:1833–1840. doi: 10.1007/s00125-005-1852-x. [DOI] [PubMed] [Google Scholar]
- 28.Rebrin K. Steil GM. van Antwerp WP. Mastrototaro JJ. Subcutaneous glucose predicts plasma glucose independent of insulin: implications for continuous monitoring. Am J Physiol. 1999;277:E561–E571. doi: 10.1152/ajpendo.1999.277.3.E561. [DOI] [PubMed] [Google Scholar]